Published online Jun 18, 2025. doi: 10.5500/wjt.v15.i2.102671
Revised: December 9, 2024
Accepted: January 23, 2025
Published online: June 18, 2025
Processing time: 119 Days and 14.7 Hours
Cytomegalovirus (CMV) prophylaxis with valganciclovir and ganciclovir is associated with elevated neutropenia and leukopenia risk in kidney transplant recipients, although the impact of these events on healthcare resource utilization (HCRU) and clinical outcomes is unclear.
To quantify clinical events and HCRU associated with neutropenia and leukope
Adult kidney transplant recipients receiving valganciclovir and/or ganciclovir prophylaxis were identified in the TriNetX database from 2012 to 2021. Patient characteristics were evaluated in the 1-year period pre-first transplant. HCRU and adjusted event rates per person-year were evaluated in follow-up year 1 and years 2-5 after first kidney transplantation among cohorts with vs without neutropenia and/or leukopenia.
Of 15398 identified patients, the average age was 52.39 years and 58.70% were male. Patients with neutropenia and/or leukopenia had greater risk of clinical events for CMV-related events, opportunistic infections, use of granulocyte colony stimulating factor, and hospitalizations (relative risk > 1 in year 1 and years 2-5). Patients with vs without neutropenia and/or leukopenia had higher HCRU in year 1 and years 2-5 post kidney transplantation, including the mean number of inpatient admissions (year 1: 3.47 vs 2.76; years 2-5: 2.70 vs 2.29) and outpatient visits (48.97 vs 34.42; 31.73 vs 15.59, respectively), as well as the mean number of labs (1654.55 vs 1182.27; 622.37 vs 327.89).
Adults receiving valganciclovir and/or ganciclovir prophylaxis post-kidney transplantation had greater risk of neutropenia and/or leukopenia, which were associated with higher clinical event rates and HCRU up to 5 years post-transplantation. These findings suggest the need for alternative prophylaxis options with lower myelosuppressive effects to improve patient outcomes.
Core Tip: This observational study examined the impact of neutropenia and leukopenia up to 5 years in kidney transplant recipients who received valganciclovir or ganciclovir as cytomegalovirus (CMV) prophylaxis. The results showed that neutropenia and/or leukopenia were associated with increased risks of CMV-related events, opportunistic infections, hospitalizations, and use of granulocyte colony-stimulating factor. Additionally, patients with neutropenia and/or leukopenia had higher healthcare resource utilization, including more inpatient admissions, outpatient visits, and labs. These findings highlight the need for alternative prophylactic treatments for CMV with fewer myelosuppressive effects to improve outcomes in kidney transplant recipients.
- Citation: Beyer AP, Moise PA, Wong M, Gao W, Xiang C, Shen P, Pavlakis M, Vincenti F, Wang W. Clinical events and healthcare resource utilization associated with neutropenia and leukopenia among adult kidney transplant recipients receiving valganciclovir. World J Transplant 2025; 15(2): 102671
- URL: https://www.wjgnet.com/2220-3230/full/v15/i2/102671.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i2.102671
Cytomegalovirus (CMV) is a widespread herpesvirus with a global seroprevalence of approximately 80% in the adult population[1]. CMV infection is typically asymptomatic and self-limited in immunocompetent individuals, becoming latent following primary infection[2]. However, CMV infection imposes an increased risk of morbidity and mortality in patients with compromised immune function, including kidney transplant recipients[3]. According to the Organ Procurement & Transplantation Network, an estimated 23315 kidney transplants were performed in the United States in 2023, comprising approximately half of all transplants[4]. In this patient population, CMV serostatus of the kidney donor and recipient is the primary risk factor for CMV disease, which is defined as infection in tissue accompanied by other clinical symptoms like fever, leukopenia, arthralgia, or thrombocytopenia[5,6]. Approximately 20% of kidney transplant recipients receive an organ from a CMV seropositive donor[6-8] and seropositivity has been increasing over time in the United States, with further increases predicted[9].
CMV DNAemia and disease can result in life-threatening complications (i.e., hepatitis, nephritis, meningitis, encephalitis, etc.), allograft rejection, and death in kidney transplant recipients[10]. A 2022 systematic review reported that the rates of CMV-related mortality were up to 5.3% among kidney and/or liver transplant recipients[11]. Therefore, CMV prevention strategies are crucial to avoid reactivation of latent infection or reinfection with a novel strain post-transplant[6,12]. Prior to the introduction of letermovir, the previous standard of care for CMV prophylaxis was antiviral therapy with valganciclovir or, less commonly, ganciclovir[12]. The use of these therapies 3 to 6 months post-kidney transplant is shown to reduce the risk of CMV infection[6,13]. However, leukopenia and neutropenia are common adverse events associated with valganciclovir and ganciclovir due to the drugs’ myelosuppressive activity[14-17], ranging from 11% to 37% among treated kidney transplant recipients[15,18-20]. Neutropenia and leukopenia can lead to interruption of CMV prophylaxis, changes in the dosing of immunosuppressants, or use of granulocyte colony stimulating factor (G-CSF)[21,22], which can impact CMV infection, opportunistic infections, transplant success, or survival outcomes[21,23]. Addi
The burden of neutropenia and leukopenia for United States kidney transplant recipients has not been established under the current standard of care for CMV prophylaxis or quantified using a large and recent dataset for this patient population. Thus, the objective of this study was to estimate rates of key clinical events and evaluate the healthcare resource utilization (HCRU) in year 1 and years 2-5 following kidney transplantation among transplant recipients who did or did not develop neutropenia and/or leukopenia after being treated with valganciclovir and/or ganciclovir.
This retrospective, observational, nationwide cohort study of adult kidney transplant recipients in the United States used electronic medical records (EMRs) from the TriNetX database. TriNetX is a global federated health research network with real-time updates of anonymized EMRs, representing over 300 million patients. TriNetX ensures data accuracy through rigorous harmonization, cleaning, and quality checks, mapping data to standardized clinical terminologies. TriNetX datasets include anonymized patient data that is pseudonymized and/or de-identified, including demographics, diag
No identifiable protected health information or data were accessed or collected during the study; thus, ethical review was not required.
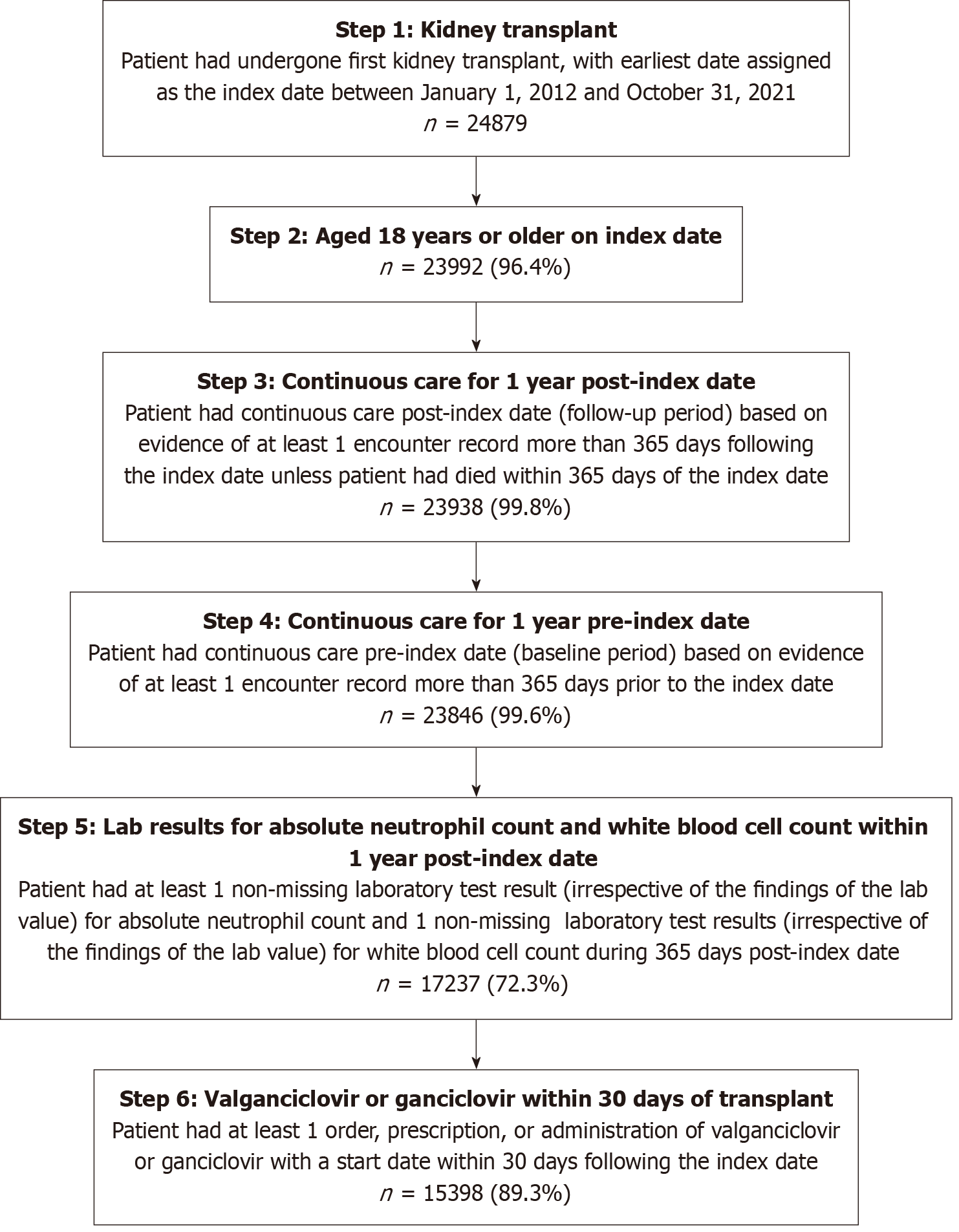
Adults who had undergone their first kidney transplant procedures between January 1, 2012 to October 31, 2021 were identified in the database. The date of the first observed kidney transplant procedure was defined as the index date. Patient demographics and clinical characteristics were evaluated during the year prior to the first kidney transplant, defined as the baseline period. Patients were followed until the end of the last encounter record for up to five years after the index date (i.e., follow-up period). The rates of clinical events and HCRU were evaluated during the follow-up period.
Patients were eligible for inclusion if they: (1) Had undergone their first kidney transplant in the United States during January 1, 2012 and October 31, 2021; (2) were aged 18 years or older at the index date; (3) had continuous observable care during the follow-up period, defined as ≥ 1 encounter record more than 365 days post-index unless the patient had died within that time; and in addition; and (4) had continuous observable care pre-index, defined as ≥ 1 encounter record more than 365 days before the index date. Patients were further required to have ≥ 1 non-missing laboratory test result (irrespective of the findings of the lab value) each for absolute neutrophil count (ANC) and white blood cell count (WBC) within 365 days post-index. Due to the nature of retrospective EMR data, not all lab values were fully recorded. Patients without documented lab values for ANC or WBC needed to evaluate the occurrence of neutropenia or leukopenia within 365 days post-index were therefore excluded. Finally, patients were required to have ≥ 1 order, prescription, or administration of valganciclovir or ganciclovir with a start date on or within 30 days after the index date.
To evaluate both the short-term and long-term impact of neutropenia and/or leukopenia, four patient cohorts were created: (1) Patients with neutropenia and/or leukopenia during year 1 of follow-up; (2) Patients with neutropenia and/or leukopenia during years 2-5 of follow-up; (3) Patients without neutropenia and leukopenia during year 1 of follow-up; and (4) Patients without neutropenia and leukopenia during years 2-5 of follow-up. The cohort with neutropenia and/or leukopenia in year 1 and years 2-5 of follow-up was independently defined and included patients who had a positive result for neutropenia (ANC < 1000/μL) and/or leukopenia (WBC < 3500/μL) during the respective timeframe[13,14]. The cohorts without neutropenia and leukopenia in year 1 and years 2-5 of follow-up were also independently defined and included patients who did not have a positive result for neutropenia and leukopenia but had a non-missing lab value during the respective timeframes.
All outcome measures were evaluated for the four patient cohorts, respectively.
Clinical outcomes: Clinical outcomes included recurring events (i.e., CMV DNAemia with a lower limit of quantification of 137 IU/mL, CMV disease based on diagnosis, opportunistic infections excluding CMV based on diagnosis, G-CSF use based on prescriptions, and all-cause rehospitalization); one-time events (i.e., acute graft rejection, graft loss, and all-cause mortality); and the proportion of patients with no non-mortality clinical events.
Adjusted clinical event rates per person-year for the recurring and one-time events were evaluated for each of the patient cohorts, after adjusting relevant baseline characteristics. The proportion of patients with no non-mortality clinical events was calculated as proportion of patients with no clinical events defined as above except for mortality.
HCRU: HCRU outcomes included inpatient admissions, emergency department (ED), outpatient, other, and lab visits after the index kidney transplantation hospitalization discharge date. Proportion of patients with each type of visit and the number of visits among those with at least one visit were assessed.
A patient observation approach was used for the HCRU assessment by year by cohort. In each year’s calculation, patients with a full year of continuous care were included. In the pooled HCRU outcomes in years 2-5, a single patient could contribute to each of years 2-5 as long as this patient had a full year of observable follow-up in each period.
Demographic characteristics reported during the baseline period for the overall patient population included sex, age at index, and United States region, etc.
Clinical characteristics reported during the baseline period included use of induction immunosuppressive therapy (7 days before to 15 days after index date), initial maintenance immunosuppressive therapy (within 3-month window post-index), calendar year of transplant, Charlson Comorbidity Index (CCI) comorbidities, and modified CCI score excluding renal disease.
Baseline characteristics not aforementioned were excluded from the covariates for one or more of the following reasons: (1) For binary variables, > 90% or < 10% prevalence and (2) Collinearity with other variables included in model.
Patient characteristics were described for the overall patient population. Means ± SD were reported for continuous variables; frequency counts and percentages were reported for categorical variables.
For recurring clinical events, adjusted clinical event rates were assessed using negative binomial models as count variables and calculated as the sum of the predicted number of events divided by the sum of person years in patient follow-up. Adjustment of baseline characteristics included the initial list of covariates: Age, sex, region, induction immunosuppressive therapies (corticosteroid therapy, thymoglobulin/anti-thymocyte globulin, basiliximab, alemtuzu
For one-time events, adjusted clinical event rates were assessed using adjusted Kaplan-Meier (KM) curves as time-to-event variables. The initial list of covariates with P < 0.05 in Cox regressions were included in the adjustment of KM curves. Parametric distributions among exponential, Weibull, log-normal, log-logistic, and generalized Gamma of the adjusted KM curves were determined based on the lowest Akaike information criterion and Bayesian information criterion values. The adjusted clinical event rate at year 1 was the adjusted event rate from KM at year 1; the adjusted clinical event rate in years 2-5 was calculated as the adjusted event rate from KM at year 5 minus the adjusted event rate from KM at year 1. The SE of the adjusted event rates was obtained by the KM curves in year 1 and was assumed to be the same in years 2-5. All analyses were performed using SAS® Studio release 9.04 or R Version 4.0.3.
A total of 15398 adults who had undergone their first kidney transplantation and who subsequently received valgan
Patient demographic and clinical characteristics are reported in Table 1. The average age of patients included in the study was 52.39 (SD: 13.18) years, approximately 60% were male, and half (51.24%) were located in the South. The most common initial induction therapy was thymoglobulin/anti-thymocyte globulin (36.17%). Almost all patients used calcineurin inhibitors as initial maintenance immunosuppressive therapy (99.31%), primarily tacrolimus (99.04%). Almost all patients used a corticosteroid therapy within 3 months post kidney transplantation (91.93%). The average modified CCI score was 1.22 (SD: 1.97) and over half (52.37%) of patients had a score of 0. The most common CCI conditions were chronic diabetes (26.74%), congestive heart failure (12.67%) and peripheral vascular disease (12.82%).
| Characteristics | n = 15398 |
| Age (years), mean (SD) | 52.39 (13.18) |
| Age category, n (%) | |
| 18 to < 45 | 4395 (28.54) |
| 45 to < 65 | 8111 (52.68) |
| 65 to < 75 | 2670 (17.34) |
| ≥ 75 | 222 (1.44) |
| Sex, n (%) | |
| Male | 9039 (58.70) |
| Female | 6359 (41.30) |
| Region1, n (%) | |
| South | 7890 (51.24) |
| Northeast | 4324 (28.08) |
| Midwest | 1951 (12.67) |
| West | 1233 (8.01) |
| Induction immunosuppressive therapy, n (%) | |
| Thymoglobulin/anti-thymocyte globulin | 5570 (36.17) |
| Basiliximab | 2236 (14.52) |
| Alemtuzumab | 1524 (9.90) |
| Rituximab | 143 (0.93) |
| Initial maintenance immunosuppressive therapy, n (%) | |
| Calcineurin inhibitors | 15292 (99.31) |
| Tacrolimus | 15250 (99.04) |
| Cyclosporine non-modified | 451 (2.93) |
| Cyclosporine modified | 0 (0.00) |
| Mycophenolic acid | 12675 (82.32) |
| Azathioprine | 330 (2.14) |
| Sirolimus | 165 (1.07) |
| Everolimus | 133 (0.86) |
| Corticosteroid therapy within 3 months post kidney transplantation, n (%) | 14156 (91.93) |
| Prednisone | 14048 (91.23) |
| Methyl prednisone | 2927 (19.01) |
| Prednisolone | 237 (1.54) |
| Calendar year of transplant, n (%) | |
| 2012 | 780 (5.07) |
| 2013 | 1063 (6.90) |
| 2014 | 1158 (7.52) |
| 2015 | 1564 (10.16) |
| 2016 | 1791 (11.63) |
| 2017 | 2087 (13.55) |
| 2018 | 2378 (15.44) |
| 2019 | 2638 (17.13) |
| 2020 | 1933 (12.55) |
| 2021 | 6 (0.04) |
| Modified CCI2, mean (SD) | 1.22 (1.97) |
| Modified CCI category, n (%) | |
| 0 | 8064 (52.37) |
| 1-2 | 4669 (30.32) |
| 3-4 | 1661 (10.79) |
| ≥ 5 | 1004 (6.52) |
| CCI comorbidity, n (%) | |
| Chronic diabetes | 4118 (26.74) |
| Congestive heart failure | 1951 (12.67) |
| Peripheral vascular disease | 1974 (12.82) |
| Chronic pulmonary disease | 1351 (8.77) |
| Mild to moderate diabetes | 1112 (7.22) |
| Mild liver disease | 1006 (6.53) |
| Cerebrovascular disease | 1000 (6.49) |
| Myocardial infarction | 732 (4.75) |
| Cancer | 704 (4.57) |
| Rheumatologic disease | 623 (4.05) |
| Moderate liver disease | 516 (3.35) |
| Metastatic solid tumor | 308 (2.00) |
| AIDS | 298 (1.94) |
| Peptic ulcer disease | 205 (1.33) |
| Hemiplegia | 79 (0.51) |
| Dementia | 27 (0.18) |
Recurring events: The rates of recurring clinical events are reported in Table 2. In year 1, 11524 (74.84%) patients with neutropenia and/or leukopenia and 3874 (25.16%) without neutropenia and leukopenia were included in the analysis; in years 2-5, 5583 (38.19%) and 9035 (61.81%) patients, respectively, were included.
| Number of events per person-year | Year 1 of follow-up with leukopenia/neutropenia, n = 11524 (A) | Year 1 of follow-up without leukopenia/neutropenia, n = 3874 (B) | Relative risk A/B | Year 2-5 of follow-up with leukopenia/neutropenia, n = 55831 (A) | Year 2-5 of follow-up without leukopenia/neutropenia, n = 90351 (B) | Relative risk A/B | ||||
| Event rate (%) | SE (%) | Event rate (%) | SE (%) | Event rate (%) | SE (%) | Event rate (%) | SE (%) | |||
| CMV DNAemia1 | 3 | 0.037 | 1.8 | 0.083 | 1.67 | 1.25 | 0.061 | 0.69 | 0.084 | 1.81 |
| CMV disease | 0.09 | 0.001 | 0.02 | 0.001 | 3.95 | 0.06 | 0.001 | 0.01 | 0 | 5.14 |
| Opportunistic infections (excluding CMV) | 2.19 | 0.009 | 1.57 | 0.012 | 1.4 | 1.15 | 0.007 | 0.76 | 0.005 | 1.52 |
| G-CSF use | 0.9 | 0.004 | 0.24 | 0.003 | 3.76 | 0.27 | 0.003 | 0.09 | 0.001 | 3.04 |
| All-cause rehospitalization | 2.24 | 0.013 | 1.52 | 0.013 | 1.47 | 0.95 | 0.011 | 0.52 | 0.005 | 1.84 |
In year 1, patients with and without neutropenia and/or leukopenia had 3.00 vs 1.80 events, respectively, per person-year for CMV DNAemia (relative risk: 1.67) and 0.09 vs 0.02 events per person-year for CMV disease (relative risk: 3.95). Patients with neutropenia and/or leukopenia also had higher rates per person-year of opportunistic infections [2.19 vs 1.57 (relative risk 1.40)], G-CSF use [0.90 vs 0.24 (3.76)], and all-cause rehospitalization [2.24 vs 1.52 (1.47)] compared to patients without neutropenia and leukopenia. In years 2-5, the relative risk between the two cohorts showed a consistent trend where patients with neutropenia and/or leukopenia continued to experience a higher number of clinical events compared to patients without neutropenia and leukopenia.
One-time events: The rates of one-time clinical events are reported in Table 3. A total of 11997 (77.91%) patients with and 3401 (22.09%) without neutropenia and leukopenia were included in the analysis. In year 1, patients with neutropenia and/or leukopenia had higher event rates for acute graft rejection [21.14% vs 13.23% (relative risk: 1.60)], graft loss [11.56% vs 7.86% (1.47)], and all-cause mortality [0.06% vs 0.02% (2.55)] compared with patients without neutropenia and leukopenia. In years 2-5, patients with neutropenia and/or leukopenia continued to have higher event rates for acute graft rejection and graft loss compared to patients without neutropenia and leukopenia. The mortality event rates increased to approximately 9% for both cohorts and the relative risk was close to 1.
| Proportion of patients with events | Year 1 of follow-up with leukopenia/neutropenia, n = 119971 (A) | Year 1 of follow-up without leukopenia/neutropenia, n = 34011 (B) | Relative risk A/B | Year 2-5 of follow-up with leukopenia/neutropenia2 (A) | Year 2-5 of follow-up without leukopenia/neutropenia2 (B) | Relative risk A/B | ||||
| Event rate (%) | SE (%) | Event rate (%) | SE (%) | Event rate (%) | SE (%) | Event rate (%) | SE (%) | |||
| Acute graft rejection | 21.14 | 0.27 | 13.23 | 0.63 | 1.6 | 13.26 | 0.27 | 10.3 | 0.63 | 1.29 |
| Graft loss | 11.56 | 0.28 | 7.86 | 1.15 | 1.47 | 11.48 | 0.28 | 6.96 | 1.15 | 1.65 |
| All-cause mortality | 0.06 | 3.21 | 0.02 | 17.69 | 2.55 | 9.04 | 3.21 | 9.46 | 17.69 | 0.96 |
Patients with no events (excluding mortality): In year 1, 17.78% of patients with neutropenia and/or leukopenia were free from any non-fatal clinical events compared to 27.54% of patients without neutropenia and leukopenia (relative risk: 0.65); in years 2-5, the proportions were 18.88% vs 32.77% (relative risk: 0.58), respectively (Table 4).
| Proportion of patients with no event (except for mortality) | Year 1 of follow-up, n = 15398 | Year 2-5 of follow-up, n = 14618 | ||||
| With leukopenia/neutropenia, n = 11524 (A), % | Without leukopenia/neutropenia, n = 3874 (B), % | Relative risk A/B | With leukopenia/neutropenia, n = 5583 (A), % | Without leukopenia/neutropenia, n = 9035 (B), % | Relative risk A/B | |
| No event | 17.78 | 27.54 | 0.65 | 18.88 | 32.77 | 0.58 |
HCRU: HCRU excluding the index hospitalization is reported in Table 5. For the year 1 analyses of HCRU, 11516 (74.83%) patients with neutropenia and/or leukopenia and 3874 (25.17%) patients without neutropenia and/or leu
| All-cause healthcare resource utilization | Year 1, n = 153901 | Year 2-5, n = 345132 | ||
| With leukopenia/neutropenia, n = 11516 | Without leukopenia/neutropenia, n = 3874 | With leukopenia/neutropenia, n = 9264 | Without leukopenia/neutropenia, n = 25249 | |
| Inpatient | ||||
| Patients with admission, n (%) | 7441 (64.61) | 2205 (56.92) | 3408 (36.79) | 6471 (25.63) |
| Number of admissions, mean ± SD (median) | 3.47 ± 5.07 (2) | 2.76 ± 3.63 (2) | 2.70 ± 3.01 (2) | 2.29 ± 2.57 (1) |
| Length of stay (days) among patients who had ≥ 1 admission, mean ± SD (median) | 6.68 ± 27.26 (4) | 7.55 ± 36.70 (4) | 7.50 ± 29.83 (4.5) | 5.92 ± 24.40 (3) |
| Emergency department/other | ||||
| Patients with visits, n (%) | 3383 (29.38) | 988 (25.50) | 1927 (20.80) | 4036 (15.98) |
| Number of visits, mean ± SD (median) | 1.81 ± 1.37 (1) | 1.74 ± 1.43 (1) | 1.87 ± 1.69 (1) | 1.77 ± 1.65 (1) |
| Outpatient | ||||
| Patients with visits, n (%) | 11304 (98.16) | 3813 (98.43) | 9130 (98.55) | 24460 (96.88) |
| Number of visits, mean ± SD (median) | 48.97 ± 37.34 (37) | 34.42 ± 28.18 (25) | 31.73 ± 27.63 (25) | 15.59 ± 18.53 (9) |
| Other3 | ||||
| Patients with other visits, n (%) | 162 (1.41) | 74 (1.91) | 122 (1.32) | 442 (1.75) |
| Number of other visits, mean ± SD (median) | 2.85 ± 7.62 (1) | 5.05 ± 12.84 (1.5) | 3.25 ± 3.84 (2) | 2.17 ± 2.11 (1) |
| Unknown | ||||
| Patients with visits, n (%) | 7702 (66.88) | 2142 (55.29) | 7473 (80.67) | 14820 (58.70) |
| Number of visits, mean ± SD (median) | 36.74 ± 31.57 (28) | 28.27 ± 30.78 (16) | 17.85 ± 18.30 (13) | 13.48 ± 16.19 (8) |
| Any labs | ||||
| Patients with any labs, n (%) | 11475 (99.64) | 3832 (98.92) | 9264 (100.00) | 25249 (100.00) |
| Number of any labs, mean ± SD (median) | 1654.55 ± 984.16 (1485) | 1182.27 ± 755.58 (1084) | 622.37 ± 706.25 (419) | 327.89 ± 374.36 (222) |
Excluding the index hospitalization, 64.61% and 56.92% of the patients with and without neutropenia and leukopenia, respectively, had an inpatient admission in year 1 post-transplant, with an average of 3.47 and 2.76 admissions of 6.68 and 7.55 days, respectively among those with at least one inpatient admission. A total of 29.38% and 25.50% of the patients with and without neutropenia and/or leukopenia, respectively, had an ED visit, with an average of 1.81 and 1.74 visits among those who had at least one ED visit. Almost all patients in both cohorts (98.16% with vs 98.43% without neu
The trend persisted in years 2-5 with patients experiencing neutropenia and/or leukopenia having a higher average number of HCRU visits compared to patients without neutropenia and leukopenia.
This retrospective cohort study described the short- and long-term clinical and HCRU burden among United States kidney transplant recipients with and without neutropenia and/or leukopenia who initiated valganciclovir and/or ganciclovir prophylaxis within 30 days after kidney transplantation. We found high risk of CMV disease and significant HCRU among this population. Within the first year post-kidney transplant among those initiated on valganciclovir and/or ganciclovir prophylaxis, approximately three-fourths of patients developed neutropenia and/or leukopenia.
Patients with neutropenia and/or leukopenia experienced higher rates of clinical events over both the short- and longer-term post-transplant periods than patients without neutropenia and leukopenia. During the first year following kidney transplantation, patients with neutropenia and/or leukopenia had 2-3 events each of CMV DNAemia, oppor
Furthermore, patients with neutropenia and/or leukopenia had higher HCRU burden. On average, patients with neutropenia and/or leukopenia incurred 0.7 more inpatient admissions and 15 more outpatient visits during year 1 post-transplant than patients without neutropenia and leukopenia, a trend which extended up to five years after transplan
The findings of a recent systematic literature review (SLR) of observational studies on the burden of neutropenia and leukopenia among adult kidney transplant recipients are broadly consistent with those of the current study[21]. The SLR of 82 studies reported that, in the first year post-transplant, up to 48% of patients experienced neutropenia and up to 83% experienced leukopenia. The authors observed an increased risk of CMV infection and disease, acute rejection, graft failure, and mortality in patients with neutropenia and/or leukopenia, similar to the present findings. The economic burden of CMV disease has been established in existing literature. A United States claims study, which included 3,258 kidney transplant recipients during 2012-2018, reported that patients who developed neutropenia following valganci
The 2020 Annual Data Report of kidney, developed by the United States Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients, identified a one-year death-censored graft failure rate of less than 5% for kidney transplant recipients, whether from living or deceased donors, and a one-year acute rejection rate of 6.8% in 2018 to 2019[27]. The current study has identified a higher one-year graft failure rate ranging from 8% to 12% and a higher one-year acute graft rejection rate ranging from 13% to 21%. While we intended to understand the discrepancies, factors that could impact transplantation success, such as recipient wait time, sensitization, mismatch (in terms of human leukocyte antigens and ABO blood group), quality of the donor kidney, warm and cold ischemic times, and the type of kidney (including considerations such as extended criteria, donation after cardiac death, donation after brain death, etc.) were not available in the current data. Furthermore, the study has identified an annual rate of 0.24 G-CSF use in patients without neutropenia and leukopenia during the first year following kidney transplantation. G-CSF might have been prescribed preventively in response to a potential decline in ANC in anticipation of neutropenia. Due to the nature of data extraction using natural language processing, there is a possibility of bias where G-CSF was noted but not actually consumed.
This study is subject to a few other limitations, some of which are inherent to retrospective studies using EMR data. First, the sample was derived from the TriNetX network, which may not be fully generalizable to the entire kidney transplantation population in the United States. Because TriNetX is not a closed system, prescriptions and encounters outside of the network might not be observed. This could result in missing data or underreporting of HCRU and clinical events. As the data cut intended to exclude patients who died within one year of kidney transplantation, this patient cohort may have lower disease severity and better observed outcomes than other populations. The mortality rates presented in this study should be interpreted with caution. Second, data abstraction relied on natural language processing, therefore the results could be impacted by any errors in data entry. Additionally, having a prescription record of valganciclovir or ganciclovir in the data may not indicate actual intake of the medication. Third, the available variables and level of detail were limited by what was reported in patients’ EMR and subsequently abstracted by TriNetX (i.e., detail for birth or death dates). Duration of valganciclovir and/or ganciclovir was not evaluated due to limited availa
Adults in the United States receiving valganciclovir and/or ganciclovir post-kidney transplantation who developed neutropenia and/or leukopenia experienced substantial clinical and economic burden, including more clinical events and higher HCRU up to five years post-transplantation, compared with patients who did not develop neutropenia and leukopenia. These findings may inform treatment decisions for clinicians and underscore the need for novel treatment options for CMV prophylaxis which are associated with lower risk of neutropenia and leukopenia to improve the outcomes of this patient population.
This study was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, United States. The study sponsor was involved in several aspects of the research, including the study design, interpretation of data, and writing of the manuscript. Medical writing assistance was provided by Shelley Batts, PhD, an independent contractor of Analysis Group, Inc. and funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, United States.
| 1. | Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, Griffiths P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol. 2019;29:e2034. [PubMed] [DOI] [Full Text] |
| 2. | La Rosa C, Diamond DJ. The immune response to human CMV. Future Virol. 2012;7:279-293. [PubMed] [DOI] [Full Text] |
| 3. | Requião-Moura LR, deMatos AC, Pacheco-Silva A. Cytomegalovirus infection in renal transplantation: clinical aspects, management and the perspectives. Einstein (Sao Paulo). 2015;13:142-148. [PubMed] [DOI] [Full Text] |
| 4. | Organ Procurement and Transplantation Network. National data: Kidney transplants 2023. [cited 21 November 2024]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. |
| 5. | Emery VC. Investigation of CMV disease in immunocompromised patients. J Clin Pathol. 2001;54:84-88. [PubMed] [DOI] [Full Text] |
| 6. | Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102:900-931. [PubMed] [DOI] [Full Text] |
| 7. | Beam E, Razonable RR. Cytomegalovirus in solid organ transplantation: epidemiology, prevention, and treatment. Curr Infect Dis Rep. 2012;14:633-641. [PubMed] [DOI] [Full Text] |
| 8. | Lentine KL, Smith JM, Miller JM, Bradbrook K, Larkin L, Weiss S, Handarova DK, Temple K, Israni AK, Snyder JJ. OPTN/SRTR 2021 Annual Data Report: Kidney. Am J Transplant. 2023;23:S21-S120. [PubMed] [DOI] [Full Text] |
| 9. | Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50:1439-1447. [PubMed] [DOI] [Full Text] |
| 10. | Azevedo LS, Pierrotti LC, Abdala E, Costa SF, Strabelli TM, Campos SV, Ramos JF, Latif AZ, Litvinov N, Maluf NZ, Caiaffa Filho HH, Pannuti CS, Lopes MH, Santos VA, Linardi Cda C, Yasuda MA, Marques HH. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70:515-523. [PubMed] [DOI] [Full Text] |
| 11. | Slavin M, Tedesco-silva H, Singh I, Sandhu A, Demuth D, Cai J. 424.7: Epidemiology of Cytomegalovirus Infection and Disease in Solid Organ Transplant Recipients in Selected Countries Outside of North America and Europe: A Systematic Review. Transplant. 2022;106:S477. [DOI] [Full Text] |
| 12. | Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13512. [PubMed] [DOI] [Full Text] |
| 13. | Humar A, Lebranchu Y, Vincenti F, Blumberg EA, Punch JD, Limaye AP, Abramowicz D, Jardine AG, Voulgari AT, Ives J, Hauser IA, Peeters P. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010;10:1228-1237. [PubMed] [DOI] [Full Text] |
| 14. | Limaye AP, Budde K, Humar A, Vincenti F, Kuypers DRJ, Carroll RP, Stauffer N, Murata Y, Strizki JM, Teal VL, Gilbert CL, Haber BA. Letermovir vs Valganciclovir for Prophylaxis of Cytomegalovirus in High-Risk Kidney Transplant Recipients: A Randomized Clinical Trial. JAMA. 2023;330:33-42. [PubMed] [DOI] [Full Text] |
| 15. | Liang X, Famure O, Li Y, Kim SJ. Incidence and Risk Factors for Leukopenia in Kidney Transplant Recipients Receiving Valganciclovir for Cytomegalovirus Prophylaxis. Prog Transplant. 2018;28:124-133. [PubMed] [DOI] [Full Text] |
| 16. | Belga S, Hernandez C, Kabbani D, Cervera C. Incidence of valganciclovir-related leukopenia and neutropenia in solid organ transplant recipients at high risk of cytomegalovirus disease. Transpl Infect Dis. 2024;26:e14227. [PubMed] [DOI] [Full Text] |
| 17. | Rostaing L, Jouve T, Terrec F, Malvezzi P, Noble J. Adverse Drug Events after Kidney Transplantation. J Pers Med. 2023;13. [PubMed] [DOI] [Full Text] |
| 18. | Hurst FP, Belur P, Nee R, Agodoa LY, Patel P, Abbott KC, Jindal RM. Poor outcomes associated with neutropenia after kidney transplantation: analysis of United States Renal Data System. Transplantation. 2011;92:36-40. [PubMed] [DOI] [Full Text] |
| 19. | Mavrakanas TA, Fournier MA, Clairoux S, Amiel JA, Tremblay ME, Vinh DC, Coursol C, Thirion DJG, Cantarovich M. Neutropenia in kidney and liver transplant recipients: Risk factors and outcomes. Clin Transplant. 2017;31. [PubMed] [DOI] [Full Text] |
| 20. | Hellemans R, Wijtvliet V, Bergs K, Philipse E, Vleut R, Massart A, Couttenye MM, Matheeussen V, Abramowicz D. A split strategy to prevent cytomegalovirus after kidney transplantation using prophylaxis in serological high-risk patients and a pre-emptive strategy in intermediate-risk patients: Combining the best of two options? Transpl Infect Dis. 2021;23:e13467. [PubMed] [DOI] [Full Text] |
| 21. | Raval AD, Kistler KD, Tang Y, Vincenti F. Burden of neutropenia and leukopenia among adult kidney transplant recipients: A systematic literature review of observational studies. Transpl Infect Dis. 2023;25:e14000. [PubMed] [DOI] [Full Text] |
| 22. | Brar S, Berry R, Raval AD, Tang Y, Vincenti F, Skartsis N. Outcomes among CMV-mismatched and highly sensitized kidney transplants recipients who develop neutropenia. Clin Transplant. 2022;36:e14583. [PubMed] [DOI] [Full Text] |
| 23. | Brum S, Nolasco F, Sousa J, Ferreira A, Possante M, Pinto JR, Barroso E, Santos JR. Leukopenia in kidney transplant patients with the association of valganciclovir and mycophenolate mofetil. Transplant Proc. 2008;40:752-754. [PubMed] [DOI] [Full Text] |
| 24. | Raval AD, Ganz M, Saravanan P, Tang Y, Santos C. 1387. Impact of Cytomegalovirus Prophylaxis on Healthcare Resource Use and Costs among Kidney Transplant Recipients: A United States Renal Data System-Medicare Linked Database Study. Open Forum Infect Dis. 2021;8:S779. [DOI] [Full Text] |
| 25. | Cheng WY, Avery RK, Thompson-Leduc P, Cheung HC, Bo T, Duh MS, Hirji I. Evaluation of treatment patterns, healthcare resource utilization, and costs among patients receiving treatment for cytomegalovirus following allogeneic hematopoietic cell or solid organ transplantation. J Med Econ. 2022;25:367-380. [PubMed] [DOI] [Full Text] |
| 26. | Turzhitsky V, Raval A, Moise P, Merchant S. Healthcare Utilization and Costs Associated With Neutropenia in Kidney Transplant Recipients Receiving Valganciclovir Prophylaxis: An Administrative Claims Database Study. J Am Soc Nephrol. 2022;33:551-552. [DOI] [Full Text] |
| 27. | Lentine KL, Smith JM, Hart A, Miller J, Skeans MA, Larkin L, Robinson A, Gauntt K, Israni AK, Hirose R, Snyder JJ. OPTN/SRTR 2020 Annual Data Report: Kidney. Am J Transplant. 2022;22 Suppl 2:21-136. [PubMed] [DOI] [Full Text] |