Published online Jun 18, 2025. doi: 10.5500/wjt.v15.i2.101005
Revised: October 25, 2024
Accepted: November 19, 2024
Published online: June 18, 2025
Processing time: 172 Days and 17.5 Hours
Lung transplantation (LT) is now an accepted therapy for end stage lung disease in appropriate patients. Atrial arrhythmias (AA) can occur after LT. Early AA after LT are most often atrial fibrillation, whereas late arrhythmias which occur many months or years after LT are often atrial tachycardia. The causes of AA are multifactorial. The review begins with a brief history of LT and AA. This review further describes the pathophysiology of the AA. The risk factors, incidence, recipient characteristics including intra-operative factors are elaborated on. Since there are no clear and specific guidelines on the management of atrial arrhythmia following LT, the recommended guidelines on the management of AA in general are often extrapolated and used in the setting of post LT arrhythmia. The strategy of rate control vs rhythm control is discussed. The pros and cons of various drug regimen, need for direct current cardioversion and catheter ablation therapies are considered. Possible methods to prevent or reduce the incidence of AA after LT are considered. The impact of AA on the short-term and long-term outcomes following LT is discussed.
Core Tip: While atrial arrhythmias (AA) after lung transplantation (LT) continue to be challenging, there has been an increase in our understanding of the mechanisms of these arrhythmias. Early arrhythmias are commonly due to atrial fibrillation and late arrhythmias are often atrial tachycardia. While the main treatment of early perioperative arrhythmia is drug therapy or cardioversion, the late stable arrhythmias can now be successfully treated by catheter ablation. Prophylactic measures to prevent arrhythmia are needed. In the absence of specific guidelines to treat AA after LT, further studies are needed to evaluate methods to prevent or reduce the incidence.
- Citation: Sunder T, Ramesh P, Kumar M. Atrial arrhythmias following lung transplantation: A state of the art review. World J Transplant 2025; 15(2): 101005
- URL: https://www.wjgnet.com/2220-3230/full/v15/i2/101005.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i2.101005
Lung transplantation (LT) is now an accepted therapy for appropriate patients with end stage lung disease (ESLD) providing both symptomatic and survival benefits. While the overall results of LT are improving due to better understanding of the mechanisms of allograft rejection, molecular diagnostics, pathophysiology of primary graft dys
While there are many reasons specific to LT to explain the above figures, atrial arrhythmias (AA) that occur following LT by its adverse effects on haemodynamics and lung pathophysiology, is an important cause for increased mortality and morbidity after LT often leading to prolonged stay in the intensive care unit or in the hospital.
Post-operative atria arrhythmia (POAA) following LT in the early post-operative period includes most commonly atrial fibrillation (AF), followed by atrial flutter (AFL), atrial tachycardia (AT), and supra ventricular tachycardia (SVT). Late AA following LT are more often AT[5]. AF are more common in the early period comprising as much as 85% to 90% of early POAA[6].
Despite the publication of many case series, case reports and meta-analyses in the current literature regarding AA after LT, there are still no clearcut guidelines for the treatment of AF following LT. There are proponents for both rate control and rhythm control strategy with pros and cons for each strategy.
In this comprehensive state of the art narrative review, the review structure is explained to aid clarity and allow seamless perusal of this manuscript. A historical perspective, followed by description of our understanding of the current scenario and eventual look at future considerations form the essential design of this review.
This review commences with a brief historical perspective of both LT and AA. AF which has been described centuries ago continues to be a formidable obstacle to this day. Then, the literature on AA in general and in the post-operative scenarios including the various strategies of AA treatments are looked at. The focus is on the various randomised trials of AF and the guidelines published for AA to see if some insights from these could be extrapolated to AA which occur following LT.
The definitions of the types of AF and LT are described; and the incidence, peak occurrence, risk factors, and the impact of AA following LT are reviewed. The mechanisms of AA are discussed. The pathophysiological effects of AA following LT and its adverse impact on the newly grafted lung particularly the effects on gas exchange and haemodynamics are elaborated on. The logical need for conversion to sinus rhythm (SR) in LT is explained.
Subsequently, we turn the attention to the currently available evidence of POAA following LT. The treatment options with outcomes are discussed in detail supported by available evidence. Finally, we conclude by looking at possible areas for further studies and research with a view to reducing or even preventing AA following LT.
The key words “lung transplantation”, “atrial arrhythmias”, “atrial fibrillation”, “atrial tachycardia”, “atrial flutter”, “catheter ablation”, “rate control”, “rhythm control”, “outcomes”, “mortality” were used to search PubMed database. Meta analyses, review articles, case series and reports were identified in English language. These articles were carefully studied and used in the preparation of this manuscript. There are 4 meta-analyses on AA following LT published so far which are listed in Table 1[5,7-9]. The great majority of the relevant literature is in the form of case series which are retrospective in nature. We identified 14 studies on catheter ablation (CA) for AA after LT most of which are single case reports or case series of small number of patients. Being a narrative review, the facts and conclusions from these articles are presented in a structured way along with an in-depth discussion. No statistical analysis was carried out.
| Ref. | Year | No. of studies | Total cases | AA | Conclusions |
| Saglietto et al[5] | 2021 | 7 | 2068 | This study looked only at late AA. Late atrial fibrillation rare–especially in double lung transplant (incidence rate only 0.9%/year) | |
| Saad et al[7] | 2017 | 12 | 3203 | 27% | Early AA increases mortality and hospital stay. Predictors of early AA are elderly age, male sex, smoking, HT, hyperlipidaemia, CAD, increased LA diameter, and restrictive lung disease |
| Waldron et al[8] | 2017 | 9 | 2653 | 29.8% | AA was associated with higher mortality, longer hospital stays, increased need for tracheostomy |
| Fan et al[9] | 2016 | 11 | 2094 | 31% | Risk factors include elderly age, male, prior AA, cystic fibrosis, interstitial lung disease, CAD, HT, hyperlipidaemia, LA size. Increases mortality and hospital stay |
LT is a complex surgical procedure and is now only just over 60 years old. After decades of experimental work in the animal laboratory, in 1963, James Hardy performed the first successful LT in humans[10]. It was a left single LT (SLT) and the patient survived only 18 days. Following this, there were attempts by surgeons, both in North America and Europe, to perform this operation[11]. There were no long term survivors and the main problem was bronchial dehiscence. Furthermore, immunosuppressive therapy was at its infancy. It was only 20 years later, in 1983, when Joel Copper from Toronto General Hospital, reported on the first successful long term survivor following LT[12] . It was a right SLT and the patient survived for 7 years following his operation. In 1986, the first en bloc double LT (DLT) was done which required cardiopulmonary bypass and tracheal anastomosis. To simply the operation further, DLT was performed as two separate sequential LT with a separate bronchial anastomosis for each lung[12]. Subsequently, this surgical procedure became standardised and was practised by surgeons across the globe.
The first ever reference to AA was with regards to AF. The historical aspects of AF has been well described by Lip and Beevers[13] in an article published in 1995. They suggest that perhaps the earliest reference to AF was by an eminent Chinese physician Huang TN Ching Su Wen between 1696 and 2598 BC. Harvey W in 1628 described AF in animals. There were other researchers who contributed along the way. Mackenzie J was the first to describe the loss of “a” wave in the pulse trace. The invention of electrocardiograph, in 1900, by Einthoven W was the pivotal point who recorded a tracing of AF. It was, then, Lewis T who first correlated the irregular pulse of AF in an electrocardiogram at the University College Hospital, London[14]. Subsequently, with technological advances and pharmacological progress, our understanding of the arrhythmia gradually improved. Despite the advances in electrophysiology and our improved understanding, there are still areas which need further research for better treatment of this elusive arrhythmia, especially after LT.
To facilitate understanding of AA in the context of LT, some of the terms are defined and elaborated further.
Based on the timing of occurrence, AA can be classified as early (peri-operative) and late AA. Most peri-operative AA are AF and are transient in nature. AF can be further classified into 4 types[15] as defined below.
Paroxysmal AF: The arrhythmia is episodic, lasting for less than 7 days and reverts to SR either spontaneously or due to intervention.
Persistent AF: The AF lasts for more than 7 days, but less than a year.
Long standing persistent AF: The arrhythmia lasts for more than a year.
Permanent AF: As the name implies, the arrhythmia is permanent not responding to any intervention and rate control strategy is followed in these patients.
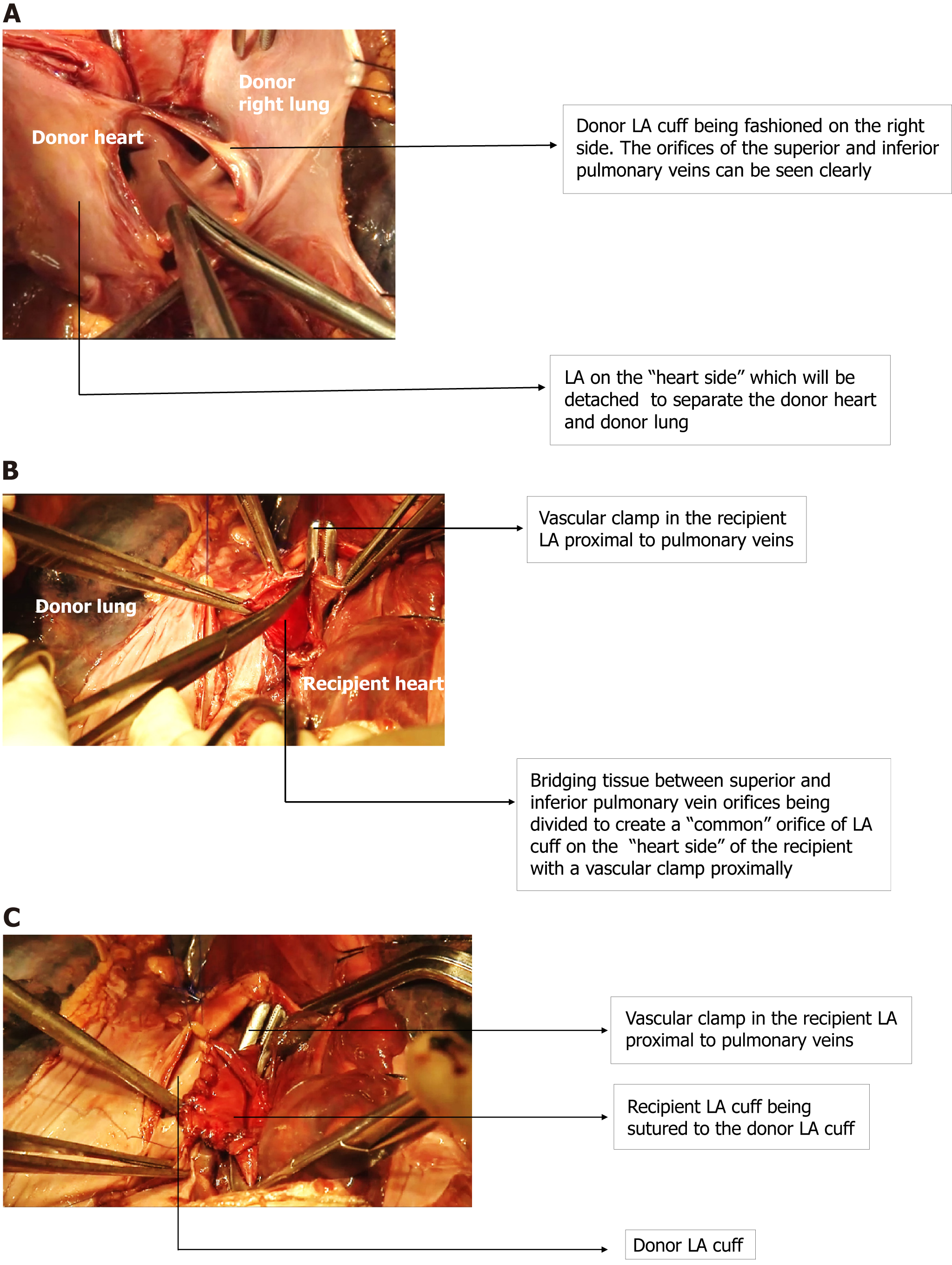
A brief description of the surgical aspect of SLT and DLT, particularly with reference to pulmonary vein (PV) and left atrial (LA) anastomosis is provided to aid the understanding of mechanisms of AA following LT.
Donor LA cuff (Figure 1A) is carefully fashioned just beyond the orifice of the two PVs (superior and inferior) and prepared to be anastomosed to a similar LA cuff in the recipient as described below.
SLT vs DLT: In SLT, one disease lung (either right or left) is explanted, and the hilum is dissected in preparation for the implantation of the donor lung. The bronchial stump and pulmonary artery (PA) stumps are fashioned, with a vascular clamp on the proximal part of the PA. The LA is dissected at the confluence of the superior and inferior PV. A vascular clamp is applied proximal to the confluence of the PV and the bridging tissue between the superior and inferior PV is divided (Figure 1B). This results in a single LA cuff which is then anastomosed (Figure 1C) to a similarly prepared donor LA cuff which receives the superior and inferior PV of the donor. This procedure, in effect, produces PV isolation (PVI) on the side of the operation. The contralateral side has the native lung with intact hilar connections with the heart.
In DLT, both the diseased lungs are explanted, and the donor lungs are sequentially implanted one after the other as described above using a LA cuff on both the donor and recipient ends. DLT, thus, results in complete PVI on both sides. Thus, establishment of surgical connection and continuity between donor and recipient PV is similar to various surgical maze procedures on LA described by Cox[16] and Cox et al[17], designed in principle to treat AF surgically.
This surgical aspect (unilateral vs bilateral PVI) explains the difference between SLT and DLT with respect to occurrences of AA following LT. Lee et al[18] report a decreased incidence of AA after LT in DLT patients, because the bilateral PV anastomosis blocks electrical impulses, whereas the incidence is higher in SLT. In contrast, however, a meta-analysis by Waldron et al[8] report a higher incidence after DLT. The authors explain the higher incidence by emphasising the fact that there is one more anastomosis with surgical oedema and inflammation–resulting in higher incidence of AA after LT.
Anatomical considerations of the left atrium: In an excellent article titled “Left atrial anatomy revisited”, Ho et al[19] describe in detail the anatomy of the left atrium which would be of interest and very valuable to arrhythmia surgeons and electrophysiologists. The LA muscular walls have the following regions–superior (roof), posterior, septal(medial), left lateral and anterior wall. The muscular fibres of the LA extend as a “sleeve” onto the external surfaces of the PV as they enter the LA. These muscular sleeves of the LA are associated with impulses originating and initiating AA. The PV orifices inside the lateral wall of the LA have an endocardial ridge separating them. The muscular sleeves of the LA wall encircle the PV. These inter-venous (between orifices of PV) muscular sleeves are closer to the epicardial surface. They crisscross from the anterior surface of the superior PV sleeve to the posterior surface of the inferior PV sleeve and vice versa. The above anatomical facts are of electrophysiological importance to both arrhythmia surgeon and electrophysiologist. The vestibule of the LA is the LA outlet surrounding the mitral annulus and the mitral isthmus is that portion between the inferior PV orifice and the attachment of the valve to the mitral leaflet.
During embryogenesis, there is enlargement of LA walls and the tissue at the ends of the PV get absorbed into the LA wall. This region becomes the posterior wall of the LA. This composite structure which includes both the LA wall along with the incorporated PV is the PV antrum. It is funnel shaped and includes parts of roof of LA and posterior LA wall between the PV. PV antrum isolation–which includes PVI and ablation of all activity in the posterior wall of LA has a higher procedural success rate with lesser incidence of recurrence[20].
Pathophysiology of AA and its adverse effects: The majority of the early POAA are AF. Hence, the pathophysiology and adverse effects of AF after LT will be considered here.
Patients who undergo LT have ESLD with resultant elevated pulmonary vascular resistance (PVR) due to hypoxia. While awaiting an organ, the blood flow across the pulmonary vascular bed gradually reduces due to the high PVR and the inflow to the LA (preload) is chronically reduced. This often results in a small cavity left ventricle with low compliance and diastolic dysfunction.
A luxuriant flow across the newly grafted lungs(with low PVR) results in sudden increase in preload to the “unprepared” left heart resulting in pulmonary congestion and pulmonary oedema.
Other adverse effects on lungs due to AA: The loss of “atrial kick” and fall in cardiac output may cause hypotension, requiring adrenergic drugs which may compound the arrhythmogenic problem. Rapid ventricular rates reduce the ventricular filling times resulting in further pulmonary congestion.
Following LT, the dual supply to the lungs are lost and the bronchial tree–notably the bronchial anastomosis relies for nutrition on retrograde blood flow in the bronchial arteries, which in turn, are fed from the vascular plexus between PA and bronchial artery.
In a best-case scenario (i.e., in a stable LT patient), it is apparent that the donor bronchial ischemia is, at best, hypoxic. This hypoxia worsens in the case of AF which reduces both the oxygen content and pressure of the blood–which can impact long term healing. Furthermore, one may speculate that hypotension and hypoxia may even, possibly, lead to the development of chronic allograft lung dysfunction (CLAD), as has been described in patients with hypoxia due to venous thromboembolism[21].
Mechanisms of atrial arrhythmia: AA occurring early are often AF, while those occurring years after LT are AT.
The pathogenesis of AA is often multifactorial. The “early” POAA which occurs in the peri-operative period are due to an “inflammatory milieu” with a background of heightened sympathetic drive. The operative trauma and resultant tissue oedema, pericarditis, electrolyte imbalance, haemodynamic instability requiring inotropes which are often arrhythmogenic result in a “milieu” conducive to the development of AA. The stretching of atrial walls which occur with fluid shifts, operative pain and proarrhythmic drugs may further contribute to the arrhythmia.
The landmark electrophysiological report in 1998 by Haïssaguerre et al[22] has demonstrated the PVs to be common sites for ectopic foci from which AA may commence. However, as demonstrated in the Figure 1, there is a complete transection and suturing of the donor and recipient LA cuff at the confluence of the PV–a surgical PVI, which is the surgical procedure for treatment of AF. Thus the scarring and fibrosis at the anastomotic site would be a barrier to the conduction of electrical impulses passing across it. Hence, logical reasoning suggests that in DLT with bilateral PVI, the incidence of AF should be much lower. However, this is not the case and, therefore, it follows that the fore mentioned “inflammatory, arrhythmogenic postoperative milieu” must play a major role in the pathogenesis of early post-operative AF (POAF).
Those AA which occur after 3 months to 12 months post LT are referred to as late AA. These are more often organised AT and not AF. With the passage of time, the early arrhythmogenic milieu settles and, early AF often reverts to SR with treatment. It is interesting to note that subsequent AA are often not AF, due to the “protective effect” of the surgical PVI done during the course of LT. This fact is supported by data from Lee et al[18], who reported only 1 instance of late AF 200 LTs (0.5% ); and See et al[23], who reported no instances of AF following LT after 1 year in 130 patients.
Chaikriangkrai et al[24] reported no difference between SLT and DLT as regards the late occurrence of AA, while Lee et al[18] report a significant reduction of late AA following DLT compared to SLT (0.5% vs 12.6%). It can be logically reasoned that in DLT the ectopic foci on both sides are blocked by bilateral PVI, whereas in SLT, the native lung has intact PV connections with LA, accounting for the increased late occurrence of AA.
The PV anastomotic site is both anti-arrhythmogenic and pro-arrhythmogenic, as discussed below.
During the LT operation, while the site of PV anastomosis, which involves a transmural suturing of the donor and recipient LA cuff (similar to the “cut and sew” Cox-Maze operation), heals and forms an electrical barrier to the conductance of impulses from ectopic sited in the PV and, hence, reduces the incidence of late AF.
Paradoxically, excess scarring in the PV anastomosis could also be foci of macro re-entrant AT[23]. In their electrophysiological study of 25 patients with AA after LT, Chaikriangkrai et al[24] report that the native PV is the main site of origin of AA. They suggest that surgical manipulation at the donor PV antrum incites an inflammatory response, which coupled with stretching of atrial walls, contributes to arrhythmogenicity. Interestingly, they also report a higher incidence of AA after DLT when compared to SLT. Uhm et al[25] describe late AT from PV anastomotic line during ultra-high density three-dimensional mapping and radio frequency (RF) CA which was performed 10 months after LT with no recurrence. Of note, they noticed electrical re-connections across anastomotic line for all 4 PVs.
Nazmul et al[26] and Sanam et al[27] report cases where electrophysiological studies have demonstrated the site of origin of AA to be from the donor PV.
Hussein et al[28] reported on reduced incidence of AF in patients taking mycophenolate as a part of their immunosuppression and suggest that its anti-fibrotic property might play a role. A randomised controlled trial (RCT)[29] de
Despite full thickness surgical scar, recovery of electrical conduction across the scar tissue has been reported–especially in patients with prior history of AF before LT.
In their elegant study, Hussein et al[28] report on the occurrence of recovery of electrical conduction across PV anastomosis following LT. Out of 755 LT patients, 51 patients had prior AF compared to 704 patients without prior AF. The incidence of late AF was 21% (11 out of 51) in patients with prior AF. In those without prior AF, the incidence of late AF was very low 2.8% (21 out of 704 patients). Recovery of electrical conduction was confirmed in patients who underwent CA. Despite ablation, AF recurred in these patients requiring further ablation.
Heart transplantation (HT), during recipient cardiectomy, involves excision of the superior vena cava, ganglionic plexus around the heart and PV. In LT, the posterior LA wall and junction of PV are left in situ[30]. This explains the lower incidence of AA following HT of 10% to 20%, when compared to LT which has an incidence of 16% to 45%, as discussed in the following section.
Recent studies on POAF: POAF has been studied extensively in surgical patients including those undergoing general surgery, cardiac surgery, and thoracic surgery. However, LT patients form a small subset and in comparison, literature of POAF in the setting of LT is limited.
A meta-analysis[31] of POAF after non cardiac surgery included 28 studies enrolling 2612816 patients. Among patients with POAF, there was an increased risk of myocardial infarction, 30-day mortality and stroke in non-cardiac surgical patients. The risk of stroke was higher in nonthoracic surgical patients.
In a recent 2023 scientific statement, Chyou et al[32] define triggers and substrates for the development of AA in acute hospital setting.
Incidence of AA following LT: While AA is reported to affect 1.5% to 2% of the general population[33], the incidence of AA following LT is higher than the incidence of AA following HT and non-transplant surgery.
The incidence of AA following LT in various case series ranges from 16%[34] to 45%[35] among adult patients and the incidence among paediatric patients ranges from 11% to 15%[36,37]. The incidence of AA following LT in the 3 meta-analyses[7-9] ranges from 27% to 31%. These incidences are higher than the incidences of AA following HT[38,39] or other non-transplant thoracic surgery.
The incidence of POAF after non transplant surgery[40] is reported as follows: (1) Overall after cardiac surgery-30%; (2) Non-cardiac thoracic surgery[41]; and (3) Non-cardiothoracic general surgery-0.39%[42].
Table 2 lists the incidences of POAF following various types of surgeries. It can be seen in the table that the highest incidence of POAF occurs in those undergoing LT, which suggests that mechanisms which maybe unique to LT may play a role[32,33,36-38,40].
| Description | Incidence (%) |
| Transplant surgery | |
| Lung transplantation[32,33] | 17 to 46 |
| Heart transplantation[36,37] | 10 to 20 |
| Thoracic surgery[38] | |
| Overall cardiac surgery | Approximately 30 |
| Coronary artery bypass surgery | Approximately 10 to 20 |
| Thoracic (non-cardiac) surgery[38] | |
| Overall | Approximately 15 |
| Pneumonectomy | Approximately 30 |
| General (non-cardiothoracic) surgery[40] | 0.39 |
The peak incidence of early AA after LT is within 5 days of the operation[24].
Older age: This risk factor is perhaps the most identified factor across many studies[43-46] and one meta-analysis[9].
Male sex: It has been identified as risk factor for AA post LT by several studies[24,34,43,47,48].
High body mass index: A few studies[24,43,44,47] identified high body mass index as a risk factor. Of note, Mason et al[49] described extremes of weight as a risk factor for AF after LT.
Recipient lung disease: Several studies have identified ILD as a risk factor. However, Barnes et al[45] reported COPD to be a risk factor for AA. In contrast, a review by Roukoz et al[6] and a meta-analysis by Fan et al[9] report low incidence of POAF in patients with cystic fibrosis. Idiopathic PA hypertension has been reported as a risk factor for AA after LT[49]. Kim et al[44] report elevated right atrial (RA) pressure and prolonged ventilation (which causes high RA pressure)to be a risk factor.
Pre transplant PA pressure: The evidence is conflicting as regards the risk of POAF in the setting of high PA pressure (PAP). Chaikriangkrai et al[24] report an inverse relationship between PAP and AF after LT, resulting in higher incidence of POAA in patients with low PAP. Mason et al[49] found no effect of PAP on the occurrence of AA after LT. In contrast to the above finding, Jesel et al[50] reported pre-transplant elevated systolic PAP as a predictor for development of late AA following LT.
Type of operation-SLT vs DLT: Some authors[24,49,51] report higher incidence of AF in patients who underwent DLT. In contrast, Lee et al[18] report low incidence of late AA after DLT.
LA enlargement: increases risk of AA after LT[48,52]. LA enlargement and higher filling pressure leads to chronic stretching of the atrial musculature leading to fibrosis and electrical remodelling; thus resulting in a higher incidence of AA.
History of prior AF: It is a risk factor[6,48] for AA after LT. Xia et al[47] compared the incidence of POAF in patients with and without Maze procedure (which was performed during LT in patients with prior AF). The incidence of POAF was still higher in the Maze group and the authors therefore recommend ligation of LA appendage in this subgroup to prevent stroke.
Table 3[6-9,35,44,48,51,53-55] and Table 4[24,45,46,49,50,56] list the various studies reporting on the effect of AA on mortality after LT. The occurrence of AA after LT has been associated with increase in mortality. Three out of the 4 meta-analyses[7-9] report an increase in mortality due to AF after LT. Furthermore, there are several studies[51,53-55] which report an adverse effect of AA on the survival following LT. Of note, there are also a few studies[24,45,46,50,56] which did not find any effect of AA after LT on mortality.
| Ref. | Year | Study type | Effect on mortality |
| Magnusson et al[53] | 2022 | Case series | Increase |
| Kim et al[44] | 2020 | Case series | Increase |
| Roukoz et al[6] | 2018 | Review | May be increased |
| Waldron et al[8] | 2017 | Meta-analysis | Increase |
| Saad et al[7] | 2017 | Meta-analysis | Increase |
| D'Angelo et al[54] | 2016 | Case series | Increase |
| Fan et al[9] | 2016 | Meta-analysis | Increase |
| Orrego et al[48] | 2014 | Case series | Increase |
| Henri et al[51] | 2012 | Case series | Increase |
| Isiadinso et al[35] | 2011 | Case series | Increase |
| Garcia et al[55] | 2011 | Case series | Increase |
Elevated systolic PAP have been found to be protective against the development of AA after LT[34]. While the authors acknowledge that the precise reason for this observation remains unknown, they propose that the higher pressures in the PA leads to a smaller LA size, volume with resultant decrease in the LA stretching and thus reduce incidence of AA post LT.
The main goals of treatment include: (1) Relief of symptoms; (2) Prevention of thromboembolism and stroke; and (3) Prevention of tachycardia induced cardiomyopathy.
After optimising general measures, these goals can be achieved by specific measures using either or both rate control and rhythm control strategy. However, achieving SR is more physiological with re-attainment of “atrial kick” and more importantly, preventing negative structural and electrical remodelling of the atria.
General measures, especially in early post-operative period, consist of addressing various factors which increase the likelihood of AA post-operatively. These measures include correction of electrolyte abnormalities–especially potassium (to maintain levels around 4.5 mEq/L) and magnesium; minimising the use of adrenergic drugs and weaning as early as possible, minimising sudden large fluid shifts, providing adequate pain relief[51], and maintaining adequate oxygenation with reassurance to allay anxiety.
Rate control methods[57] use drugs which block conduction across atrio ventricular node and include beta blockers (BB), calcium channel blockers (CCB) and digoxin. Rate control was the preferred strategy in the early 2000s with nu
Rhythm control methods include anti-arrhythmic drugs (AAD), electrical cardioversion and CA. With improvements and advancements, rhythm control strategies started gaining popularity, with clinicians re-visiting rhythm control methods.
Guidelines for treatment of AF detailing the various options in different clinical scenarios have been provided by the American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS), European Society of Cardiology and Canadian Cardiovascular Society (CCS). In an excellent review article, Cheung et al[63] has published in 2021 an updated comparison of the guidelines for the management of AF by AHA[64], CCS[65] and ESC[66]. Although there are similar recommendations by all three societies, the authors point out many differences between the guidelines and highlight the existing uncertainty in the management of AF.
More recently, the 2023 guidelines for the management of AF by ACC, AHA and HRS was published in 2024[67]. These guidelines updated the earlier 2019 and 2016 AHA guidelines for management of AF. These guidelines provide a class IIa recommendation for the use of BB and posterior pericardiotomy to prevent POAF.
While rate control strategy has been shown to be non-inferior to rhythm control strategy, with no difference in outcomes in the general population, there is a trend towards rhythm control of AA recently. A recent article, in 2024, debates rate control strategy vs rhythm control strategy in the management of AF[68]. The authors conclude that rate control strategy would be appropriate in elderly patients, dilated LA, with multiple comorbidities and long-standing persistent AF. On the other hand, the authors recommend rhythm control strategy in young symptomatic patients with heart failure and in patients who are intolerant of rate control medications.
Han et al[69], in a recent meta-analysis and systematic review analysing early rhythm control vs rate control, conclude that adopting early rhythm control is associated with lower risk of heart failure, stroke, and mortality.
Table 5 lists the studies detailing the different treatment strategies for AA after LT[8,23,46,48,49,51,54,56]. A meta-analysis by Waldron et al[8], out of 791 patients with AF, 62% were treated with rate control drugs, while rhythm control drugs were used only in 47%. Several studies[23,48,49,54,56] use rate control strategy as first line for AA following LT. A study by Silva et al[46] used rhythm control as the first line, while Henri et al[51] used both strategies equally.
| Ref. | Year | Study type | Lung transplantation patients | Atrial fibrillation patients | Rate control | Rhythm control | Direct current cardioversion |
| Campos Silva et al[46] | 2018 | Case series | 74 | 32 (43) | 6 | 72 | 25 |
| Waldron et al[8] | 2017 | Meta-analysis | 2653 | 791 (30) | 62 | 47 | 37 |
| D'Angelo et al[54] | 2016 | Case series | 652 | 198 (30) | 42 | 6 | 33 |
| Raghavan et al[56] | 2015 | Case series | 131 | 46 (35) | 87 | 53 | 35 |
| Orrego et al[48] | 2014 | Case series | 366 | 93 (25) | 40 | 28 | 13 |
| Henri et al[51] | 2012 | Case series | 224 | 65 (29) | 40 | 46 | 28 |
| See et al[23] | 2009 | Case series | 127 | 40 (32) | 56 | 15 | 15 |
| Mason et al[49] | 2007 | Case series | 333 | 68 (20) | 27 | 8 | 36 |
In the early 2000s, rate control therapy for AA was preferentially pursued.
The main drugs used for controlling ventricular rate includes: (1) BB; (2) Non dihydropyridine CCB (ND-CCB); and (3) Digoxin.
While digoxin is often used as a reserve, the targets of rate control differ between the guidelines. The American guidelines[64] recommend a target HR < 80/mt while the European[66] and Canadian[70] guidelines recommend HR around 110/mt.
Rate control is recommended in asymptomatic patients, elderly patients, those with markedly enlarged LA, long standing AF, those in HF (especially CCB).
BB: These agents work as both rate and rhythm control agents. The 2023 AHA guidelines provide a class IIa recom
ND-CCB: Drugs such as Veerapamil and CCB achieve rate control by their effects on blocking conduction across the atrioventricular node. They are best avoided in patients with heart failure. These drugs may increase the serum levels of tacrolimus, a potent anti-rejection drug belonging to the calcineurin inhibitor class, and therefore need to be used with caution in LT patients. Frequent monitoring of tacrolimus levels may be required, if these drugs have to be used.
Digoxin: Digoxin has been used for more than 2 centuries[72] as a cardiac drug. It received approved by Food and Drug Administration for treatment of AF in 1954 and has been used for several decades since then[73]. Two Meta-analyses[74,75] report that use of digoxin in AF is associated with higher mortality. It is, therefore, only recommended as a second line therapy in patients who are intolerant of BB or ND-CCB, although the RATE-AF RCT[76] showed no difference between digoxin and bisoprolol.
This includes the following: (1) Electrical direct current (DC) cardioversion; (2) AAD; and (3) CA.
Electrical DC cardioversion: DC cardioversion was first reported as a therapy for AF in 1963[77]. It is most helpful in cases of haemodynamic instability in the immediate post-operative setting. Intravenous heparin or low molecular weight heparin is given prior to DC cardioversion. If available, a trans oesophageal echocardiogram to rule out thrombus in LA appendage is recommended. Electrical cardioversion for AF has been discussed elaborately in prior publications[78,79].
AAD: While the list of AAD are extensive, the following discussion will be limited to a few of the commonly used drugs.
Amiodarone: While amiodarone has an excellent rhythm controlling property in restoring and maintaining SR after AF[80] and is commonly used in the general population, its side effects can be significant. Pulmonary toxicity in particular is worrisome[81], especially in patients who have had LT as these lungs are “precious”.
Table 6 lists some of the publications discussing the use of amiodarone in AA following LT[34,35,43-45,53,81]. There are many studies which demonstrate safety of use in LT populations[43] and some authors[45] use amiodarone as the first-line of drug therapy in patients with AA after LT. Some studies do not mention any amiodarone related side effects[34,44], despite 89% (24/27) of patients with AA taking amiodarone[34]. However, there are also studies which caution against the use of amiodarone in patients after LT in view of increased mortality with use of amiodarone for AA after LT. Magnusson et al[53] noted an increase in mortality in patients taking amiodarone for AA after LT, but note that there was no relation of amiodarone intake and development of CLAD. Isiadinso et al[35] reports a high mortality of 64% (24/38 patients) who were treated with amiodarone for AA after LT.
| Ref. | Year | Study type | Atrial fibrillation patients | Amiodarone use | Notes |
| Magnusson et al[53] | 2022 | Case series | 159 | 63 (40) | Amiodarone increase mortality but not chronic lung allograft dysfunction |
| Hathaway et al[43] | 2021 | Case series | 102 | 77 (79) | No difference between amiodarone and others. Safe to use |
| Kim et al[44] | 2020 | Case series | 46 | 6 (13) | No mention of any amiodarone related adverse effect |
| Barnes et al[45] | 2020 | Case control study | 100 | 48 (alone), 26 (combination) | Recommend amiodarone as the first drug to be used |
| Malik et al[34] | 2013 | Case series | 27 | 24 (88.9) | No mention of any amiodarone related adverse effect |
| Isiadinso et al[35] | 2011 | Case series | 62 | 38 (65) | 24 out of 38 (64%) of patients treated with amiodarone died |
| Diaz-Guzman et al[81] | 2009 | Case report | 1 | 1 patient | Amiodarone pulmonary toxicity–resolved with cessation and steroids |
Amiodarone pulmonary toxicity: The concerns of pulmonary toxicity[82] is the main issue, in addition to the other side effects of amiodarone such as hepatic, thyroid and ocular side effects. A prospective trial, reported in 1994, which compared amiodarone and verapamil in thoracic surgery patients had to be stopped due to life threatening adult respiratory distress syndrome in the amiodarone arm[83]. In an editorial, Mathru et al[84] discusses the possible mechanisms of amiodarone pulmonary toxicity (APT). In the post-operative lung, physiologic changes in the fluid status such increase pulmonary capillary pressure, alterations in endothelium, and impaired lymphatic flow may increase the likelihood of APT. High oxygen concentrations during lung operations may further accentuate the acute lung injury due to amiodarone. Epithelial–mesenchymal transition (EMT) is an event which leads to pulmonary fibrosis[85]. Weng et al[86] suggest that amiodarone may cause pulmonary fibrosis by the EMT and transforming growth factor β1 which can be increased by amiodarone. Klebsiella pneumoniae has been shown to trigger EMT in airway epithelial cells inducing fibrosis[87]. Thus, one can speculate that a co-existent infection with Klebsiella can, possibly, exacerbate the fibrotic response in the lungs.
The risk factors for developing APT include older age and duration of amiodarone therapy[88]. Daily amiodarone consumption of 400 mg for greater than 2 months or 200 mg for more than 2 years increases the risk of APT[89,90]. The diagnosis of APT is by exclusion. Prompt cessation of the drug is mandatory. Steroids help in resolution and may be needed for months to prevent recurrence.
Moreover, in the transplant population where immunosuppression is mandatory, the use of amiodarone can possibly increase the risk of infection.
In appropriate patients, flecainide is used as a first line drug for SVT/AF in some units. It must not be used in patients without coronary artery or structural heart disease due to increased mortality. Of note, the CAST trial[91] was stopped before completion due to increased mortality in the flecainide arm. Furthermore, it is vital that AV nodal blocker (BB or CCB) is administered concurrently with flecainide to prevent rapid ventricular rate. Echt et al[92] describe the pharmacology, the guidelines for administration and use of flecainide.
Sotalol is used as rhythm control drug. In a meta-analysis by Kerin et al[93], sotalol was found superior to placebo and amiodarone in restoring ad maintaining SR. However, it causes QT prolongation and torsade de pointes as reported in PAFAC trial[94,95]. The QT interval of each patient must be checked prior to administration of this drug.
Ibutilide is used as a single dose (1 mg) intravenous drug for rapid termination of AF or AFL[96]. A second dose may be repeated if there is no response in 30 minutes. It can be used in patients with structural heart disease, however, it causes QT prolongation and hence avoided in patients with QT interval more than 440 msec.
Anticoagulation strategy during DC cardioversion: Anticoagulation prior to DC cardioversion is needed to reduce the incidence of stroke[97]. In haemodynamically unstable patients due to AF with fast ventricular rate of short duration (less than 48 hours), intravenous heparin or subcutaneous low molecular weight heparin can be used prior to DC cardio
CA: Following surgical ablation operations such as maze procedure, first described by Cox in 1987[101], less invasive ablative procedures have developed. One of the pioneers in developing CA is Scheinman and Rutherford[102] who initially used DC for ablation. Subsequently, radiofrequency energy was developed. The history and development of CA has been well chronicled[102-104].
As regards CA for AA following LT, the publications are limited to case series and case reports only. LT offers unique insights to the understanding of mechanisms of AA and the role of CA in their treatment. CA are used when medical therapy fails and are mostly done for late AA, most of which are AT and AFL. An review article on management of AA after lung transplant[105] considers electrophysiological interventions on late AA after LT, in addition to medical management.
Case series on CA for AA after LT: Table 7 lists the case series of patients with AA after LT who were treated by CA[23,24,28,52,106]. There are 5 case series[23,24,28,52,106] published on CA for AA after LT. These case series which were published between 2009 and 2021 include 55 patients. As regards timing of intervention, CA were often done more than 6 months after LT, except 1 patient who had CA as early as 28 days after LT. Most of the AA were AT. Recurrence of AT have been reported[23,28,52], requiring re-ablation. Freedom from recurrence of AA has been reported in 75% of patients in 3 case series[23,28,106].
| Ref. | Year | LT patients | AA patients, n (% of LT) | CA patients, n (% of AA) | Time to CA | Outcome |
| Mariani et al[106] | 2021 | 15 | 75% free from recurrence at median of 19 months (6-86 months) | |||
| Hussein et al[28] | 2017 | 755 | 32 (4.2) | 8 (25) | 43 months (20-54 months) | At 3 years, 6 (75) stayed in SR. 2 patients had recurrence and 1 had re-do CA |
| Chaikriangkrai et al[24] | 2015 | 293 | Early 48 (16.3), late 42 (14.3) | 25 (8.5) | 80% of late AA were from left atrium. 20% of late AA were from right atrial | |
| Azadani et al[52] | 2011 | 269 | 35 (13) | 3 (8.6) | Patient (no. 1) = 1 year. Patient (no. 2) = 28 days. Patient (no. 3) = 18 months | Patient (no. 1) = no recurrence at 1 months. Patient (no. 2) = no recurrence at 6 months. Patient (no. 3) = recurrence at 8 months and had second ablation |
| See et al[23] | 2009 | 127 | 40 (31.5) | 4 (12) | 2 patients reverted to SR. 1 patient had 3 prior ablations. All 4 patients remained free of AA, 3 without anti arrhythmic drugs |
Case reports on CA for AA after LT: Table 8 lists the individual case reports where CA was used for AA after LT. There are 9 case reports of CA for AA after LT published till date[25-27,107-111].
| Ref. | Year | Type of atrial arrhythmias | How long after lung transplantation | Site of foci | Type of catheter ablation | Outcome | Notes |
| Guan et al[109] | 2020 | AT | 10 years | Left PV-LA anastomosis | Radiofrequency | AT terminated | No recurrence in 3 months |
| Uhm et al[25] | 2018 | AT | 10 months | PV anastomotic line–left side | RFCA | Reverted | No recurrence in 6 months. Electrical reconnection in all 4 PVs |
| Baykaner and Cooper[110] | 2018 | AFL | 7 years | Left PV site | RFCA | Reverted | |
| Itoh and Yamada[111] | 2017 | AFL | 14 years | PV-LA right | RFCA | Reverted | Electrical reconnection noted |
| Sanam et al[27] | 2015 | AFL | 9 years | Donor PV | RFCA | Reverted | |
| Nazmul et al[26] | 2011 | AT | 1 year | Left upper PV | RFCA | Reverted | No recurrence in 4 years |
| Nguyen and Marcus[107] | 2010 | AFL | 2 months | PV-LA | RFCA | Reverted | No recurrence in 6 months |
| Sacher et al[108] | 2008 | AT | 10 months | LA | RFCA | Reverted |
Timing of CA after LT: Among these 9 case reports, some case reports[25,26,107,108] describe CA done within 18 months of LT (ranging from 2 months to 18 months). There are also case reports[27,109-111] which describe CA done many years (ranging from 7 years to 14 years) after LT. While the all patients reverted to SR, electrical reconnection of PV across suture lines were demonstrated in 2 case reports[25,111].
These studies demonstrate that CA has now evolved to be a safe procedure for those patients who develop late AA not responding to medical therapy.
Methods to prevent AA after LT: General measures such as meticulous attention to electrolyte levels, oxygenation, pain relief, optimal fluid balance and minimal use of adrenergic drugs when needed would prevent precipitation of AA, in the setting of a “pro-arrhythmogenic milieu”.
Specific methods aimed at preventing or even reducing the incidence of AA after LT have not been successful. The incidence continues to remain the same even now. Some centres report that prophylactic use of drugs such as ND-CCB (diltiazem), BB (metoprolol) and digoxin has not been effective in reducing the incidence of AA after LT. Whited et al[112] reported a pilot study where intra-operative application of amiodarone gel was performed on the PV anastomotic suture line. This small study appeared to reduce the incidence of POAA when compared to historical controls. Further data is needed prior to consideration as a prophylactic measure.
In a letter to the editor, Bazaz et al[113] describe an intra-operative technique during PV anastomosis where they used a bipolar radiofrequency clamp to “electrically” isolate the PV anastomotic line. They used this technique in a 65-year-old man who had intra-operative AF, but no AF for until 2 years follow-up after DLT.
Narayan[114] in his critical appraisal of posterior left pericardiotomy for prevention of AF (PALACS) trial[115], concludes by confirming that “it is an important trial in the right direction” towards preventing POAF, by a simple procedure which prevents pericardial collection and POAF. This simple procedure while not reported in LT may be considered to see if the prophylactic effect of this procedure is effective in LT patients as well.
Attention to the general measures as described above must prioritise the post-operative management in preventing AA.
In the event of AA, all corrective general measures-including potassium and magnesium level optimisation, fluid balance, oxygenation and pain relief are undertaken.
In case of stable patients, rate control strategy may be undertaken safely along with anticoagulation. BB would be the first line drugs. In case of haemodynamic instability, electrical DC cardioversion may be considered. Intravenous dose of ibutilide may also be considered, provided the QT intervals are not prolonged.
Rhythm control is needed when rate control is ineffective or in the presence of heart failure. Flecainide would be a preferred drug–provided there is no structural heart disease or coronary artery disease and concomitant AV nodal blocking drug must be administered.
Amiodarone, although used as a first line drug in many units, is preferably avoided. It may only be reserved until other options have been considered and exhausted, given its risk profile, including mortality.
Late AA when not responding to AAD or presence of intolerable side effects, may be referred for CA, which has been demonstrated to be effective and safe in experienced units.
After undertaking all the general measures such as electrolyte correction, optimising oxygenation, allaying anxiety, and providing pain relief; attention is then turned to drug therapy. Frequently a combination of drugs may be required in some patients with AF after LT.
Flecainide and BB: Flecainide often requires the use of BB or CCB because of likelihood of tachycardia. Also, structural heart disease must be ruled out, prior to initiating therapy with flecainide. They must be avoided in patients with prolonged QT interval.
Flecainide and CCB: Patients intolerant of BB may be treated with CCB. However, CCB are best avoided in patients with heart failure. Concomitant use of CCB with tacrolimus can increase serum levels of tacrolimus and this drug combination is avoided if possible. In case of necessity to use CCB, serum tacrolimus levels must be monitored. Furthermore, patients with LT have a propensity to develop paralytic ileus postoperatively due to neuropraxia of vagal nerves. Since CCBs like verapamil causes constipation due to smooth muscle relaxation, abdominal distention must be watched out for.
Amiodarone: It is used as drug of first choice for AF after LT in some units[45]. However, it is avoided in many units in view of its pulmonary toxicity. Given that the transplanted lungs are precious, many units avoid amiodarone as a therapy for AF. However, it may have to be used in cases of resistant AF, when a watchful policy has to be adopted.
Digoxin: By virtue of its positive ionotropic effect, it can be used in patients with heart failure.
The use of either rate or rhythm control has been discussed so far. This section deals with clinical situations at a high risk of developing AA or those having persistent AF despite treatment.
Patient at high risk of developing AF: It is well known that patients with dilated LA, systemic hypertension and ven
Persistent AF: Some patients do not respond to the usual drugs and rate control has to be achieved because long standing tachycardia, by itself, can depress left ventricular function. In such patients, attempts must be made to identify any correctable cause. Pericardial effusion can be a cause of persistent AA and an echo cardiogram is mandatory to rule out the same. Significant pericardial collections may require drainage percutaneously or surgically. Co-existent sepsis, renal or liver failure have to be treated.
Some patients may need a combination of drugs including BB, flecainide, amiodarone, digoxin, and sotalol. Review of the drug chart daily and looking for the drug interaction and safety profile becomes mandatory. Patients with acute liver failure must not be given amiodarone. Combination of beta blocker, amiodarone or digoxin need constant monitoring of the heart rate and to avoid significant bradycardia. DC cardioversion may be needed after ensuring all general measures have been taken. In stable patients anticoagulation must be given for a few weeks prior to DC cardioversion. Transoesophageal echocardiogram is an alternate approach to rule out LA clot prior to DC cardioversion in patients who can’t wait long.
A survey on early AF after LT, reported by Simon et al[117], reported that standardised and protocolised treatment was practised only in 4 of 12 units. Given that the incidence is still common, this study emphasises the need for further research to develop protocols for prevention, early recognition and treatment of this common condition after LT.
During medical management, the rhythm control strategy is more physiological and logically more appealing. Unfortunately, it suffers from significant adverse effects of AAD. Development of newer molecules of AAD without their side effects would be of great benefit to this group of patients and further research involving prospective safety studies is needed.
“Out-of-the-box” lateral thinking in the surgical steps of LT, particularly in relation to PV anastomosis might lead operative steps which can be critical in preventing POAF. RF ablation lesions applied during surgery maybe refined further, without adding to the complexity of the LT procedure.
There are lacunae in our current understanding of AA, particularly in electrophysiology. LT offers an unique opportunity for researchers to study mechanisms of AA and response to ablation. While CA techniques result in conversion to SR occurs, CA techniques suffer from recurrences and need for further intervention and drugs post intervention. Evaluation of possible role of antifibrotics after CA to prevent recurrences and strategies to prevent electrical reconnection which leads to recurrences could be a line of future research.
Understanding electrical reconnection and their timing and anatomical locations can help in further refinement of ablation techniques with better outcomes in the future.
AA after LT continues to occur and is an important cause of morbidity and even mortality. With improving outcomes following LT, older and sicker patients with risk factors for POAF are being transplanted more often. The incidence and adverse influence of AA will be considerable. Further research is needed in areas that would minimise the incidence, morbidity and mortality associated with AA after LT. Meticulous attention to detail in ensuring that all general preventive measures have been done is the first step. Tailoring the therapy for each individual patient, based on their characteristics and co-morbidities would ensure that the most appropriate therapy is provided.
| 1. | Hariharan S, Israni AK, Danovitch G. Long-Term Survival after Kidney Transplantation. N Engl J Med. 2021;385:729-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 373] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 2. | Kaltenmeier C, Jorgensen D, Dharmayan S, Ayloo S, Rachakonda V, Geller DA, Tohme S, Molinari M. The liver transplant risk score prognosticates the outcomes of liver transplant recipients at listing. HPB (Oxford). 2021;23:927-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Hsich EM, Blackstone EH, Thuita LW, McNamara DM, Rogers JG, Yancy CW, Goldberg LR, Valapour M, Xu G, Ishwaran H. Heart Transplantation: An In-Depth Survival Analysis. JACC Heart Fail. 2020;8:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Bos S, Vos R, Van Raemdonck DE, Verleden GM. Survival in adult lung transplantation: where are we in 2020? Curr Opin Organ Transplant. 2020;25:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 5. | Saglietto A, Matta M, Gaita F, De Ferrari GM, Anselmino M. Late atrial arrhythmias after lung transplantation: a meta-analysis. J Cardiovasc Med (Hagerstown). 2020;21:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Roukoz H, Benditt DG. Atrial arrhythmias after lung transplantation. Trends Cardiovasc Med. 2018;28:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Saad M, Elgendy IY, Mentias A, Abdelaziz HK, Barakat AF, Abuzaid A, Elgendy AY, Mojadidi MK, Chandrashekaran S, Mahmoud AN. Incidence, Predictors, and Outcomes of Early Atrial Arrhythmias After Lung Transplant: A Systematic Review and Meta-Analysis. JACC Clin Electrophysiol. 2017;3:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Waldron NH, Klinger RY, Hartwig MG, Snyder LD, Daubert JP, Mathew JP. Adverse outcomes associated with postoperative atrial arrhythmias after lung transplantation: A meta-analysis and systematic review of the literature. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Fan J, Zhou K, Li S, Du H, Che G. Incidence, risk factors and prognosis of postoperative atrial arrhythmias after lung transplantation: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2016;23:790-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Hardy JD, Webb WR, Dalton ML Jr, Walker GR Jr. Lung homotransplantation in man. JAMA. 1963;186:1065-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 400] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Wildevuur CR, Benfield JR. A review of 23 human lung transplantations by 20 surgeons. Ann Thorac Surg. 1970;9:489-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Venuta F, Van Raemdonck D. History of lung transplantation. J Thorac Dis. 2017;9:5458-5471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Lip GY, Beevers DG. ABC of atrial fibrillation. History, epidemiology, and importance of atrial fibrillation. BMJ. 1995;311:1361-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Aronson JK. One hundred years of atrial fibrillation. Br J Clin Pharmacol. 2005;60:345-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Nayak S, Natarajan B, Pai RG. Etiology, Pathology, and Classification of Atrial Fibrillation. Int J Angiol. 2020;29:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Cox JL. The longstanding, persistent confusion surrounding surgery for atrial fibrillation. J Thorac Cardiovasc Surg. 2010;139:1374-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Cox JL, Ad N, Palazzo T, Fitzpatrick S, Suyderhoud JP, DeGroot KW, Pirovic EA, Lou HC, Duvall WZ, Kim YD. Current status of the Maze procedure for the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2000;12:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 192] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Lee G, Wu H, Kalman JM, Esmore D, Williams T, Snell G, Kistler PM. Atrial fibrillation following lung transplantation: double but not single lung transplant is associated with long-term freedom from paroxysmal atrial fibrillation. Eur Heart J. 2010;31:2774-2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Ho SY, Cabrera JA, Sanchez-Quintana D. Left atrial anatomy revisited. Circ Arrhythm Electrophysiol. 2012;5:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 266] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | Kanj M, Wazni O, Natale A. Pulmonary vein antrum isolation. Heart Rhythm. 2007;4:S73-S79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Magin JC, Xu C, Peskoe S, Dorry M, Frankel CW, Dahhan T, Snyder LD. The Association of Post-Lung Transplant Pulmonary Embolism With the Development of Chronic Lung Allograft Dysfunction. Transplant Direct. 2024;10:e1572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5432] [Cited by in RCA: 5400] [Article Influence: 200.0] [Reference Citation Analysis (0)] |
| 23. | See VY, Roberts-Thomson KC, Stevenson WG, Camp PC, Koplan BA. Atrial arrhythmias after lung transplantation: epidemiology, mechanisms at electrophysiology study, and outcomes. Circ Arrhythm Electrophysiol. 2009;2:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Chaikriangkrai K, Jyothula S, Jhun HY, Chang SM, Graviss EA, Shuraih M, Rami TG, Dave AS, Valderrábano M. Incidence, Risk Factors, Prognosis, and Electrophysiological Mechanisms of Atrial Arrhythmias after Lung Transplantation. JACC Clin Electrophysiol. 2015;1:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Uhm JS, Park MS, Joung B, Pak HN, Paik HC, Lee MH. Intra-atrial reentrant tachycardia originating from the pulmonary vein cuff anastomosis in a lung transplantation patient: Ultra-high-density 3-dimensional mapping. HeartRhythm Case Rep. 2018;4:152-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Nazmul M, Munger TM, Powell BD. Atrial tachycardia originating from a donor pulmonary vein in a lung transplant recipient. Circulation. 2011;124:1288-1289. [PubMed] [DOI] [Full Text] |
| 27. | Sanam K, Holmes D, Shah D, Foster N. Late atrial tachycardia originating from donor pulmonary vein in a double lung transplant recipient. HeartRhythm Case Rep. 2015;1:490-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Hussein AA, Panchabhai TS, Budev MM, Tarakji K, Barakat AF, Saliba W, Lindsay B, Wazni OM. Atrial Fibrillation and Pulmonary Venous Electrical Conduction Recovery After Full Surgical Resection and Anastomosis of the Pulmonary Veins: Insights From Follow-Up and Ablation Procedures in Lung Transplant Recipients. JACC Clin Electrophysiol. 2017;3:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Koyama T, Tada H, Sekiguchi Y, Arimoto T, Yamasaki H, Kuroki K, Machino T, Tajiri K, Zhu XD, Kanemoto-Igarashi M, Sugiyasu A, Kuga K, Nakata Y, Aonuma K. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol. 2010;56:1463-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Dizon JM, Chen K, Bacchetta M, Argenziano M, Mancini D, Biviano A, Sonett J, Garan H. A comparison of atrial arrhythmias after heart or double-lung transplantation at a single center: insights into the mechanism of post-operative atrial fibrillation. J Am Coll Cardiol. 2009;54:2043-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | AlTurki A, Marafi M, Proietti R, Cardinale D, Blackwell R, Dorian P, Bessissow A, Vieira L, Greiss I, Essebag V, Healey JS, Huynh T. Major Adverse Cardiovascular Events Associated With Postoperative Atrial Fibrillation After Noncardiac Surgery: A Systematic Review and Meta-Analysis. Circ Arrhythm Electrophysiol. 2020;13:e007437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 32. | Chyou JY, Barkoudah E, Dukes JW, Goldstein LB, Joglar JA, Lee AM, Lubitz SA, Marill KA, Sneed KB, Streur MM, Wong GC, Gopinathannair R; American Heart Association Acute Cardiac Care and General Cardiology Committee, Electrocardiography and Arrhythmias Committee, and Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Cardiovascular and Stroke Nursing; and Stroke Council. Atrial Fibrillation Occurring During Acute Hospitalization: A Scientific Statement From the American Heart Association. Circulation. 2023;147:e676-e698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 33. | Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5167] [Cited by in RCA: 4885] [Article Influence: 542.8] [Reference Citation Analysis (0)] |
| 34. | Malik A, Hsu JC, Hoopes C, Itinarelli G, Marcus GM. Elevated pulmonary artery systolic pressures are associated with a lower risk of atrial fibrillation following lung transplantation. J Electrocardiol. 2013;46:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Isiadinso I, Meshkov AB, Gaughan J, Sandhu P, Lim S, Cordova F, Criner G. Atrial arrhythmias after lung and heart-lung transplant: effects on short-term mortality and the influence of amiodarone. J Heart Lung Transplant. 2011;30:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Gandhi SK, Bromberg BI, Mallory GB, Huddleston CB. Atrial flutter: a newly recognized complication of pediatric lung transplantation. J Thorac Cardiovasc Surg. 1996;112:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Sill J, Baskar S, Zang H, Spar D, Iliopoulos I, Morales DLS, Hayes D Jr, Koh W. Atrial arrhythmias following lung transplant: a single pediatric center experience. Front Pediatr. 2023;11:1161129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 38. | Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Darche FF, Thomas D, Bruckner T, Doesch AO, Katus HA, Ehlermann P. Elevated pre-transplant pulmonary vascular resistance is associated with early post-transplant atrial fibrillation and mortality. ESC Heart Fail. 2020;7:176-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Ferretto S, Giuliani I, Sanavia T, Bottio T, Fraiese AP, Gambino A, Tarzia V, Toscano G, Iliceto S, Gerosa G, Leoni L. Atrial fibrillation after orthotopic heart transplantatation: Pathophysiology and clinical impact. Int J Cardiol Heart Vasc. 2021;32:100710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Gaudino M, Di Franco A, Rong LQ, Piccini J, Mack M. Postoperative atrial fibrillation: from mechanisms to treatment. Eur Heart J. 2023;44:1020-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 96] [Reference Citation Analysis (0)] |
| 41. | Fabiani I, Colombo A, Bacchiani G, Cipolla CM, Cardinale DM. Incidence, Management, Prevention and Outcome of Post-Operative Atrial Fibrillation in Thoracic Surgical Oncology. J Clin Med. 2019;9:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Sohn GH, Shin DH, Byun KM, Han HJ, Cho SJ, Song YB, Kim JH, On YK, Kim JS. The incidence and predictors of postoperative atrial fibrillation after noncardiothoracic surgery. Korean Circ J. 2009;39:100-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Hathaway T, Klipsch E, Rachwan R, Kutkut I, Roe D, Hage C, Mangus R. Amiodarone Use in Lung Transplant Recipients with New Onset Atrial Arrhythmias. J Heart Lung Transpl. 2021;40:S375-S376. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Kim BG, Uhm JS, Yang PS, Yu HT, Kim TH, Joung B, Pak HN, Kim SY, Park MS, Lee JG, Paik HC, Lee MH. Clinical significance of postoperative atrial arrhythmias in patients who underwent lung transplantation. Korean J Intern Med. 2020;35:897-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Barnes H, Gurry G, McGiffin D, Westall G, Levin K, Paraskeva M, Whitford H, Williams T, Snell G. Atrial Flutter and Fibrillation Following Lung Transplantation: Incidence, Associations and a Suggested Therapeutic Algorithm. Heart Lung Circ. 2020;29:1484-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Campos Silva SI, Caires N, Dias Coelho R, Santos AS, Rosa R, Lopes T, Alfarroba S, Calvinho P, Semedo L, Cardoso J, Fragata J. Atrial arrhythmias after lung transplantation: prevalence, risk factors, management and outcome. Eur Respir J. 2018;Suppl 62:321. [DOI] [Full Text] |
| 47. | Xia Y, Patel S, Lee S, Villamater R, Biniwale R, Ardehali A. Postoperative Atrial Fibrillation in Lung Transplant Recipients with and without Concomitant Modified Maze. J Heart Lung Transpl. 2022;41:S266. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Orrego CM, Cordero-Reyes AM, Estep JD, Seethamraju H, Scheinin S, Loebe M, Torre-Amione G. Atrial arrhythmias after lung transplant: underlying mechanisms, risk factors, and prognosis. J Heart Lung Transplant. 2014;33:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Mason DP, Marsh DH, Alster JM, Murthy SC, McNeill AM, Budev MM, Mehta AC, Pettersson GB, Blackstone EH. Atrial fibrillation after lung transplantation: timing, risk factors, and treatment. Ann Thorac Surg. 2007;84:1878-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Jesel L, Barraud J, Lim HS, Marzak H, Messas N, Hirschi S, Santelmo N, Olland A, Falcoz PE, Massard G, Kindo M, Ohlmann P, Chauvin M, Morel O, Kessler R. Early and Late Atrial Arrhythmias After Lung Transplantation - Incidence, Predictive Factors and Impact on Mortality. Circ J. 2017;81:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Henri C, Giraldeau G, Dorais M, Cloutier AS, Girard F, Noiseux N, Ferraro P, Rinfret S. Atrial fibrillation after pulmonary transplantation: incidence, impact on mortality, treatment effectiveness, and risk factors. Circ Arrhythm Electrophysiol. 2012;5:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Azadani PN, Kumar UN, Yang Y, Scheinman MM, Hoopes CW, Marcus GM, Rifkin C, Olgin JE, Lee BK. Frequency of atrial flutter after adult lung transplantation. Am J Cardiol. 2011;107:922-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Magnusson JM, Bobbio E, Danielsson C, Wallinder A, Dellgren G, Bollano E. A Retrospective Study of Posttransplant Amiodarone Exposition on Clad Development and Survival After Lung Transplantation. Transplant Proc. 2022;54:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 54. | D'Angelo AM, Chan EG, Hayanga JW, Odell DD, Pilewski J, Crespo M, Morrell M, Shigemura N, Luketich J, Bermudez C, Althouse AD, D'Cunha J. Atrial arrhythmias after lung transplantation: Incidence and risk factors in 652 lung transplant recipients. J Thorac Cardiovasc Surg. 2016;152:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Garcia S, Canoniero M, Sattiraju S, Chen LY, Adkisson W, Hertz M, Benditt DG. Atrial Fibrillation After Lung Transplantation: Incidence, Predictors and Long-Term Implications. J Atr Fibrillation. 2011;4:363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Raghavan D, Gao A, Ahn C, Torres F, Mohanka M, Bollineni S, Peltz M, Wait M, Ring S, Kaza V. Contemporary analysis of incidence of post-operative atrial fibrillation, its predictors, and association with clinical outcomes in lung transplantation. J Heart Lung Transplant. 2015;34:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Alobaida M, Alrumayh A. Rate control strategies for atrial fibrillation. Ann Med. 2021;53:682-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Deshpande R, Al Khadra Y, Al-Tamimi R, Albast N, Labedi M. Atrial fibrillation: Rate control or rhythm control? Cleve Clin J Med. 2022;89:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 59. | Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2961] [Cited by in RCA: 2817] [Article Influence: 122.5] [Reference Citation Analysis (0)] |
| 60. | Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, Walter S, Tebbe U; STAF Investigators. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 640] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 61. | Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation--Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 732] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 62. | Hagens VE, Crijns HJ, Van Veldhuisen DJ, Van Den Berg MP, Rienstra M, Ranchor AV, Bosker HA, Kamp O, Tijssen JG, Veeger NJ, Van Gelder IC; RAte Control versus Electrical cardioversion for persistent atrial fibrillation study group. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J. 2005;149:1106-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 63. | Cheung CC, Nattel S, Macle L, Andrade JG. Management of Atrial Fibrillation in 2021: An Updated Comparison of the Current CCS/CHRS, ESC, and AHA/ACC/HRS Guidelines. Can J Cardiol. 2021;37:1607-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 64. | January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125-e151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1944] [Article Influence: 324.0] [Reference Citation Analysis (0)] |
| 65. | Deyell MW, AbdelWahab A, Angaran P, Essebag V, Glover B, Gula LJ, Khoo C, Lane C, Nault I, Nery PB, Rivard L, Slawnych MP, Tulloch HL, Sapp JL; Members of the Secondary Panel. 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Position Statement on the Management of Ventricular Tachycardia and Fibrillation in Patients With Structural Heart Disease. Can J Cardiol. 2020;36:822-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 66. | Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3176] [Cited by in RCA: 6469] [Article Influence: 1617.3] [Reference Citation Analysis (0)] |
| 67. | Writing Committee Members; Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, Deswal A, Eckhardt LL, Goldberger ZD, Gopinathannair R, Gorenek B, Hess PL, Hlatky M, Hogan G, Ibeh C, Indik JH, Kido K, Kusumoto F, Link MS, Linta KT, Marcus GM, McCarthy PM, Patel N, Patton KK, Perez MV, Piccini JP, Russo AM, Sanders P, Streur MM, Thomas KL, Times S, Tisdale JE, Valente AM, Van Wagoner DR. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2024;83:109-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 318] [Article Influence: 318.0] [Reference Citation Analysis (0)] |
| 68. | Shin ED, Tran HN, Ramalingam ND, Liu T, Fan E. Rate Versus Rhythm Control for Atrial Fibrillation. Perm J. 2024;28:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 69. | Han S, Jia R, Cen Z, Guo R, Zhao S, Bai Y, Xie M, Cui K. Early rhythm control vs. rate control in atrial fibrillation: A systematic review and meta-analysis. Front Cardiovasc Med. 2023;10:978637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 70. | Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, Cox JL, Dorian P, Gladstone DJ, Healey JS, Khairy P, Leblanc K, McMurtry MS, Mitchell LB, Nair GM, Nattel S, Parkash R, Pilote L, Sandhu RK, Sarrazin JF, Sharma M, Skanes AC, Talajic M, Tsang TSM, Verma A, Verma S, Whitlock R, Wyse DG, Macle L; Members of the Secondary Panel. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2020;36:1847-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 397] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 71. | Branch KR, Harris A, Nguyen D, Lease ED, Rho R, Parmar B, Koprowicz K, Mulligan MS. Atrial Arrhythmia Common After Lung Transplantation and Reduced With Beta Blocker Use. J Am Coll Cardiol. 2020;75:467. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 72. | Khandelwal R, Vagha JD, Meshram RJ, Patel A. A Comprehensive Review on Unveiling the Journey of Digoxin: Past, Present, and Future Perspectives. Cureus. 2024;16:e56755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 73. | Scalese MJ, Salvatore DJ. Role of Digoxin in Atrial Fibrillation. J Pharm Pract. 2017;30:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 74. | Qureshi W, O'Neal WT, Soliman EZ, Al-Mallah MH. Systematic review and meta-analysis of mortality and digoxin use in atrial fibrillation. Cardiol J. 2016;23:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Wang ZQ, Zhang R, Chen MT, Wang QS, Zhang Y, Huang XH, Wang J, Yan JH, Li YG. Digoxin Is Associated With Increased All-cause Mortality in Patients With Atrial Fibrillation Regardless of Concomitant Heart Failure: A Meta-analysis. J Cardiovasc Pharmacol. 2015;66:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Kotecha D, Bunting KV, Gill SK, Mehta S, Stanbury M, Jones JC, Haynes S, Calvert MJ, Deeks JJ, Steeds RP, Strauss VY, Rahimi K, Camm AJ, Griffith M, Lip GYH, Townend JN, Kirchhof P; Rate Control Therapy Evaluation in Permanent Atrial Fibrillation (RATE-AF) Team. Effect of Digoxin vs Bisoprolol for Heart Rate Control in Atrial Fibrillation on Patient-Reported Quality of Life: The RATE-AF Randomized Clinical Trial. JAMA. 2020;324:2497-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 77. | Lown B, Perlroth MG, Kaidbey S, Abe T, Harken DE. "Cardioversion" of atrial fibrillation. A report on the treatment of 65 episodes in 50 patients. N Engl J Med. 1963;269:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 198] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Nusair M, Flaker GC, Chockalingam A. Electric cardioversion of atrial fibrillation. Mo Med. 2010;107:59-64. [PubMed] |
| 79. | Carpenter A, Sargent S. DC cardioversion of atrial fibrillation and atrial flutter in the emergency department: improving specialist protocols for the generalist. BMJ Open Qual. 2018;7:e000260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | Istratoaie S, Sabin O, Vesa ŞC, Cismaru G, Donca VI, Buzoianu AD. Efficacy of amiodarone for the prevention of atrial fibrillation recurrence after cardioversion. Cardiovasc J Afr. 2021;32:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 81. | Diaz-Guzman E, Mireles-Cabodevila E, Arrossi A, Kanne JP, Budev M. Amiodarone pulmonary toxicity after lung transplantation. J Heart Lung Transplant. 2008;27:1059-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Scaramozzino MU Sr, Sapone G, Plastina UR, Nucara M. Amiodarone-Induced Lung Toxicity: A Case Initially Not Correctly Framed. Cureus. 2023;15:e36818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 83. | Van Mieghem W, Coolen L, Malysse I, Lacquet LM, Deneffe GJ, Demedts MG. Amiodarone and the development of ARDS after lung surgery. Chest. 1994;105:1642-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 117] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Mathru M, McDaniel LB. Primum non nocere. Is the therapy worse than the disease? Chest. 1994;105:1634-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 85. | Salton F, Volpe MC, Confalonieri M. Epithelial⁻Mesenchymal Transition in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Medicina (Kaunas). 2019;55:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 86. | Weng J, Chen H, Wu H, Tu M, Wang Z, Chen D, Wang Z, Chen C. Amiodarone induces epithelial-mesenchymal transition in A549 cells via activation of TGF-β1. Drug Chem Toxicol. 2020;43:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Leone L, Mazzetta F, Martinelli D, Valente S, Alimandi M, Raffa S, Santino I. Klebsiella pneumoniae Is Able to Trigger Epithelial-Mesenchymal Transition Process in Cultured Airway Epithelial Cells. PLoS One. 2016;11:e0146365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Nacca N, Bhamidipati CM, Yuhico LS, Pinnamaneni S, Szombathy T. Severe amiodarone induced pulmonary toxicity. J Thorac Dis. 2012;4:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 89. | Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J. 2009;16:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 90. | Ott MC, Khoor A, Leventhal JP, Paterick TE, Burger CD. Pulmonary toxicity in patients receiving low-dose amiodarone. Chest. 2003;123:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 91. | Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2258] [Cited by in RCA: 2061] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 92. | Echt DS, Ruskin JN. Use of Flecainide for the Treatment of Atrial Fibrillation. Am J Cardiol. 2020;125:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 93. | Kerin NZ, Jacob S. The efficacy of sotalol in preventing postoperative atrial fibrillation: a meta-analysis. Am J Med. 2011;124:875.e1-875.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | Fetsch T, Bauer P, Engberding R, Koch HP, Lukl J, Meinertz T, Oeff M, Seipel L, Trappe HJ, Treese N, Breithardt G; Prevention of Atrial Fibrillation after Cardioversion Investigators. Prevention of atrial fibrillation after cardioversion: results of the PAFAC trial. Eur Heart J. 2004;25:1385-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 95. | Shelton R. Prevention of atrial fibrillation after cardioversion: results of the PAFAC trial. Eur Heart J. 2004;25:2174; author reply 2174-2174; author reply 2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 96. | Murray KT. Ibutilide. Circulation. 1998;97:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 114] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 97. | Lucà F, Giubilato S, Di Fusco SA, Piccioni L, Rao CM, Iorio A, Cipolletta L, D'Elia E, Gelsomino S, Rossini R, Colivicchi F, Gulizia MM. Anticoagulation in Atrial Fibrillation Cardioversion: What Is Crucial to Take into Account. J Clin Med. 2021;10:3212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 98. | Venuta F, Rendina EA, De Giacomo T, Ciccone AM, Mercadante E, Coloni GF. Esophageal perforation after sequential double-lung transplantation. Chest. 2000;117:285-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 99. | Kerbaul F, Renard S, Guidon C, Gouin F, Villacorta J, Collart F, Mouly-Bandini A, Kreitmann B, Métras D, Ville E, Doddoli C. Acute esophagoarterial perforation and hemorrhagic shock during transesophageal echocardiography that occurs after heart-lung transplantation. J Heart Lung Transplant. 2004;23:509-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 100. | van Vugt SPG, Brouwer MA. Periprocedural anticoagulation in atrial fibrillation: Update on electrical cardioversion and ablation. Neth Heart J. 2018;26:352-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 101. | Cox JL. The first Maze procedure. J Thorac Cardiovasc Surg. 2011;141:1093-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 102. | Scheinman MA, Rutherford JD. The Development of Cardiac Arrhythmia Ablation: A Conversation With Melvin A. Scheinman, MD. Circulation. 2017;135:1191-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 103. | Yoshida K, Aonuma K. Catheter ablation of atrial fibrillation: Past, present, and future directions. J Arrythm. 2012;2:83-90. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 104. | McCarthy PM, Cox JL, Kislitsina ON, Kruse J, Churyla A, Malaisrie SC, Mehta CK. Surgery and Catheter Ablation for Atrial Fibrillation: History, Current Practice, and Future Directions. J Clin Med. 2021;11:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Marazzato J, Eikermann M, Di Biase L. Management of Atrial Arrhythmias After Lung Transplant. JACC Clin Electrophysiol. 2023;9:1824-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 106. | Mariani MV, Pothineni NVK, Arkles J, Deo R, Frankel D, Supple G, Garcia F, Lin D, Hyman MC, Kumareswaran R, Riley M, Nazarian S, Schaller RD, Epstein AE, Bermudez C, Dixit S, Callans D, Marchlinski FE, Santangeli P. Catheter ablation of atrial arrhythmias following lung transplant: Electrophysiological findings and outcomes. J Cardiovasc Electrophysiol. 2021;32:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 107. | Nguyen DT, Marcus GM. A Case of Atrial Arrhythmia After Lung Transplant. Card Electrophysiol Clin. 2010;2:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 108. | Sacher F, Vest J, Raymond JM, Stevenson WG. Incessant donor-to-recipient atrial tachycardia after bilateral lung transplantation. Heart Rhythm. 2008;5:149-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 109. | Guan F, Suna G, Thomas MF, Inci I, Regg CA, Saguner AM. Catheter ablation of left atrial macroreentrant tachycardia after bilateral lung transplantation. Cardiovasc Med. 2020;. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 110. | Baykaner T, Cooper JM. Atypical flutter following lung transplantation involving recipient-to-donor tissue connections. HeartRhythm Case Rep. 2018;4:548-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 111. | Itoh T, Yamada T. Left atrial and pulmonary vein flutter associated with double electrical connections after a lung transplantation. Europace. 2017;19:855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 112. | Whited W, Smith S, Rice J, Trivedi J, Fox M, van Berkel V. Intra-Operative Amiodarone-Releasing Hydrogel to Prevent Post Lung Transplant Atrial Fibrillation: A Pilot Clinical Trial. J Heart Lung Transplant. 2020;39:S161. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 113. | Bazaz R, Salizzoni S, Bonde P, Espinoza A, Toyoda Y. A novel strategy for prevention of post-operative atrial arrhythmias in patients undergoing lung transplantation. J Heart Lung Transplant. 2010;29:713-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 114. | Narayan P. Posterior left pericardiotomy for the prevention of atrial fibrillation: evidence from the PALACS trial. Indian J Thorac Cardiovasc Surg. 2022;38:226-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 115. | Gaudino M, Sanna T, Ballman KV, Robinson NB, Hameed I, Audisio K, Rahouma M, Di Franco A, Soletti GJ, Lau C, Rong LQ, Massetti M, Gillinov M, Ad N, Voisine P, DiMaio JM, Chikwe J, Fremes SE, Crea F, Puskas JD, Girardi L; PALACS Investigators. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: an adaptive, single-centre, single-blind, randomised, controlled trial. Lancet. 2021;398:2075-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 116. | Lopes LA, Agrawal DK. Post-Operative Atrial Fibrillation: Current Treatments and Etiologies for a Persistent Surgical Complication. J Surg Res (Houst). 2022;5:159-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 117. | Simon J, Anderson R, Craig S. Early Post-Operative Atrial Fibrillation after Lung Transplantation. J Heart Lung Transplant. 2020;39:S310. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |