Published online Jun 18, 2025. doi: 10.5500/wjt.v15.i2.98055
Revised: November 10, 2024
Accepted: January 23, 2025
Published online: June 18, 2025
Processing time: 249 Days and 18.2 Hours
Pancreatic cystic lesions are common in patients eligible for solid organ transplan
To evaluate the prevalence of pancreatic cystic lesions and the risk of cyst progression in immunosuppressed patients.
A systematic literature search was performed in relevant databases. Studies reporting either on the prevalence and/or the incidence of pancreatic cyst progression compared to a control group were implemented in the first systematic review and meta-analysis on this topic.
The prevalence of pancreatic cystic lesions was comparable with 7% (95%CI: 5%-11%) in the immunosuppressed cohort and 9% (95%CI: 5%-16%) in the control cohort. The mean cyst size increase in the immunosuppression group was 3.2 mm (range 1.0-5.2mm) compared to 3.5 mm (1.0-6.9) in the control group (standar
Immunosuppression does not increase the prevalence of pancreatic cystic lesions, nor does it increase the risk of cyst progression in terms of cyst size and development of resection criteria. Therefore, pancreatic cystic lesions in transplant candidates should not be a contraindication for solid organ transplantation.
Core Tip: Immunosuppression does not increase the prevalence of pancreatic cystic lesions, nor does it increase the risk cyst progression in terms of size and development and resection criteria or worrisome features.
- Citation: Kießler M, Friess H, Assfalg V. Prevalence and risk of progression of pancreatic cystic lesions in immunosuppressed patients: A systematic review and meta-analysis. World J Transplant 2025; 15(2): 98055
- URL: https://www.wjgnet.com/2220-3230/full/v15/i2/98055.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i2.98055
Organ transplantation improves patient survival and quality of life and is considered the gold standard in the treatment of patients with end-stage organ failure. However, it comes at the cost of the need for lifelong immunosuppression, which, among other side effects, increases the risk of developing cancer[1,2].
Pancreatic cystic lesions are an increasingly common incidental finding, with up to 8% of all patients undergoing an abdominal computed tomography (CT) or magnetic resonance imaging (MRI) scan[3]. Pancreatic cystic lesions are a rather heterogeneous group, including intraductal papillary mucinous neoplasm (IPMN), mucinous cystic neoplasm (MCN), serous cystic adenomas, pseudocysts, cystic pancreatic neuroendocrine tumors, and solid pseudopapillary neoplasms. IPMNs and MCNs are of particular interest because they are considered precancerous lesions, with approximately 8% of all pancreatic cancers arising from one of these two[4,5].
It is reasonable to assume that immunosuppression may also promote the development and/or progression of pancreatic cystic lesions. It is for the above mentioned frequency not unusual for physicians to encounter a patient with cystic pancreatic lesions in preparation for organ transplantation. But so far there are no guidelines or comprehensive studies to guide doctors. Therefore, the aim of this study was to assess and summarize the current literature on the prevalence of cystic pancreatic lesions and the risk of cyst progression in patients under immunosuppression for the first time in a systematic review and meta-analysis.
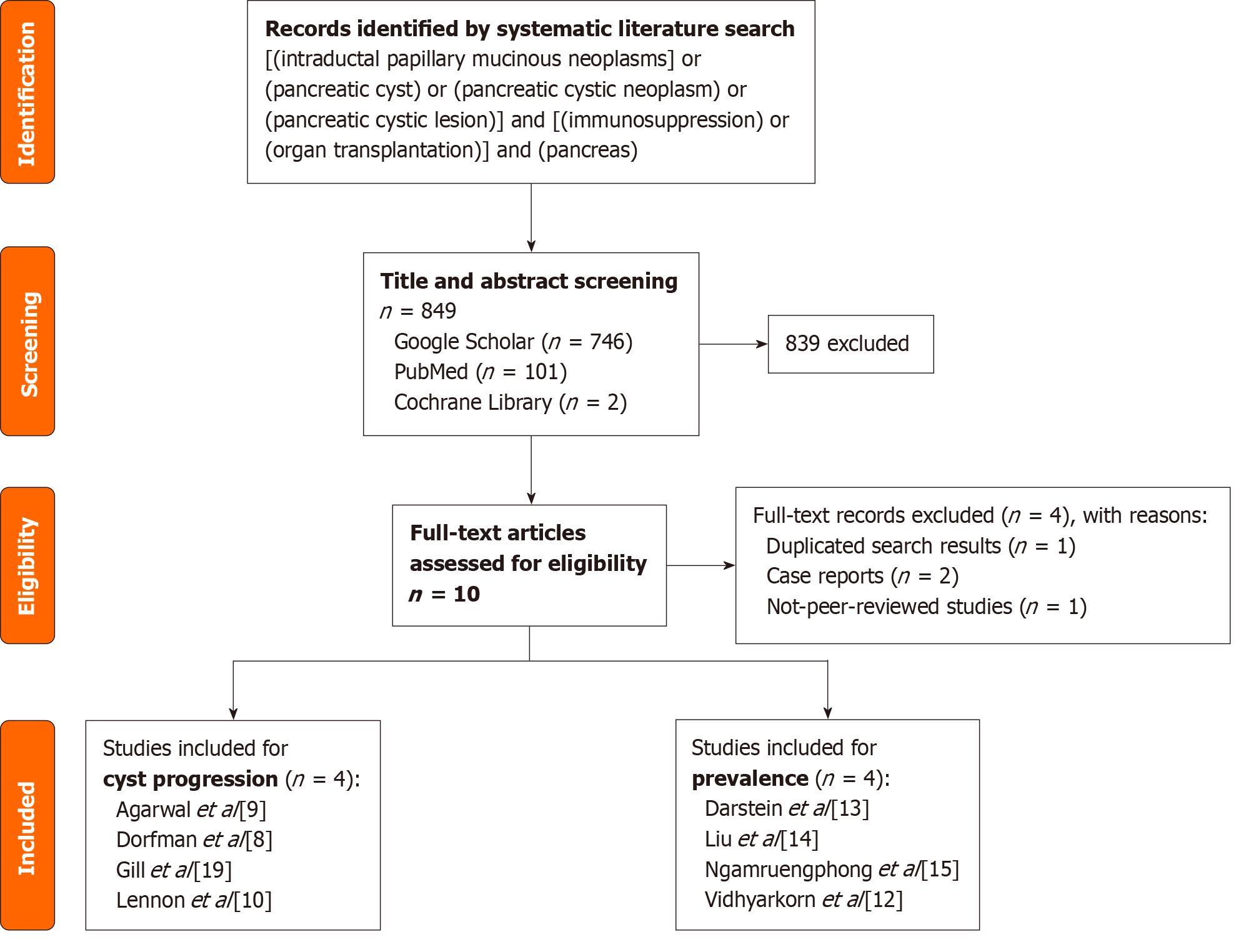
A systematic literature search for was performed using the following terms in relevant databases (Google Scholar, PubMed, Cochrane Library): [“Intraductal Papillary Mucinous Neoplasms” OR “Pancreatic cyst” OR “Pancreatic cystic neoplasm”] AND [“Immunosuppression” OR “organ transplantation”] AND [“pancreas”]. In conducting the search in PubMed and the Cochrane Library, the search terms were entered without quotation marks. In contrast, quotation marks were used when entering the search terms in Google Scholar.
A total of 953 records were identified. Duplicate studies, case reports, and no-peer-reviewed studies were excluded. The subsequent title and abstract screening identified 10 studies for full-text screening. Two studies reported on the prevalence of pancreatic cystic lesions in immunosuppressed patients and the incidence of cyst progression in comparison to a control group. Another two studies reported only on the prevalence of cystic pancreatic lesions under immunosuppression. And again, two studies reported on the incidence of cyst progression compared to a control group. Thus, two comparison groups could be formed with four studies each.
To evaluate the prevalence of pancreatic cystic lesions in non-immunosuppressed patients, a systematic literature search was initiated. However, a recent meta-analysis, including a total of 17 studies that addressed this very question. It was therefore decided to include these studies for comparison. One study from this group was excluded due to diffe
The meta-analysis was performed using the meta package in R[6,7]. The prevalence of cystic pancreatic lesions in the immunosuppression group and in the control group were pooled using the generalized linear mixed models method implemented in the meta package using the metaprop function[6,7]. For the increase in cyst size, the standardized mean difference between the cyst size increase in the immunosuppression group and the control group was calculated using the Hedges' g method. When standard deviation was not reported in the publication, it was calculated from given P values[8,9] or from the given interquartile range[10] using methods published by Wan et al[11]. The increase in cyst size was then analyzed using the metacont function of the meta package. For the meta-analysis on cyst progression to the primary outcome, the number of events in both groups was extracted from the full texts. The primary outcome was defined as the meeting resection criteria or the development of worrisome features (for more details, see Table 1). The relative risk was pooled using the Mantel-Haenszel method implemented in the metabin function of the meta package[6].
| Agarwal et al[9], 2016 | |
| Patients n (IS/ control) | 26/26 |
| Mean Age in years (IS/control) | 60/63 |
| Reason for IS | Solid organ transplantation (35.9%), autoimmune disorders (33.3%), inflammatory bowel disease (15.4%), other conditions (15.4%) |
| Follow up in months (IS/control) | 24.3/21.5 |
| Type of included cysts | IPMN, MCN |
| Primary outcome | 1. Obstructive jaundice in a patient with cystic lesion of the head of the pancreas |
| 2. Enhancing solid components within the cyst | |
| 3. Main pancreatic duct diameter measuring 10 mm or larger | |
| 4. Cyst progression to 30 mm or larger | |
| 5. Development of thickened/enhancing cyst walls | |
| 6. Increase of main pancreatic duct size 5-9 mm | |
| 7. Development of non-enhancing mural nodules | |
| 8. Abrupt change in caliber of the pancreatic duct with distal pancreatic atrophy | |
| Dorfman et al[8], 2016 | |
| Patients n (IS/control) | 16/131 |
| Mean age in years (IS/control) | 61/70 |
| Type of included cysts | IPMN |
| Reason for IS | Liver transplantation |
| Follow up in months (IS/control) | 31/31 |
| Primary outcome | Development of: |
| 1. Presence of mural nodules | |
| 2.Cyst size of ≥ 30 mm | |
| 3. Thickened cyst walls | |
| 4. Pancreatic duct diameter of 5 to 9 mm | |
| 5. Abrupt change in the duct caliber with distal pancreatic atrophy and lymphadenopathy | |
| Gill et al[16], 2009 | |
| Patients n (IS/control) | 33/57 |
| Mean age in years (IS/control) | 63/68 |
| Reason for IS | Solid organ transplantation [liver (81.8%), kidney (15.2%), heart (3.0%)] |
| Follow up in months (IS/control) | 29/28 |
| Type of included cysts | IPMN |
| Primary outcome | Consensus indication for resection (i.e., cyst-related symptoms, cyst size > 30 mm, mural nodules, positive cytology) |
| Lennon et al[10], 2014 | |
| Patients n (IS/control) | 23/274 |
| Mean age in years (IS/control) | 56.8/63.7 |
| Reason for IS | Liver transplantation |
| Follow up in months (IS/control) | 53.7/24 |
| Type of included cysts | IPMN |
| Primary outcome | 1. Jaundice secondary to the pancreatic cyst |
| 2. Mural nodule or solid component within the cyst | |
| 3. Cytology suspicious or diagnostic for malignancy | |
| 4. Cyst diameter 3 cm | |
| 5. Main pancreatic duct involvement (pancreatic duct diameter 5 mm) | |
Eggers test was performed to rule out potential publication bias. For cyst size increase (intercept: -30.6, 95%CI: -55.0 to -6.3, P value 0.13) and progression to the primary outcome (intercept: -0.69, 95%CI: -3.60 to 2.22, P value 0.69) the Eggers test did not reveal any significant asymmetry.
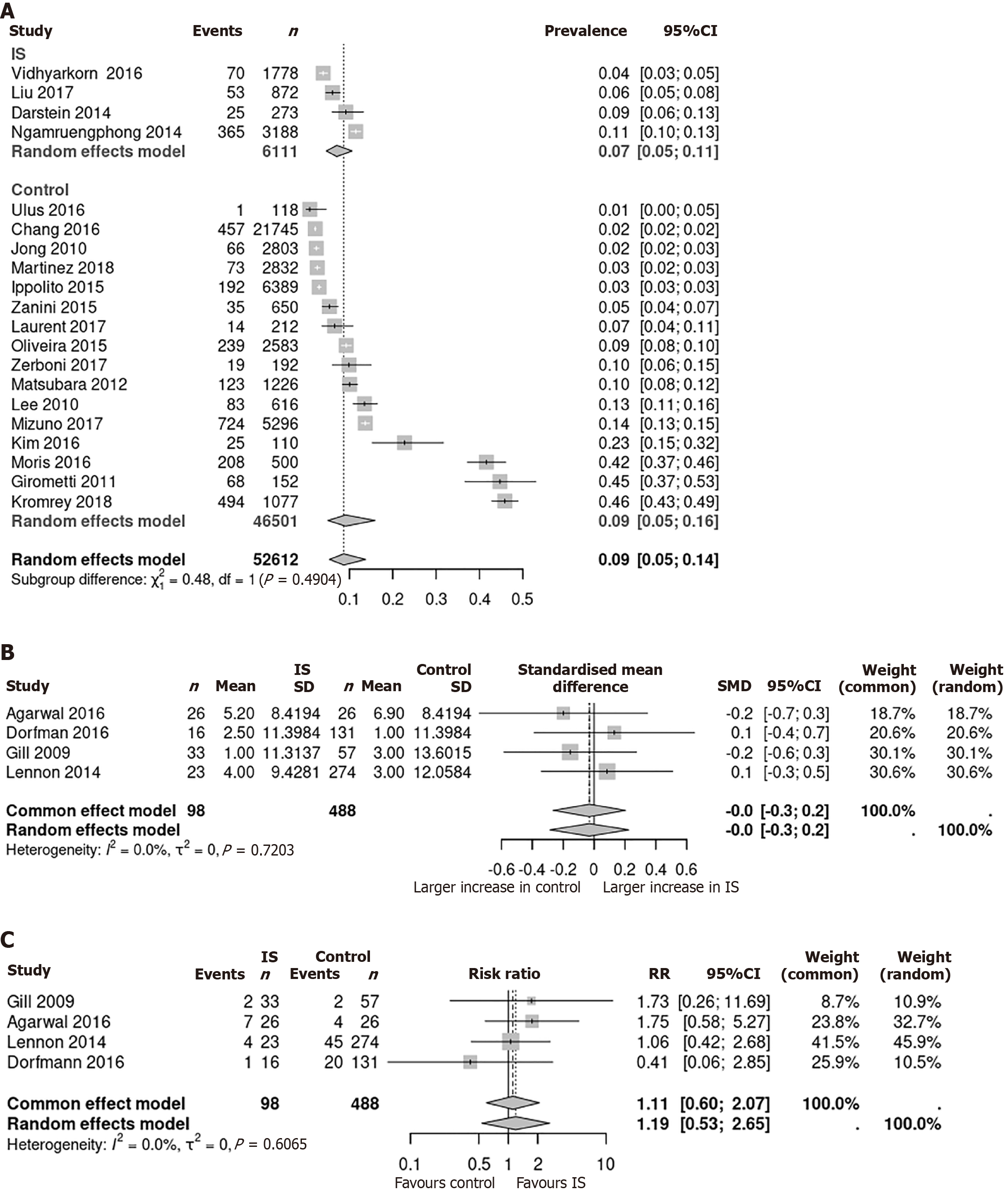
Four studies have reported the prevalence of pancreatic lesions in patients under immunosuppression[12-15]. These studies included a total number of 6111 patients. Within this group, 513 patients were diagnosed with at least one cystic pancreatic lesion by CT or MRI imaging. The reason for immunosuppression was liver transplantation in three of the four studies (Darstein et al[13], Liu et al[14], Vidhyarkorn et al[12]) and transplantation of liver or combined liver-kidney (56.2%), kidney alone (28.2%), lung (10.3%), heart (5.2%) in the study by Ngamruengphong et al[15].
For comparison with the prevalence in a healthy, non-immunosuppressed population, we included the studies identified in a meta-analysis by Zerboni et al[3]. One study was excluded because endosonographic ultrasound was used for cyst detection for comparability. The remaining 16 studies included 46501 patients, and 2821 patients had at least one cystic pancreatic lesion detected by either MRI or CT imaging.
In immunosuppressed patients, the pooled prevalence was 7% (95%CI: 5%-11%). The mean prevalence of cystic pancreatic lesions in non-immunosuppressed individuals was 9% (95%CI: 5%-16%). There was no significant difference between the two cohorts (P = 0.49; Figure 2A).
Study population: A literature search identified four studies of 586 patients, comparing the incidence of cyst progression in an immunosuppressed cohort (n = 98) with a healthy control group (n = 488). Mean follow-up ranged from 24.3 to 53.7 months in the immunosuppressed group and from 21.5 to 31 months in the control group. In three of four studies, all cysts were characterized as IPMN. Agarwal et al[9] combined IPMNs and MCNs in their study without providing information on the proportion of each. The reason for immunosuppression was liver transplantation in the studies of Dorfman et al[8] and Lennon et al[10]. In the study of Gill et al[16], patients were immunosuppressed due to transplan
The primary outcome was defined as the development of resection criteria either due to either cyst-related symptoms or worrisome features. A complete list of resection criteria is summarized in Table 1.
Increase in cyst size in patients under immunosuppression vs control: The cyst size increase was measured in all four identified studies. It ranged from 1.0 to 5.2 mm in the immunosuppressed subgroup and from 1.0 to 6.9 mm in the control group, respectively. The standardized mean difference between cyst size increase in immunosuppressed and non-immunosuppressed patients was calculated and ranged from -0.2 to 0.1 mm. Using a fixed and random effects model, a pooled effect size of 0.0 mm (95%CI: -0.3 to 0.2, P value 0.72) was calculated (Figure 2B).
Progression to the primary outcome under immunosuppression vs control: There were 14 events (14.2%) in the immunosuppression group, compared with 71 (14.5%) events in the control group. The relative risk of cyst progression ranged from 0.41 to 1.75 in the individual studies. A pooled relative risk was 1.11 (95%CI: 0.6 to 2.07) in the fixed-effect-model and 1.19 (95%CI: 0.53 to 2.65) in the random-effect-model (Figure 2C).
MCNs and IPMNs are precancerous lesions, and approximately 8% of all pancreatic cancers arise from one of the two[17]. Immunosuppression is a well-recognized risk factor for the development of malignant disease[1,2]. Immunosuppression, accompanying organ transplantation therefore poses a risk to organ recipients. Pancreatic cystic lesions, including MCNs and IPMNs, are found frequently in patients on the organ transplantation waiting list. For example, the study by Laurent et al[18] estimated an incidence of 6.6% in patients on the waiting list for a liver transplantation. At present, the data on cyst progression under immunosuppression is rather limited.
In order to answer the question of the influence of immunosuppression on cystic pancreatic lesions, we have put forward the following two hypotheses: First, if immunosuppression promotes the development of pancreatic lesions, the incidence of these lesions is expected to be higher in the immunosuppressed cohort. Second, if immunosuppression accelerates the progression of existing pancreatic cystic lesions, we should see a difference in the cyst growth and the incidence of development of worrisome features or malignant degeneration.
However, our results did not show a difference in the incidence of pancreatic cystic lesions between immunosuppressed and non-immunosuppressed patients. Regarding cystic progression under immunosuppression, we evaluated the two following two parameters: (1) Increase in cyst size over time; and (2) Development of resection criteria or worrisome features. For both parameters we did not find a significant increase in the immunosuppressed cohort. It can be concluded that immunosuppression does not increase the growth rate of pancreatic cysts or the risk of cyst progression.
However, some limitations of this study must be mentioned. First, the number of included studies is relatively small with four studies for each parameter evaluated (cyst size increase, progression to primary outcome). Additionally, in one of the four studies, the reason for immunosuppression included multiple reasons (e.g., organ transplantation, intestinal bowel disease, autoimmune disorders, and others), and the agent, rate, and dose of which immunosuppressive drugs are administered to these patients are different. Moreover, all of the included studies are of retrospective in nature with the inherent risk of a hindsight bias. With the increasing variety in immunosuppressive agents, the effects on pancreatic cystic lesions need more in-depth analysis, preferably in prospective trials.
An inherent problem of pancreatic cystic lesions is that in most cases the final diagnosis can only be made by the pathologist after pancreatic resection. However, the included studies rely on radiologic diagnosis and are therefore at risk of misdiagnosing a lesion and thus hiding the effect of immunosuppression on the lesion.
Here, we performed the first comprehensive systematic review and meta-analysis on the topic of pancreatic cystic lesions in patients under immunosuppression. Although our study is certainly flawed by the limitations mentioned above, it comprehensively summarizes the current literature on this topic and shows that immunosuppression does not increase the prevalence of pancreatic cystic lesions, nor does it increase the risk of cyst progression in terms of size and development of resection criteria or worrisome features.
| 1. | Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1090] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 2. | Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10:1889-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 346] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 3. | Zerboni G, Signoretti M, Crippa S, Falconi M, Arcidiacono PG, Capurso G. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology. 2019;19:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 4. | Keane MG, Afghani E. A Review of the Diagnosis and Management of Premalignant Pancreatic Cystic Lesions. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Stark A, Donahue TR, Reber HA, Hines OJ. Pancreatic Cyst Disease: A Review. JAMA. 2016;315:1882-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 6. | Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1954] [Cited by in RCA: 3078] [Article Influence: 513.0] [Reference Citation Analysis (0)] |
| 7. | R Core Team. R: A language and environment for statistical computing. 2018. [cited 10 November 2024]. Available from: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing. |
| 8. | Dorfman V, Verna EC, Poneros JM, Sethi A, Allendorf JD, Gress FG, Schrope BA, Chabot JA, Gonda TA. Progression of Incidental Intraductal Papillary Mucinous Neoplasms of the Pancreas in Liver Transplant Recipients. Pancreas. 2016;45:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Agarwal A, Scott FI, Ahmad NA, Chandrasekhara V. Chronic immunosuppression does not potentiate the malignant progression of mucinous pancreatic cystic lesions. Pancreatology. 2016;16:900-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Lennon AM, Victor D, Zaheer A, Ostovaneh MR, Jeh J, Law JK, Rezaee N, Molin MD, Ahn YJ, Wu W, Khashab MA, Girotra M, Ahuja N, Makary MA, Weiss MJ, Hirose K, Goggins M, Hruban RH, Cameron A, Wolfgang CL, Singh VK, Gurakar A. Liver transplant patients have a risk of progression similar to that of sporadic patients with branch duct intraductal papillary mucinous neoplasms. Liver Transpl. 2014;20:1462-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3433] [Cited by in RCA: 6921] [Article Influence: 629.2] [Reference Citation Analysis (0)] |
| 12. | Vidhyarkorn S, Siripongsakun S, Yu J, Sayre J, Agopian VG, Durazo F, Lu DS. Longterm follow-up of small pancreatic cystic lesions in liver transplant recipients. Liver Transpl. 2017;23:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Darstein F, König C, Hoppe-Lotichius M, Grimm D, Knapstein J, Mittler J, Lang H, Galle PR, Zimmermann T. Impact of pancreatic comorbidities in patients with end-stage liver disease on outcome after liver transplantation. Eur J Intern Med. 2014;25:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Liu K, Joshi V, van Camp L, Yang QW, Baars JE, Strasser SI, McCaughan GW, Majumdar A, Saxena P, Kaffes AJ. Prevalence and outcomes of pancreatic cystic neoplasms in liver transplant recipients. World J Gastroenterol. 2017;23:8526-8532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Ngamruengphong S, Seeger K, Mccrone L, Felgueroso MM, Garrison S, Pungpapong S, Keaveny A, Raimondo M. Prevalence and Outcomes of Cystic Neoplasm of the Pancreas in Immunosuppressed Patients With Solid Organ Transplantation. Am J Gastroenterol. 2014;109:S69. [DOI] [Full Text] |
| 16. | Gill KR, Pelaez-Luna M, Keaveny A, Woodward TA, Wallace MB, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, Pearson RK, Petersen BT, Topazian MD, Vege SS, Kendrick M, Farnell MB, Raimondo M. Branch duct intraductal papillary mucinous neoplasm of the pancreas in solid organ transplant recipients. Am J Gastroenterol. 2009;104:1256-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Le H, Ziogas A, Rhee JM, Lee JG, Lipkin SM, Zell JA. A population-based, descriptive analysis of malignant intraductal papillary mucinous neoplasms of the pancreas. Cancer Epidemiol Biomarkers Prev. 2008;17:2737-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Laurent L, Vullierme MP, Rebours V, Maire F, Hentic O, Francoz C, Durand F, Ruszniewski P, Lévy P. Estimation of the prevalence of intraductal papillary mucinous neoplasm of the pancreas in the French population through patients waiting for liver transplantation. United European Gastroenterol J. 2017;5:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |