Published online Mar 18, 2025. doi: 10.5500/wjt.v15.i1.97598
Revised: October 3, 2024
Accepted: October 15, 2024
Published online: March 18, 2025
Processing time: 176 Days and 23.4 Hours
Transplant teams often hesitate to use the right kidney (RK) in living donor (LD) transplants due to the complexities of anastomosing the short, thin-walled right renal veins, which can potentially lead to graft loss or graft dysfunction. Never
To compare transplant outcomes between recipients of RK and LK while examining the factors that influence these outcomes.
We retrospectively analyzed data from adult patients who received LD kidney transplants involving meticulous patient selection and surgical techniques at our center from January 2020 to December 2023. We included all kidney donors who were over 18, fit to donate, and had undergone diethylenetriamine pentaacetic acid split function and/or computed tomography based volumetry. The variables examined comprised donor and recipient demographics, and outcome measures included technical graft loss (TGL), delayed or slow graft function (SGF), and post-transplant serum creatinine (SC) trends. We used a logistic regression model to assess the likelihood of adverse outcomes considering the donor kidney side.
Of the 250 transplants performed during the period, 56 (22%) were RKs. The recipient demographics and transplant factors were comparable for the right and LKs, except that the donor warm and cold ischemia time were shorter for RKs. TGL and SGF each occurred in 2% (n = 1) of RKs and 0.5% (n = 1) of LKs, the difference being insignificant. These complications, however, were not related to the venous anastomosis. One RK (2%) developed delayed graft function after 48 hours, which was attributable to postoperative hypoxia rather than the surgical technique. The post-transplant SC trend and mean SC at the last follow-up were similar across both kidney sides.
The donor kidney side has little impact on post-transplant adverse events and graft function in LD transplants, provided that careful patient selection and precise surgical techniques are employed.
Core Tip: Donor right kidneys (RKs) are associated with technical difficulty, and have a higher incidence of delayed graft function and peri-operative graft loss. Short length and thin wall of the renal vein makes the venous anastomosis challenging. We focus on the selection criteria for RKs and how to manage short veins during bench work, analyze our experience with RKs, and compare outcomes with left kidneys in living donor transplantation.
- Citation: Khan T, Ahmad N, Iqbal Q, Hassan M, Asnath L, Khan N, Shakeel S. Comparative study of living donor kidney transplants: Right vs left. World J Transplant 2025; 15(1): 97598
- URL: https://www.wjgnet.com/2220-3230/full/v15/i1/97598.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i1.97598
Kidney transplantation is widely regarded as the best treatment option for end-stage renal disease due to its positive impact on patient survival and quality of life[1]. However, the procedure’s success depends on multiple factors, the most important being the vascular anastomoses between the donor kidney and the recipient vessels.
Anastomosing short and thin-walled veins of right kidneys (RKs) to the recipient’s veins presents more challenges than left kidneys (LKs), which typically have more favorable characteristics; consequently, RKs are more prone to complications[2-5]. Such a risk is increased if the kidney recovery is laparoscopic, where the application of clips and staples shortens the vein, and the option of acquiring a caval cuff to make up for the loss in length is not available[4,6,7]. Grafts with renal veins shorter than 2.5 cm are particularly vulnerable—more so in obese recipients or if the donor kidney is large. The complexities associated with RKs have led to the preference for LKs and their preponderance in living donor (LD) transplants[6]. We analyzed the short-term outcomes of RK and LK transplants in our LD transplant series, which involved meticulous patient selection and surgical technique. We omitted long-term results because complications related to technical issues manifest in the early post-transplant period.
We gathered data from hospital records of LD transplants performed at the Kidney Transplant Unit of Rehman Medical Institute in Peshawar, Pakistan, between January 2020 and December 2023. The data were divided into two groups based on the donor kidney side to determine its impact on transplant outcomes. It included donor and recipient demographics with information about the donor kidney’s side, split function, number of arteries, donor nephrectomy types, open [open donor nephrectomy (ODN)] or laparoscopic [laparoscopic donor nephrectomy (LDN)], ischemia times, and immunosuppressant protocols. Outcome measures included rates of immediate graft function (IGF), slow graft function (SGF), delayed graft function (DGF), technical graft loss (TGL), and for graft function, we recorded number of hours it took for the pre-transplant serum creatinine (SC) to decrease by 50% (SC50), SC on day 7 after surgery (SCD7), days taken to normalize SC, and SC at last follow-up (LSC).
We included all kidney donors who were over 18, fit to donate, and had undergone diethylenetriamine pentaacetic acid (DTPA) split function and/or computed tomography (CT) based volumetry. Our sample size was 250, sampling was convenience type, and multiple colleagues were involved in data collection after being trained on the forms and coding used for various variables before data collection. All the information collected were rechecked to minimize errors and avoid bias.
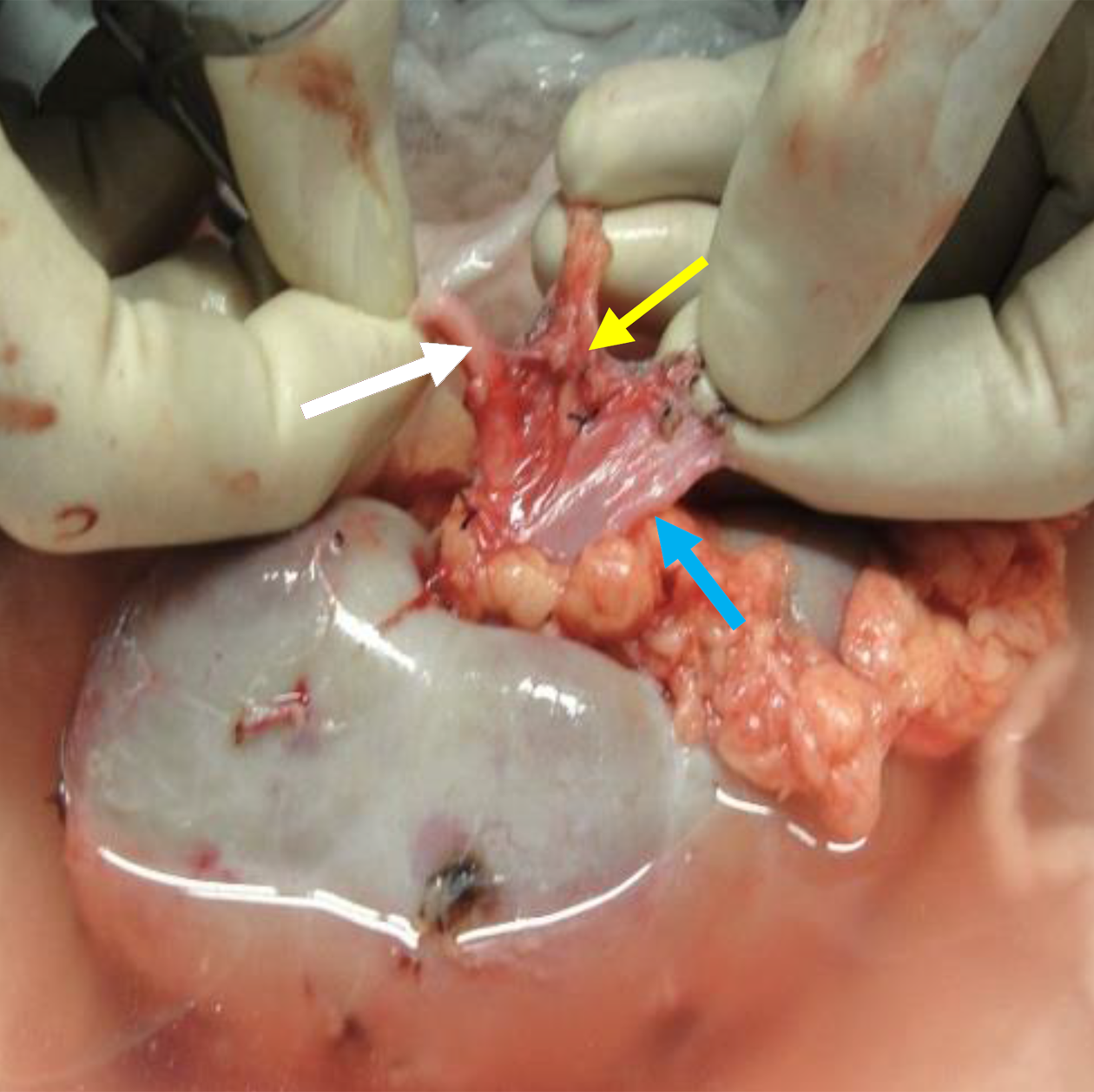
Our choice of donor kidney depended on its vascular anatomy, DTPA-based split function, and CT-based volumetry; we opted for kidneys with a lower function and smaller volume (difference > 15 cc); however, if the right renal vein (RRV) was less than 2.5 cm, we opted for LKs. When both kidneys were approximately the same size, we preferred the RK if the LK had multiple arteries, provided the RRV had the minimum threshold length. In thin females, we even accepted a 2 cm length because their iliac veins are more superficial, and valuable length could be gained by careful circumferential dissection of the RRV in the renal hilum (Figure 1). The right renal artery, generally longer than the vein, was shortened to match the length of the vein, as described elsewhere[2,6].
We used the following operational definitions. Donor warm ischemia (DWI) was defined as time from clamping the renal artery to cold flushing of the graft. Cold ischemia time (CIT) was defined as duration that the graft was stored in a cold solution. Recipient warm ischemia was defined as time from starting vascular anastomosis to graft reperfusion. TGL was defined as graft removal within 7 days of transplantation. DGF was defined as the need for dialysis in the 1st week after transplant. SGF was defined as SC > 3 mg/dL on day 5 after transplant.
All information gathered from the patient file reviews and electronic medical records was entered into a proforma and then transferred to an excel sheet. All recorded variables were compared between left or right-side nephrectomy to observe the differences in outcomes.
All grafts were placed in the retroperitoneum with end-to-side anastomosis between the graft vessels and the recipient external iliac vessels or to more proximal vessels. Ice-filled swabs and cold saline irrigation kept the graft cool during the entire implantation procedure. Multiple arteries were implanted individually or placed on a common ostium. The vesico-ureteric anastomosis was extravesical, and fashioned over a stent.
Data collection and analysis utilized Microsoft Excel 2013 and Statistical Package for the Social Sciences version 25 (IBM Corp., Armonk, NY, United States). Qualitative data are described as frequencies and percentages, while quantitative data are expressed as mean with standard deviation. The independent sample t-test was employed to assess the statistical significance of differences between groups. Additionally, a linear regression model was utilized to evaluate the influence of confounding variables on outcomes. A post hoc subgroup analysis was performed based on the donor nephrectomy technique, (open or laparoscopic), donor relation (related or unrelated), and immunosuppression protocol (steroid based or steroid sparing). Missing data were addressed through exclusion, and patients lost to follow-up or who died were censored as graft loss. All P values were based on two-sided tests and significance was set at a P value < 0.05.
A total of 250 LD kidney recipients and donors who satisfied the eligibility criteria were included in the study. Table 1 provides details about donor and recipient demographics and Table 2 shows transplant-related ischemia times. Among donors, 126 (50.4%) were male and 124 (49.6%) were female. The mean age of RK donors was 35.1 ± 10.5 years and 33.6 ± 10.1 years for LKs. Two hundred and thirteen (85.2%) recipients were male and thirty-seven (14.7%) were female, with seven females who received RKs (13%) and 30 (16%) who received LKs. The mean age of recipients who received RKs was 37 ± 11.6 years and 38.7 ± 11.3 years for LKs.
| Parameters | Living donor kidneys (n = 250) | ||
| Right kidney | Left kidney | P value | |
| Donor age in years | 35.1 ± 10.5 | 33.6 ± 10.1 | 0.36 |
| Donor sex | Males = 28 (50), females = 28 (50) | Males = 98 (51), females = 96 (49) | 0.9 |
| Mean donor weight in kg | 65.9 ± 8.8 | 66.2 ± 11 | 0.9 |
| Mean recipient age in years | 37 ± 11.6 | 38.7 ± 11.3 | 0.32 |
| Recipient sex | Males = 49 (87), females = 7 (13) | Males = 164 (84), females = 30 (16) | 0.6 |
| Mean recipient body mass index | 23.6 ± 3.5 | 24.4 ± 3.8 | 0.1 |
| Mean human leukocyte antigen mismatches, n ± SD | 5 ± 2.9 | 5.5 ± 2.9 | 0.32 |
| Donor kidney split function | 47 ± 2 | 50 ± 2 | 0.000 |
| Donor arteries multiplicity | Single = 51 (91), multiple = 5 (9) | Single = 161 (84), multiple = 31 (16) | 0.17 |
| Donor type | LRD = 42 (75), LURD = 14 (25) | LRD = 155 (80), LURD = 39 (20) | 0.43 |
| Donor nephrectomy type | ODN = 53 (95), LDN = 3 (5) | ODN = 182 (73), LDN = 68 (27) | 0.000 |
| Immunosuppressant, steroid sparing | 6 (10.7) | 20 (10.3) | 0.9 |
| Mean cold ischemia time in minutes | 40.1 ± 16.6 | 48.6 ± 23.6 | 0.01 |
| Mean donor warm ischemia in minutes | 2.1 ± 0.9 | 2.7 ± 1.7 | 0.008 |
| Mean recipient warm ischemia in minutes | 44.2 ± 5.2 | 45.3 ± 8.3 | 0.36 |
| Mean donor warm ischemia of cohort in minutes | ODN = 1.8 ± 0.9, LDN = 4.7 ± 1.2 | 0.000 | |
| Mean cold ischemia time of cohort in minutes | ODN = 41.5 ± 18.3, LDN = 60.4 ± 26.8 | 0.00 | |
| Parameter | Right kidney | Left kidney | P value |
| Immediate graft function | 53 (96) | 192 (99) | 0.15 |
| Slow graft function | 1 (1.7) | 1 (0.5) | |
| Delayed graft function | 1 (1.7) | 0 | |
| Technical graft loss | 1 (1.7) | 1 (0.5) | |
| Mean pre-transplant SC in mg/dL | 5.90 ± 2.05 | 5.78 ± 1.57 | 0.7 |
| Mean time in hours to 50% fall in pre-transplant SC | 17.36 ± 7.38 | 17.82 ± 10.15 | 0.8 |
| Mean SC on post-transplant day 7 in mg/dL | 1.107 ± 0.61 | 1.073 ± 0.42 | 0.6 |
| Mean post-transplant time in days to normal SC | 4.13 ± 2.848 | 4.11 ± 2.774 | 0.9 |
| Mean SC on last follow-up in mg/dL | 1.033 ± 0.217 | 1.038 ± 0.443 | 0.9 |
The total number of transplants was 250, comprising 194 recipients (78%) who received LKs and 56 who received (22%) RKs. One hundred and eighty-two (73%) of the kidneys were recovered by ODN and sixty-eight (27%) by LDN. Of the latter, 65 (95%) were LKs and 3 (5%) were RKs. Compared to LKs, RKs had significantly smaller mean volumes (47 ± 2 vs 50 ± 2). Six (11%) RKs and thirty (15%) LKs had multiple arteries. Mean donor age, sex, weight, and artery multiplicity, along with mean human leukocyte antigen mismatches with recipients were not statistically different between the two groups. The RK DWI and CIT were significantly shorter than those of LKs (2.1 ± 0.9 vs 2.7 ± 1.7 and 40.1 ± 16.6 vs 48.6 ± 23.6, respectively), primarily because RKs were recovered by ODN, which also require less bench work. Significantly shorter DWI and CIT were recorded for ODN compared to LDN (1.81 ± 0.9 vs 4.7 ± 1.2 and 41.5 ± 18.2 vs 60.4 ± 26.8, respectively). The difference in rewarming ischemia (RWI) between right and LKs, or in ODN and LDN was not significant (Table 2).
As shown in Table 2, 96% of recipients of RKs and 99% of LKs had IGF; 1.7% of RKs and none of the LKs had SGF, while 1.7% of RKs and 0.5% of LKs had DGF; the difference was not statistically significant in either case. For both kidneys, SC50, SCD7, and LSC were comparable (Table 3).
| Parameter | Frequency | SC to decrease by 50% in hours | P value | Normalize SC | P value | SC at last follow-up | P value | SC on day 7 after surgery in days | P value | |
| Open donor nephrectomy | RK | 53 (95) | 17.9 ± 10.2 | 0.6 | 4 ± 2.7 | 0.4 | 0.9 ± 0.2 | 0.02 | 1 ± 0.5 | 0.9 |
| LK | 129 (67) | |||||||||
| Total | 182 (73) | |||||||||
| Laparoscopic donor nephrectomy | RK | 3 (5) | 17.2 ± 7.5 | 4.4 ± 2.8 | 1.1 ± 0.6 | 1 ± 0.3 | ||||
| LK | 65 (33) | |||||||||
| Total | 68 (27) | |||||||||
| Living related donor | RK | 42 (75) | 17.3 ± 10 | 0.2 | 4 ± 2.7 | 0.2 | 0.99 | 0.000 | 1.0 ± 0.4 | 0.005 |
| LK | 155 (80) | |||||||||
| Total | 197 (79) | |||||||||
| Living unrelated donor | RK | 14 (25) | 19.1 ± 7 | 4.6 ± 2.9 | 1.2 | 1.2 ± 0.8 | ||||
| LK | 39 (20) | |||||||||
| Total | 53 (21) | |||||||||
| Steroid sparing immunosuppression | RK | 6 (11) | 15 ± 0.7 | 0.13 | 3.5 ± 2.4 | 0.23 | 0.97 ± 0.2 | 0.36 | 0.9 ± 0.4 | 0.07 |
| LK | 20 (10) | |||||||||
| Total | 26 (10) | |||||||||
| Steroid based immunosuppression | RK | 50 (89) | 18 ± 0.9 | 4.2 ± 2.8 | 1 ± 0.4 | 1.1 ± 0.5 | ||||
| LK | 174 (90) | |||||||||
| Total | 224 (90) | |||||||||
There were two TGLs, one LK and one RK, both caused by arterial bleeding; one was an anastomotic blowout in an LK because of an infected graft recovered by LDN, and the other was a cautery burn away from the anastomosis in an RK.
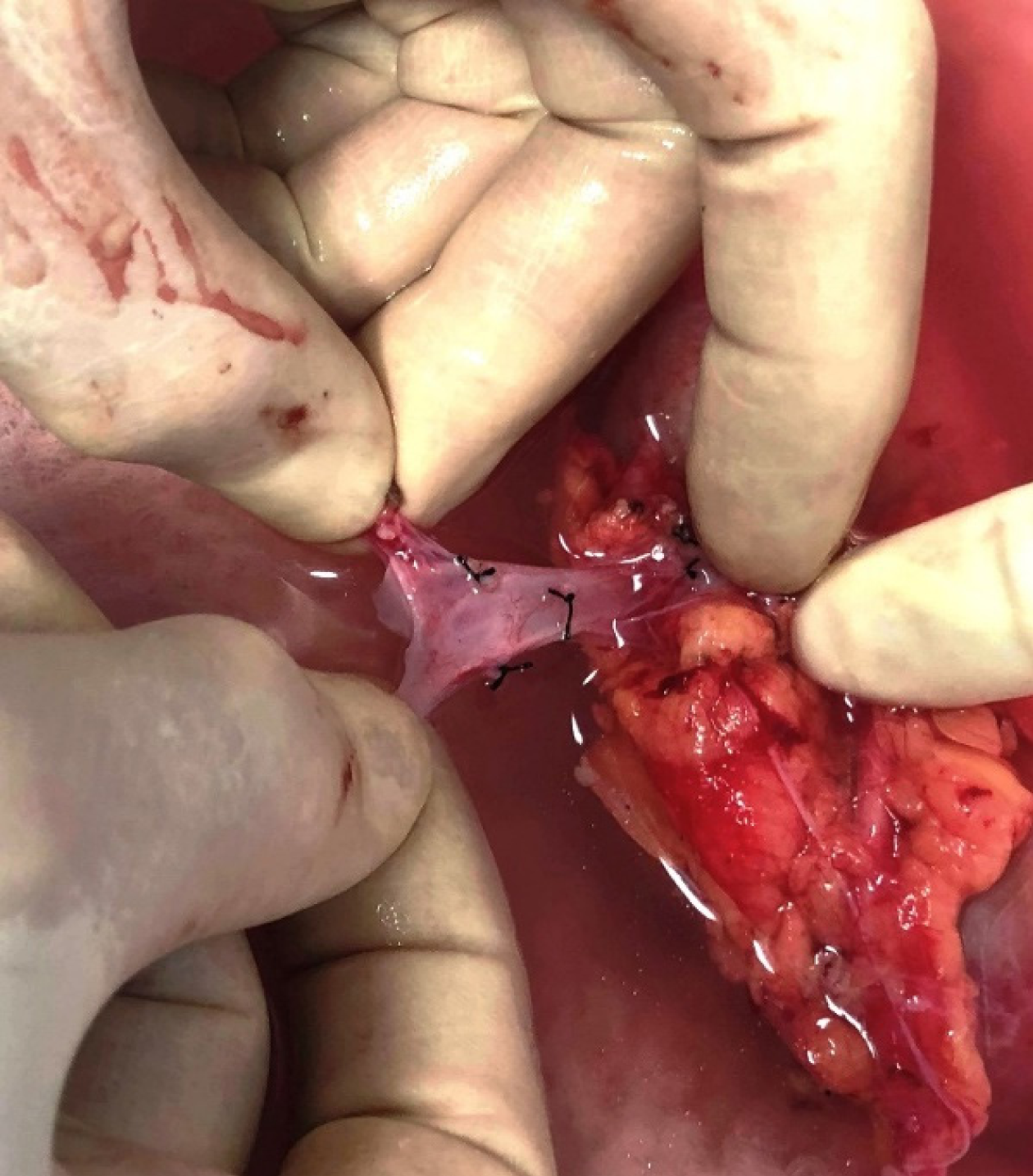
This study is the first such report from Pakistan that addresses the impact of right vs left donor kidney on TGL, delayed and SGF, and post-transplant SC trends. There is substantial evidence that RK recipients may have worse outcomes than LK recipients in LD transplantation[2,4,8-10]. The main reason is the variable length of the RRV, which is usually short with thin, fragile walls (Figure 2), making venous anastomosis difficult, especially in obese recipients with limited exposure. Lifting the graft to access the anastomotic site risks tearing a stretched, fragile vein, and attempts at repair may further extend the tear. Dealing with this can prolong warm ischemia and risks DGF, but rushing the anastomosis to save time may result in partial or complete venous occlusion and thrombosis. If bleeding ensues because the anastomosis is not perfect, the perfused, swollen graft hampers access and control of bleeding becomes challenging. A clear preference among transplant teams for LKs is thus quite understandable[2,6], and the surge in LDN, more suitable for LKs than RKs, has further boosted this preference.
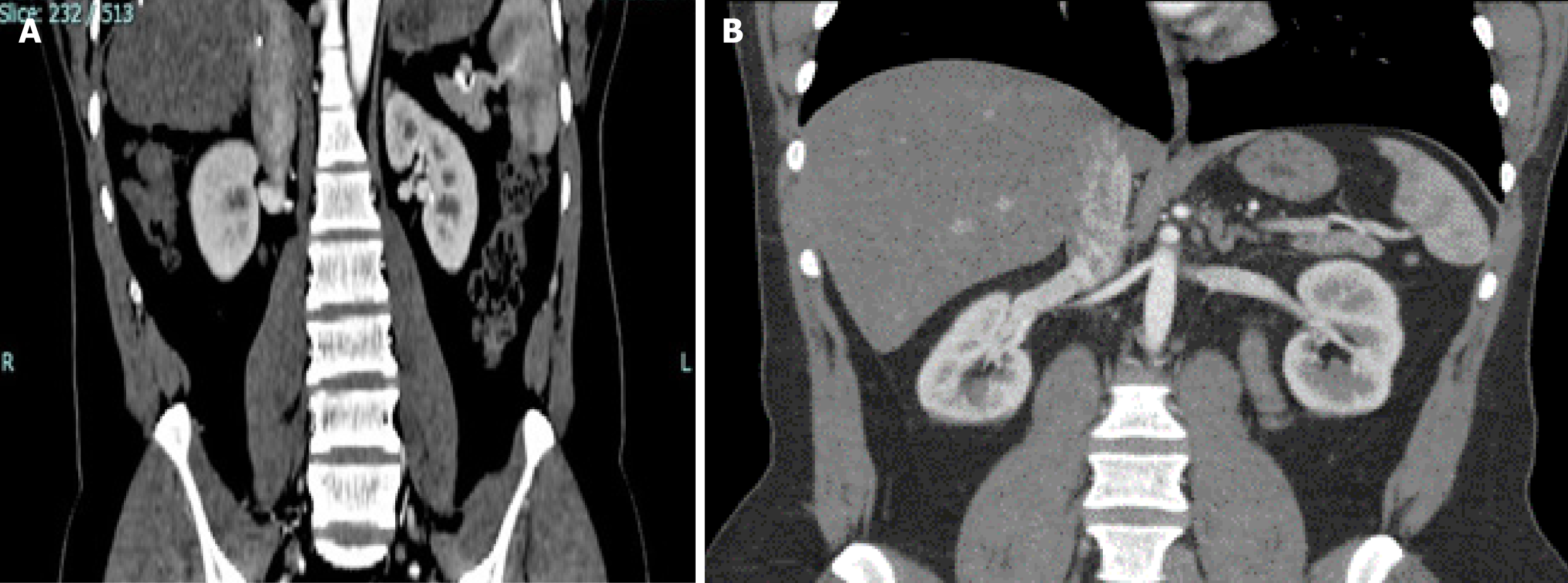
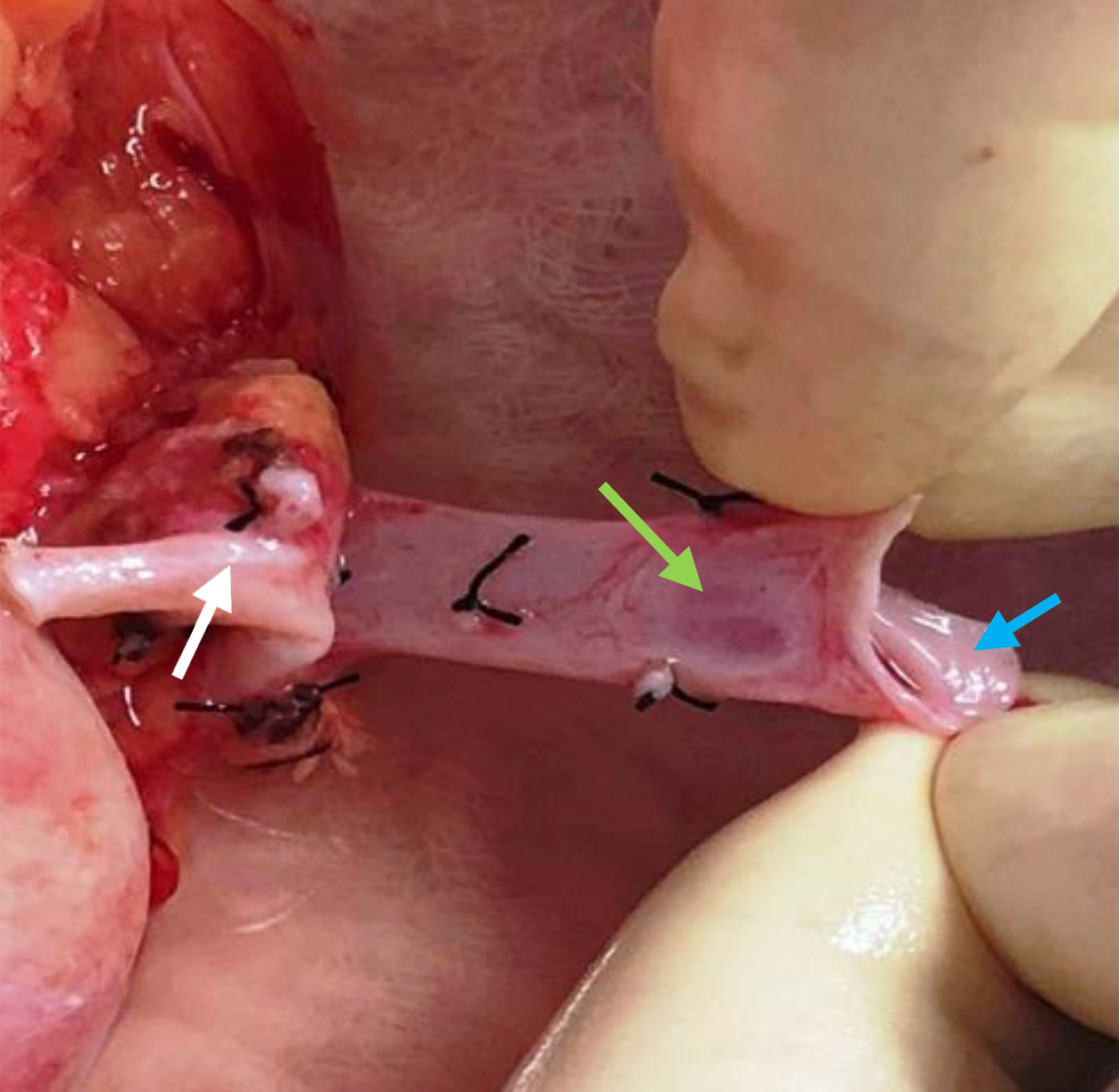
Nevertheless, under certain circumstances, RKs are a better choice for transplantation. This is commonly the case when the LK vascular anatomy is unfavorable or when there are concerns about compromising the donor residual renal function by removing a larger LK. Thus, it is prudent for transplant surgeons to be aware of the complexities of RK transplantation and the strategies to cope with them. The choice of RKs for transplantation must be a deliberate and a calculated one, based on the verifiable length of the RRV on CT imaging. A tip is to note the angle at which the RRV joins the inferior vena cava (IVC): A horizontal entry makes the length shorter than when it enters at an obtuse angle (Figure 3). We advocate a minimum vein length of 2.5 cm to perform a safe venous anastomosis; severe technical problems can arise with a shorter length, which cannot be mitigated with renal vein augmentation from the adjacent IVC in LDs[11]. A caval cuff can be taken in LDs, but only during ODN, because claims of acquiring a caval cuff in LDN with an Endo-TA stapler remain unsubstantiated[12]. A useful technique to gain a valuable centimeter of length during bench work is to mobilize the renal vein circumferentially and separate it from the artery by ligating and dividing all tributaries and lymphatic tissue between the two (Figure 1). In cases with short donor RRVs, fully mobilizing the recipient external iliac vein helps to lift the vein up, making anastomosis easier as it decreases the distance between the two venotomies and reduces tension on the anastomosis. Another critical point about RRV is identifying a thin oval area in its ventral wall that looks like a structural defect (Figure 4). Suturing through it requires the surgeon and the assistant to be cautious and gentle; any inadvertent tear here is exceedingly difficult to repair and could end up with narrowing, thrombosis, and graft loss[6]. The Achilles heel of an RK is its vein and not the artery, which can be shortened to match vein length even when disproportionately longer. Other considerations in selecting RKs for transplantation include the recipient’s body mass index and the donor kidney size. In obese patients, the challenge of anastomosing a short donor RRV is exacerbated by the deep-seated iliac veins, whereas a large kidney restricts access to the anastomosis site following reperfusion.
In our series, we conducted a comprehensive preoperative assessment of the donor RRV to be sure of its length, and if short, we adopted the techniques described above: First, to increase the vein length during bench work; and second, to fully mobilize the recipient external iliac vein. We used a fine Prolene 6/0 suture with a C-1 needle for the anastomosis, paired with a lightweight Castroviejo needle holder. This choice results in smaller needle holes, reduces venous wall consumption, and ensures precise and smooth suturing. In contrast, a conventional needle holder is bulkier and prone to uncontrolled movements during unlocking, with the potential to tear the vein along the needle tract, and troublesome bleeding after clamp removal. Attention to detail explains why the venous anastomoses in our series were uneventful, as evidenced by there being no differences in RWI between the RKs and LKs. It also accounted for the low incidence of adverse post-transplant events and similar trends in post-transplant SC in both kidneys. DWI and CIT were shorter for RKs than LKs, as the former was mostly recovered by ODN (96% vs 50%), which is a faster method than LDN, and kidneys thus recovered require less bench preparation. Due to the limited cases for analysis, we could not confirm whether these factors influenced the transplant results.
Studies comparing outcomes of right and LK transplants have shown varying results. Analyses of the Organ Procurement and Transplant Network and Australian and New Zealand Dialysis and Transplantation data also supported the association between RK transplants and increased risks of DGF and graft loss[3,5]. Another large registry-based study found that recipients of RKs had a higher risk of DGF and graft loss than those receiving LKs; 36% of transplants with RKs experienced primary non-function, compared to only 9% with LKs[10]. On the contrary, two meta-analyses failed to identify a significant association between the incidence of DGF and graft loss in RK grafts, despite the exclusive use of LDN in the studies analyzed[13,14]. This was unexpected and difficult to explain, because application of staples in laparoscopic recovery consumes length, and makes the anastomosis difficult. The contradictory findings of the reported studies and the findings in our series suggest that there may be other factors beyond vein length that play a role in determining graft outcome in RK transplantation. Identifying these factors would necessitate a more extensive and inclusive study involving a comprehensive dataset encompassing all relevant variables.
The studies that showed RKs resulted in more technical losses and poorer early graft function than LKs did not differ in longer-term results[3,6,8,10]. This is because technical complications manifest in the early postoperative period, and after grafts exhibit immediate function, the side of the kidney does not have a role in longer-term function. Our study further substantiates this observation by demonstrating that the right and LKs perform comparably despite the former having a lesser volume. It demonstrates that the differences in right and LKs are not in their nephron characteristics but in their vascular anatomy. In other words, the longer-term outcome depends on factors other than laterality, such as graft quality, rejection episodes, drug toxicity, drug compliance and disease recurrence. However, early setbacks always have consequences, and grafts that recover from DGF have poorer outcomes than grafts with immediate function, irrespective of whether they are right or left[2,15].
We imagine that the observed inferior outcomes associated with RK transplants may stem from inaccuracies in preoperative donor assessment of RRV length, limited surgical proficiency, and unfamiliarity with the techniques required to manage challenging operative situations. When faced with the dilemma of choosing between an RK with borderline vein length and an LK with multiple arteries, opting for LK may be safer and less risky for a surgeon not well-versed in dealing with fragile, short veins; studies suggest that transplants involving multiple arteries yield better outcomes than those involving kidneys with borderline-length veins[16]. Last, SCs in our recipients who received kidneys recovered by LDN were higher than those receiving kidneys recovered by ODN, suggesting that because RKs were recovered predominantly by ODN, this may have contributed positively to our results.
The main strength of our study is that it represents the largest cohort of LD transplants from Pakistan and is the first to report the impact of selecting right donor kidneys on graft outcomes and provide an insight for transplant surgeons when selecting RKs for transplantation. However, it is also important to recognize limitations of our study: It is retrospective in nature and carried out at a single center. A larger prospective multi-centric study would better identify factors other than donor vein length for disparity in outcomes between groups.
Contrary to common perception, our study shows that the donor kidney side has little impact on post-transplant adverse events and graft function in LD transplantation, provided careful patient selection and precise surgical techniques are employed.
| 1. | Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, Taylor RS. Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study. Health Technol Assess. 2005;9:1-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Özdemir-van Brunschot DM, van Laarhoven CJ, van der Jagt MF, Hoitsma AJ, Warlé MC. Is the Reluctance for the Implantation of Right Donor Kidneys Justified? World J Surg. 2016;40:471-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Mogulla MR, Bhattacharjya S, Clayton PA. Risk factors for and outcomes of delayed graft function in live donor kidney transplantation - a retrospective study. Transpl Int. 2019;32:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Tsoulfas G, Agorastou P, Ko D, Hertl M, Elias N, Cosimi AB, Kawai T. Laparoscopic living donor nephrectomy: is there a difference between using a left or a right kidney? Transplant Proc. 2012;44:2706-2708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Khalil A, Mujtaba MA, Taber TE, Yaqub MS, Goggins W, Powelson J, Sundaram C, Sharfuddin AA. Trends and outcomes in right vs. left living donor nephrectomy: an analysis of the OPTN/UNOS database of donor and recipient outcomes--should we be doing more right-sided nephrectomies? Clin Transplant. 2016;30:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Khan TT, Ahmad N, Siddique K, Fourtounas K. Implantation of Right Kidneys: Is the Risk of Technical Graft Loss Real? World J Surg. 2018;42:1536-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Liu KL, Chiang YJ, Wang HH, Chu SH. Techniques of vascular control in laparoscopic donor nephrectomy. Transplant Proc. 2008;40:2342-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Adler JT, Markmann JF, Yeh H. Renal allograft thrombosis after living donor transplantation: risk factors and obstacles to retransplantation. Clin Transplant. 2016;30:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Carolan C, Tingle SJ, Thompson ER, Sen G, Wilson CH. Comparing outcomes in right versus left kidney transplantation: A systematic review and meta-analysis. Clin Transplant. 2021;35:e14475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Dobrijevic ELK, Au EHK, Rogers NM, Clayton PA, Wong G, Allen RDM. Association Between Side of Living Kidney Donation and Post-Transplant Outcomes. Transpl Int. 2022;35:10117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Khan TFT, Baig MA, Zahid R, Mousa D. Right Renal Vein Augmentation in Deceased Donor Kidney Transplantation: Importance of the Contiguous Inferior Vena Cava. UIJ. 2010;3. [DOI] [Full Text] |
| 12. | Modi P, Kadam G, Devra A. Obtaining cuff of inferior vena cava by use of the Endo-TA stapler in retroperitoneoscopic right-side donor nephrectomy. Urology. 2007;69:832-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Wang K, Zhang P, Xu X, Fan M. Right Versus Left Laparoscopic Living-Donor Nephrectomy: A Meta-Analysis. Exp Clin Transplant. 2015;13:214-226. [PubMed] |
| 14. | Liu N, Wazir R, Wang J, Wang KJ. Maximizing the donor pool: left versus right laparoscopic live donor nephrectomy--systematic review and meta-analysis. Int Urol Nephrol. 2014;46:1511-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Nogueira JM, Haririan A, Jacobs SC, Weir MR, Hurley HA, Al-Qudah HS, Phelan M, Drachenberg CB, Bartlett ST, Cooper M. The detrimental effect of poor early graft function after laparoscopic live donor nephrectomy on graft outcomes. Am J Transplant. 2009;9:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Moreno-Alarcón C, Server-Pastor G, López-González PÁ, López-Cubillana P, Ruiz-Morcillo JC, Doñate-Iñíguez G, Olarte-Barragán EH, Gómez-Gómez GA. Must we still be worried about multiple arteries in kidney transplantation? Nephrourol Mon. 2013;5:692-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |