Published online Jun 18, 2025. doi: 10.5500/wjt.v15.i2.98228
Revised: October 8, 2024
Accepted: December 9, 2024
Published online: June 18, 2025
Processing time: 237 Days and 20 Hours
In patients with chronic liver disease or hepatic dysfunction with sarcopenia, there is an increased risk of frailty as measured by functional impairment, making frailty a vital predictor of post-transplant mortality.
To investigate the effects of frailty on mortality after liver transplantation.
A retrospective review of post-transplant outcomes in liver transplant recipients assessed frailty using Karnofsky Performance Score. Data from the Scientific Registry of Transplant Recipients database for 37427 liver transplant recipients was used.
Of 82.7% frail patients, 42.7% were severely frail and 40% were moderately frail (P < 0.001) at the time of transplantation. Compared with non-frail patients, post-transplant mortality in frail patients was significantly higher at 12 months [odds ratio (OR) = 1.94, P = 0.02)]. Secondary analysis of the data revealed that liver grafts from donation after circulatory death (DCD) were more likely to be associated with frail patients at transplant (OR = 1.86, P < 0.001). Furthermore, a donor history of hypertension was associated with a lower likelihood of frailty in the recipient at the time of transplant (OR = 0.65, P = 0.03).
Recipient frailty is associated with increased mortality at 12 months following liver transplantation, and liver transplants from donors with DCD are associated with increased frailty of the liver transplant recipient.
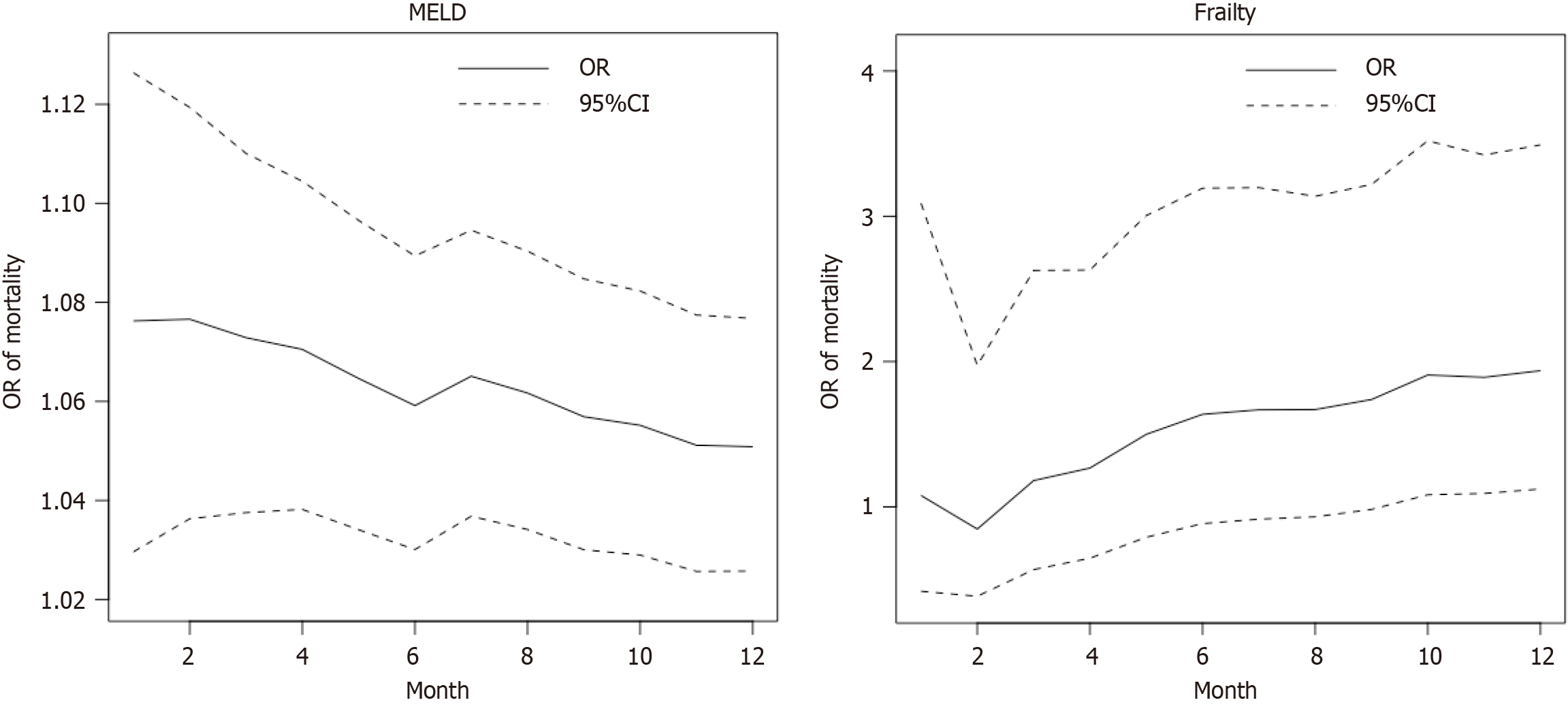
Core Tip: In this study, we utilized an updated Karnofsky Performance Score, and intentionally converted the frailty assessment binary (frail vs non-frail) and enhancing reliability over more nuanced frailty assessments. Our study observed that frail recipients had significantly higher post-transplant mortality at 12 months, a critical period when transplant centers' monitoring traditionally wanes. Moreover, our study also showed a declining trend of the association between the model for end-stage liver disease score and post-transplant mortality, but an increasing trend between frailty and post-transplant mortality, from 1 month to 12 months post-transplant.
- Citation: Balogh J, Mubashir T, Li Y, Digbeu BD, Hegde N, Pour FM, Rezapour M, Lai HY, West K, Chaudhry RA, Williams GW, Maroufy V. Effect of frailty as measured by functional impairment on long-term outcomes in liver transplantation in the United States. World J Transplant 2025; 15(2): 98228
- URL: https://www.wjgnet.com/2220-3230/full/v15/i2/98228.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i2.98228
Chronic liver disease and cirrhosis account for approximately 35000 deaths each year in the United States, and liver cirrhosis is the ninth leading cause of death in the United States[1]. Currently, more than 14000 patients are registered on the transplant waiting list at any given time. More than 3000 either die or are de-listed annually[2]. Donor-related factors have been shown to affect post-transplant outcomes. Several studies have shown various factors, including age, can negatively impact post-transplant mortality[3].
Frailty is a complex syndrome marked by disturbances across multiple physiological systems, originally identified in aging populations[4] and later recognized in patients with cirrhosis[5]. This syndrome encompasses not only physical frailty but also cognitive, emotional, and psychosocial dimensions[5]. The prevalence of frailty as defined by the Fried Frailty Index[5] in patients seeking liver transplantation ranges between 17% to 49%[6], and frailty associated with liver cirrhosis has also been linked to greater waitlist mortality and increased hospital length of stay[7,8].
Frailty assessment aims to estimate biological age that correlates with quality of life, hospital admissions, and mortality[9]. Several frailty tools have been used in the liver transplant population. The Fried Frailty Index defines frailty as a clinical syndrome in which at least three of the following components are present: (1) Unintentional weight loss (10 pounds in the past year); (2) Self-reported exhaustion; (3) Weakness (as measured by grip strength); (4) Walking speed; and (5) Low physical activity[5]. The Liver Frailty Index is an objective measure of physical frailty utilizing grip strength, chair stands, and balance[4]. The Karnofsky Performance Score (KPS) rates a patient's overall performance status on a scale from 0–100 in increments of 10, where 0 indicates a moribund state and 100 represents excellent health[10]. No single frailty tool has emerged in the literature as a standardized tool for liver transplant patients, and it is recommended for transplant centers to incorporate a frailty tool for liver transplant patients, and to use frailty as one of many objective measures in determining transplant candidacy[4]. These standardized tools focus on physical frailty, and lack cognitive, social, and emotional aspects, in order to objectively define frailty[4].
Currently, the KPS is the only measure used by the United Network for Organ Sharing/Organ Procurement and Transplantation Network (OPTN) to approximate frailty. In patients awaiting liver transplantation, there is a proven association between functional status and mortality, both pre-transplant and post-transplant[11,12]. Furthermore, severe functional impairment at the time of liver transplantation, as indicated by a low KPS, is strongly linked to increased mortality and/or graft failure within one year after the transplant[13-15]. Assessment of frailty in potential transplant candidates helps identify patients who would benefit from preoperative interventions, thus modifying post-transplant outcomes. While the KPS is advantageous due to its simplicity and speed, it captures only one facet of the broader concept of frailty—functional status—and its subjective nature may lead to biased assessments[16].
Our study aimed to evaluate the risk of post-transplant mortality in frail and non-frail individuals by analyzing recipient and donor characteristics using the KPS scale (as a metric for frailty).
A retrospective review of post-transplant outcomes in 37427 liver transplant recipients who underwent frailty assessment at transplant was conducted. The Institutional Review Board of the University of Texas Health Science Center at Houston does not require studies that use anonymized databases retrospectively to attain approval from this board.
For this study, data from the Scientific Registry of Transplant Recipients (SRTR) database were used. The SRTR database receives data directly collected by the OPTN and is supplemented by data from the Centers for Medicare and Medicaid Services and the National Technical Information Service Death Master File. The SRTR makes data available after honoring all requirements regarding patients’ privacy in accordance with the Final Rule by the United States Department of Health and Human Services.
Adults aged ≥ 18 years listed as waiting for a liver transplant between January 1, 2006, and November 27, 2019 (n = 139563) were queried. The study was also limited to subjects who had deceased organ donation only (n = 69881) and received whole-liver transplants only (n = 68397).
Of the 68397 participants, those with a history of malignancy (n = 11827) and pancreas islet transplantation (n = 1488) were excluded. Participants who did not have a specified type of cirrhosis diagnosis [type B, type C, autoimmune, stea
Since April 2005, transplant centers have recorded functional status using the KPS scale, which is expressed in 10% increments[17]. The KPS scale was further classified into three categories: (1) Normal; (2) Moderate; and (3) Severe, according to the patient’s ability to work at 80%-100%, 50%-70%, and 10%-40% ratings, respectively[18]. Patients at transplant were classified into frail and non-frail groups according to the updated KPS scale. Subjects in KPS ‘moderate’ and ‘severe’ categories (according to the patient’s ability to work as 10%-70% of rating) were classified into the frail group and subjects in KPS ‘normal’ categories (according to the patient’s ability to work as 80%-100% rating) were classified into the non-frail group.
The primary outcome was mortality within 1 month, 6 months, and 12 months after the liver transplant, respectively, but frailty was not associated with survival at 1 month and 6 months. Factors associated with frailty at transplant were also examined as secondary outcomes.
The recipient variables collected at the listing that could influence the outcomes were considered. These variables included age (≥ 18 years), gender, race/ethnicity (white, black, Hispanic, and others), body mass index (BMI), primary cirrhosis diagnosis (viral, alcohol-associated, NASH, other/mixed), model for end-stage liver disease (MELD) score, warm ischemic time, and death status.
Donor information included donor age (≥ 18 years), gender, race/ethnicity (white, black, Hispanic, and other), BMI, ABO blood, deceased donation pathway [donation after brain death (DBD) or donation after circulatory death (DCD)], cause of death (anoxia, cerebrovascular/stroke, dead trauma, central nervous system tumor, and other), cigarette use, history of cocaine, diabetes, hypertension, warm ischemic time, and risk of donor’s organ.
The selected baseline characteristics were analyzed based on physical frailty. Categorical variables were reported as frequencies and proportions, and continuous variables were reported as means and SD. Baseline recipient and donor characteristics were compared between frail and non-frail patients using unpaired t-tests for continuous variables when the normality assumption was met, the Wilcoxon rank sum test for continuous variables when the normality assumption did not hold, and Pearson’s χ2 tests for categorical variables. Multivariable logistic regression was applied to determine the association between either frailty or death status and the selected covariates. Furthermore, to assess the robustness of our analyses and address the missing values issue we conducted a sensitivity analysis. All P values were considered statistically significant at a level of 0.05. Analyses were performed using RStudio (version 1.3).
Among all the 37427 recipients, the prevalence of frailty in subjects who underwent frailty assessment at transplant was 82.7% (30943 patients). The frail group was younger (P < 0.001), had a higher proportion of females (P < 0.001), a larger representation of Hispanic individuals (P < 0.001), a higher mean MELD score, and a longer average warm ischemic time (Table 1). Males comprised 66.9% of the frail group compared to 77.1% in the non-frail group (P < 0.001). Hispanics and African Americans were 16.0% and 8.4% in the frail group vs 12.0% and 10.1% in the non-frail group (P < 0.001). There was a significantly higher proportion of alcohol-associated cirrhosis and NASH, but lower rates of viral cirrhosis diagnosis (34.4% vs 52.0%) and unadjusted death post-liver transplant (21.0% vs 22.3%, P = 0.022) among frail recipients
| Frailty status, recipients’ characteristics | Total (n = 37427) | Frail group (n = 30943) | Non-frail group (n = 6484) | P value |
| Age groups | < 0.001a | |||
| 18-34 years | 957 (2.6) | 850 (2.7) | 107 (1.7) | |
| 35-49 years | 7092 (18.9) | 6075 (19.6) | 1017 (15.7) | |
| 50-64 years | 23529 (62.9) | 19248 (62.2) | 4281 (66.0) | |
| > 64 years | 5849 (15.6) | 4770 (15.4) | 1079 (16.6) | |
| Gender | < 0.001a | |||
| Male | 25696 (68.7) | 20700 (66.9) | 4996 (77.1) | |
| Female | 11731 (31.3) | 10243 (33.1) | 1488 (22.9) | |
| Race | < 0.001a | |||
| White | 26542 (70.9) | 21993 (71.1) | 4549 (70.2) | |
| Black or African American | 3267 (8.7) | 2611 (8.4) | 656 (10.1) | |
| Hispanic Latino | 5720 (15.3) | 4942 (16.0) | 778 (12.0) | |
| Others | 1898 (5.1) | 1397 (4.5) | 501 (7.7) | |
| Body mass index (kg/m2), mean (SD) | 29.1 (6.0) | 29.1 (6.1) | 29.0 (5.5) | 0.429 |
| Primary diagnosis | < 0.001a | |||
| Viral | 14003 (37.4) | 10629 (34.4) | 3374 (52.0) | |
| Alcohol-associated | 10940 (29.2) | 9624 (31.1) | 1316 (20.3) | |
| Non-alcoholic steatohepatitis | 7132 (19.1) | 6181 (20.0) | 951 (14.7) | |
| Other/mixed | 5352 (14.3) | 4509 (14.6) | 843 (13.0) | |
| Model for end-stage liver disease score, unit, mean (SD) | 23.3 (10.0) | 24.7 (9.8) | 16.7 (7.6) | < 0.001a |
| Ventilator | < 0.001a | |||
| No | 35831 (95.7) | 29357 (94.9) | 6474 (99.8) | |
| Yes | 1596 (4.3) | 1586 (5.1) | 10 (0.2) | |
| Warm ischemic time, minute, mean (SD) | 40.4 (20.1) | 40.6 (20.6) | 39.8 (18.4) | 0.029a |
| Death indicator after transplant | 0.022a | |||
| Death | 7955 (21.3) | 6508 (21.0) | 1447 (22.3) | |
| Non-death | 29472 (78.7) | 24435 (79.0) | 5037 (77.7) | |
| Functional status at transplant in Karnofsky Performance Score scale | - | |||
| Normal | 6484 (17.3) | 0 (0.0) | 6484 (100.0) | |
| Moderate | 14957 (40.0) | 14957 (48.3) | 0 (0.0) | |
| Severe | 15986 (42.7) | 15986 (51.7) | 0 (0.0) |
The frail group exhibited significant associations with the following donor characteristics: (1) Younger age (P < 0.001); (2) A higher representation of Hispanic Latino and African American donors; (3) A higher prevalence of O blood type; (4) Specific causes of death such as anoxia and head trauma (P < 0.001); (5) Reduced cigarette use within the last 6 months; (6) A lower incidence of hypertension history; (7) A higher incidence of cocaine use history; (8) An increased risk level of the organ donor; and (9) A shorter warm ischemic time. Regarding the causes of death, anoxia and stroke accounted for 32.0% and 34.9% of donor deaths in the frail group compared to 27.7% and 39.9% in the non-frail group (Table 2).
| Recipient status, donor characteristics | Total (n = 37427) | Frail (n = 30943) | Non-frail (n = 6484) | P value |
| Age group | < 0.001a | |||
| 18-34 years | 12966 (34.6) | 10955 (35.4) | 2011 (31.0) | |
| 35-49 years | 10829 (28.9) | 8912 (28.8) | 1917 (29.6) | |
| 50-64 years | 10478 (28.0) | 8580 (27.7) | 1898 (29.3) | |
| > 64 years | 3154 (8.4) | 2496 (8.1) | 658 (10.1) | |
| Gender | 0.758 | |||
| Male | 22517 (60.2) | 18605 (60.1) | 3912 (60.3) | |
| Female | 14910 (39.8) | 12338 (39.9) | 2572 (39.7) | |
| Race | < 0.001a | |||
| White | 24549 (65.6) | 20265 (65.5) | 4284 (66.1) | |
| Black or African American | 6703 (17.9) | 5565 (18.0) | 1138 (17.6) | |
| Hispanic Latino | 4878 (13.0) | 4120 (13.3) | 758 (11.7) | |
| Others | 1297 (3.5) | 993 (3.2) | 304 (4.7) | |
| Body mass index (kg/m2), mean (SD) | 28.1 (6.5) | 28.1 (6.5) | 28.0 (6.5) | 0.378 |
| ABO blood group | < 0.001a | |||
| A group | 14889 (39.8) | 12313 (39.8) | 2576 (39.7) | |
| AB group | 503 (1.3) | 380 (1.2) | 123 (1.9) | |
| B group | 4520 (12.1) | 3545 (11.5) | 975 (15.0) | |
| O group | 17515 (46.8) | 14705 (47.5) | 2810 (43.3) | |
| Donation pathway | 0.414 | |||
| Donation after brain death | 35319 (94.4) | 29214 (94.4) | 6105 (94.2) | |
| Donation after circulatory death | 2108 (5.6) | 1729 (5.6) | 379 (5.8) | |
| Cause of death | < 0.001a | |||
| Anoxia | 11695 (31.2) | 9900 (32.0) | 1795 (27.7) | |
| Cerebrovascular/stroke | 13389 (35.8) | 10802 (34.9) | 2587 (39.9) | |
| Head trauma | 11366 (30.4) | 9448 (30.5) | 1918 (29.6) | |
| Central nervous system tumor | 166 (0.4) | 126 (0.4) | 40 (0.6) | |
| Others | 811 (2.2) | 667 (2.2) | 144 (2.2) | |
| Cigarette use in last 6 months | < 0.001a | |||
| No | 29766 (79.5) | 24762 (80.0) | 5004 (77.2) | |
| Yes | 7661 (20.5) | 6181 (20.0) | 1480 (22.8) | |
| History of cocaine | 0.001a | |||
| No | 30223 (80.8) | 24895 (80.5) | 5328 (82.2) | |
| Yes | 7204 (19.2) | 6048 (19.5) | 1156 (17.8) | |
| History of diabetes | 0.174 | |||
| No | 32921 (88.0) | 27250 (88.1) | 5671 (87.5) | |
| Yes | 4506 (12.0) | 3693 (11.9) | 813 (12.5) | |
| History of hypertension | < 0.001a | |||
| No | 23610 (63.1) | 19677 (63.6) | 3933 (60.7) | |
| Yes | 13817 (36.9) | 11266 (36.4) | 2551 (39.3) | |
| High risk for organ donor | ||||
| No | 29843 (79.7) | 24477 (79.1) | 5366 (82.8) | |
| Yes | 7584 (20.3) | 6466 (20.9) | 1118 (17.2) | |
| Warm ischemic time, minute, mean (SD) | 3.8 (8.3) | 3.5 (7.8) | 5.9 (10.5) | < 0.001a |
Frail recipients at transplant exhibited a significantly higher odds ratio (OR) of post-transplant mortality at the 12-month compared to non-frail recipients (OR = 1.89; P = 0.03; 95%CI: 1.09-3.41) as shown in Table 3. A higher MELD score was associated with increased risk of 1-month, 6-month, and 12-month mortality following liver transplant, with respective ORs of 1.06 (P = 0.01; 95%CI: 1.02–1.12), 1.05 (P < 0.001; 95%CI: 1.02-1.08), and 1.04 (P < 0.001; 95%CI: 1.02-1.07). Compared with female patients, males had a higher risk of 6-month (P = 0.04; 95%CI: 1.04-3.39) and 12-month (P = 0.03; 95%CI: 1.07-2.98) mortality following liver transplantation. Compared with white recipients, the adjusted OR for 1-month mortality for African Americans was significantly higher (OR = 3.36; P = 0.02; 95%CI: 1.12-9.14). Patients with chronic viral cirrhosis had a significantly higher risk of post-transplant mortality at 12 months (OR = 1.98; P = 0.02; 95%CI: 1.15-3.54) compared to patients with chronic alcohol-associated cirrhosis.
| Variables | 1 month | 6 months | 12 months | |||||||||
| OR | L | U | P value | OR | L | U | P value | OR | L | U | P value | |
| Donation pathway (ref: Donation after brain death) | ||||||||||||
| Donation after circulatory death | 1.16 | 0.48 | 3.03 | 0.75 | 1.08 | 0.62 | 1.95 | 0.79 | 1.01 | 0.62 | 1.69 | 0.95 |
| Donor's age group (ref: > 64 years) | ||||||||||||
| 18-34 years | - | - | - | - | 0.66 | 0.15 | 4.58 | 0.61 | 0.37 | 0.12 | 1.47 | 0.12 |
| 35-49 years | - | - | - | - | 0.61 | 0.14 | 4.24 | 0.55 | 0.44 | 0.14 | 1.71 | 0.19 |
| 50-64 years | - | - | - | - | 1.41 | 0.34 | 9.71 | 0.67 | 0.88 | 0.28 | 3.43 | 0.84 |
| Donor continued use cigarette in past 6 month (ref: No) | ||||||||||||
| Yes | 0.79 | 0.26 | 2.08 | 0.66 | 0.78 | 0.40 | 1.45 | 0.45 | 0.68 | 0.38 | 1.19 | 0.19 |
| Donor's history of cocaine use (ref: No) | ||||||||||||
| Yes | 1.39 | 0.46 | 3.65 | 0.52 | 0.50 | 0.21 | 1.04 | 0.09 | 0.59 | 0.29 | 1.09 | 0.11 |
| Donor gender (ref: Female) | ||||||||||||
| Male | 1.18 | 0.52 | 2.89 | 0.70 | 1.38 | 0.82 | 2.40 | 0.24 | 1.41 | 0.89 | 2.28 | 0.15 |
| Donor BMI (kg/m2) | 1.04 | 0.96 | 1.12 | 0.28 | 0.99 | 0.94 | 1.04 | 0.75 | 0.99 | 0.94 | 1.03 | 0.51 |
| Donor's history of diabetes (ref: No) | ||||||||||||
| Yes | 2.56 | 0.59 | 9.13 | 0.17 | 1.78 | 0.68 | 4.23 | 0.21 | 1.59 | 0.67 | 3.50 | 0.26 |
| Donor's history of hypertension (ref: No) | ||||||||||||
| Yes | 2.02 | 0.76 | 5.17 | 0.15 | 1.39 | 0.74 | 2.54 | 0.29 | 1.36 | 0.79 | 2.30 | 0.26 |
| High risk for organ donor (ref: No) | ||||||||||||
| Yes | 0.93 | 0.24 | 2.80 | 0.91 | 0.86 | 0.36 | 1.83 | 0.71 | 1.19 | 0.60 | 2.21 | 0.60 |
| Donor's race (ref: White) | ||||||||||||
| Black or African American | 0.36 | 0.05 | 1.40 | 0.20 | 0.84 | 0.33 | 1.84 | 0.68 | 0.66 | 0.28 | 1.37 | 0.30 |
| Hispanic Latino | 0.23 | 0.01 | 1.23 | 0.17 | 0.67 | 0.22 | 1.67 | 0.43 | 0.72 | 0.28 | 1.59 | 0.45 |
| Others | 0.63 | 0.03 | 3.71 | 0.68 | 1.95 | 0.53 | 5.64 | 0.26 | 1.30 | 0.36 | 3.63 | 0.65 |
| Donor warm ischemic time | 0.99 | 0.95 | 1.03 | 0.73 | 1.01 | 0.98 | 1.03 | 0.40 | 1.01 | 0.99 | 1.03 | 0.35 |
| Recipient's age group (ref: > 64 years) | ||||||||||||
| 18-34 years | 3.86 | 0.33 | 45.54 | 0.26 | 1.55 | 0.20 | 7.94 | 0.63 | 1.83 | 0.34 | 7.71 | 0.44 |
| 35-49 years | 1.44 | 0.29 | 10.60 | 0.68 | 0.91 | 0.39 | 2.23 | 0.83 | 0.71 | 0.34 | 1.53 | 0.37 |
| 50-64 years | 1.80 | 0.48 | 11.91 | 0.45 | 0.92 | 0.45 | 2.02 | 0.82 | 0.79 | 0.43 | 1.52 | 0.46 |
| Recipient gender (ref: Female) | ||||||||||||
| Male | 1.27 | 0.53 | 3.28 | 0.61 | 1.84 | 1.04 | 3.39 | 0.04 | 1.76 | 1.07 | 2.98 | 0.03 |
| Recipient race (ref: White) | ||||||||||||
| Black or African American | 3.36 | 1.12 | 9.14 | 0.02 | 1.62 | 0.73 | 3.32 | 0.21 | 1.32 | 0.64 | 2.54 | 0.43 |
| Hispanic Latino | 2.80 | 0.99 | 7.27 | 0.04 | 1.01 | 0.48 | 1.96 | 0.98 | 0.95 | 0.50 | 1.71 | 0.86 |
| Others | - | - | - | - | 1.11 | 0.25 | 3.46 | 0.88 | 1.03 | 0.29 | 2.87 | 0.96 |
| Recipient primary diagnosis (ref: Alcohol-associated) | ||||||||||||
| Non-alcoholic steatohepatitis | 2.37 | 0.48 | 11.44 | 0.27 | 1.95 | 0.79 | 4.71 | 0.14 | 1.43 | 0.63 | 3.15 | 0.38 |
| Other/mixed | 6.06 | 1.86 | 24.03 | a | 2.07 | 0.92 | 4.62 | 0.08 | 1.92 | 0.95 | 3.90 | 0.07 |
| Viral | 1.94 | 0.63 | 7.33 | 0.28 | 1.87 | 1.00 | 3.67 | 0.06 | 1.98 | 1.15 | 3.54 | 0.02 |
| Model for end-stage liver disease score | 1.06 | 1.02 | 1.12 | 0.01 | 1.05 | 1.02 | 1.08 | 0.00 | 1.04 | 1.02 | 1.07 | a |
| Recipient BMI (kg/m2) | 1.02 | 0.96 | 1.09 | 0.50 | 1.00 | 0.96 | 1.05 | 0.95 | 1.02 | 0.98 | 1.05 | 0.41 |
| Recipient warm ischemic time | 1.00 | 0.98 | 1.02 | 0.81 | 0.99 | 0.98 | 1.00 | 0.21 | 0.99 | 0.98 | 1.00 | 0.32 |
| Ventilator (ref: No) | ||||||||||||
| Yes | 3.93 | 0.83 | 15.26 | 0.06 | 3.91 | 1.28 | 11.13 | 0.01 | 4.73 | 1.71 | 12.71 | a |
| Frail status at transplant (non-frailty) | ||||||||||||
| Frailty | 1.04 | 0.40 | 2.99 | 0.94 | 1.60 | 0.86 | 3.12 | 0.15 | 1.89 | 1.09 | 3.41 | 0.03 |
Although a higher MELD score is linked to increased odds of mortality within 1-12 months following a liver trans
The frailty at transplant showed a significant association with factors, including receiving livers from DCD donors compared to DBD donors (OR = 1.86; P < 0.001; 95%CI: 1.30-2.65), an increasing MELD score (OR = 1.10; P < 0.001; 95%CI: 1.08-1.13), donor without the history of hypertension (OR = 0.65; P = 0.03; 95%CI: 0.44-0.96), and a shorter duration of recipients’ warm ischemic time (OR = 0.989; P = 0.002; 95%CI: 0.983-0.996) (Table 4).
| Variables | Odds ratio | The lower boundary of the 95%CI, i.e., 2.5% of CI | The upper boundary of the 95%CI, i.e., 97.5% of CI | P value | Coefficient | SE | Statistics values |
| Donation pathway (ref: Donation after brain death) | |||||||
| Donation after circulatory death | 1.86 | 1.30 | 2.65 | < 0.001a | 0.62 | 0.18 | 3.44 |
| Donor's age group (ref: > 64 years) | |||||||
| 18-34 years | 1.19 | 0.48 | 2.99 | 0.70 | 0.18 | 0.46 | 0.38 |
| 35-49 years | 1.53 | 0.62 | 3.79 | 0.36 | 0.42 | 0.46 | 0.92 |
| 50-64 years | 1.05 | 0.42 | 2.63 | 0.92 | 0.05 | 0.47 | 0.10 |
| Donor continued use cigarette in past 6 month (ref: No) | |||||||
| Yes | 1.09 | 0.73 | 1.63 | 0.68 | 0.08 | 0.20 | 0.41 |
| Donor's history of cocaine use (ref: No) | |||||||
| Yes | 1.15 | 0.75 | 1.77 | 0.53 | 0.14 | 0.22 | 0.63 |
| Donor gender (ref: Female) | |||||||
| Male | 1.09 | 0.78 | 1.50 | 0.62 | 0.08 | 0.16 | 0.50 |
| Donor BMI (kg/m2) | 0.99 | 0.96 | 1.02 | 0.49 | -0.01 | 0.01 | -0.69 |
| Donor's history of diabetes (ref: No) | |||||||
| Yes | 0.78 | 0.42 | 1.47 | 0.43 | -0.25 | 0.32 | -0.78 |
| Donor's history of hypertension (ref: No) | |||||||
| Yes | 0.65 | 0.44 | 0.96 | 0.03a | -0.43 | 0.20 | -2.20 |
| High risk for organ donor (ref: No) | |||||||
| Yes | 1.11 | 0.69 | 1.81 | 0.68 | 0.10 | 0.25 | 0.42 |
| Donor's race (ref: White) | |||||||
| Black or African American | 1.19 | 0.72 | 2.01 | 0.51 | 0.17 | 0.26 | 0.66 |
| Hispanic Latino | 0.75 | 0.43 | 1.34 | 0.32 | -0.29 | 0.29 | -0.99 |
| Others | 0.53 | 0.23 | 1.29 | 0.15 | -0.63 | 0.43 | -1.44 |
| Donor warm ischemic time (minutes) | 1.00 | 0.99 | 1.02 | 0.85 | 0.00 | 0.01 | 0.19 |
| Recipient's age group (ref: > 64 years) | |||||||
| 18-34 years | 0.65 | 0.16 | 2.90 | 0.55 | -0.43 | 0.72 | -0.60 |
| 35-49 years | 0.83 | 0.48 | 1.44 | 0.51 | -0.18 | 0.28 | -0.65 |
| 50-64 years | 1.12 | 0.69 | 1.79 | 0.63 | 0.11 | 0.24 | 0.48 |
| Recipient gender (ref: Female) | |||||||
| Male | 0.80 | 0.56 | 1.13 | 0.21 | -0.23 | 0.18 | -1.26 |
| Recipient race (ref: White) | |||||||
| Black or African American | 0.65 | 0.39 | 1.10 | 0.10 | -0.42 | 0.26 | -1.63 |
| Hispanic Latino | 1.62 | 1.00 | 2.71 | 0.06 | 0.48 | 0.25 | 1.90 |
| Others | 1.26 | 0.59 | 2.90 | 0.56 | 0.23 | 0.40 | 0.58 |
| Recipient primary diagnosis (ref: Alcohol-associated) | |||||||
| Non-alcoholic steatohepatitis | 0.95 | 0.53 | 1.70 | 0.85 | -0.06 | 0.30 | -0.19 |
| Other/mixed | 0.87 | 0.52 | 1.46 | 0.60 | -0.14 | 0.26 | -0.52 |
| Viral | 0.79 | 0.53 | 1.17 | 0.24 | -0.24 | 0.20 | -1.19 |
| Model for end-stage liver disease score | 1.10 | 1.08 | 1.13 | < 0.001a | 0.10 | 0.01 | 8.79 |
| Recipient BMI (kg/m2) | 1.01 | 0.98 | 1.04 | 0.65 | 0.01 | 0.01 | 0.46 |
| Recipient warm ischemic time (minutes) | 0.989 | 0.983 | 0.996 | 0.002a | -0.01 | 0.00 | -3.08 |
Since there are significant missing values for the variable Recipient warm ischemic time, we conducted a sensitivity analysis to confirm the consistency of our analysis. Specifically, we excluded this variable from the multivariable analysis to include all the recipients in the analysis. This analysis confirmed the significant association of frailty with mortality at 1 month, 6 months, and 12 months (OR = 1.57, OR = 1.48, and OR = 1.64, respectively).
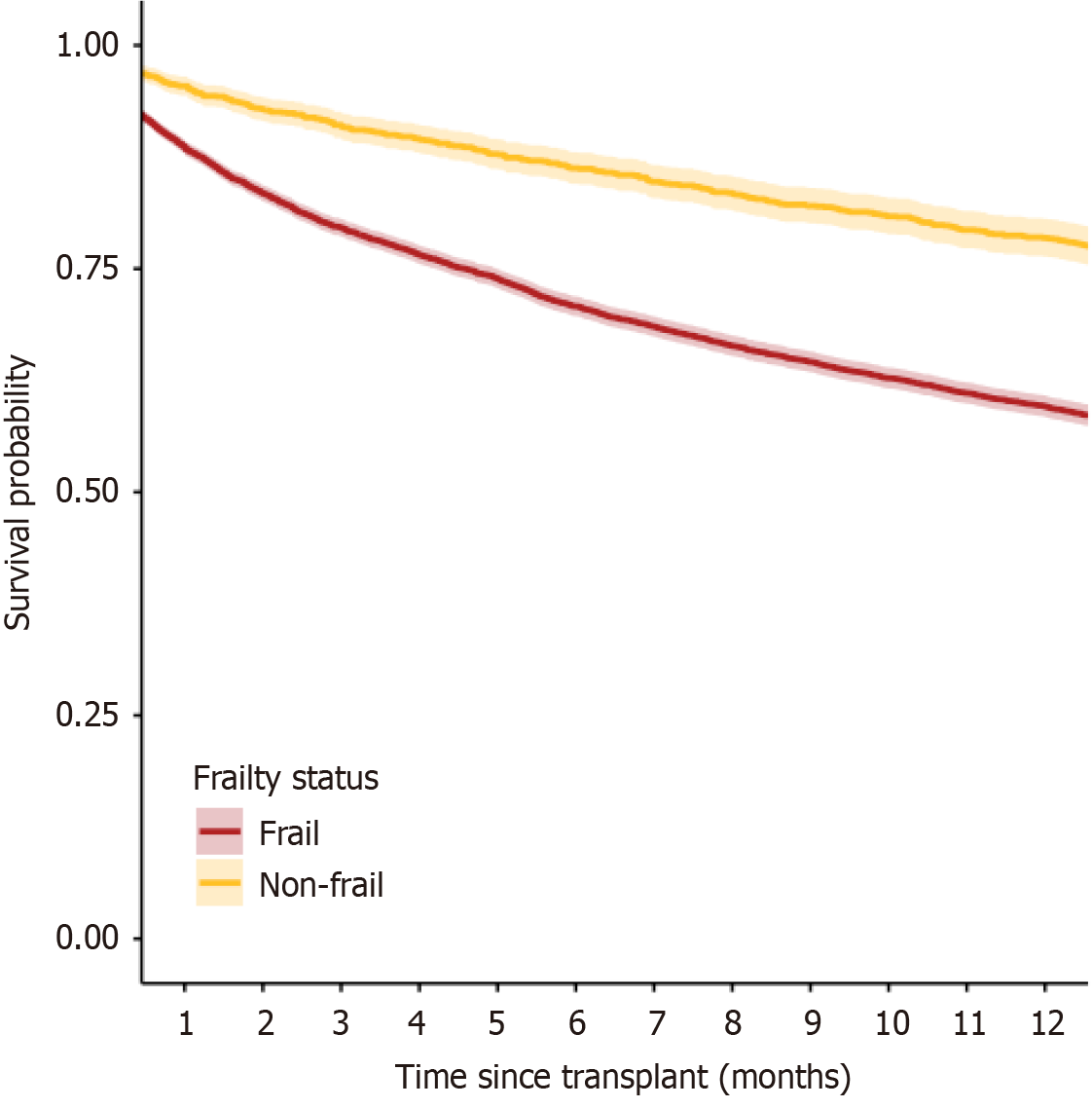
Furthermore, although we were interested in estimating OR for mortality, two further confirm the robustness of our results we included a survival cure for the two frailty subgroups (Figure 2), presenting a significant difference between the survival rates between the two groups within 12 months following liver transplant.
Frailty, a decline in physical capabilities and functional status contributes significantly to morbidity and prolonged hospitalization[17]. This study specifically aimed to assess the impact of the frailty on 1-month, 6-month, and 12-month mortality post-liver transplantation in patients with chronic liver cirrhosis. Data from the SRTR database, validated for both pediatric and adult transplant candidates, helped inform our analysis[18-20].
In this study, we utilized an updated KPS, and intentionally converted the frailty assessment to a binary format (frail vs non-frail) in order to enhance its reliability over more nuanced frailty assessment results in the database. An important finding from Klein et al[21] is the significant variability in interrater reliability of the KPS, which led to a reduction in frailty categories from 10 to 4, enhancing the tool's utility by simplifying its scale. Previous research, involving 114 patients, supported our approach by demonstrating that non-frail patients with a median MELD score of 16 had notably better survival outcomes post-transplant[21]. Our analysis mirrored these previously published approaches and was consistent with the existing literature in that it demonstrated that frail recipients had significantly higher post-transplant mortality at 12 months, a critical period when transplant centers' monitoring traditionally wanes[11,22].
Our study also showed a declining trend of the association between MELD score and post-transplant mortality, but an increasing trend between frailty and post-transplant mortality, from 1 month to 12 months post-transplant. This pattern is consistent with what we see clinically, in that higher MELD score patients are more likely to receive an organ due to accepted acuity protocols. Unlike MELD score, frailty is a potentially modifiable risk factor. These trends demonstrate a potential area of intervention through nutrition optimization and physical and occupational therapies in order to improve long-term post-transplant outcomes.
The current study showed a significant difference in warm ischemic time between frail and non-frail recipients, although it cannot be reliably evaluated prior to the transplant. Other studies also included cold ischemic time (CIT) to reflect on the importance of transportation distance for allocating organs and impacting recipient and graft survival outcomes[23]. Paterno et al[24] found longer CIT negatively impacted postoperative liver transplants, while a slightly longer warm ischemic time (up to 40 minutes) was not associated with graft loss. Therefore, it is important to note that the warm ischemic time differential that we observe in this case may not be clinically significant considering the magnitude of the observed change.
The outcomes of the current study showed a safeguarding association between donors with a history of hypertension and non-frail patients. Although chronic hypertension history did not differ significantly in terms of mortality in any of the shorter time intervals in this study, a retrospective study showed donor hypertension as the only donor risk factor significantly related to 1-month mortality due to graft failure[25]. Further prospective study is warranted to evaluate this phenomenon. However, based on our findings, chronic hypertension should not necessarily be viewed as a negative factor for long-term graft function.
Secondary analysis of the data revealed that DCD liver grafts were more likely to be transplanted in frail patients[26]. A critical shortage of organs has prompted an increase in the use of DCD donors for liver transplantation[27]. This critical shortage, coupled with the increased number of patients currently awaiting liver transplantation[28], can result in transplant centers accepting a donor organ that may be better suited to other recipients. It is plausible that centers are more prone to accept DCD grafts for frail patients as these patients are likely in increased clinical duress and therefore the risk of accepting a DCD organ would be outweighed by the possibility of not receiving a graft at all. Several studies have evaluated the outcomes of liver transplants using DCD organs. A prospective analysis in the United Kingdom found that both graft loss and recipient mortality were approximately twice as high in DCD livers than in DBD livers. They concluded that the results of their investigation could help shape policies for the use of DCD liver grafts[29].
Croome et al[30] evaluated DCD grafts from donors aged > 50 years and concluded that optimizing recipient selection criteria and minimizing CIT would improve outcomes. Dutkowski et al[31] created a scoring system to detect unfavorable combinations of donor and recipient factors. They concluded that DCD liver grafts should be used in patients with a balance of risk score ≤ 9[31]. DCD grafts do increase the number of organs available for transplantation; however, our research demonstrated that these grafts were being utilized in patients with a higher frailty score. Our study suggests that these grafts may be better utilized in non-frail patients awaiting liver transplantation. Utilization of normothermic regional perfusion (in situ and ex situ) to evaluate the graft’s ability to overcome the functional warm ischemia time may aid in both short-term and long-term organ function when DCD grafts are transplanted in frail patients.
A key strength of our study is the use of a large, multicenter database from the SRTR, which collects data from various transplant centers across different geographic regions. This diverse and comprehensive dataset enhances the generalizability and rigor of our findings, reducing potential biases associated with single-center studies.
The extensive coverage of demographic, clinical, and outcome variables allows for a nuanced analysis of frailty's impact on liver transplant outcomes. Such detailed insights are crucial for refining clinical practices and developing policies to improve outcomes for frail patients. Moreover, the geographic diversity ensures that our results are relevant to different healthcare systems, enhancing the external validity and contributing significantly to the literature on frailty in liver transplantation. This broad scope aids in guiding future research toward targeted interventions and management strategies for this complex condition.
However, the retrospective nature of the data, varying definitions of frailty, limited knowledge of DCD donor clinical characteristics, and the absence of histological data to assess graft function post-transplant all pose challenges to interpreting our findings fully. Moreover, while the impact of warm ischemic times emerged as a notable factor, its clinical significance remains uncertain without direct histological confirmation. It is also noted that while the SRTR database is comprehensive in scope since the data obtained is required to be submitted by transplanting centers, there is some distrust among clinicians about the accuracy of nuanced data such as frailty scores in the SRTR database.
Despite the challenges associated with large database research, our findings contribute to the understanding of how frailty influences liver transplant outcomes. Additionally, our study emphasizes the need for refined assessment tech
In conclusion, as documented in the SRTR database, recipient frailty may be associated with increased mortality at 12 months following liver transplantation to a degree similar to the increased mortality associated with the MELD score. Liver transplants from donors with DCD additionally may be associated with increased frailty of the recipient. Chronic hypertension in organ donors is associated with lower frailty in liver transplant recipients. Further prospective studies are warranted to evaluate these findings.
| 1. | Medscape. Cirrhosis: Practice Essentials, Overview, Etiology. 2021. Available from: https://emedicine.medscape.com/article/185856-overview?form=fpf. |
| 2. | Mahmud N. Selection for Liver Transplantation: Indications and Evaluation. Curr Hepatol Rep. 2020;19:203-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Cuende N, Miranda B, Cañón JF, Garrido G, Matesanz R. Donor characteristics associated with liver graft survival. Transplantation. 2005;79:1445-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Lai JC, Sonnenday CJ, Tapper EB, Duarte-Rojo A, Dunn MA, Bernal W, Carey EJ, Dasarathy S, Kamath BM, Kappus MR, Montano-Loza AJ, Nagai S, Tandon P. Frailty in liver transplantation: An expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant. 2019;19:1896-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 5. | Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13384] [Cited by in RCA: 15964] [Article Influence: 665.2] [Reference Citation Analysis (1)] |
| 6. | Laube R, Wang H, Park L, Heyman JK, Vidot H, Majumdar A, Strasser SI, McCaughan GW, Liu K. Frailty in advanced liver disease. Liver Int. 2018;38:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Lai JC, Rahimi RS, Verna EC, Kappus MR, Dunn MA, McAdams-DeMarco M, Haugen CE, Volk ML, Duarte-Rojo A, Ganger DR, O'Leary JG, Dodge JL, Ladner D, Segev DL. Frailty Associated With Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology. 2019;156:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 8. | Sundaram V, Lim J, Tholey DM, Iriana S, Kim I, Manne V, Nissen NN, Klein AS, Tran TT, Ayoub WS, Schlansky B. The Braden Scale, A standard tool for assessing pressure ulcer risk, predicts early outcomes after liver transplantation. Liver Transpl. 2017;23:1153-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Rowe R, Iqbal J, Murali-Krishnan R, Sultan A, Orme R, Briffa N, Denvir M, Gunn J. Role of frailty assessment in patients undergoing cardiac interventions. Open Heart. 2014;1:e000033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45:2220-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 11. | Orman ES, Ghabril M, Chalasani N. Poor Performance Status Is Associated With Increased Mortality in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Perito ER, Bucuvalas J, Lai JC. Functional status at listing predicts waitlist and posttransplant mortality in pediatric liver transplant candidates. Am J Transplant. 2019;19:1388-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Dolgin NH, Movahedi B, Anderson FA, Brüggenwirth IM, Martins PN, Bozorgzadeh A. Impact of recipient functional status on 1-year liver transplant outcomes. World J Transplant. 2019;9:145-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 14. | Dolgin NH, Martins PN, Movahedi B, Lapane KL, Anderson FA, Bozorgzadeh A. Functional status predicts postoperative mortality after liver transplantation. Clin Transplant. 2016;30:1403-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Thuluvath PJ, Thuluvath AJ, Savva Y. Karnofsky performance status before and after liver transplantation predicts graft and patient survival. J Hepatol. 2018;69:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Wang CW, Lai JC. Reporting functional status in UNOS: The weakness of the Karnofsky Performance Status Scale. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 391] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 18. | Luo X, Mogul DB, Massie AB, Ishaque T, Bridges JFP, Segev DL. Predicting chance of liver transplantation for pediatric wait-list candidates. Pediatr Transplant. 2019;23:e13542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Haugen CE, Thomas AG, Chu NM, Shaffer AA, Norman SP, Bingaman AW, Segev DL, McAdams-DeMarco M. Prevalence of frailty among kidney transplant candidates and recipients in the United States: Estimates from a National Registry and Multicenter Cohort Study. Am J Transplant. 2020;20:1170-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Aggarwal R, Jackson S, Lemke NT, Trager L, Shumway SJ, Kelly RF, Hertz M, Huddleston SJ. Time since primary transplant and poor functional status predict survival after redo lung transplant. J Thorac Dis. 2022;14:3819-3830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Klein CG, Malamutmann E, Latuske J, Tagay S, Dörri N, Teufel M, Paul A, Oezcelik A. Frailty as a predictive factor for survival after liver transplantation, especially for patients with MELD≤15-a prospective study. Langenbecks Arch Surg. 2021;406:1963-1969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Kremer WM, Nagel M, Reuter M, Hilscher M, Michel M, Kaps L, Labenz J, Galle PR, Sprinzl MF, Wörns MA, Labenz C. Validation of the Clinical Frailty Scale for the Prediction of Mortality in Patients With Liver Cirrhosis. Clin Transl Gastroenterol. 2020;11:e00211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Stahl JE, Kreke JE, Malek FA, Schaefer AJ, Vacanti J. Consequences of cold-ischemia time on primary nonfunction and patient and graft survival in liver transplantation: a meta-analysis. PLoS One. 2008;3:e2468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Paterno F, Guarrera JV, Wima K, Diwan T, Cuffy MC, Anwar N, Woodle ES, Shah S. Clinical Implications of Donor Warm and Cold Ischemia Time in Donor After Circulatory Death Liver Transplantation. Liver Transpl. 2019;25:1342-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Rana A, Kaplan B, Jie T, Porubsky M, Habib S, Rilo H, Gruessner AC, Gruessner RW. A critical analysis of early death after adult liver transplants. Clin Transplant. 2013;27:E448-E453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Lai JC, Covinsky KE, Hayssen H, Lizaola B, Dodge JL, Roberts JP, Terrault NA, Feng S. Clinician assessments of health status predict mortality in patients with end-stage liver disease awaiting liver transplantation. Liver Int. 2015;35:2167-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | United Network for Organ Sharing. US Organ Transplantation. Available from: https://unos.org/. |
| 28. | Health Resources & Services Administration. Detailed Description of Data. Available from: https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description. |
| 29. | Callaghan CJ, Charman SC, Muiesan P, Powell JJ, Gimson AE, van der Meulen JH; UK Liver Transplant Audit. Outcomes of transplantation of livers from donation after circulatory death donors in the UK: a cohort study. BMJ Open. 2013;3:e003287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Croome KP, Mathur AK, Lee DD, Moss AA, Rosen CB, Heimbach JK, Taner CB. Outcomes of Donation After Circulatory Death Liver Grafts From Donors 50 Years or Older: A Multicenter Analysis. Transplantation. 2018;102:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 31. | Dutkowski P, Schlegel A, Schadde E, Oberkofler C, Müllhaupt B, Clavien P. Risk Assessment in DCD Liver Transplantation-Which Recipient Should Be Selected? Transplantation. 2012;94:155. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |