Published online Jun 18, 2025. doi: 10.5500/wjt.v15.i2.98616
Revised: November 15, 2024
Accepted: December 11, 2024
Published online: June 18, 2025
Processing time: 235 Days and 10.4 Hours
Pediatric living-donor liver transplantation is considered a safe alternative for the treatment of children with end-stage liver disease. Experienced tertiary centers and specialized medical staff are necessary to ensure compatible long-term sur
To report the results and the 10-year learning curve of a pediatric living-donor liver transplantation program.
We conducted a retrospective cohort study of pediatric recipients from 2013 to 2023. Post-transplant outcomes and patient survival rates were compared between two 5-year periods of the program.
A total of 25 and 48 patients underwent transplantation in the first (2013-2017) and second period (2018-2023), respectively. Portal vein and hepatic artery thrombosis occurred in 11 (15.1%) and seven (9.6%) patients, respectively. Biliary complications were observed in 39 of 73 patients (53.4%). A lower warm ischemia time was observed in the second period compared to the first (32.6 ± 8.6 minutes vs 38.4 ± 9.8 minutes, P = 0.018, respectively). Patient survival rates at 1 and 5 years were 84% in the first period and 91.7% in the second period, with no significant difference (P = 0.32).
The reported indications and outcomes align with the current literature. Our findings provide crucial evidence regarding the feasibility of establishing a living donor program with consistent results over time.
Core Tip: We present findings related to the implementation of a pediatric living-donor liver transplant center in Brazil, reporting the results of the first 10 years of this program. Complication rates and post-transplant survival are comparable to those obtained at high-volume centers worldwide.
- Citation: Farina Junior MA, Utz-Melere M, da Silva CS, Nader LS, Trein CS, Lucchese AM, Machry M, Mariano R, Ferreira CT, Kalil AN, Feier FH. Ten years of a pediatric living donor liver transplantation program in Brazil. World J Transplant 2025; 15(2): 98616
- URL: https://www.wjgnet.com/2220-3230/full/v15/i2/98616.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i2.98616
Liver transplantation is crucial for the treatment of children with end-stage liver disease and liver metabolic disorders. The indications for pediatric liver transplantation have expanded, but the number of donors cannot keep up with the growing demand[1]. Due to varying donor availability between countries, living-donor liver transplantation (LDLT) has become increasingly favored for pediatric patients. In Brazil, living donors account for 66% of liver transplants in children[2], with reported survival rates of approximately 90% for this transplant modality[3].
The assessment of a patient for liver transplantation conducted by a multidisciplinary team aims to review and evaluate indications for transplantation, treatment alternatives, contraindications to transplantation, active infections, immunological status, dysfunction of other organs (such as cardiac, pulmonary, and renal), nutritional and metabolic status, and the psychosocial condition of the child and parents[4]. The primary focus of caring for children with end-stage liver disease, once centered around securing a liver transplant, has now shifted towards long-term follow-up, preventing complications related to immunosuppression, and promoting normal growth as much as possible[4]. This shift has resulted in long-term survival rates exceeding 85% in most high-volume pediatric transplant centers[5].
The long-term outcomes of living-donor grafts are slightly superior to those of deceased donor grafts despite the risks to the healthy donors[3]. The decision to opt for a living-donor graft is complex and influenced by factors such as reci
Establishing a pediatric LDLT program is a complex undertaking that requires collaboration among multiple experts in pediatric hepatology, liver surgery, pediatric surgery, interventional radiology, and anesthesiology. Surgical data cor
This study was a retrospective, single-center cohort analysis of patients who underwent LDLT at Santa Casa de Porto Alegre, Brazil, a tertiary care center. Data were collected from the database of children who underwent LDLT at our institution between 2013 and 2023. By analyzing a decade's worth of data, this approach enables the identification of factors influencing transplant success and the overall health of recipients over time.
We analyzed various demographic and perioperative variables, including sex, weight, age at the time of LDLT, Pediatric End-Stage Liver Disease (PELD) score, Model for End-Stage Liver Disease score, graft-to-recipient weight ratio (GRWR), and ischemia times. The selection process ensured a representative sample of the pediatric population affected by end-stage liver disease, enabling a comprehensive analysis of outcomes. Additionally, postoperative outcomes were assessed, focusing on the incidence of complications (e.g., vascular and biliary), duration of hospitalization and intensive care unit stay, and occurrence of rejection. Ethical approval for this study was obtained from the hospital ethics committee.
ABO blood group compatibility dictated recipient and donor selection, ensuring that only compatible blood transplantations were performed throughout the study period. Grafts were implanted orthotopically using the “piggyback tech
Immunosuppression predominantly involved tacrolimus (FK 506, Prograf; Astellas Pharma, Tokyo, Japan) and steroids, with efforts made to discontinue steroids by the third month post-transplantation, if feasible. Basiliximab (Simulect; Novartis, Basel, Switzerland) was used to induce immunosuppression in most recipients and became routinely used after 2018.
Doppler ultrasound was performed routinely on the first postoperative day and subsequently as deemed necessary based on clinical evaluation. Any vascular or biliary abnormalities detected on Doppler ultrasound were confirmed using contrast imaging such as computed tomography or magnetic resonance imaging. All patients received routine anticoagulation, including intravenous prophylactic heparin, when the international normalized ratio was less than 2.5, the platelet count exceeded 50000, and the partial thromboplastin time was normal. Once oral intake was possible, patients were switched to aspirin at a dose of 5 mg/kg/day.
Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) monitoring involves serial assessment of the viral load using polymerase chain reaction[7]. Either oral valganciclovir or intravenous ganciclovir was administered for CMV prophy
Statistical analysis involved summarizing continuous variables using means ± SD or medians [(interquartile ranges) IQR], depending on the data distribution characteristics. Group comparisons for continuous variables were conducted using Student’s t-test or the Mann-Whitney test if normality assumptions were not met. Categorical variables were presented as numbers and percentages, with differences between groups assessed using the χ2 or Fisher’s exact test as appropriate.
Survival analyses for both patients and grafts were performed using the Kaplan-Meier product-limit estimator, and comparisons between patient subgroups were evaluated using the two-sided log-rank test. Multivariate Cox regression analysis was performed to adjust for potential confounding factors. Variables identified with a significance level of P < 0.1 in univariate analysis, along with those deemed clinically significant, were included in the regression model. Statistical analyses were performed using the SPSS version 21.0 (IBM Corp., Armonk, NY, United States). A significance level of alpha (α) = 0.05 was used for all statistical analysis. The study was reviewed by our expert biostatistician Alvaro Rosler.
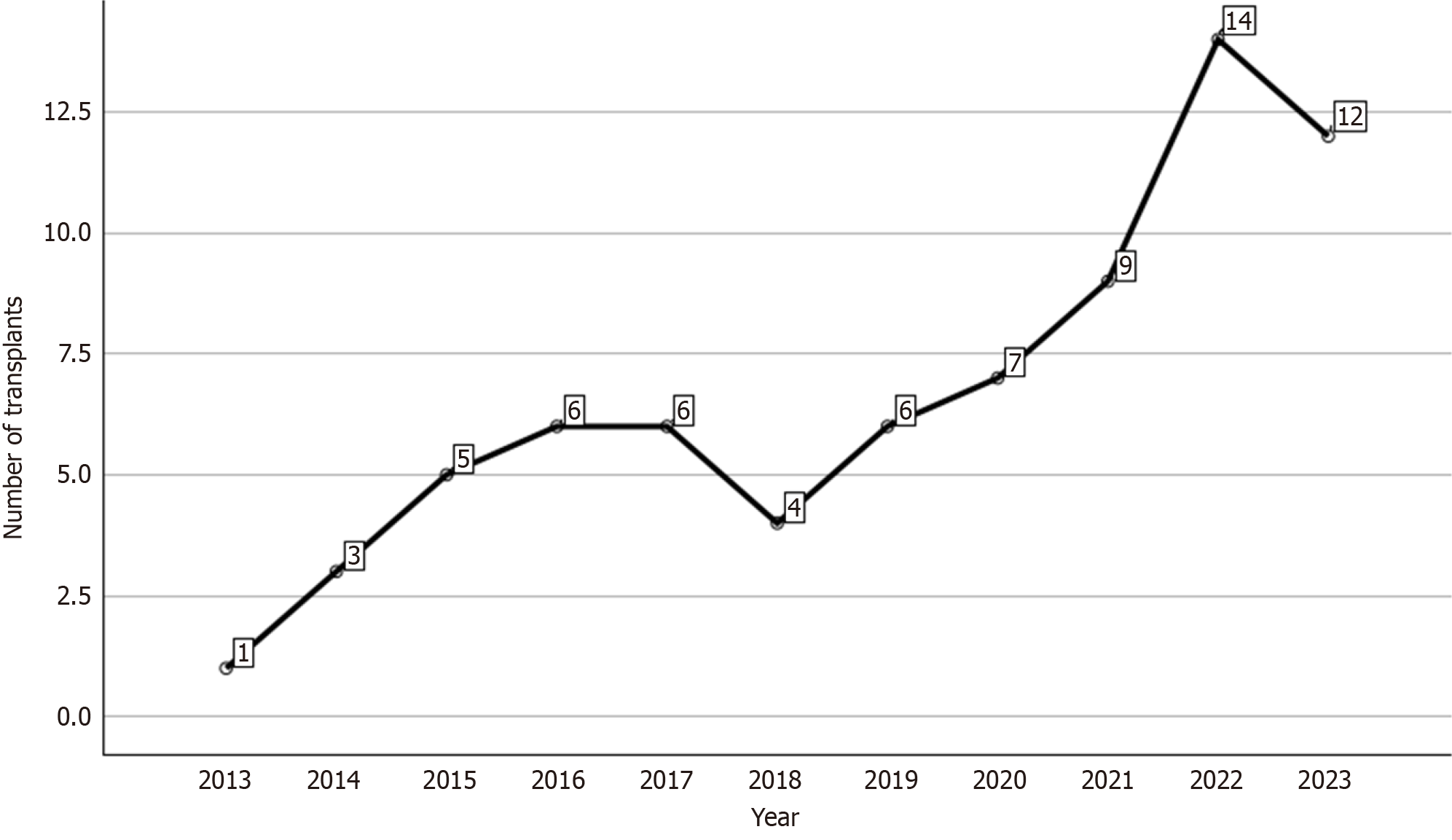
Between December 2013 and December 2023, 73 pediatric LDLT procedures were performed at our hospital. During this period, our team has progressively increased the number of such procedures. In Brazil, the majority of liver transplants are funded by the government, and patients are typically referred to the nearest transplant center in their region. There are only a few active pediatric liver transplantation centers in Brazil. The first two centers with the highest volumes are located in the state of São Paulo, followed by two other centers, including ours, situated in the state of Rio Grande do Sul. Figure 1 illustrates the yearly distribution of LDLT procedures performed at our center.
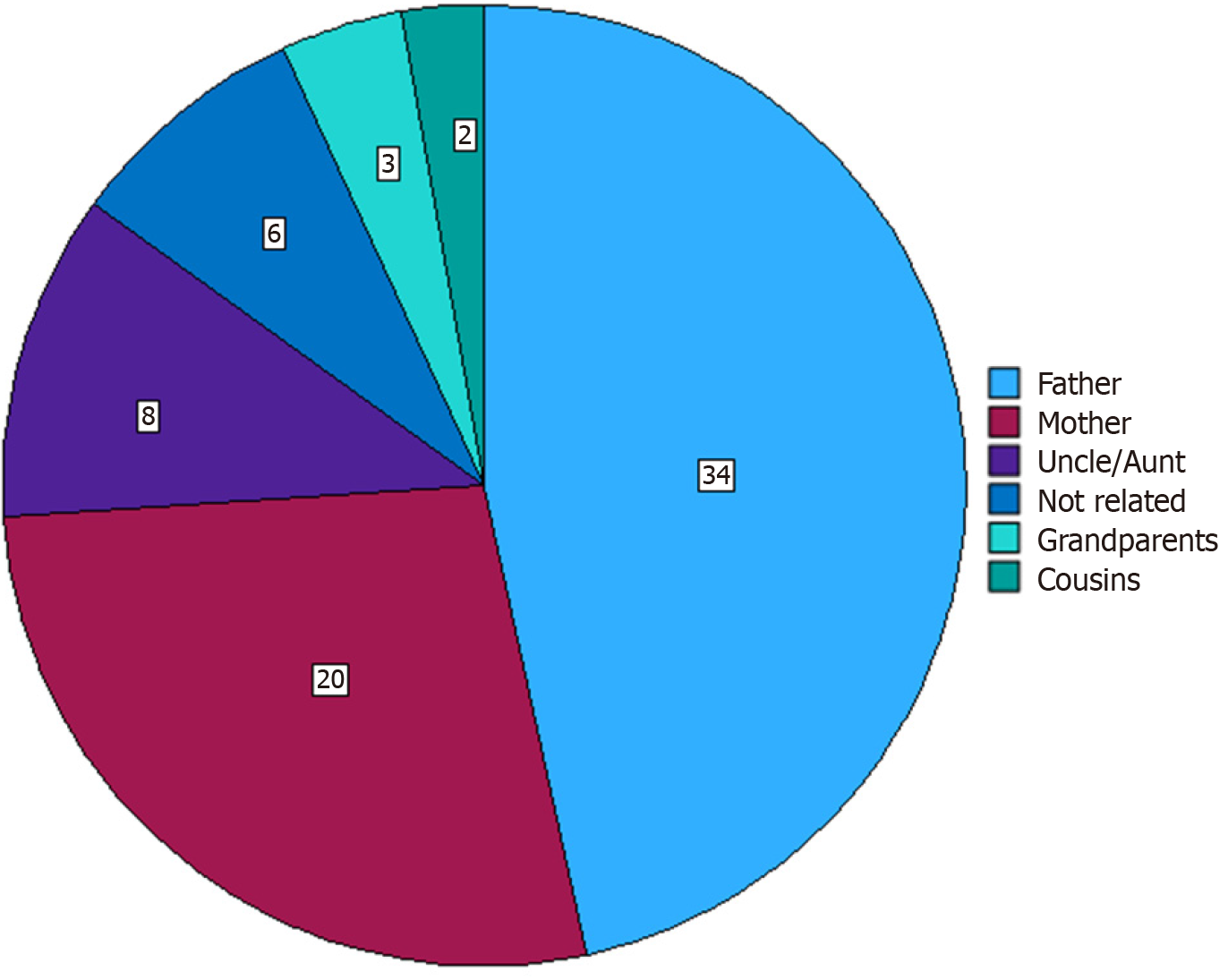
Of the total number of patients, 39 (53.4%) were male. The median weight of the recipients at the time of transplant was 12.13 kg (IQR: 3.4-56 kg). The median Z-scores for height and age and weight and age were -1.17 (IQR: -5.38 to 1.86) and -1.65 (IQR: -6.27 to 2.48), respectively. The median PELD score was 17 (IQR: 1-46). Among all donors, 67 (91.8%) were related to the recipients, with fathers being the most common donors, as shown in Figure 2.
A total of 41 (56.2%) patients underwent transplantation for biliary atresia (BA), and 18 had previously undergone Kasai portoenterostomy. The patient and graft survival rates were both 89% at 1 and 5 years. Two patients required retransplantation, one due to small-for-size syndrome and the other due to primary non-function, but both eventually died from liver failure. Overall, eight patients died during follow-up, with five deaths occurring within 30 days post-transplantation. Causes of death included liver failure (two patients), intravascular coagulation disorder (two patients), sepsis (one patient), pulmonary edema (one patient), strangulated diaphragmatic hernia (one patient), and acute viral bronchiolitis (one patient).
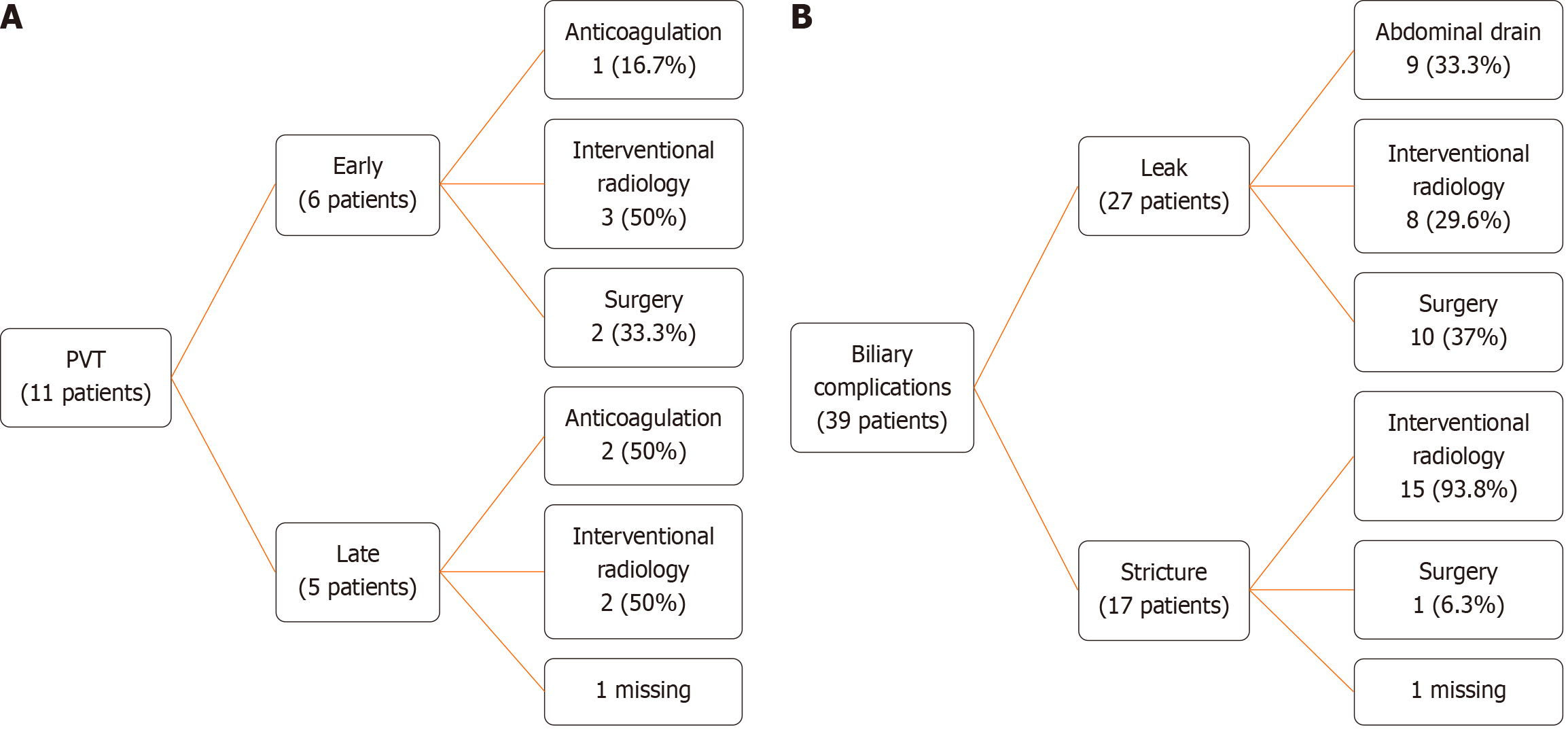
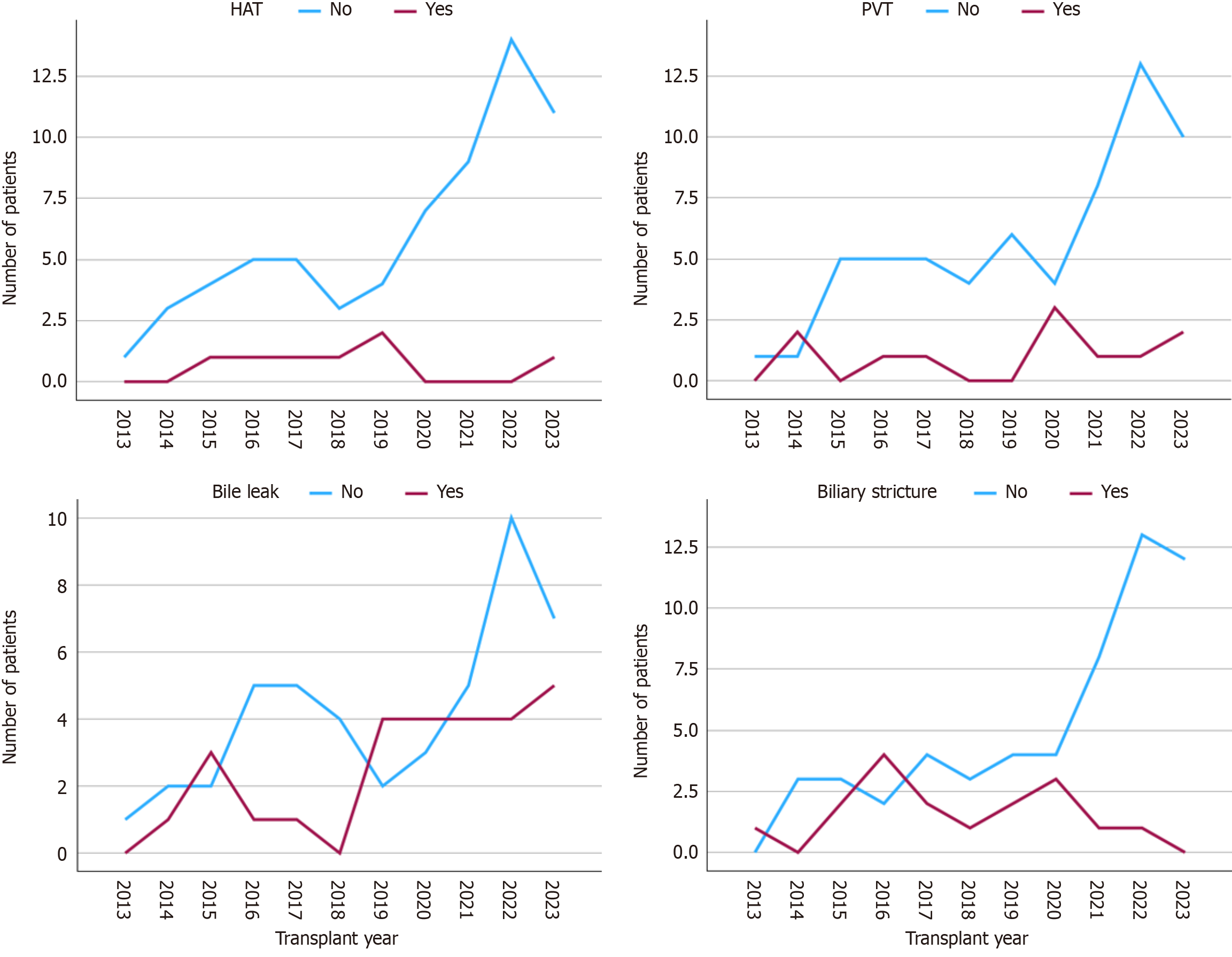
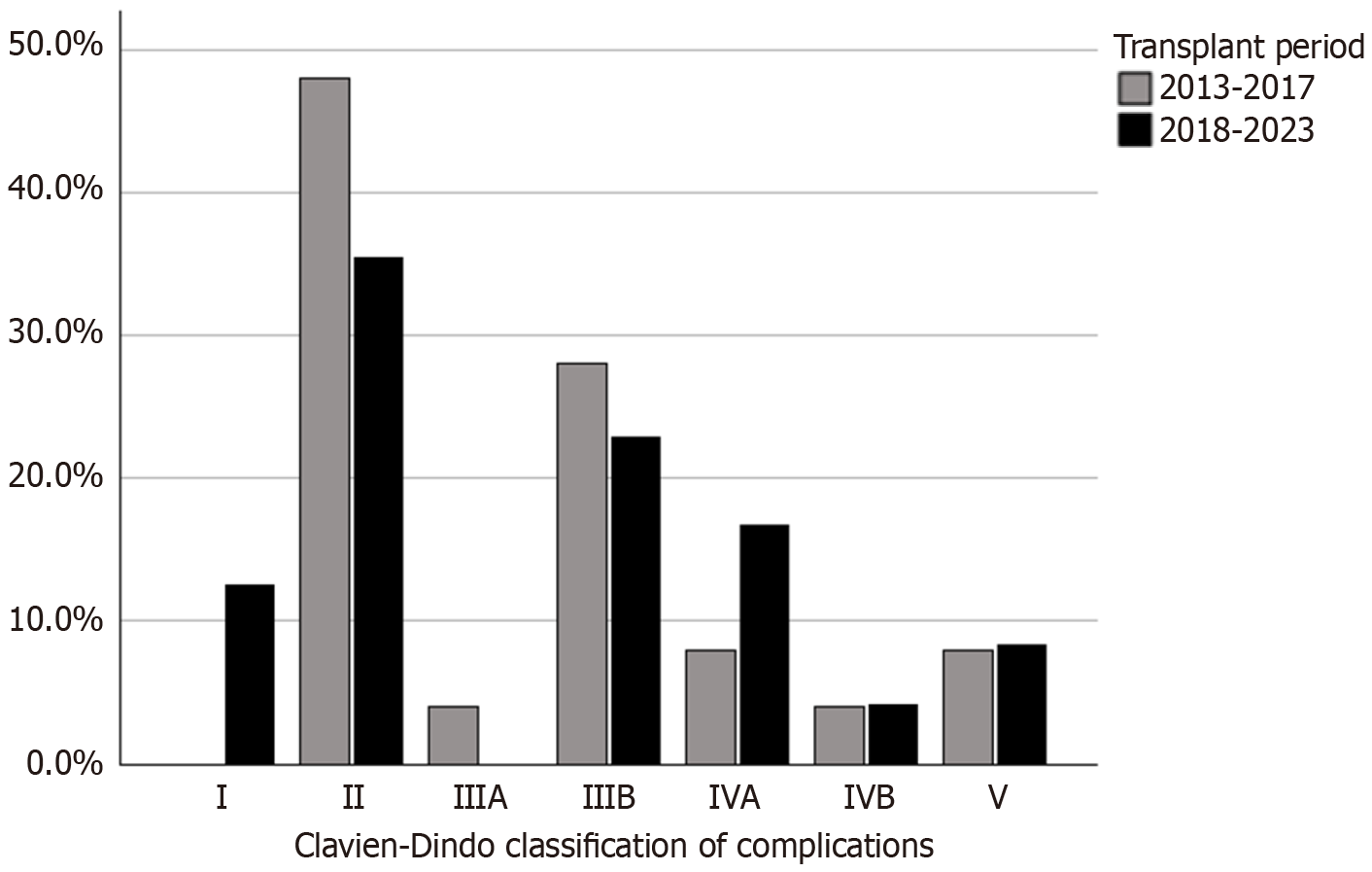
Three patients developed post-transplantation lymphoproliferative disorder (PTLD). Postoperative complications were classified using the Clavien-Dindo system[8]. Class I (8.2%), II (39.7%), III (26.1%), IV (17.8%), and V (8.2%). Portal vein thrombosis occurred in 11 (15.1%) patients, with six presenting early and five presenting late (Figure 3A). Eleven patients were diagnosed with portal vein stenosis, but only four (36.4%) required angioplasty; the others were monitored through routine examinations and imaging without clinical repercussions. Hepatic artery thrombosis (HAT) occurred in seven (9.6%) patients, but no graft was lost due to HAT.
Biliary complications were observed in 39 of 73 patients (53.4%): 27/73 (37%) had bile leaks and 17/73 (23.3%) had biliary strictures, with 5/73 patients (6.8%) experiencing both (Figure 3B). A total of 22/73 grafts (30%) presented with double biliary ducts. On such occasions, both ducts were anastomosed, either in a single or double jejunal orifice. A plasty was not performed in our cohort.
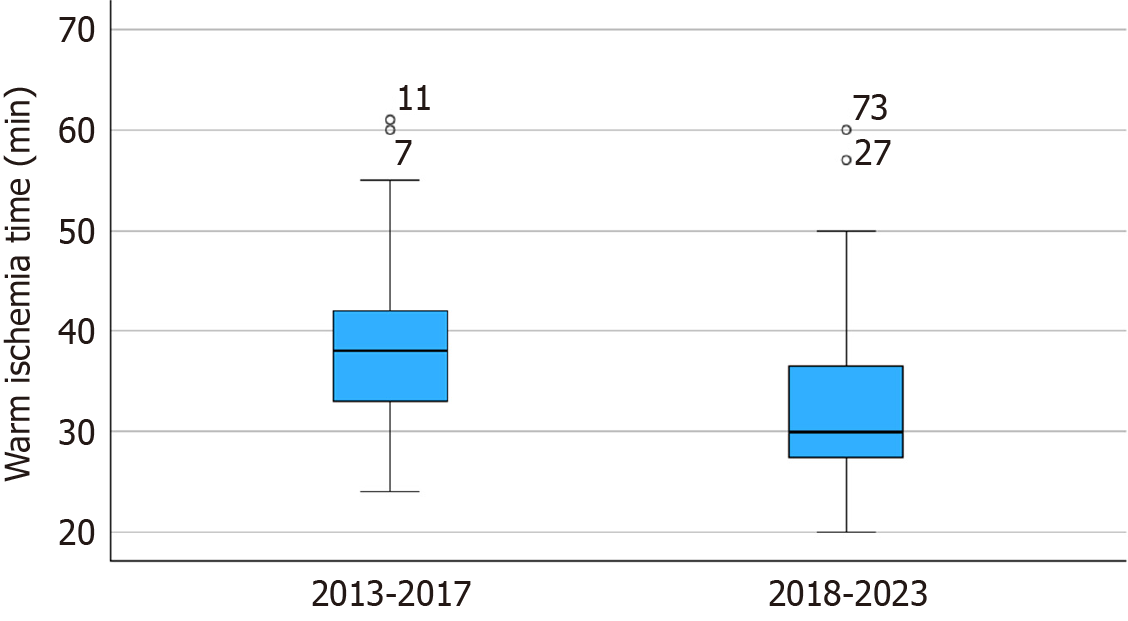
To compare the results over the years, patients were divided into two groups based on a 5-year period. A total of 25 patients underwent transplantation in the first period (2013-2017) and 48 in the second period (2018-2023). Table 1 outlines the demographic and intraoperative variables. The only variable with significant differences between the two periods was the warm ischemia time (WIT). A lower WIT was observed in the second period when compared to the first (32.6 ± 8.6 minutes vs 38.4 ± 9.8 minutes, P = 0.018, respectively), reflecting the center’s learning curve (Figure 4).
| First period (2013-2017), n = 25 | Second period (2018–2023), n = 48 | P value | |
| Sex, male | 14 (56) | 25 (52.1) | 0.8 |
| Age at LT, months, median (IQR) | 11.2 (7.8-20.7) | 12 (6.9-58.1) | 0.64 |
| Weight at LT, kg, median (IQR) | 7.6 (6.35-11.25) | 7.36 (6.4-17.6) | 0.67 |
| Z-score weight/age, median (IQR) | -1.33 (-2.85 to 0.45) | -1.1 (-2.31 to -0.19) | 0.74 |
| Z-score height/age, median (IQR) | -1.68 (-2.6 to -0.75) | -1.63 (-3.58 to -0.73) | 0.95 |
| PELD/MELD | 17 (8.5-23) | 17 (6-24) | 0.99 |
| Liver graft | 0.26 | ||
| LLS | 23 (92) | 47 (97.9) | |
| LL | 2 (8) | 1 (2.1) | |
| GRWR, %, mean ± SD | 3.38 ± 1.23 | 3.1 ± 1.45 | 0.39 |
| TIT, minute, mean ± SD | 120.9 ± 42.64 | 129.4 ± 51.53 | 0.46 |
| CIT, minute, median (IQR) | 75 (57-105) | 90 (66.5-111.5) | 0.25 |
| WIT, minute, mean ± SD | 38.4 ± 9.8 | 32.6 ± 8.6 | 0.018 |
| PBCT, mL/kg, median (IQR) | 14.2 (0-24.2) | 17.7 (1.42-24.5) | 0.59 |
| Hospital stay, days, median (IQR) | 23 (16-41) | 23 (16-31.7) | 0.37 |
| ICU stay, days, median (IQR) | 8 (7-12) | 8 (5-16.5) | 0.87 |
Regarding post-transplant outcomes, there was a lower frequency of acute cellular rejection (4.2% vs 24%, P = 0.01), CMV infection (58.3% vs 80%, P = 0.07), and EBV infection (41.7% vs 76%, P = 0.007) in the second period. Regarding surgical complications, only the incidence of biliary strictures was significantly lower during the second period (40% in the first period vs 14.6% in the second, P = 0.02; Table 2).
| First period (2013-2017), n = 25 | Second period (2018-2023), n = 48 | P value | |
| Acute rejection | 6 (24) | 2 (4.2) | 0.01 |
| Chronic rejection | 1 (4) | 0 (0) | 0.34 |
| CMV infection | 20 (80) | 28 (58.3) | 0.07 |
| EBV infection | 19 (76) | 20 (41.7) | 0.007 |
| PTLD | 1 (4) | 2 (4.2) | 1 |
| HAT | 4 (16) | 3 (6.3) | 0.22 |
| Portal vein graft | 3 (12) | 12 (25) | 0.23 |
| PVT | 4 (16) | 7 (14.6) | 1 |
| PV stenosis | 3 (12) | 8 (17.6) | 0.73 |
| HV stenosis | 2 (8) | 0 (0) | 0.11 |
| Bile leak | 6 (24) | 21 (43.8) | 0.12 |
| Biliary strictures | 10 (40) | 7 (14.6) | 0.02 |
| Reoperation | 7 (28) | 15 (31.3) | 1 |
| Retransplant | 2 (8) | 0 (0) | 0.11 |
| Death | 4 (16) | 4 (8.3) | |
| Clavien-Dindo classification of complications1 | 0.34 | ||
| I | 0 | 6 (12.5) | |
| II | 12 (48) | 17 (35.4) | |
| IIIA | 1 (4) | 0 | |
| IIIB | 7 (28) | 11 (29) | |
| IVA | 2 (8) | 8 (16.7) | |
| IVB | 1 (4) | 2 (4.2) | |
| V | 2 (8) | 4 (8.3) |
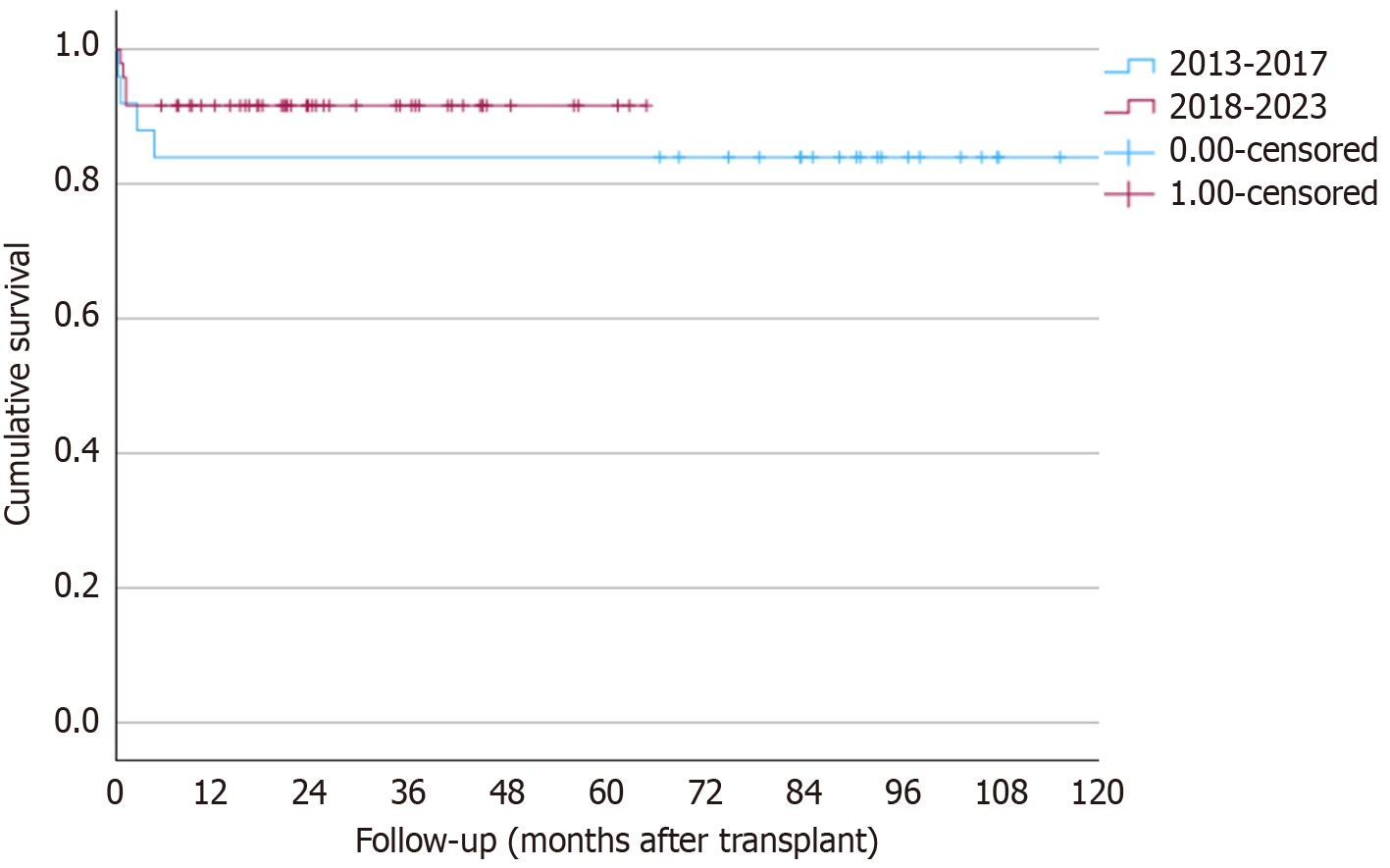
The frequency of surgical complications distributed along the years is shown in Figure 5. There was a reduction in those frequencies during the study period for all complications, particularly for vascular complications. This reflects the center’s learning curve. Complications according to the Clavien-Dindo classification are illustrated in Figure 6, showing no significant differences between periods (P = 0.32). There was an increase in patient survival rates at 1 and 5 years from 84% in the first period to 91.7% in the second period. This difference, however, was not statistically significant (P = 0.32; Figure 7).
Logistic regression analysis was performed to evaluate the variables associated with patient survival. A WIT greater than 35 minutes was identified as a risk factor for patient death [hazard ratio (HR) = 5.48, 95%CI: 1.02-29.36, P = 0.047] as was a GRWR lower than 1.3% (HR = 5.9, 95%CI: 1.12-31.01, P = 0.036). In the multivariate model, both parameters lost statistical significance at an α of 0.05 (Table 3).
| P value | HR | 95%CI | |
| GRWR < 1.3% | 0.066 | 5.12 | 0.89 to 29.3 |
| WIT > 35 minutes | 0.068 | 4.96 | 0.88 to 27.7 |
In Brazil, LDLT accounts for 4%-8% of all liver transplant procedures, with the majority (60%) being performed in the pediatric population[9]. This highlights the critical role of LDLT as a viable option for children suffering from end-stage liver disease, particularly given the limited availability of deceased donor organs. The pediatric population often faces unique challenges, including the need for age-appropriate donor-recipient matching and the management of postope
In our case series, 56% of transplants were due to BA, followed by tumors and metabolic causes. Similarly, the mul
Pediatric liver transplantation poses significant technical challenges due to the small size of the vessels and the biliary tree. There is often a discrepancy in the diameter between the donor and recipient portal vein and hepatic artery, particularly in the early postoperative period, leading to vascular and biliary complications[13]. These complications can significantly impact patient outcomes and require vigilant monitoring and intervention. Understanding these challenges is essential for identifying potential complications early and implementing strategies to address them effectively, thereby improving overall success rates in pediatric liver transplantation[14].
HAT is the most common vascular adverse event in the pediatric population, with an incidence ranging from approximately 1% to 26%[12,14]. The development of early post-transplant arterial thrombosis is a significant cause of graft loss and mortality as it can lead to fulminant graft failure or bile duct necrosis. Urgent revascularization may prevent the need for re-transplantation; however, early diagnosis is essential for the success of this strategy. If thrombosis occurs > 30 days post-transplantation, the clinical presentation may be silent or may result in progressive bile duct injury[15,16].
Our approach to HAT has been previously reported, and none of the patients who developed this complication in our series required re-transplantation or died due to HAT[17]. The advantage of using a normal liver with a low cold ische
Portal vein complications after LDLT are reported to occur in 9%-14% of cases[18,19]. In our study, portal vein throm
Biliary complications, including leaks and strictures, are a significant cause of morbidity after liver transplantation, with a higher incidence following LDLT, often being considered as the “Achilles’ heel” of pediatric transplantation. In pediatric recipients, the incidence ranges from 6% to 40%[6]. In our study, 37% of patients experienced bile leaks and 23.3% had biliary strictures. One-third of these leaks originated from the cut surface area of the liver and were managed with abdominal drain maintenance. The role of interventional radiology in the treatment of biliary complications was evident in our cohort. This finding supports evidence in the literature that highlights its efficacy in the treatment of anastomotic strictures and bile leaks, this approach often reduces the necessity for surgical intervention[21]. Graft retrieval techniques and the frequency of double ducts in liver grafts can partially explain the higher rates[22].
Controlling the immune response and reducing the risk of infection are crucial for reducing the morbidity and mortality associated with liver transplantation[23]. The success of solid-organ transplantation is largely due to the control of acute rejection mediated by T cells. The reported incidence of acute rejection is 49.7%, with 9% of patients experiencing chronic rejection. The use of immunosuppressants targeting the T-cell response has helped reduce the graft loss rate and the incidence of acute cellular rejection[24]. In our case series, only 11% of patients experienced acute rejection, and 1.4% developed chronic rejection.
PTLD is reported in 5%-15% of children post-transplant, with 90% of cases occurring after EBV infection. The risk is higher among patients with primary EBV disease, affecting up to 75% of susceptible children within the first 6 months post-transplantation. Pre-transplant serological evaluation and post-transplant monitoring are essential and routinely performed at our center[25,26]. The continuous monitorization and medical practice refinement over the years are reflected in the lower incidences of both CMV and EBV infections in the second period.
In a study by Seda-Neto et al[12], patient and graft survival rates were 90.6% and 83.6% at 12 months and 88.7% and 80.8% at 60 months[27]. In our study, when comparing the two periods at our center, improvements over time and the growing expertise of the group were evident. This was demonstrated by a lower WIT and fewer complications, with significant reductions in biliary strictures and post-transplant viral infections. Patient survival was also higher during the second period, although the difference was not statistically significant. The current survival rate of 91.7% is comparable to that of high-volume centers worldwide[11,24,27-29].
Pediatric liver transplantation is a complex and challenging procedure that has profoundly transformed the lives of numerous children worldwide. Advances in surgical techniques, broadening of donor options, and refinements in post-transplant care have significantly enhanced the prognosis of children with severe liver diseases.
Our risk assessment analysis was limited by the constraints of a single-center design and a small sample size, which may compromise statistical power. However, the identified risk factors such as WIT and GRWR, which indicate the risk of small-for-size syndrome, are consistent with findings from larger studies involving living-donor transplants[4,29]. Additionally, the retrospective nature of the study introduces potential biases, such as incomplete data capture and variability in follow-up intervals, which could affect the accuracy of complication reporting and long-term outcome assessment. Furthermore, as a single-center analysis, our findings may reflect specific institutional practices and surgeon experience, potentially limiting their applicability to other settings. Future multi-center prospective studies with larger sample sizes would be beneficial to confirm these associations and provide a more comprehensive understanding of risk factors in pediatric living donor liver transplantation.
The results of this study mirror those reported in the literature regarding indications, survival rates, and post-transplant complications. A higher risk of vascular and biliary complications is compatible with LDLT. These findings provide crucial evidence regarding the feasibility of establishing a living donor program with consistent results over time. This study has provided evidence on the epidemiological profile and allowed for the identification of the most common complications, which provides support for the decision-making process of the team in planning and implementing effective interventions aimed at the success of surgery and improvement in long-term survival of these patients.
The authors are deeply grateful to the patients and their families for their trust, collaboration, and resilience, which were essential throughout this journey. We would also like to thank all those involved in the pediatric gastroenterology service, including the multidisciplinary, anesthetic, and surgical teams of the Pediatric Liver Transplantation Unit at Hospital Santa Casa de Misericórdia de Porto Alegre/RS and Hospital da Criança Santo Antônio – Complexo Santa Casa de Misericórdia de Porto Alegre, for their invaluable support and dedication.
| 1. | Kohli R, Cortes M, Heaton ND, Dhawan A. Liver transplantation in children: state of the art and future perspectives. Arch Dis Child. 2018;103:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Associação Brasileira de Transplante de Órgãos. Transplante De Fígado. 2024. [cited 27 November 2024]. Available from: https://site.abto.org.br/transplante-de-figado-2/. |
| 3. | Seda Neto J, Costa CM, Pugliese R, Vincenzi R, Benavides MR, Travassos NPR, de Oliveira CMV, Roda K, Fernandes DP, Kondo M, Fonseca EA. Living Donor Whole and Partial Liver Grafts, Deceased Donor Whole Liver and SPLIT: Outcome Comparison. J Pediatr Surg. 2024;59:1784-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 4. | Spada M, Riva S, Maggiore G, Cintorino D, Gridelli B. Pediatric liver transplantation. World J Gastroenterol. 2009;15:648-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 125] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (2)] |
| 5. | Lauterio A, Di Sandro S, Concone G, De Carlis R, Giacomoni A, De Carlis L. Current status and perspectives in split liver transplantation. World J Gastroenterol. 2015;21:11003-11015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Hackl C, Schlitt HJ, Melter M, Knoppke B, Loss M. Current developments in pediatric liver transplantation. World J Hepatol. 2015;7:1509-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Yilmaz ZB, Memisoglu F, Akbulut S. Management of cytomegalovirus infection after liver transplantation. World J Transplant. 2024;14:93209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (3)] |
| 8. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24776] [Article Influence: 1179.8] [Reference Citation Analysis (0)] |
| 9. | Andraus W, Canedo BF, D'Alburquerque LA. Living donor liver transplantation in Brazil-current state. Hepatobiliary Surg Nutr. 2016;5:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Polat KY, Yazar Ş, Aslan S, Kargı A, Selimoğlu A, Gürbulak B, Astarcıoğlu İ. Comparing the Outcomes of Pediatric Liver Transplantation. Transplant Proc. 2023;55:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Elisofon SA, Magee JC, Ng VL, Horslen SP, Fioravanti V, Economides J, Erinjeri J, Anand R, Mazariegos GV; Society of Pediatric Liver Transplantation Research Group. Society of pediatric liver transplantation: Current registry status 2011-2018. Pediatr Transplant. 2020;24:e13605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 12. | Seda-Neto J, Antunes da Fonseca E, Pugliese R, Candido HL, Benavides MR, Carballo Afonso R, Neiva R, Porta G, Miura IK, Teng HW, Iwase FC, Rodrigues ML, Carneiro de Albuquerque LA, Kondo M, Chapchap P. Twenty Years of Experience in Pediatric Living Donor Liver Transplantation: Focus on Hepatic Artery Reconstruction, Complications, and Outcomes. Transplantation. 2016;100:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Jara P, Hierro L. [Childhood liver transplantation. Long-term results]. Gastroenterol Hepatol. 2010;33:398-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Broering DC, Kim JS, Mueller T, Fischer L, Ganschow R, Bicak T, Mueller L, Hillert C, Wilms C, Hinrichs B, Helmke K, Pothmann W, Burdelski M, Rogiers X. One hundred thirty-two consecutive pediatric liver transplants without hospital mortality: lessons learned and outlook for the future. Ann Surg. 2004;240:1002-1012; discussion 1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9:746-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 375] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 16. | Stefanowicz M, Kaliciński P, Kowalewski G, Kowalski A, Ciopiński M, Szymczak M, Kwiecińska A, Patkowski W, Zieniewicz K, Grzelak I, Kamińska D, Ismail H. The Impact of Hepatic Artery Thrombosis on the Outcome of Pediatric Living Donor Liver Transplantations. Children (Basel). 2023;10:340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Feier FH, Melere MU, Trein CS, da Silva CS, Lucchese A, Horbe A, Tonet F, Ricachinevsky C, Ferreira CT, Chedid MF, Kalil AN. Early hepatic arterial thrombosis in liver transplantation: Systemic intravenous alteplase as a potential rescue treatment after failed surgical revascularization. Pediatr Transplant. 2021;25:e13902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Ueda M, Oike F, Kasahara M, Ogura Y, Ogawa K, Haga H, Takada Y, Egawa H, Tanaka K, Uemoto S. Portal vein complications in pediatric living donor liver transplantation using left-side grafts. Am J Transplant. 2008;8:2097-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Yabuta M, Shibata T, Shibata T, Shinozuka K, Isoda H, Okamoto S, Uemoto S, Togashi K. Long-term outcome of percutaneous transhepatic balloon angioplasty for portal vein stenosis after pediatric living donor liver transplantation: a single institute's experience. J Vasc Interv Radiol. 2014;25:1406-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Neto JS, Fonseca EA, Feier FH, Pugliese R, Candido HL, Benavides MR, Porta G, Miura IK, Danesi VB, Guimaraes T, Porta A, Borges C, Godoy A, Kondo M, Chapchap P. Analysis of factors associated with portal vein thrombosis in pediatric living donor liver transplant recipients. Liver Transpl. 2014;20:1157-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Lee AY, Lehrman ED, Perito ER, Kerlan RK Jr, Kohi MP, Kolli KP, Taylor AG, Ostroff JW, Kang SM, Roberts JP, Rhee S, Rosenthal P, Fidelman N. Non-operative management of biliary complications after Liver Transplantation in pediatric patients: A 30-year experience. Pediatr Transplant. 2021;25:e14028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Hsiao CY, Ho CM, Wu YM, Ho MC, Hu RH, Lee PH. Biliary Complication in Pediatric Liver Transplantation: a Single-Center 15-Year Experience. J Gastrointest Surg. 2019;23:751-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Lee BT, Fiel MI, Schiano TD. Antibody-mediated rejection of the liver allograft: An update and a clinico-pathological perspective. J Hepatol. 2021;75:1203-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Ng VL, Alonso EM, Bucuvalas JC, Cohen G, Limbers CA, Varni JW, Mazariegos G, Magee J, McDiarmid SV, Anand R; Studies of Pediatric Liver Transplantation (SPLIT) Research Group. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: report of the studies of pediatric liver transplantation experience. J Pediatr. 2012;160:820-6.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Baker A, Frauca Remacha E, Torres Canizales J, Bravo-Gallego LY, Fitzpatrick E, Alonso Melgar A, Muñoz Bartolo G, Garcia Guereta L, Ramos Boluda E, Mozo Y, Broniszczak D, Jarmużek W, Kalicinski P, Maecker-Kolhoff B, Carlens J, Baumann U, Roy C, Chardot C, Benetti E, Cananzi M, Calore E, Dello Strologo L, Candusso M, Lopes MF, Brito MJ, Gonçalves C, Do Carmo C, Stephenne X, Wennberg L, Stone R, Rascon J, Lindemans C, Turkiewicz D, Giraldi E, Nicastro E, D'Antiga L, Ackermann O, Jara Vega P. Current Practices on Diagnosis, Prevention and Treatment of Post-Transplant Lymphoproliferative Disorder in Pediatric Patients after Solid Organ Transplantation: Results of ERN TransplantChild Healthcare Working Group Survey. Children (Basel). 2021;8:661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Marie E, Navallas M, Navarro OM, Punnett A, Shammas A, Gupta A, Chami R, Shroff MM, Vali R. Posttransplant Lymphoproliferative Disorder in Children: A 360-degree Perspective. Radiographics. 2020;40:241-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Neto JS, Pugliese R, Fonseca EA, Vincenzi R, Pugliese V, Candido H, Stein AB, Benavides M, Ketzer B, Teng H, Porta G, Miura IK, Baggio V, Guimaraes T, Porta A, Rodrigues CA, Carnevale FC, Carone E, Kondo M, Chapchap P. Four hundred thirty consecutive pediatric living donor liver transplants: variables associated with posttransplant patient and graft survival. Liver Transpl. 2012;18:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Venick RS, Farmer DG, Soto JR, Vargas J, Yersiz H, Kaldas FM, Agopian VG, Hiatt JR, McDiarmid SV, Busuttil RW. One Thousand Pediatric Liver Transplants During Thirty Years: Lessons Learned. J Am Coll Surg. 2018;226:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 29. | Kasahara M, Sakamoto S. Optimal graft size in pediatric living donor liver transplantation: How are children different from adults? Pediatr Transplant. 2023;27:e14543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |