Published online Jun 18, 2025. doi: 10.5500/wjt.v15.i2.101517
Revised: November 8, 2024
Accepted: December 25, 2024
Published online: June 18, 2025
Processing time: 156 Days and 21.3 Hours
C3 glomerulopathies (C3G) are a rare cause of kidney failure resulting from complement dysregulation. Small studies demonstrate a high rate of recurrence and poor outcomes in kidney transplantation. Treatment efficacy in this setting with eculizumab, a terminal complement inhibitor, is largely unknown.
To determine the outcomes of kidney transplantation in patients with C3G and the potential impact of eculizumab.
We retrospectively studied kidney transplant recipients who underwent a post-transplant biopsy confirming C3G between January 1, 1993 and December 31, 2023 at a single center. Only the first episode of kidney transplant was reviewed. The electronic medical records were reviewed for post-transplant allograft function, indication for biopsy, time to biopsy from transplant, time to allograft failure from transplantation, post-C3G treatment, complement laboratory testing, and concurrent malignancy/infection. Reports, and when available slides and immunofluorescence/electron microscopic images, were re-reviewed by a renal pathologist.
A total of fifteen patients were included in this study. Fourteen patients had suspected recurrent disease, with a pre-transplant native kidney report of C3G. One patient developed de novo C3G. Median post kidney transplant clinical follow up time was 91 months. Median time to recurrence was 7 months with median graft survival of 48 months post kidney transplantation. The most common index biopsy pattern of injury was endocapillary proliferative glomerulonephritis (often with exudative features) with or without mesangial hypercellularity (56%) followed by membranoproliferative glomerulonephritis (25%). Most patients developed membranoproliferative glomerulonephritis pattern of injury on follow up biopsies (63%). Seven patients with recurrent disease received treatment with eculizumab with a median graft survival of 73 months, with five functioning grafts by the end of the study period. Seven patients with recurrent disease did not receive therapy, and all lost their graft with a median graft survival of 22 months (P = 0.003).
C3G following kidney transplantation is mostly a recurrent disorder with a poor prognosis in untreated patients. Untreated recurrence has a poor prognosis with median allograft survival < 2 years. Early treatment with eculizumab may improve transplant outcomes in patients with recurrent C3G.
Core Tip: C3 glomerulopathy is a highly recurrent disease in kidney transplant recipients, resulting in premature allograft loss. In this single center retrospective observational study recipients with underling C3 glomerulopathy experienced early recurrence with a median time of 7 months post kidney transplantation. The most common finding on kidney biopsy was endocapillary proliferative glomerulonephritis. Treatment with eculizumab was associated with a median graft survival of 73 months, compared to a median graft survival of 22 months in untreated patient with recurrent C3 glomerulopathy.
- Citation: Zuckerman J, Pham PT, Parakkal M, Velazquez AF, Sarkar M, Pablos MA, Bunnapradist S, Lum EL. C3 glomerulopathy post kidney transplantation: A single center experience. World J Transplant 2025; 15(2): 101517
- URL: https://www.wjgnet.com/2220-3230/full/v15/i2/101517.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i2.101517
C3 glomerulopathies (C3G) comprise a group of kidney disorders with a shared mechanism of alternative complement pathway dysregulation. These entities present with overactivation of the complement system, resulting in predominant C3 deposition and inflammation within the glomeruli and often membranoproliferative pattern on light microscopy[1-4]. Two distinct forms of C3G have been described, dense deposit disease (DDD) and C3 glomerulonephritis (C3GN), which are distinguished by differences in deposits on electron microscopy (EM)[5]. Kidney transplantation in individuals with C3G does not address the underlying complement dysregulation. Thus, recurrent disease post kidney transplantation is common, occurring in > 50% of patients. C3G post kidney transplantation carries a poor prognosis, with high rates of early allograft loss[6-8]. Treatment with steroids and mycophenolate mofetil, common for native kidney C3G, is limited in kidney transplant recipients who are often already on these medications[9]. Addressing the complement system directly with eculizumab, a terminal complement inhibitor, targets the underlying complement overactivation and may improve outcomes[10-12]. Large scale analysis of C3G in kidney transplant recipients are limited due the absence of C3G as a selectable diagnosis on the United Network for Organ Sharing (UNOS) transplant candidate registration forms. In this retrospective observational study, we describe the occurrence and prognosis of post kidney transplant C3G in fifteen kidney transplant recipients at a single center.
We reviewed a pathology database containing all kidney transplant biopsies performed at our center between January 1, 1993 and December 31, 2023 with a diagnosis of C3G or possible C3G. Biopsy reports and when available light microscopy slides, immunofluorescence (IF), and electron microscopic studies were re-reviewed by a renal pathologist to confirm C3G or likely C3G diagnosis. This study was approved by the institutional review board of University of California Los Angeles. The clinical and research activities reported are consistent with the principles of the Declaration of Istanbul. A total of eighteen patients were identified, fifteen of whom had clinical follow up at the center following kidney transplant biopsy and were included in the study. The cohort was divided into three groups based on whether post C3G was likely to be recurrent disease or de novo disease, based on pretransplant medical records, and in cases of likely recurrent disease whether treatment was provided. Clinical parameters were obtained through the electronic medical records. Four patients with allograft loss to recurrent disease underwent re-transplantation, but only their first episode was included in the analysis. Demographic information included: Age at kidney transplant, gender, documented immunosuppression treatment prior to kidney transplantation, and primary cause of end-stage kidney disease as assessed through the UNOS transplant candidate registration form. Post-transplant outcomes included: Time to confirmed C3G post kidney transplantation, documented C3G treatment if received, presence of post-transplant lymphoproliferative disorder (PTLD), patient survival, allograft survival defined as return to dialysis or re-transplan
Definitive disease recurrence was based on pathology findings and defined as IF C3 staining with an intensity of ≥ 2+ (scale 0-4) if no EM was available or if EM was performed and confirmed the presence of deposits. The IF pattern was considered C3-only when there was no staining for immunoglobulins (Igs) and C3-dominant when C3 staining was at least two semi-quantitative units greater in magnitude than Igs. Index biopsy was defined as the first post-transplant biopsy that showed recurrence. In some biopsies the IF staining pattern did not meet consensus threshold for C3 staining. However, as in each case subsequent biopsies met consensus criteria and thus the first biopsy with any GN was considered the index biopsy. Such biopsies were considered to be likely C3 recurrences at the early time points. DDD was defined by the presence of a preponderance of highly electron dense intramembranous deposits in the electron microscopic studies; all other cases were classified as C3GN. Definitions of light and electron microscopic lesions were as described by Haas et al[13]. Determination of concurrent rejection was based on Banff 2022 criteria when sufficient information was available for re-review[14].
The primary outcomes of interest were allograft survival and time to allograft loss following kidney transplantation.
Patients were compared using descriptive statistics, including counts and percentages for categorical variables and mean, median and standard deviation for numerical variables. Kaplan-Meier event free survival analyses with log-rank test were computed using DATAtab: Online Statistics Calculator (https://datatab.net). Analyses were based on each episode of kidney transplantation. Re-transplantations were considered separate events for recurrence time estimates, index biopsy pathology calculations, and post-index biopsy survival evaluations. The de novo case was excluded from these analyses.
A total of fifteen patients were identified through the pathology records with post-transplant C3G on biopsy and clinical follow up (Table 1). The median age at transplant was 21 years and with seven females and eight males in the cohort. Fourteen patients had a documented native kidney disease of either membranoproliferative GN (MPGN), DDD, or G3GN (93.3%), suggesting recurrent disease post kidney transplantation was the most common reason for C3G in a post kidney transplant biopsy. Eight patients had native biopsy reports and/or biopsy slides available for review which all demonstrated MPGN pattern of injury. The remaining patient diagnosis were determined from patient notes and listed diagnoses on UNOS forms. Only one patient had crescents (DDD-6) in their native biopsy. Six of these patients had IF staining demonstrating C3 only or C3 dominance. One patient had IgG/C3 co-dominant staining not meeting C3G consensus criteria, and their native disease is classified as immune complex mediated MPGN. One patient (DDD-6) did not have IF tissue. However, typical highly electron dense intra-membranous deposits were present on EM. One patient (C3GN-5) had a monoclonal gammopathy (IgG-lambda) at time of native disease occurrence with a bone marrow biopsy demonstrating a small population of clonal plasma cells. Eleven patients received their kidney transplant after 2007, following eculizumab approval for paroxysmal nocturnal hemoglobinuria. Eight patients received living donor kidney transplants. Seven received lymphocyte depleting agents for induction, seven received interleukin-2 blockade, and one received no induction agent.
| ID | Age | Sex | Native kidney diagnosis | Native kidney biopsy findings on re-examination | Transplant year | Allograft type | Induction | Biopsy Indication | Maintenance immunotherapy at bx | Serum creatinine (mg/dL) at bx | Proteinuria at bx | Time to diagnosis of C3G post kidney transplant (months) | Post-transplant C3G treatment | Graft/Patient survival from time of transplant (months) | Clinical follow up time from transplant (months) | Renal function at last follow-up (mg/dL) |
| C3GN-1 | 40 | F | MPGN type 1 | LM: MPGN; IF: C3 (4+); EM: Mesangial/subepithelial (some large)/subendothelial deposits | 2018 | DDRT | ATG | Hematuria | Pred/fk/mmf | 1.44, eGFR 47 | UPC 0.2 | 10 | Duodenal PTLD | 60 | 64 | ESRD |
| DDD-1 | 70 | M | MPGN type 1 | Unknown | 2015 | DDRT | ATG | AKI, hematuria | Pred/fk | 2.9, eGFR 26 | UPC 0.4 | 7 | Crypto meningitis | 18 | 18 | Cr 0.93 - Death |
| DDD-2 | 27 | M | DDD | Unknown | 2005 (first), 2010 (second) | LURT | Simulect | Protocol | Pred/fk/mmf | NA | UPC 0.8 | 3 (first); 31 (second) | None | 11 | 109 | Retransplant Cr 1.4 |
| DDD-3 | 37 | M | DDD | Unknown | 2007 | DDRT | ATG | Hematuria | Pred/fk/mmf | 2.5, eGFR | UPC 0.365 | 23 | None | 56 | 148 | ESRD |
| DDD-4 | 16 | M | DDD | Unknown | 1991 (first), 1995 (second) | LRRT | None | Proteinuria, graft dysfunction | Pred/imuran | 1.3 | 2+ | 25 | None | 47 | 53 | ESRD |
| DDD-5 | 11 | M | DDD | LM: MPGN with approximately 53% active crescents; IF: C3 (4+); EM: Intramembranous, mesangial deposits | 1992 (first), 3/2002 (second) | DDRT | Simulect | Hematuria, proteinuria | Pred/cyclo/imuran | NA | 3+ blood, 3+ protein | 2 (first), 4 (fecond) | None | 19 | 95 | ESRD |
| DDD-6 | 11 | F | DDD | LM: MPGN; no IF tissue; EM: Intramembranous deposits | 2008 (first), 2023 (second) | DDRT | Simulect | Protocol | Pred/fk/mmf | 0.6 | NA | 6 | None | 11 | 188 | Retransplant Cr 0.85 |
| C3GN-2 | 44 | M | MPGN | Unknown | 2012 | LURT | Campth | Protocol | Fk/mmf | 1.3 | NA | 24 | Steroids × 2 weeks | 140 | 140 | 2.3 |
| C3GN-3 | 21 | M | C3GN | LM: MPGN; IF: C3 (4+); EM: Mesangial, subendothelial, intramembranous, hump-like deposits | 2019 | LURT | ATG | Proteinuria | Pred/fk/mmf | 1.38 | UPC 0.1 | 4 | Eculizumab | 47 | 47 | 1.34 |
| C3GN-4 | 19 | F | C3GN | LM: MPGN; IF C3(2-3+); IgM (1-2+); EM: Mesangial, intramembranous, subendothelial, subepithelial, hump like deposits | 2017 | DDRT | ATG | AKI, proteinuria | Pred/fk/mmf | 1.15 | UPC 1.6 | 5 | Eculizumab | 73 | 73 | 3.8 |
| C3GN-5 | 46 | F | C3GN | LM: MPGN, IF: C3(4+) EM: Subendothelial and mesangial deposits | 2014 | LURT | Simulect | Proteinuria, AKI | Pred/fk/mmf | 2.8 | 3+ | 2 | Pheresis+ eculiuzmab × 1 year (6 months after diagnosis) | 41 | 82 | ESRD |
| DDD-7 | 15 | M | MPGN type 1 | Unknown | 2004 | LRRT | Simulect | Protocol | Pred/fk/mmf | 1.2 | UPC 0.1 | 60 | Eculizumab in 2023 with graft dysfunction and proteinuria | 230 | 230 | 1.93 |
| DDD-8 | 22 | F | DDD | LM: MPGN; IF: C3 (4+), IgG (trace), IgM (1+), and kappa and lambda light chains (both 1+); EM: Mesangial, intramembranous, and hump-like deposits | 2016 | LRRT | Simulect | Proteinuria | Pred/fk/mmf | 0.7 | 3+ hematuria, UPC 0.1 | 2 | Eculizumab | 89 | 91 | 1.25 |
| C3GN-6 | 21 | F | MPGN type 1 | LM: MPGN; IF: C3 (4+), IgG (3-4+), IgA (1+), IgM (2+), C1q (2+), kappa light chain (1+), and lambda light chain (2+); EM: Subendothelial and mesangial deposits | 2012 | DDRT | ATG | AKI, hematuria, proteinuria | Pred/fk/mmf | 2.7 | 4.2 g | 11 | Pheresis + eculizumab (start 1 year after for only 3 months) | 48 | 77 | ESRD |
| C3GN-7 | 17 | F | Crescentic glomerulonephritis consistent with granulomatosis with polyangiitis; PR3+/ C-ANCA | Unknown | 2009 | LURT | Daclizumab | AKI with proteinuria during PTLD | Pred/fk | 2.2 | 1.5 g | 153 | Treatment steroids/rituximab for PTLD dx 14 months prior | 159 | 159 | AKI-CRRT - Death |
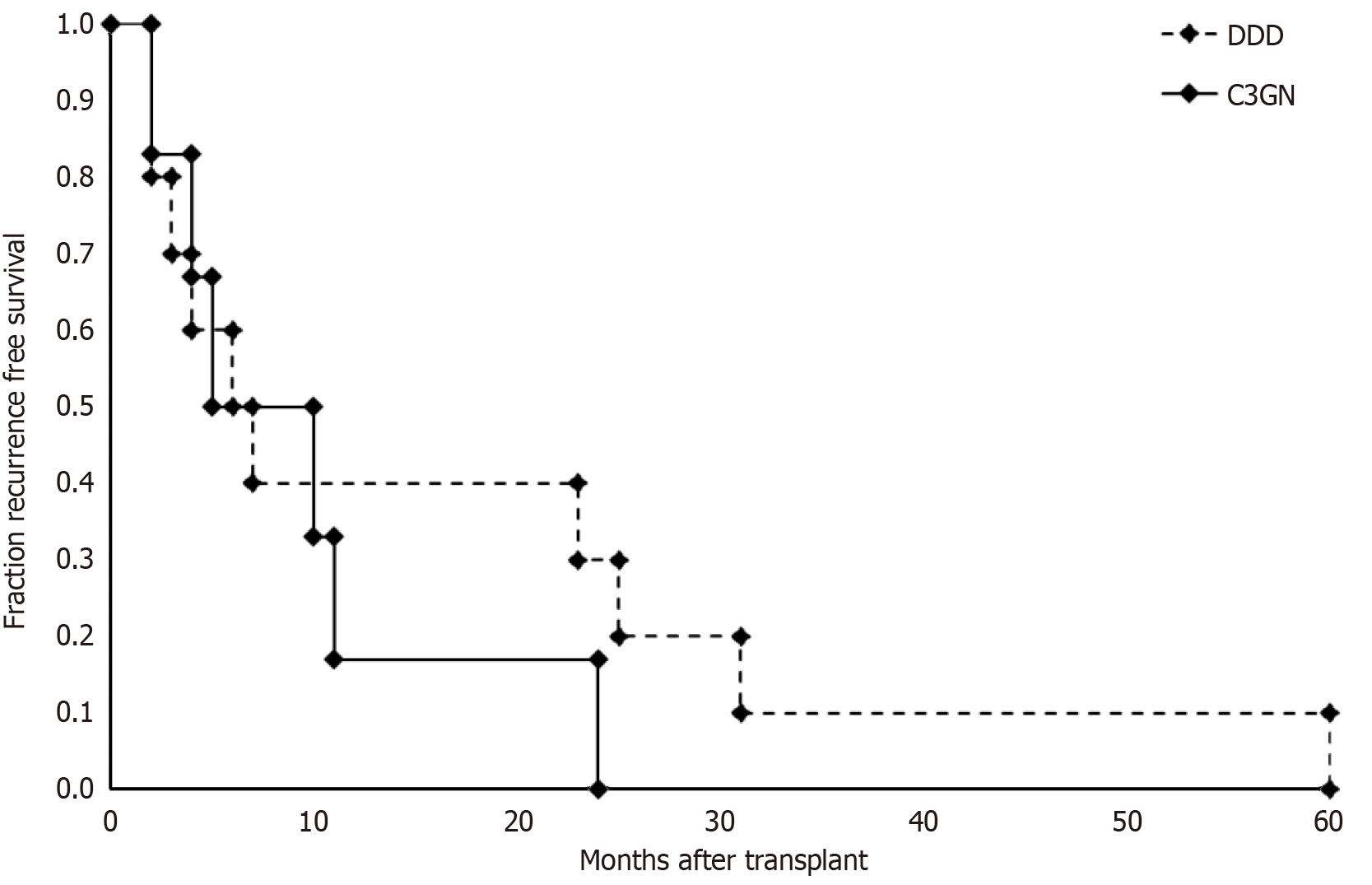
The median time from transplant to kidney transplant biopsy with features consistent with C3G was 6.5 months. Median recurrence time for DDD (6.5 months) and C3GN (7.5 months) were similar (Figure 1). The overall median graft survival was 47 months post kidney transplantation. Ten patients (66.7%) experiences graft failure by the end of study with a median clinical follow up time of 91 months post kidney transplantation. Four patients underwent re-trans
Most index biopsies with C3 diagnoses were done for cause and four per protocol. Ten patients had more than one post-transplant biopsy (range 1-9). Table 2 summarizes the transplant kidney biopsies findings (see Supplementary Table 1 for individual biopsy information). The most common light microscopic pattern of injury on index biopsy was pure endocapillary proliferative GN (31%), followed by mesangial and endocapillary proliferative GN patterns (25%), MPGN pattern (25%), mesangial proliferative GN only (13%), and no hypercellularity (13%). On index biopsy, 19% of cases demonstra
| Parameter | Index biopsy | Any biopsy |
| Light microscopy pattern | ||
| No hypercellularity | 3 (19) | 3 (19) |
| Mesangial proliferative GN only | 2 (13) | 3 (19) |
| Endocapillary proliferative GN only | 5 (31) | 5 (31) |
| Mesangial and endocapillary proliferative GN | 4 (25) | 4 (25) |
| Membranoproliferative GN | 5 (31) | 10 (63) |
| Exudative features | 4 (25) | 4 (25) |
| Active crescents | 3 (19) | 5 (31) |
| Segmental glomerular scars | 5 (31) | 9 (56) |
| Concurrent biopsy findings | ||
| Cellular rejection | 6 (38) | 8 (50) |
| Antibody mediated rejection | 2 (13) | 4 (25) |
| Thrombotic microangiopathy | 1 (6) | 3 (19) |
| Acute/chronic CNI toxicity | 2 (13) | 5 (31) |
| Arterio/arteriolosclerosis | 4 (25) | 4 (25) |
| BK virus nephropathy | 1 (6) | 1 (6) |
| Acute tubular injury | 2 (13) | 4 (25) |
| None | 3 (19) | 3 (19) |
| Immunofluorescence findings | ||
| C3 only | 7 (44) | 9 (56) |
| C3 dominant | 9 (56) | 9 (56) |
| Non-C3 dominant GN | 1 (6) | 1 (6) |
| Electron microscopy findings | ||
| Mesangial deposits | 12 (75) | 13 (81) |
| Subendothelial deposits | 7 (44) | 9 (56) |
| Subepithelial | 7 (44) | 10 (63) |
| Intra-membranous deposits | 12 (75) | 13 (81) |
| Tubular basement membrane deposits | 7 (44) | 7 (44) |
| Subepithelial “hump-like” deposits | 5 (31) | 7 (44) |
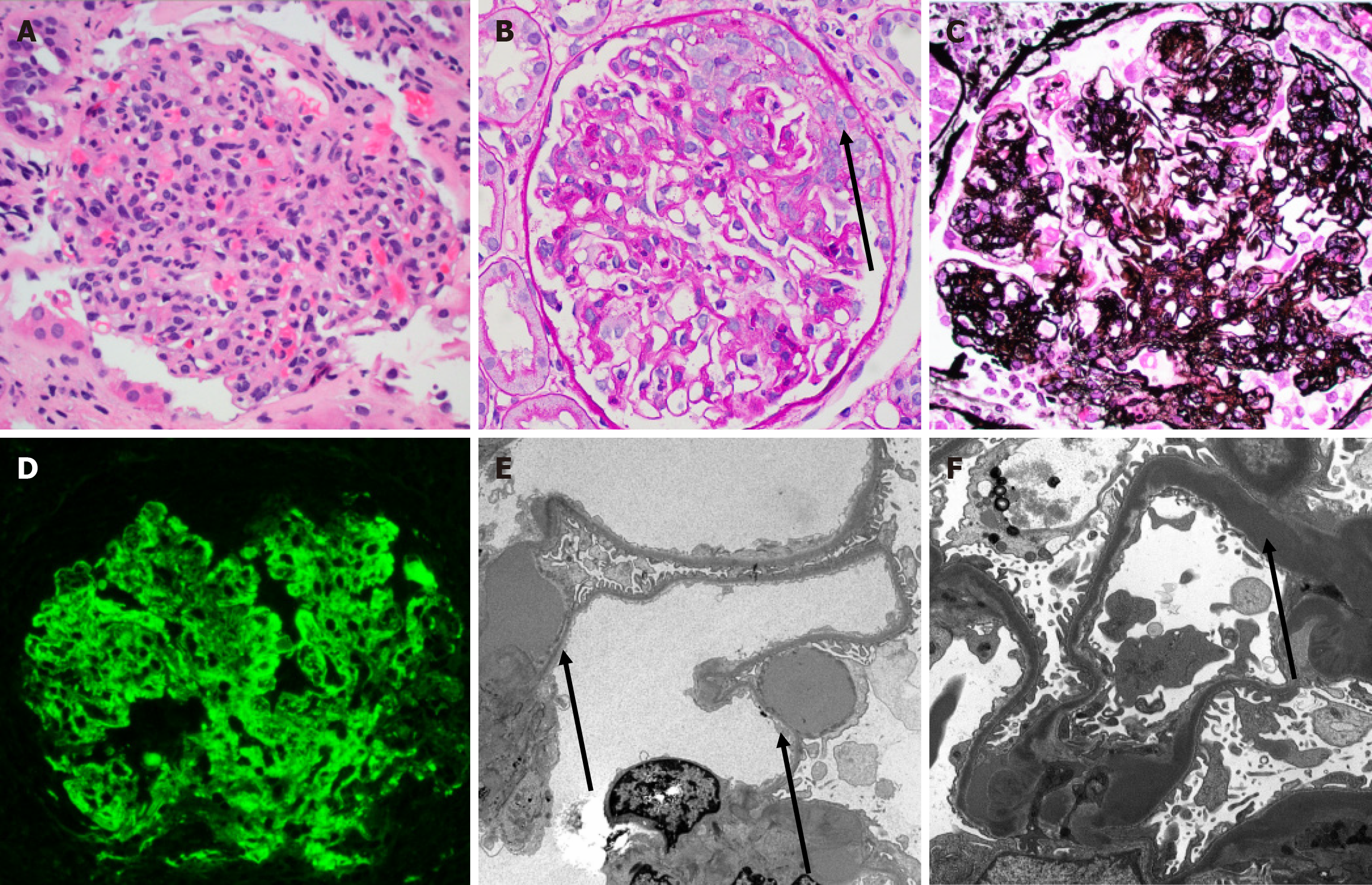
IF studies of index biopsies demonstrated 44% to be C3 only (Figure 2D), 50% to be C3 dominant. One index biopsy (C3GN-2) showed an immune complex (non-C3 dominant) GN which subsequently became C3 dominant on follow up biopsies. When follow up biopsies were available, all cases trended towards C3 only staining with other reagent staining becoming less prominent over time. The most common non-C3 staining of deposits was for IgG and IgM. Three patients (C3GN-2, C3GN-7, and C3GN-5, including the patient with the monoclonal gammopathy) had IF studies performed on paraffin tissue following pronase digestion without evidence of masked deposits. The IF staining patterns of post-transplant recurrences were generally similar to the native disease where data was available (i.e., C3 dominant/C3 only status matched).
Electron microscopic studies demonstrated mesangial and intramembranous deposits to be the most common overall (> 75% of biopsies). Greater than 50% of biopsies also displayed varying degrees of subendothelial and subepithelial deposits. Interestingly, 44% of patients demonstrated subepithelial “hump-like” deposits on EM (Figure 2E). All DDD cases demonstrated highly osmiophilic intra-membranous deposits typical for this diagnosis including biopsies at 2 months post-transplant (Figure 2F). Two patients (DDD-7 and DDD-2) showed EM features with overlapping DDD/G3GN features. In patient DDD-7, the initial deposition was characteristic for DDD. However, on subsequent biopsies the EM analysis demonstrated features more typical for C3GN.
In patients treated with eculizumab, there was no appreciable resolution of C3 deposition, nor definite effect on light or electron microscopic features. One patient (C3GN-6) demonstrated IgG-kappa light chain positive deposits consistent with eculizumab deposition[15]. Other pathologic findings included predominantly mild cellular rejection (50%). Features of antibody mediated rejection (25%), BK virus nephropathy (6%), acute or chronic calcineurin inhibitor toxicity (31%), and acute tubular injury (25%). Microangiopathic injury (19%) was present in a subset of biopsies; interestingly, one patient developed a severe early de novo thrombotic microangiopathy before subsequently developing recurrent C3GN.
Seven patients did not receive treatment for C3G following biopsy diagnosis. The median time to diagnosis post kidney transplant was 7 months with a median graft survival (not death censored) of 22 months post kidney transplantation. Two of the patients had an underlying reported diagnosis of MPGN-1 and were diagnosed with recurrence post kidney transplantation following persistent hematuria, native kidney pathology slides were not available for review for these patients. Neither received therapy due to underlying medical conditions, malignancy for patient C3GN-1 and cryptococcal meningitis for patient DDD-1. Patient C3GN-1was diagnosed with PTLD 2 months following C3GN diagnosis and underwent treatment with dexamethasone and rituximab with graft loss 60 months post kidney transplantation. Patient DDD-1 expired from complications from cryptococcal meningitis 18 months after transplantation with a serum creatinine unchanged from his baseline. Of the four patients who underwent repeat kidney transplantation, 3 had recurrence of C3G including 2 with allograft failure. One patient (DDD-2) had a functioning allograft 44 months post-transplant before being lost to follow up. One patient (DDD-6) received a second kidney transplant and was initiated on eculizumab at the time of repeat transplantation and continued to have allograft function for 11 months at the end of the study without evidence of disease recurrence.
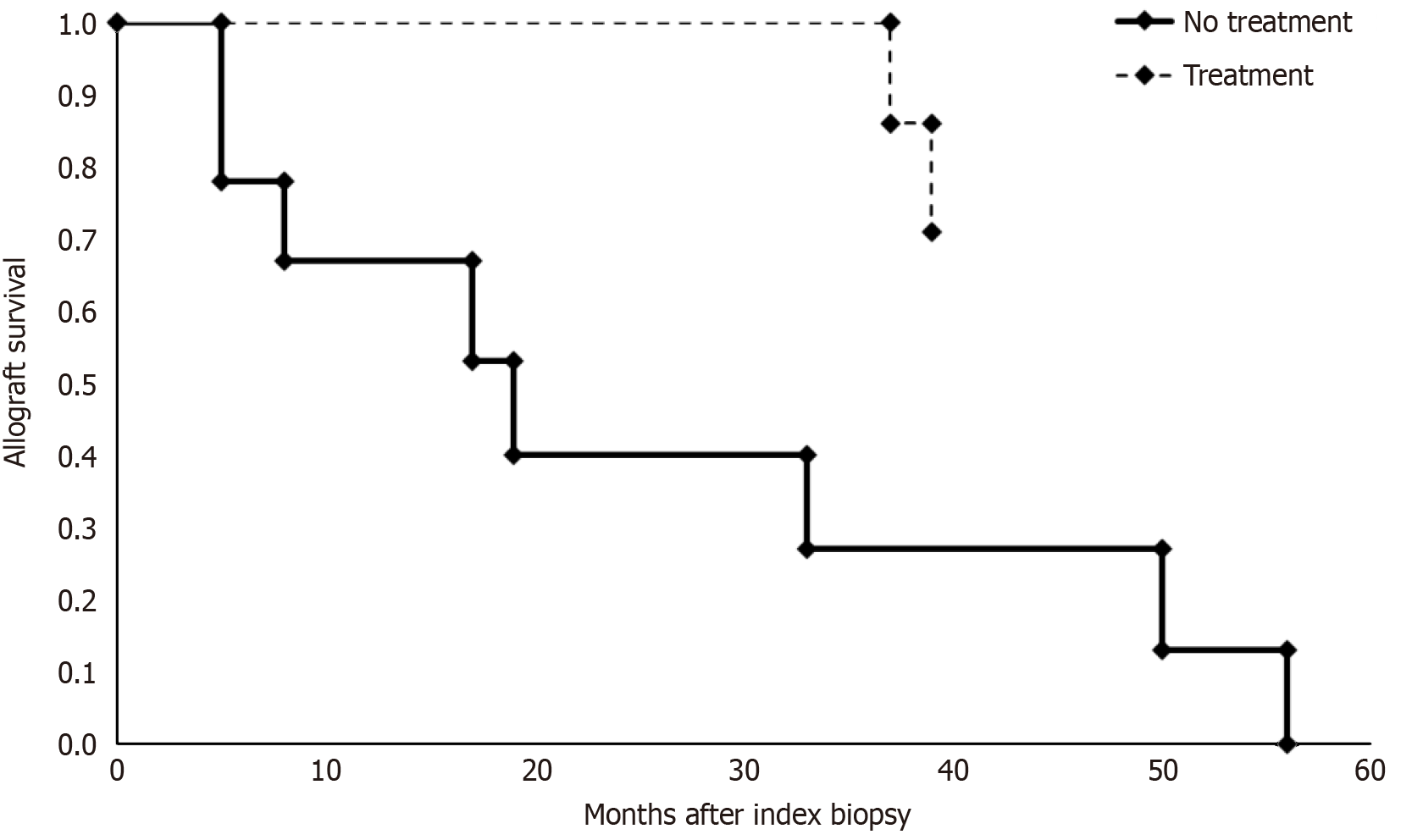
Seven patients underwent treatment for C3G following kidney transplant biopsy. The median time to diagnosis was 5 months post kidney transplantation with a median graft survival of 73 months by the end of study period. Five treated patients had a function graft by the end of the study period. Two were diagnosed on protocol biopsies and received minimal therapy initially with long term function until the end of the study period. One of these patients developed slowly progressive disease and was initiated on eculizumab 14 years after initial biopsy recurrence. Six patients received eculizumab therapy with four having long term response and continued allograft function at the end of the study period. Two patients (patients C3GN-5/C3GN-6) received delayed eculizumab treatment at least 6 months following the initial diagnosis and had significant impaired allograft function at the time of biopsy. Both patients lost their allograft within 4 years of kidney transplantation. The allograft survival analysis following index biopsy was performed between untreated and treated cohorts (Figure 3) which demonstrated a significantly improved allograft survival in the treatment group (P = 0.003, log-rank test). Excluding the patient C3GN-2 who did not receive complement blockade, the improved outcome in the treatment group was persistent (P = 0.009, log-rank test). The time to index biopsy recurrence was similar between the treated and untreated cohorts (p = 0.872, log-rank test) (Supplementary Figure 1).
One patient was believed to have de novo C3GN presumably developed in the setting of PTLD as their underlying cause of kidney disease was anti-neutrophil cytoplasmic antibodies vasculitis. This patient expired due to progressive malig
The occurrence of C3G post kidney transplantation most often represents early recurrent post kidney transplantation disease. In this cohort, recurrence occurred within 2 years of kidney transplantation and untreated disease was associated with a significant reduction in graft survival, with median graft survival < 2 years post kidney transplantation. Conversely, individuals with treated disease had a median graft survival of 73 months with five patients having ongoing graft function at the end of the study period. Our study results mirror those of the three largest single center studies previously published[16-18]. The Mayo group reviewed 21 kidney transplant recipients within a C3G registry for kidney transplant outcomes[16]. A total of 14 patients (66.7%) developed biopsy proven recurrent disease. The median time to recurrence was 28 months and graft failure occurred in 50% of patients with recurrence with a median time of 77 months after kidney transplantation. This study did not include treatment for patients with recurrent disease, presumably due to lack of availability of terminal complement inhibitors during the study time frame, 1996-2010. In a single center study, Regunathan-Shenk et al[16] reviewed 19 patients (12 C3GN and 7 DDD) with confirmed recurrent C3G as part of a C3G registry. Recurrence occurred in 84.2% of transplant recipients with a median time to recurrence of 14 months for C3GN and 15 months for DDD. Graft failure was more frequent, especially for patients with DDD, with a median time to failure of 42 months. Tarragón et al[18] studied 18 patients with native kidney failure attributed to C3G (12 C3GN and 6 DDD) in the early post-transplant period and found C3G recurrence occurred in 89% of patients (11 with C3GN and 5 with DDD) at a median of 33 days after transplantation. This study demonstrated relatively favorable short-term outcomes, possi
In line with prior C3G transplant series[7,19] the current series demonstrates only a subset of index biopsies with MPGN injury pattern (25%) with the majority showing mesangial, endocapillary, or mesangial and endocapillary proliferative GN. Interestingly, our study showed a higher degree of “active” GN at index biopsy than reported in other studies with 56% demonstrating the presence of endocapillary hypercellularity including 25% with exudative features as well as 19% with at least focal active crescents or necrosis. Crescents were present both at early and late time points post recurrence, although exudative features tending to occur at early recurrence timepoints. The reason for increased GN activity in this cohort is unclear but could be related to a predominance of for cause biopsies. Most cases with multiple biopsies showed progression to MPGN pattern of injury over time which may reflect the more aggressive nature of this cohort’s disease or indicate a final common pathway that most C3G will progress to over time.
In our cohort, the IF studies with C3 dominant staining on index biopsy all trended towards C3 only staining over time when multiple biopsies were available. It is known that a subset of immune complex MPGN are related to abnormalities in alternative complement pathway and cases which “switch” between immune complex and C3 dominant GN on subsequent biopsies have been reported. Treatment did not influence C3 deposition which remained either persistent or enhanced in patients following treatment. The electron microscopic studies demonstrated deposits in all cases of recurrent disease. Nearly all cases of DDD had dense intra-membranous deposits in their index recurrence biopsy which persisted on follow up biopsies. Case DDD-7 demonstrated a shift from predominantly DDD morphology to a more C3GN electron microscopic picture over the course of several years. As this patient received complement blockade, it is uncertain if this represents a disease intrinsic phenomenon or a treatment effect. Cases of C3GN/DDD overlap have been reported[20]. The electron microscopic studies also demonstrated subepithelial “hump-like” deposits which mimic those seen in classic post-infectious GN in a high percentage of both DDD and C3GN cases. Such deposits occurred both early and at later timepoints of recurrence. These data illustrate that such subepithelial deposits (as well as acute exudative features on light microscopy as noted above) are not useful in differentiating post-infectious GN from C3G.
This series also included a case of apparent de novo C3GN in a patient with PTLD. De novo C3G is a rare but reported occurrence[21]. To our knowledge this is the first case potentially associated with PTLD. An additional patient developed recurrent C3GN in the setting of PTLD. However, given the C3GN persisted and resulted in allograft failure 4 years after treatment of PTLD, the two findings are not favored to be related in the second case.
In native kidney disease the first line of treatment for C3G is a combination of steroids and mycophenolate mofetil[22]. However, most kidney transplant recipients in the United States are on an immunosuppression regimen including these agents and recurrence through these medications could be categorized as refractory disease. The underlying cause of C3G is dysregulation of the complement system and complement inhibitors may be of greater benefit in treating individuals with G3N, and their use is recommended for refractory disease by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative. Eculizumab was approved for paroxysmal nocturnal hemoglobinuria in 2007.
The effect of eculizumab therapy was found to be inconsistent in the aforementioned study by Regunathan-Shenk et al[16]. Eight patients received eculizumab, with response in only 50% of those treated. A larger meta-analysis patients treated for recurrent disease post kidney transplantation, either with eculizumab, plasma exchange, or rituximab were reviewed[23]. Eculizumab appeared to be the most effective therapy amongst those reviewed with a 32% graft loss. However, several of the included studies involved large cohorts of DDD before the year 2000. Treatment response appeared to be improved in our study, especially when eculizumab was initiated early and with preserved graft function. In our study, 40 % of patients received eculizumab therapy with a significant improvement in graft survival compared to those who did not receive therapy. Of the patients who did not receive therapy, 5 had disease prior to the availability of eculizumab and the other 2 had underlying malignancy and infection limiting administration of eculizumab. Additionally, patient C3GN-5 may had a monoclonal paraprotein which could have been a driver their disease[24]. Patient C3GN-6 had a high aggressive recurrence with a crescentic presentation which may have limited effectiveness of complement blockade due to established glomerular injury. Patients with DDD had the worst clinical course with 3 of the 5 undergoing repeat transplantation with allograft loss within 1 year of transplantation. Of interest is patient DDD-6 who lost her first transplant from recurrent DDD and received a second transplant. Eculizumab was initiated at time of her repeat kidney transplant and she continues to have stable graft function without proteinuria or hematuria 11 months post-transplant, the end of the study period.
The primary limitation of this study is the low number of patients analyzed. This is primarily due to the rare nature of this disease and our sample size is one of the largest to date. Genetic analysis, evaluation of soluble membrane attack complex, complement evaluation, and evaluation for monoclonal gammopathies were also notably absent in the majority of patients in this cohort, factors which may influence therapeutic decisions. Genetic and serological testing in C3G may permit for targeted therapies with improved efficacy by addressing the specific component of complement dysregulation. For example, monoclonal gammopathies may respond better to plasma cell therapies such as rituximab or daratumumab directed against the B cell clone. Factor H deficiencies or genetic abnormalities may respond to plasma exchange. Identification of C3 nephritic factors may respond to novel C3 inhibitors. Other limitations of our study include non-standardized biopsy timing, variable patient follow up times, absence of native kidney reports for review in a subset of cases, absence of pathology slides/IF/and EM images for review in a subset of cases. As biopsies were mostly done for indication, subclinical recurrence disease could not be excluded in many cases. Lastly, as only a subset of cases had slides available for direct re-review, the new C3 histologic activity/chronicity indexes were not calculated as the majority of biopsy reports did not provide sufficient detail for accurate calculation[25]. Further studies using larger cohorts, extended follow up times, complete genetic and complement studies, and new complement inhibitors would improve our knowledge. Identification of C3G and its impact on transplant outcomes has been limited due to the absence of its diagnosis on the UNOS candidate forms. This has restricted its understanding to single center evaluations and the authors recommend adding C3G as a diagnostic criteria code.
C3G post kidney transplant is often a recurrent disease and is associated with premature allograft loss. Eculizumab appears to be an effective therapy for improving outcomes and larger multicenter studies using new complement inhibitors should be considered.
| 1. | Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, Nasr SH, Theis JD, Dogan A, Smith RJ. C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 2012;82:465-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 2. | Zhang Y, Nester CM, Martin B, Skjoedt MO, Meyer NC, Shao D, Borsa N, Palarasah Y, Smith RJ. Defining the complement biomarker profile of C3 glomerulopathy. Clin J Am Soc Nephrol. 2014;9:1876-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Michels MAHM, Wijnsma KL, Kurvers RAJ, Westra D, Schreuder MF, van Wijk JAE, Bouts AHM, Gracchi V, Engels FAPT, Keijzer-Veen MG, Dorresteijn EM, Volokhina EB, van den Heuvel LPWJ, van de Kar NCAJ. Long-term follow-up including extensive complement analysis of a pediatric C3 glomerulopathy cohort. Pediatr Nephrol. 2022;37:601-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Medjeral-Thomas NR, O'Shaughnessy MM, O'Regan JA, Traynor C, Flanagan M, Wong L, Teoh CW, Awan A, Waldron M, Cairns T, O'Kelly P, Dorman AM, Pickering MC, Conlon PJ, Cook HT. C3 glomerulopathy: clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol. 2014;9:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | Pickering MC, D'Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Frémeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodríguez de Córdoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, Cook HT. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 449] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 6. | Garg N, Zhang Y, Nicholson-Weller A, Khankin EV, Borsa NG, Meyer NC, McDermott S, Stillman IE, Rennke HG, Smith RJ, Pavlakis M. C3 glomerulonephritis secondary to mutations in factors H and I: rapid recurrence in deceased donor kidney transplant effectively treated with eculizumab. Nephrol Dial Transplant. 2018;33:2260-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Kumar A, Ramachandran R, Rawat A, Das R, Rayat CS, Kenwar DB, Sharma A, Gupta KL, Nada R. Poor allograft outcome in Indian patients with post-transplant C3 glomerulopathy. Clin Kidney J. 2021;14:291-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Wong L, Moran S, Lavin PJ, Dorman AM, Conlon PJ. Kidney transplant outcomes in familial C3 glomerulopathy. Clin Kidney J. 2016;9:403-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Avasare RS, Canetta PA, Bomback AS, Marasa M, Caliskan Y, Ozluk Y, Li Y, Gharavi AG, Appel GB. Mycophenolate Mofetil in Combination with Steroids for Treatment of C3 Glomerulopathy: A Case Series. Clin J Am Soc Nephrol. 2018;13:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Gurkan S, Fyfe B, Weiss L, Xiao X, Zhang Y, Smith RJ. Eculizumab and recurrent C3 glomerulonephritis. Pediatr Nephrol. 2013;28:1975-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Moog P, Jost PJ, Büttner-Herold M. Eculizumab as salvage therapy for recurrent monoclonal gammopathy-induced C3 glomerulopathy in a kidney allograft. BMC Nephrol. 2018;19:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Rousset-Rouvière C, Cailliez M, Garaix F, Bruno D, Laurent D, Tsimaratos M. Rituximab fails where eculizumab restores renal function in C3nef-related DDD. Pediatr Nephrol. 2014;29:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Haas M, Seshan SV, Barisoni L, Amann K, Bajema IM, Becker JU, Joh K, Ljubanovic D, Roberts ISD, Roelofs JJ, Sethi S, Zeng C, Jennette JC. Consensus definitions for glomerular lesions by light and electron microscopy: recommendations from a working group of the Renal Pathology Society. Kidney Int. 2020;98:1120-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Naesens M, Roufosse C, Haas M, Lefaucheur C, Mannon RB, Adam BA, Aubert O, Böhmig GA, Callemeyn J, Clahsen-van Groningen M, Cornell LD, Demetris AJ, Drachenberg CB, Einecke G, Fogo AB, Gibson IW, Halloran P, Hidalgo LG, Horsfield C, Huang E, Kikić Ž, Kozakowski N, Nankivell B, Rabant M, Randhawa P, Riella LV, Sapir-Pichhadze R, Schinstock C, Solez K, Tambur AR, Thaunat O, Wiebe C, Zielinski D, Colvin R, Loupy A, Mengel M. The Banff 2022 Kidney Meeting Report: Reappraisal of microvascular inflammation and the role of biopsy-based transcript diagnostics. Am J Transplant. 2024;24:338-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 103] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 15. | Herlitz LC, Bomback AS, Markowitz GS, Stokes MB, Smith RN, Colvin RB, Appel GB, D'Agati VD. Pathology after eculizumab in dense deposit disease and C3 GN. J Am Soc Nephrol. 2012;23:1229-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Regunathan-Shenk R, Avasare RS, Ahn W, Canetta PA, Cohen DJ, Appel GB, Bomback AS. Kidney Transplantation in C3 Glomerulopathy: A Case Series. Am J Kidney Dis. 2019;73:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Uffing A, Hullekes F, Riella LV, Hogan JJ. Recurrent Glomerular Disease after Kidney Transplantation: Diagnostic and Management Dilemmas. Clin J Am Soc Nephrol. 2021;16:1730-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Tarragón B, Peleg Y, Jagannathan G, Sekulic M, Chang JH, Cohen DJ, Crew RJ, Dube GK, Fernandez HE, Husain SA, Mohan S, Morris HK, Appel GB, Jadav P, Santoriello D, Kudose S, Stokes MB, Batal I, Bomback AS. C3 Glomerulopathy Recurs Early after Kidney Transplantation in Serial Biopsies Performed within the First 2 Years after Transplantation. Clin J Am Soc Nephrol. 2024;19:1005-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 19. | Zand L, Lorenz EC, Cosio FG, Fervenza FC, Nasr SH, Gandhi MJ, Smith RJ, Sethi S. Clinical findings, pathology, and outcomes of C3GN after kidney transplantation. J Am Soc Nephrol. 2014;25:1110-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Sethi S, Fervenza FC, Smith RJ, Haas M. Overlap of ultrastructural findings in C3 glomerulonephritis and dense deposit disease. Kidney Int. 2015;88:1449-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Abbas F, El Kossi M, Jin JK, Sharma A, Halawa A. De novo glomerular diseases after renal transplantation: How is it different from recurrent glomerular diseases? World J Transplant. 2017;7:285-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Obata S, Vaz de Castro PAS, Riella LV, Cravedi P. Recurrent C3 glomerulopathy after kidney transplantation. Transplant Rev (Orlando). 2024;38:100839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Gonzalez Suarez ML, Thongprayoon C, Mao MA, Leeaphorn N, Bathini T, Cheungpasitporn W. Outcomes of Kidney Transplant Patients with Atypical Hemolytic Uremic Syndrome Treated with Eculizumab: A Systematic Review and Meta-Analysis. J Clin Med. 2019;8:919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Leung N, Bridoux F, Batuman V, Chaidos A, Cockwell P, D'Agati VD, Dispenzieri A, Fervenza FC, Fermand JP, Gibbs S, Gillmore JD, Herrera GA, Jaccard A, Jevremovic D, Kastritis E, Kukreti V, Kyle RA, Lachmann HJ, Larsen CP, Ludwig H, Markowitz GS, Merlini G, Mollee P, Picken MM, Rajkumar VS, Royal V, Sanders PW, Sethi S, Venner CP, Voorhees PM, Wechalekar AD, Weiss BM, Nasr SH. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 2019;15:45-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 348] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 25. | Caravaca-Fontán F, Trujillo H, Alonso M, Díaz-Encarnación M, Cabello V, Ariceta G, Quintana LF, Marco H, Barros X, Ramos N, Rodríguez-Mendiola N, Cruz S, Fernández-Juárez G, Rodríguez E, de la Cerda F, Pérez de José A, López I, Fernández L, Pérez Gómez V, Ávila A, Bravo L, Lumbreras J, Allende N, Sanchez de la Nieta MD, Olea T, Melgosa M, Huerta A, Miquel R, Mon C, Fraga G, de Lorenzo A, Draibe J, González F, Shabaka A, Illescas ML, Calvo C, Oviedo V, Da Silva I, Goicoechea de Jorge E, Caravaca F, Praga M; C3G Study Group of the Spanish Group for the Study of Glomerular Diseases (GLOSEN). Validation of a Histologic Scoring Index for C3 Glomerulopathy. Am J Kidney Dis. 2021;77:684-695.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |