Published online Dec 18, 2024. doi: 10.5500/wjt.v14.i4.97612
Revised: July 17, 2024
Accepted: August 2, 2024
Published online: December 18, 2024
Processing time: 108 Days and 16.4 Hours
Liver grafts from donation after circulatory death (DCD) are associated with a higher risk of early graft dysfunction, determined by the warm ischemia and cold ischemia times. It is essential to have precise criteria to identify this complication in order to guide therapeutic strategies.
To validate different graft and recipient survival scores in patients undergoing liver transplantation (LT) with DCD grafts.
A retrospective and observational unicentric study was conducted on 65 LT patients with grafts obtained from controlled DCD donors from November 2013 to November 2022. The United Kingdom (UK) risk score, early allograft dysfunction (EAD) Olthoff score, and model for early allograft function (MEAF) score were used to evaluate the risk of graft and recipient survival post-transplant. For survival analysis purposes, we used the Kaplan-Meier method, and the differences between subgroups were compared using the log-rank (Mantel-Cox) test.
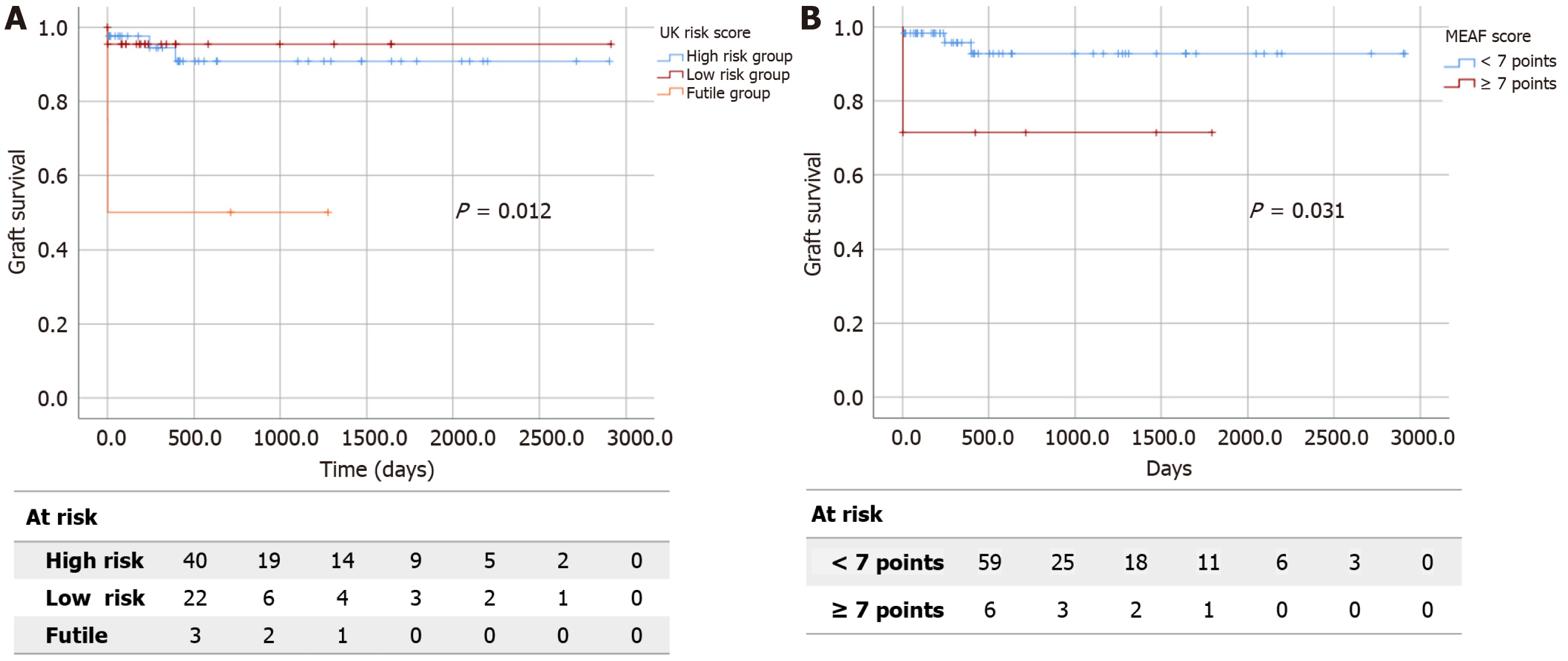
Sixty-five patients were included in the study. The UK risk score did not demonstrate predictive capacity for recipient or graft survival. However, in donors aged over 70 years old (18.4%), it significantly predicted graft survival (P < 0.05). According to Kaplan-Meier survival curves, graft survival rates at 6 months, 2 years, and 5 years in the futility group dramatically decreased to 50% compared to the other groups (log-rank 8.806, P < 0.05). The EAD Olthoff and MEAF scores did not demonstrate predictive capacity for recipient or graft survival. Based on Kaplan-Meier survival curves, patients with a MEAF score ≥ 7 had a lower graft survival rate at 6 months, 2 years, and 5 years compared to patients with a lower MEAF score (log-rank 4.667, P < 0.05).
In our series, both UK DCD risk score and MEAF score showed predictive capability for graft survival.
Core Tip: Controlled donation after circulatory death (DCD) grafts are increasingly being used in liver transplantation, and in some countries, more than one‐third of the deceased donor liver transplants are performed with DCD grafts. Nonetheless, DCD grafts are not risk-free. Despite the increased complexity in predicting outcomes for liver transplant recipients from DCD, risk scores for allograft failure remain a valuable tool in assisting clinicians in identifying patients at higher risk for adverse outcomes and guiding decision-making in post-transplant care.
- Citation: Mohamed Chairi MH, Mogollón González M, Triguero Cabrera J, Segura Jiménez I, Villegas Herrera MT, Villar del Moral JM. Risk scores for allograft failure: Are they still useful in liver recipients from donation after circulatory death? World J Transplant 2024; 14(4): 97612
- URL: https://www.wjgnet.com/2220-3230/full/v14/i4/97612.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i4.97612
Liver transplantation (LT) represents the treatment of choice in the end stage of certain liver diseases when all other therapeutic alternatives have been exhausted[1]. The incorporation of donation after circulatory death (DCD) has enabled an increase in organ availability to meet the growing demand, leading to an increase in the number of transplants. Five categories of DCD have been defined based on the context of the death. In uncontrolled DCD categories I and II, circulatory death occurs suddenly and unexpectedly without medical presence or control and without success after cardiopulmonary resuscitation maneuvers. In controlled DCD (cDCD) categories III, IV, and V, circulatory death occurs in a hospital setting and under medical control[2]. Currently, cDCD is steadily and gradually expanding. According to donation and transplant activity data in Spain for 2022, DCD accounted for a 38% increase compared to the previous year, representing 42% of the current total donations, with over 92% of cases being cDCD[3,4].
Grafts from cDCD donors are associated with a higher risk of early graft dysfunction, mainly related to the length of warm ischemia time and cold ischemia time (CIT). Early allograft dysfunction (EAD) dramatically influences graft and patient outcomes. Several studies have shown that the most favorable outcomes in LT using grafts from cDCD donors are associated with young donors, short functional warm ischemia times (FWIT; ≤ 12 min), and limited CIT (< 6 h). Precise criteria are essential to identify complications and guiding therapeutic strategies[5].
The aim of the following study was to validate the currently available LT outcome scores for both graft and recipient survival in cDCD.
A retrospective and observational unicentric study was conducted on LT patients with grafts obtained from cDCD donors from November 2013 to November 2022. The study has been reviewed and approved by the regional Research Ethics Committee. All study participants or their legal guardian provided informed written consent prior to study enrollment. Exclusion criteria were: super-rapid recovery technique, insufficient data, and retrieval procedure performed by other transplant teams. Within the study group, three parameters were defined to build a high-risk cohort: Donors aged ≥ 70, CIT ≥ 6 h, and FWIT ≥ 12 min. Both graft and recipient survival were evaluated.
We analyzed the performance in our series of the United Kingdom (UK) DCD risk score, Olthoff definition and model for early allograft function (MEAF) score. Scores were calculated as described in their respective original publications[1,6,7]. UK DCD risk score stratifies cDCD liver recipients according to seven variables and aims to predict 1-year graft survival. Donor age > 60 years, cold ischemia > 6 h, and recipient lab model for end-stage liver disease score > 25 were assigned 2 points each; donor body mass index > 25, functional warm ischemia > 20-30 min, and recipient age > 60 years 3 points; functional warm ischemia > 30 min 6 point and retransplantation 9 points. Scores of 0-5 are ‘‘low risk” (1-year graft survival > 95%), 6–10 ‘‘high-risk” (1-year graft survival > 85%), and > 10 ‘‘futile” (1-year graft survival < 40%)[6]. Olthoff defined the presence or absence of EAD within the first 7 post-operative days, based on levels of aminotransferases, bilirubin and international normalized ratio[7]. The MEAF score developed by Pareja et al[1] consists of a nonlinear regression model based on the peak values of three post-operative laboratory parameters: The maximum of alanine aminotransferase and international normalized ratio within the first 3 days and the bilirubin on the third post-operative day. In this case, we considered a score of 7 to mark the high-risk group based on our previous experience[8].
Data were expressed as median and interquartile range for continuous variables and as percentages for categorical variables. The Kolmogorov-Smirnov test was used to assess the normality of each continuous variable. Univariate analysis was performed using χ2 or Fisher tests for categorical data and Mann-Whitney U or Kruskall-Wallis tests for continuous data. For survival analysis, we used the Kaplan-Meier method, and the differences between subgroups were compared using the log-rank (Mantel-Cox) test. IBM SPSS Statistics 20.0 (IBM Corp., Armonk, NY, United States) was used for statistical analysis. A P < 0.05 was considered statistically significant.
A total of 348 LTs were conducted in this period of time, 72 from cDCD. Seven patients were excluded: 2 were super-rapid recovery technique, 1 had insufficient data, and 4 were performed by other transplant teams. Sixty-five patients fulfilled inclusion criteria. Forty-three (66.1%) were transplanted for alcohol-associated cirrhosis [of which 18 (27.7%) had subsidiary hepatocarcinoma], 11 (16.9%) for cirrhosis due to hepatitis C virus [6 (9.2%) with hepatocarcinoma], 4 (6.1%) for cirrhosis due to hepatitis B virus [3 (4.6%) with hepatocarcinoma], 6 (9.2%) for primary biliary cholangitis and 1 (1.5%) for cryptogenetic cirrhosis.
In the donor group, 60% were men. The main cause of death (58.4%) was stroke. Among the recipients, 81.5% were male, with a median age of 57 (52-63) years.
The median operative time was 340 (325-420) min. The median number of transfused red blood cell packs was 5 (2-7). Regarding vessel reconstruction, temporary portocaval shunt was performed in almost half (46.8%) of the transplants; in 90.7% of cases, a single end-to-end vascular reconstruction was performed; in the remaining 9.3% of cases, multiple reconstructions were required due to vascular anomalies present in the donor, the recipient, or both.
Functional WIT was > 12 min in 46 donors (63.1%), CIT was ≥ 6 h in 13 patients (20%), and 12 donors were older than 70 (18.5%). According to UK DCD risk score, four patients (6.2%) were classified in the “futile” group, and 23 patients (35.4%) were categorized as the “high risk” group. Twelve patients (20%) developed EAD according to Olthoff criteria, and seven (11.6%) scored ≥ 7 points (indicating severe dysfunction) according to the MEAF score.
The patients were monitored after surgery with a median clinical follow-up of 13 (5.2-42.7) months. At the time of analysis, 15 patients (23.1%) had died. The overall survival rate was 89.1% at 1 year, 76.2% at 3 years, and 74% at 5 years. In contrast, the graft survival rates at 1 year, 3 years and 5 years were 84.2%, 69.4%, and 67.9%, respectively.
Regarding the global predictive capacity of UK DCD risk score for survival, we did not observe a significant correlation between group risk and patient (P = 0.173) or graft survival (P = 0.162). However, in donors aged over 70 years (18.4%), the score significantly predicted graft survival (P < 0.05) and exhibited a trend towards significance in recipient survival
| Survival | Donors aged ≥ 70 years | Cold ischemia time ≥ 6 h | Functional warm ischemia time ≥ 12 min | ||||||||
| Low risk, n = 5 | High risk, n = 7 | P value | Low risk, n = 6 | High risk, n = 4 | Futile, n = 3 | P value | Low risk, n = 24 | High risk, n = 18 | Futile, n = 4 | P value | |
| Graft survival in days | 420 (306-1636) | 236 (1-395) | 0.0481 | 987 (11-1657) | 592 (47-1480) | 2 (1-3) | 0.3302 | 505 (179-1469) | 357 (1.2-1134) | 218 (100-685) | 0.3402 |
| Overall survival in months | 13 (9-53) | 7 (1-12) | 0.0881 | 32 (3.7-53) | 19 (1.5-47.7) | 8 (6-9) | 0.8472 | 16 (5-48) | 15 (8-36) | 7 (2.7-22) | 0.6782 |
The EAD Olthoff and MEAF scores did not demonstrate predictive capacity for recipient or graft survival, either globally or in any risk subgroup (Tables 2 and 3). However, focusing on survival analysis among subgroups, patients with a MEAF score ≥ 7 displayed poorer 6-month (98.2% vs 71.4%), 2-year (92% vs 71%) and 5-year (92% vs 0%) graft survival rate than patients with a lower MEAF score (log-rank 4.667, P < 0.05; Figure 1B).
| Survival | Donors aged ≥ 70 years | Cold ischemia time ≥ 6 h | Functional warm ischemia time ≥ 12 min | ||||||
| Meet, n = 2 | Does not meet, n = 10 | P value | Meet, n = 7 | Does not meet, n = 6 | P value | Meet, n = 19 | Does not meet, n = 27 | P value | |
| Graft survival in days | 210 (1-220) | 319 (265-569) | 0.5821 | 505 (2-1641) | 998 (100-1556) | 0.5302 | 400 (123-907) | 505 (107-1641) | 0.4432 |
| Overall survival in months | 6.5 (1-7) | 10 (8-18) | 0.4821 | 16 (5-53) | 32 (3-50) | 0.9652 | 12.5 (3.5-29) | 16 (5-53) | 0.8542 |
| Survival | Donors aged ≥ 70 years | Cold ischemia time ≥ 6 h | Functional warm ischemia time ≥ 12 min | ||||||
| MEAF < 7, n = 10 | MEAF ≥ 7, n = 2 | P value | MEAF < 7, n = 8 | MEAF ≥ 7, n = 5 | P value | MEAF < 7, n = 38 | MEAF ≥ 7, n = 8 | P value | |
| Graft survival in days | 420 (410-430) | 315 (219-563) | 0.6271 | 751 (57-1642) | 357 (2-1280) | 0.4612 | 420 (2-1091) | 409 (153-1279) | 0.6662 |
| Overall survival in months | 13 (2-18) | 10 (6.5-18) | 0.7271 | 24 (1.5-53) | 15.5 (5.7-41.7) | 0.8082 | 13 (4-35) | 13 (4.5-41) | 0.4322 |
LT risk scoring systems offer valuable insights for predicting outcomes, a key point in the era of more widespread use of extended criteria donor livers and potentially reduced graft quality. Nevertheless, there is no consensus on the clinical criteria to define EAD and no one score is considered the gold standard. According to our results, both MEAF and UK DCD risk score have shown an association with graft survival prediction, though not with patient survival. In our sample, the threshold defined by Olthoff score did not exhibit an association with either graft survival or overall survival.
UK DCD risk score was designed to predict risk and futility in the context of cDCD LT[6]; however, its application requires caution. Several variables included in the score are not directly related to the donor and graft itself. For instance, assigning a score of 9 to the retransplantation item implies considering nearly all retransplantations as futile. Consequently, some critical risk factors may be downplayed or omitted entirely in the final risk stratification score.
Pareja et al[1] introduced the MEAF score to assess the severity of EAD. This model demonstrates a significant association with both patient and graft outcomes (in our data, only with graft outcomes), enabling survival estimation for a concrete recipient. Despite MEAF utilizing a continuous concept, it does not capture the trend or rate of change in variables. Cases with gradually increasing laboratory test values post-LT differ from cases with a rapid decline, even if they initially exhibited similar results.
This study presents several limitations that must be considered when interpreting the results. Firstly, it is important to highlight that the study design is non-randomized and relies on historical data, which may introduce selection bias and confounding variables that could impact the validity of the findings. Moreover, the median duration of follow-up is relatively short, which limits the ability to assess long-term outcomes and the durability of the risk scores for predicting allograft failure in liver transplant recipients. Additionally, the sample size of patients included in the study is relatively small, which may limit the generalizability of the results and the statistical power to detect significant associations. Furthermore, while the study focuses on the utility of these three risk scores for predicting allograft failure, UK DCD risk score, Olthoff definition and MEAF score, it would be valuable to explore the potential use of other risk assessment tools or scores in this population to improve the accuracy and reliability of predicting graft survival. By incorporating alternative risk scores or predictive models, researchers may be able to enhance the predictive capacity and clinical utility of risk assessment in liver transplant recipients receiving livers from DCD.
In our series, both UK DCD risk score and MEAF score showed predictive capability for graft survival. They can help in the election of the best matching donor/recipient, and they can become a useful tool in informed decision-making. Larger sample sizes, a long duration of follow-up, prospective studies, and the application of additional prognostic scores are necessary for more robust results.
The authors would like to extend their heartfelt gratitude to the dedicated team of liver transplantation specialists at Virgen de las Nieves University Hospital for their tireless dedication and exceptional patient care. Their unwavering commitment to excellence in liver transplantation has resulted in countless lives being saved and transformed. We thank them for all for their hard work, dedication, and commitment to improving the lives of others through liver trans
| 1. | Pareja E, Cortes M, Hervás D, Mir J, Valdivieso A, Castell JV, Lahoz A. A score model for the continuous grading of early allograft dysfunction severity. Liver Transpl. 2015;21:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Le Dinh H, de Roover A, Kaba A, Lauwick S, Joris J, Delwaide J, Honoré P, Meurisse M, Detry O. Donation after cardio-circulatory death liver transplantation. World J Gastroenterol. 2012;18:4491-4506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Feo M, Miñambres E, Suberviola B, Campos-Fernández S, Sánchez-Arguiano J, Kislikova M, Ballesteros MA. Controlled Donation After Circulatory Death Program: Analysis and Results at a Tertiary Care Hospital. Transplant Proc. 2022;54:70-72. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Lomero M, Gardiner D, Coll E, Haase-Kromwijk B, Procaccio F, Immer F, Gabbasova L, Antoine C, Jushinskis J, Lynch N, Foss S, Bolotinha C, Ashkenazi T, Colenbie L, Zuckermann A, Adamec M, Czerwiński J, Karčiauskaitė S, Ström H, López-Fraga M, Dominguez-Gil B; European Committee on Organ Transplantation of the Council of Europe (CD-P-TO). Donation after circulatory death today: an updated overview of the European landscape. Transpl Int. 2020;33:76-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 5. | van Leeuwen OB, Bodewes SB, Porte RJ, de Meijer VE. Excellent long-term outcomes after sequential hypothermic and normothermic machine perfusion challenges the importance of functional donor warm ischemia time in DCD liver transplantation. J Hepatol. 2023;79:e244-e245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Schlegel A, Kalisvaart M, Scalera I, Laing RW, Mergental H, Mirza DF, Perera T, Isaac J, Dutkowski P, Muiesan P. The UK DCD Risk Score: A new proposal to define futility in donation-after-circulatory-death liver transplantation. J Hepatol. 2018;68:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 7. | Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 875] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 8. | Brea-Gómez E, Villar-Quintana R, Plata-Illescas C, Zambudio-Carroll N, Lopez-Garrido MA, Nogueras-Lopez F, Muffak-Granero K, Becerra-Massare A, Villegas-Herrera MT, Segura Jiménez I, Muñoz Pérez N, Villar-Del-Moral JM. Analysis of the Predictive Ability for Graft Loss and Mortality of Two Criteria for Early Allograft Dysfunction After Liver Transplantation. Transplant Proc. 2018;50:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |