Published online Dec 18, 2024. doi: 10.5500/wjt.v14.i4.96244
Revised: June 3, 2024
Accepted: June 25, 2024
Published online: December 18, 2024
Processing time: 142 Days and 21.5 Hours
Although the benefits of exercise for kidney transplant recipients (KTRs) have been widely demonstrated, these patients experience several barriers in undertaking a structured exercise program in hospital and non-hospital facilities.
To compare the effects of a supervised moderate-intensity gym-based intervention with a home-based low-intensity walking program on exercise capacity in KTRs.
KTRs were asked to choose between two six-month programs. The first group performed a low-intensity interval walking intervention at home-based exercise intervention (HBex). The second group performed a supervised training program at an adapted physical activity gym (Sgym), including aerobic and resistance training. The outcomes, collected at baseline and at the end of the programs, included the 6-minute walking test, the peak oxygen consumption (VO2peak) during a treadmill test, the 5-time sit-to-stand test, and blood pressure.
Seventeen patients agreed to participate and self-selected into the HBex (n = 9) and Sgym (n = 8) groups. Two patients in the Sgym group dropped out because of familial problems. At baseline, patients in the HBex group were significantly older and had lower walking distance, VO2peak, and lower limb strength. Primary outcome changes were significantly greater in the HBex group than in the Sgym group (52 ± 23 m vs 8 ± 34; P = 0.005). No other significant differences between groups were observed. Both groups improved most of the outcomes in the within-group comparisons, with significant variations in VO2 peak.
Six-month moderate-intensity supervised or low-intensity home-based training programs effectively improved exercise capacity in KTRs. Gym-based programs combine aerobic and resistance training; however, in-home walking may be proposed for frail KTRs.
Core Tip: This nonrandomized pragmatic pilot study highlights the significance of implementing structured exercise programs in kidney transplant recipients (KTRs). This study, for the first time, provided the possibility of choosing the preferred training strategy for patients. This fact led to high adherence to the programs and superimposable results in terms of exercise capacity. Home-based low-intensity programs may be a useful option for more deconditioned KTRs.
- Citation: Crepaldi A, Piva G, Lamberti N, Felisatti M, Pomidori L, Battaglia Y, Manfredini F, Storari A, López-Soto PJ. Supervised vs home-based exercise program in kidney transplant recipients: A pilot pragmatic non-randomized study. World J Transplant 2024; 14(4): 96244
- URL: https://www.wjgnet.com/2220-3230/full/v14/i4/96244.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i4.96244
The number of people living with chronic kidney disease (CKD) worldwide is estimated to be 843. 6 million[1], and long-term sedentary behavior seems to increase the risk of CKD, particularly in women[2]. Altered levels of albuminuria and estimated glomerular filtration rate (eGFR) were observed in people with inactive lifestyles, representing generalized endothelial dysfunction and poor renal functioning[2]. At the final stage of CKD, dialysis treatment or kidney repla
In both patients with ESKD and KTRs, physical exercise is an effective nonpharmacological strategy for reducing cardiovascular risk factors, metabolic diseases, and infections[12]. However, exercise prescriptions for solid organ transplantation recipients are only occasionally extensively covered in academic courses for healthcare professionals, creating a further cultural barrier to the development of exercise programs for KTRs[13]. Indeed, a structured exercise training program that includes aerobic training, resistance training, or both has been shown to induce several short-term benefits, such as improved cardiorespiratory fitness, muscle mass, lower limb muscular endurance capacity, and quality of life. Long-term benefits of physical activity, such as reduced arterial stiffness and a lower hospitalization rate, have also been reported[14]. In Italian KTRs, the eGFR was found to be greater in active patients, particularly in elderly patients aged 51-70 years[15]. Zhang et al[16], in their recent systematic review of the efficacy of exercise intervention in KTRs, suggested that a well-planned physical activity program could improve the blood lipid profile and renal function by reducing creatinine and urea levels. For these reasons, a structured exercise intervention should be part of the standard clinical care for KTRs to improve clinical outcomes, physical functioning, and quality of life.
Although the benefits of exercise in KTRs have been widely demonstrated, these patients experience several barriers in undertaking a structured exercise program. In particular, low energy levels, chronic fatigue, lack of time, fear of harming the graft, and difficulty accessing training programs have been reported[14,17,18]. The prevalence and causes of fatigue are still controversial topics in KTRs due to the lack of longitudinal studies, but their effects on quality of life, functional impairment, and immunosuppression therapy adherence are well established[19].
Intensity, duration, and frequency are three cardinal parameters for training programming, and the appropriate exercise prescription for KTRs is still debated[20]. Recent reviews suggest that a structured and personalized training program lasting from 20 to 60 minutes for sessions performed at least 3-4 times a week for 3-6 months could significantly impact the health status of these patients[21]. However, an optimal intervention modality still needs to be defined, and new studies are warranted to identify specific intervention protocols[21].
In this study, we hypothesize that a simple daily unsupervised structured home-based walking program could be beneficial for KTRs. The aim of this pragmatic nonrandomized study was to compare the effects of a traditional moderate-intensity gym-based supervised intervention with those of a low-intensity home-based walking program on functional outcomes in a KTR population.
This prospective pilot pragmatic nonrandomized clinical study was conducted at the Department of Rehabilitation Medicine and Nephrology, Dialysis and Transplantation at the University Hospital of Ferrara, Italy. The study was approved by the Ethics Committee Area Vasta Emilia Centrale with approval number 49/Sper/19 and was conducted between January 2019 and March 2020.
KTRs who could be included in the study were contacted to evaluate possible enrollment if they met the following inclusion criteria: males and females > 18 years, previous kidney transplant > 1 year, any class of medication allowed, and provision of informed consent. Patients who met the following criteria were excluded: uncontrolled hypertension, symptoms of angina pectoris, severe osteoarticular disorders, or unstable laboratory parameters compromising transplanted kidney function.
All patients provided written informed consent to participate in the study.
Every patient could choose between two six-month exercise programs. The first group performed the pain-free home-based walking program (Home-Based Group – HBex) already described elsewhere[22], which consists of interval walking sessions starting from 60% of the usual walking speed calculated from the results of a 6-minute walking test (6MWT), with an incremental speed progression every week until reaching the patient’s baseline velocity. Walking sessions were organized in 1-minute walking bouts separated by 1-minute seated rest breaks to be repeated 8 to 10 consecutive times, twice daily. A training diary was used to assess the number of sessions performed.
The second group chose an individual supervised training program at an adapted physical activity gym (Supervised Training Group – Sgym): Every session included aerobic work on a treadmill or cycle ergometer and strength exercises for lower and upper body training (leg extension, pectoral machine, lat machine, leg curl, abductor machine). Each workout was one hour long and was repeated three times per week. The progression of exercise during weeks was defined by the exercise specialist who conducted the training sessions, which ranged from 40%-50% to 65%-75% of a person’s maximal exercise capacity. Program adherence corresponded to the number of training sessions completed compared to the expected number of sessions.
Outcome measures were collected at baseline and at the end of the six-month training program. The baseline physical activity level of every participant was assessed using the Rapid Assessment of Physical Activity (RAPA) questionnaire[23].
The primary outcome was the 6-minute walking distance (6MWD) measured during the 6MWT. Patients were instructed to walk along a 22-meter corridor to cover the greatest possible distance. Participants were asked to report any symptoms they experienced during the test; the distance of the first reported symptom was recorded as the pain-free walking distance (PFWD). They were allowed to stop and rest if necessary and start again by the end of the time. At the end of the test, the 6MWD was recorded.
The secondary outcomes included the following:
Body mass index (BMI) was measured through a standard scale and obtained by dividing weight by height squared.
An incremental treadmill protocol was used to calculate the maximum oxygen consumption (VO2max). Patients underwent the Balke treadmill test[24] or the Manfredini treadmill test[25] according to the initial physical activity level recorded on the RAPA questionnaire. Patients undergoing Balke's test walked at a constant speed (4.8 km/h for women and 5.3 km/h for men), increasing the incline by 2.5% every 2 minutes. The test ends when the effort is perceived as no longer sustainable by the patient. The PeakVO2 was calculated by the formula (time × 1.444) + 14.99 (male) or (time x 1.38) + 5.22 (female). Patients undergoing the Manfredini test start walking at a speed of 1.5 km/h, which is increased every 10 m by 0.1 km/h. The test ends when the speed increase is no longer sustainable. VO2 max was calculated using the American College of Sports Medicine formula[26].
5-time sit-to-stand test (5t-STS) for lower limb strength assessment. Patients were asked to stand up and sit down five times with their arms crossed on their chests as quickly as possible. The time needed to complete the task was recorded[27].
Ambulatory blood pressure was measured according to international guidelines. Both systolic and diastolic blood pressures were recorded (SPB and DPB, respectively).
Testing sessions were performed for both groups in the hospital in the same temperature-controlled environment by expert assessors who were not blinded to patient allocation.
The data distribution was verified with the Shapiro-Wilk test. Baseline comparisons between the two groups were carried out with the χ2 test for categorical variables and Student’s t test or the Mann-Whitney U test for continuous variables, as appropriate.
Between-group differences at the end of the training programs were assessed with Student’s t tests or Mann-Whitney U tests according to the data distribution. Within-group comparisons were performed with paired-samples t tests or Wilcoxon tests.
According to the baseline imbalances between the two groups, a subsequent analysis of covariance (ANCOVA) was performed for all the primary and secondary outcomes. All the parameters that presented a significant difference at baseline in the between-group comparisons were included as covariates in the ANCOVA. A P value < 0. 05 was considered to indicate statistical significance.
Statistical analysis was performed with MedCalc Statistical Software version 22. 016 (MedCalc Software Ltd., Ostend, Belgium; https://www.medcalc.org; 2023).
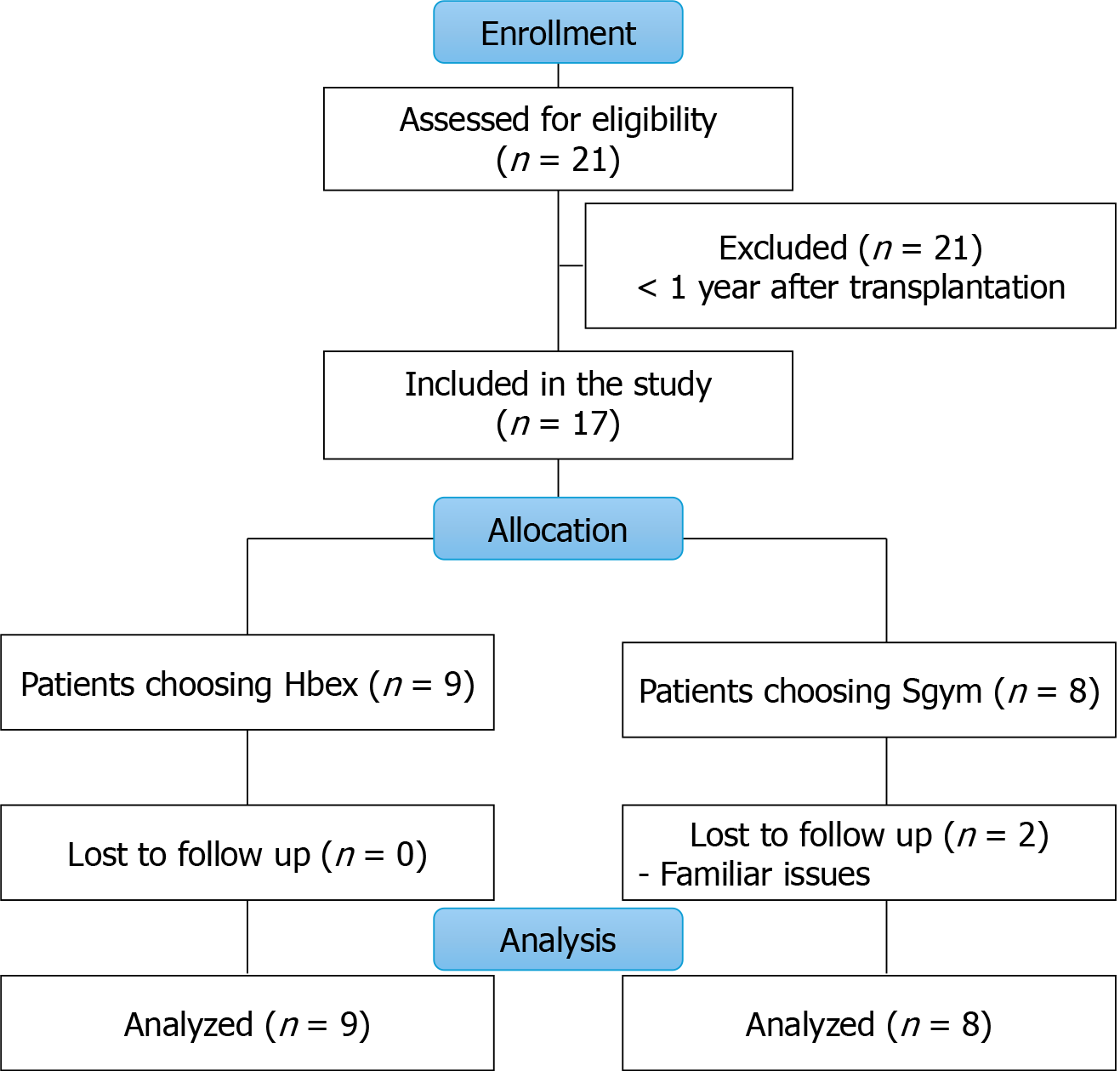
Twenty-one KTRs were contacted for possible inclusion in the study. Four patients were excluded because they had undergone transplantation within the previous 1 year.
Seventeen patients were included in the study and asked to choose a home-based or supervised program at the gym. Nine patients chose the home-based exercise intervention (HBex), and eight patients preferred the Sgym. All patients started the programs, but two patients in the Sgym dropped out because of personal problems. The flow diagram of the study is shown in Figure 1.
At baseline, the Sgym group presented more significantly impaired kidney function despite being younger and more active, according to the RAPA score.
In addition, patients who chose the HBex had significantly lower exercise capacity in terms of walking distance, VO2peak, and lower limb strength. A baseline comparison of the two groups is reported in Table 1.
| HBex, N = 9 | Sgym, N = 6 | P value | |
| Age, years (mean ± SD) | 63 ± 3 | 55 ± 11 | 0.06 |
| Male sex | 6 (67) | 3 (50) | 0.53 |
| Dialysis vintage, month (mean ± SD) | 24 ± 15 | 23 ± 20 | 0.85 |
| Age of transplantation (mean ± SD) | 56 ± 8 | 52 ± 10 | 0.15 |
| Risk factors and comorbidities | |||
| Smokers | 0 | 1 | 0.41 |
| Hypertension | 9 | 6 | 1 |
| Diabetes | 1 | 0 | 0.41 |
| Hyperlipidemia | 4 | 4 | 1 |
| Charlson index (mean ± SD) | 4 ± 2 | 5 ± 4 | 0.52 |
| Medications | |||
| Ace-inhibitors | 2 | 1 | 0.8 |
| Diuretics | 2 | 0 | 0.24 |
| Beta-blockers | 8 | 5 | 0.19 |
| Calcium antagonists | 1 | 3 | 0.22 |
| Antiplatelets therapy | 7 | 5 | 0.37 |
| Statins | 7 | 4 | 0.16 |
| Laboratory parameters (mean ± SD) | |||
| Hemoglobin, g/dL | 12.6 ± 1.1 | 12.5 ± 1.6 | 0.88 |
| Serum creatinine, mg/dL | 1.06 ± 0.25 | 1.64 ± 0.64 | 0.028 |
| Sodium mmol/L | 127.1 ± 33.2 | 140.0 ± 1.8 | 0.47 |
| Potassium mEq/L | 4.2 ± 0.4 | 4.0 ± 0.2 | 0.76 |
| PTH mg/mL | 71 ± 33 | 136 ± 72 | 0.07 |
| RAPA questionnaire (mean ± SD) | 3.3 ± 3.8 | 6.7 ± 3.1 | 0.1 |
| 6MWD (m) | 356 ± 113 | 478 ± 53 | 0.030a |
| PFWD (m) | 278 ± 172 | 442 ± 71 | 0.047a |
| VO2peak (ml/100 g/min) | 18.8 ± 8.3 | 21.9 ± 3.1 | 0.41 |
| 5T-STS | 18.3±16.2 | 11.0 ± 1.9 | 0.3 |
In both groups, all patients completed the proposed intervention without any adverse events. No falls or injuries were reported during the six months.
The Hbex group executed 83% ± 9% of the home walking sessions concerning the prescribed ones, walking for a mean of 96 ± 12 minutes per week and corresponding to 120 ± 8 km in the entire 6-month period. The average speed was 80 steps/min, corresponding to a mean value of 2.8 kmh-1. The rating of perceived exertion (RPE) rate during the training was 2 ± 1 out of 10.
The 6 people in the Sgym group who completed the program attended 64 ± 11 training sessions at the gym, corresponding to an adherence rate of 90% ± 7%. Throughout the entire program, the mean walking speed on the treadmill was 3.0 kmh-1 (range 1.8-4.6), while the mean external load during the bilateral leg extension movement was 18 kg (range 5-40).
The adherence rate was comparable between the two groups (P = 0. 24).
Primary outcome changes were significantly greater in the Hbex group (+ 52 ± 23 meters) than in the Sgym group (+ 8 ± 34 m), even after correction for baseline imbalances (mean change 55 ± 34 m; P = 0. 005).
Moreover, the Hbex group exhibited more significant improvements in most of the outcomes, although the differences did not reach statistical significance. In contrast, the Sgym group showed a greater reduction in systolic blood pressure than did the HBex group (-6 ± 3 mmHg vs -4 ± 5 mmHg), although the difference was not significant. The data are reported in Table 2.
| HBex, N = 9 | Sgym, N = 6 | Between group Ancova | |||||
| T0 | T6 | Δ | T0 | T6 | Δ | Bonferroni-Corrected P value | |
| 6MWD (M) | 356 ± 113 | 408 ± 107a | +52 ± 23 | 478 ± 53 | 488 ± 68 | +8 ± 34 | 0.005 |
| PFWD (M) | 278 ± 172 | 366 ± 147a | +97 ± 88 | 442 ± 71 | 459 ± 78 | +17 ± 31 | 0.25 |
| VO2peak (Ml/100 g/Min) | 18.8 ± 8.3 | 21.9 ± 8.1a | +3.1 ± 2.2 | 21.9 ± 3.1 | 22.8 ± 3.4a | +0.9 ± 0.7 | 0.26 |
| 5t-Sts (S) | 18.3 ± 16.2 | 13.3 ± 8.6 | -5.0 ± 7.9 | 11.0 ± 1.9 | 10.3 ± 2.2 | -0.7 ± 1.5 | 0.60 |
| Systolic blood Pressure (mmHg) | 146 ± 21 | 142 ± 14 | -4 ± 5 | 142 ± 17 | 136 ± 15 | -6 ± 3 | 0.57 |
| Diastolic blood pressure (mmHg) | 74 ± 8 | 74 ± 8 | 0 ± 2 | 72 ± 7 | 75 ± 5 | 3 ± 2 | 0.18 |
| BMI (Kgm-2) | 28.3 ± 8 | 28.2 ± 8.2 | -0.1 ± 0,5 | 24.2 ± 2.4 | 24.5 ± 3.0 | 0.4 ± 1.4 | 0.22 |
Considering the within-group analysis of changes at the end of the exercise program, both groups exhibited significant changes in VO2peak (Hbex: 3.1 ± 2.2 mlO2/kg/min; Sgym: 0.9 ± 0.7 mlO2/kg/min). Patients in the Hbex group also exhibited significantly improved walking capacity, as measured through the 6MWD (Table 2).
Favorable, albeit not significant, improvements in lower limb strength and systolic blood pressure were also observed in both groups. No changes occurred in BMI or diastolic blood pressure were observed (Table 2).
In the present study, we confirmed the effectiveness of exercise programs for improving physical fitness in a single-center population of KTRs. Indeed, following kidney transplantation, structured or unstructured training programs represent a valuable option for reducing intervention-related risk factors such as cardiovascular and metabolic disease and hypomobility[20] by improving cardiorespiratory fitness and arterial stiffness in particular[14,28].
In our study, the majority of the enrolled subjects improved in terms of most of the clinical outcomes concerning baseline, as frequently reported in the literature[16] and thanks to the high adherence to the proposed protocol. However, the novelty of the trial is the possibility for patients to decide the setting where to exercise and, accordingly, the training program to be performed. Indeed, at the baseline visit, every patient was free to select the exercise option that he/she preferred, according to his/her feelings, availability of funds, and transport. To the best of our knowledge, only a previous pilot trial in the literature in solid organ transplant recipients allowed patients to choose the setting of training programs, offering home-based, hospital-based, or community gym solutions[29]. Our study revealed that younger and more physically active patients preferred a supervised program at a gym center to improve or at least maintain their fitness status. On the other hand, the patients who opted for a home training protocol were older and more deconditioned, in contrast with the findings of Ribeiro et al[29]. This, on the one hand, causes baseline imbalances between groups’ characteristics but, on the other hand, poses the first important issue to be solved, which is a potential barrier to participation in physical activity for frail KTRs. Indeed, as recently reported[30], more frail patients are usually excluded from habitual training programs. However, individuals in financial difficulties or who cannot be transported to training/rehabilitation centers should have access to exercise to improve their fitness level.
Home-based exercise programs for KTRs may represent a useful opportunity to overcome many of the abovementioned barriers and improve patients’ participation in physical activity[17]. In the literature, only a few studies have reported the effectiveness of home-based programs in KTRs[31,32], as recently highlighted in a systematic review[33]. In particular, Michou et al[32], who enrolled a population with comorbidities similar to those of the patients in our study, observed improvements in aerobic capacity and lower limb strength comparable to those observed in our trial. Further evidence should be provided by two current ongoing clinical trials employing home-based exercise modalities[34,35]. However, in all these reported interventions, the home-based programs included both aerobic and resistance training. In those programs, patients were required to walk or cycle at a moderate intensity for at least 20 to 40 consecutive minutes, exposing them to barriers related to their baseline frailty level or weather issues. The walking program proposed in this study is significantly different regarding training features and organizational issues. First, the slow interval walking training employed, which was previously validated in several frail populations, including ESKD patients[22,36-39] whose improvement was significantly greater than that of patients who followed traditional walking advice[40], allows people with frailty to participate because of its slow pace and frequent rest breaks. Second, the program can be performed every day inside the home, overcoming all the barriers related to outdoor execution, the necessity of transportation, costs, etc[41]. Nonetheless, despite the lower exercise intensity performed by the HBex, the improvements in terms of peak VO2 were comparable to the values observed by Michou et al[32] and other values reported in the literature[33].
On the other hand, in addition to improving exercise capacity, supervised exercise programs have also been shown to be effective at improving quality of life of KTRs[42]. In a multicenter study, twelve months of supervised aerobic and resistance training improved physiological variables related to physical fitness and cardiovascular risks without affecting renal function[43]. Supervised combined training was safe and effective in increasing aerobic capacity, muscle strength, and quality of life and improving glucose metabolism in stable liver transplant recipients[44]. Additionally, supervised high-intensity interval training performed during stationary cycling was safe and effective at improving oxygen consumption[34], although the authors reported that participants struggled to achieve the desired intensity. However, some of the analyzed studies reported low adherence despite a lower exercise intensity, with a dropout rate of 45%[45].
In our study, except for the 6-minute walking distance results, both programs significantly improved most of the outcome parameters without any significant superiority to the other programs. This is important, considering that an improvement in exercise parameters (as measured in our study) was closely correlated with a lower risk of death from all causes and morbidity[46].
Finally, a further aspect worthy of mention is the recorded changes in systolic blood pressure. Indeed, in KTRs, the control of arterial hypertension is a fundamental factor in the prevention of adverse cardiovascular and renal outcomes, considering that hypertension is a major contributing factor to graft failure and cardiovascular morbidity and mortality[47]. Among nonpharmacologic treatments, exercise is considered a fundamental lifestyle change in KTRs to control blood pressure[48]. Although not statistically significant, in our sample, a reduction of 5 mmHg in systolic blood pressure was observed in both groups, demonstrating the protective effect of both exercise protocols.
In addition, the psychological aspects of KTRs also need to be considered. First, on the one hand, KTRs reported increased independence toward a “normal life” after transplantation, but on the other hand, they lived with anxiety about their new kidney failure[49]. The improvement of their physical functioning, as obtained in our study after both treatments, may also significantly contribute to controlling cardiovascular risk factors among the modifiable factors responsible for graft failure[50].
This study has several limitations. First, for technical reasons, the study was not previously registered on a public platform. Moreover, the lack of randomization led to baseline imbalances between groups, but on the other hand, the possibility of choosing the exercise program improved participation. Other limitations, including dietary control during the training period, may have affected some outcomes despite all patients receiving tailored dietary indications from the nephrology team.
A 6-month moderate-intensity supervised or low-intensity home-based training program was equally effective at improving exercise capacity in KTRs, likely because of the high adherence of the patients. Gym-based exercise programs are needed to perform combined aerobic and resistance training; however, in-home interval walking options may be proposed for frail patients to improve their functional status.
Further randomized studies with larger samples are needed to confirm our reported preliminary data and define the most appropriate training solution for each patient who underwent kidney transplantation.
| 1. | Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 2022;12:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 1325] [Article Influence: 441.7] [Reference Citation Analysis (0)] |
| 2. | Jang YS, Park YS, Kim H, Hurh K, Park EC, Jang SY. Association between sedentary behavior and chronic kidney disease in Korean adults. BMC Public Health. 2023;23:306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Voora S, Adey DB. Management of Kidney Transplant Recipients by General Nephrologists: Core Curriculum 2019. Am J Kidney Dis. 2019;73:866-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4332] [Cited by in RCA: 3807] [Article Influence: 761.4] [Reference Citation Analysis (0)] |
| 5. | Czyżewski L, Sańko-Resmer J, Wyzgał J, Kurowski A. Assessment of health-related quality of life of patients after kidney transplantation in comparison with hemodialysis and peritoneal dialysis. Ann Transplant. 2014;19:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Schoot TS, Goto NA, van Marum RJ, Hilbrands LB, Kerckhoffs APM. Dialysis or kidney transplantation in older adults? A systematic review summarizing functional, psychological, and quality of life-related outcomes after start of kidney replacement therapy. Int Urol Nephrol. 2022;54:2891-2900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Battaglia Y, Martino E, Piazza G, Cojocaru E, Massarenti S, Peron L, Storari A, Grassi L. Abnormal Illness Behavior, Alexithymia, Demoralization, and Other Clinically Relevant Psychosocial Syndromes in Kidney Transplant Recipients: A Comparative Study of the Diagnostic Criteria for Psychosomatic Research System versus ICD-10 Psychiatric Nosology. Psychother Psychosom. 2018;87:375-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Eckardt KU, Berns JS, Rocco MV, Kasiske BL. Definition and classification of CKD: the debate should be about patient prognosis--a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Kang AW, Garber CE, Eaton CB, Risica PM, Bostom AG. Physical Activity and Cardiovascular Risk among Kidney Transplant Patients. Med Sci Sports Exerc. 2019;51:1154-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Gibson CA, Gupta A, Naik A, Sullivan DK, Doshi M, Backes J, Harvey S, Lee J, Mount R, Valentine H, Shaffer K. Developing a Healthy Lifestyle Program for Recent Kidney Transplant Recipients. Prog Transplant. 2023;33:193-200. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Birdwell KA, Park M. Post-Transplant Cardiovascular Disease. Clin J Am Soc Nephrol. 2021;16:1878-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Janaudis-Ferreira T, Mathur S, Konidis S, Tansey CM, Beaurepaire C. Outcomes in randomized controlled trials of exercise interventions in solid organ transplant. World J Transplant. 2016;6:774-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 13. | Negreanu K, Wang ZQ, Campanelli J, Zappia A, Massierer D, Spahija J, Janaudis-Ferreira T. Inclusion of Exercise Prescription in Solid Organ Transplant in Physical Therapy Curricula Across Canadian Universities: A National Survey. Physiother Can. 2022;74:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | De Smet S, Van Craenenbroeck AH. Exercise training in patients after kidney transplantation. Clin Kidney J. 2021;14:ii15-ii24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Masiero L, Puoti F, Bellis L, Lombardini L, Totti V, Angelini ML, Spazzoli A, Nanni Costa A, Cardillo M, Sella G, Mosconi G. Physical activity and renal function in the Italian kidney transplant population. Ren Fail. 2020;42:1192-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Zhang D, Yu L, Xia B, Zhang X, Liang P, Hu X. Systematic review and meta-analysis of the efficacy of exercise intervention in kidney transplant recipients. World J Urol. 2023;41:3449-3469. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Hannan M, Bronas UG. Barriers to exercise for patients with renal disease: an integrative review. J Nephrol. 2017;30:729-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Goedendorp MM, Hoitsma AJ, Bloot L, Bleijenberg G, Knoop H. Severe fatigue after kidney transplantation: a highly prevalent, disabling and multifactorial symptom. Transpl Int. 2013;26:1007-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Bossola M, Arena M, Urciuolo F, Antocicco M, Pepe G, Calabrò GE, Cianfrocca C, Stasio ED. Fatigue in Kidney Transplantation: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 20. | Calella P, Hernández-Sánchez S, Garofalo C, Ruiz JR, Carrero JJ, Bellizzi V. Exercise training in kidney transplant recipients: a systematic review. J Nephrol. 2019;32:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Whitsett M, Serper M. Exercise Interventions for Transplant Recipients. Curr Transpl Rep. 2021;8:111-117. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Manfredini F, Mallamaci F, D'Arrigo G, Baggetta R, Bolignano D, Torino C, Lamberti N, Bertoli S, Ciurlino D, Rocca-Rey L, Barillà A, Battaglia Y, Rapanà RM, Zuccalà A, Bonanno G, Fatuzzo P, Rapisarda F, Rastelli S, Fabrizi F, Messa P, De Paola L, Lombardi L, Cupisti A, Fuiano G, Lucisano G, Summaria C, Felisatti M, Pozzato E, Malagoni AM, Castellino P, Aucella F, Abd ElHafeez S, Provenzano PF, Tripepi G, Catizone L, Zoccali C. Exercise in Patients on Dialysis: A Multicenter, Randomized Clinical Trial. J Am Soc Nephrol. 2017;28:1259-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 268] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 23. | Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PubMed] |
| 24. | Balke B, Ware RW. The present status of physical fitness in the Air Force. Proj Rep USAF Sch Aviat Med. 1959;59:1-9. [PubMed] |
| 25. | Manfredini F, Conconi F, Malagoni AM, Manfredini R, Basaglia N, Mascoli F, Liboni A, Zamboni P. Training guided by pain threshold speed. Effects of a home-based program on claudication. Int Angiol. 2004;23:379-387. [PubMed] |
| 26. | Pescatello LS, Arena R, Riebe D, Thompson PD. Medicine AC of S. In ACSM's Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; 9th ed.; Jonathan KE; 2014; 72-76. |
| 27. | Whitney SL, Wrisley DM, Marchetti GF, Gee MA, Redfern MS, Furman JM. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the Five-Times-Sit-to-Stand Test. Phys Ther. 2005;85:1034-1045. [PubMed] [DOI] [Full Text] |
| 28. | Chen G, Gao L, Li X. Effects of exercise training on cardiovascular risk factors in kidney transplant recipients: a systematic review and meta-analysis. Ren Fail. 2019;41:408-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Ribeiro PAB, Gradassi M, Martin SM, Leenknegt J, Baudet M, Le V, Pomey MP, Räkel A, Tournoux F. Clinical Implementation of Different Strategies for Exercise-Based Rehabilitation in Kidney and Liver Transplant Recipients: A Pilot Study. Arq Bras Cardiol. 2022;119:246-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Bennett PN, Bohm C, Harasemiw O, Brown L, Gabrys I, Jegatheesan D, Johnson DW, Lambert K, Lightfoot CJ, MacRae J, Meade A, Parker K, Scholes-Robertson N, Stewart K, Tarca B, Verdin N, Wang AY, Warren M, West M, Zimmerman D, Li PK, Thompson S. Physical activity and exercise in peritoneal dialysis: International Society for Peritoneal Dialysis and the Global Renal Exercise Network practice recommendations. Perit Dial Int. 2022;42:8-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Orlandi G, Sofi F, Moscarelli L, Cirami L, Mancini S, Stefani L. Exercise Prescription in Renal Transplant Recipients: From Sports Medicine Toward Multidisciplinary Aspects: A Pilot Study. J Funct Morphol Kinesiol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Michou V, Nikodimopoulou M, Deligiannis A, Kouidi E. Metabolic and functional effects of exercise training in diabetic kidney transplant recipients. World J Transplant. 2022;12:184-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (4)] |
| 33. | Yoo J, Ruppar T, Wilbur J, Miller A, Westrick JC. Effects of Home-Based Exercise on Frailty in Patients With End-Stage Renal Disease: Systematic Review. Biol Res Nurs. 2022;24:48-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Billany RE, Smith AC, Hutchinson GM, Graham-Brown MPM, Nixon DGD, Bishop NC. Feasibility and acceptability of high-intensity interval training and moderate-intensity continuous training in kidney transplant recipients: the PACE-KD study. Pilot Feasibility Stud. 2022;8:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 35. | Vandecruys M, De Smet S, Cornelissen V, Raes J, De Geest S, Naesens M, Leunis S, De Beir J, Vanden Wyngaert K, Nagler E, Glorieux G, Van Biesen W, Calders P, Monbaliu D, Van Craenenbroeck A. MO590: A Home-Based Exercise and Physical Activity Intervention After Kidney Transplantation: Impact of Exercise Intensity. The Phoenix-Kidney Study Protocol. Nephrol Dial Transpl. 2022;37. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Lamberti N, Straudi S, Malagoni AM, Argirò M, Felisatti M, Nardini E, Zambon C, Basaglia N, Manfredini F. Effects of low-intensity endurance and resistance training on mobility in chronic stroke survivors: a pilot randomized controlled study. Eur J Phys Rehabil Med. 2017;53:228-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Malagoni AM, Cavazza S, Ferraresi G, Grassi G, Felisatti M, Lamberti N, Basaglia N, Manfredini F. Effects of a "test in-train out" walking program versus supervised standard rehabilitation in chronic stroke patients: a feasibility and pilot randomized study. Eur J Phys Rehabil Med. 2016;52:279-287. [PubMed] |
| 38. | Lamberti N, Straudi S, Donadi M, Tanaka H, Basaglia N, Manfredini F. Effectiveness of blood flow-restricted slow walking on mobility in severe multiple sclerosis: A pilot randomized trial. Scand J Med Sci Sports. 2020;30:1999-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Lamberti N, Malagoni AM, Ficarra V, Basaglia N, Manfredini R, Zamboni P, Mascoli F, Manfredini F. Structured Home-Based Exercise Versus Invasive Treatment: A Mission Impossible? A Pilot Randomized Study in Elderly Patients With Intermittent Claudication. Angiology. 2016;67:772-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Manfredini F, Traina L, Ficarra V, Gandolfi G, Argentoni A, Straudi S, Gasbarro V, Lamberti N. A "test in-train out" program versus a "go home and walk" intervention for home-based exercise therapy in patients with peripheral artery disease: A randomized controlled trial. Scand J Med Sci Sports. 2024;34:e14584. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Lamberti N, Straudi S, Manfredini R, De Giorgi A, Gasbarro V, Zamboni P, Manfredini F. Don't stop walking: the in-home rehabilitation program for peripheral artery disease patients during the COVID-19 pandemic. Intern Emerg Med. 2021;16:1307-1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Lima PS, de Campos AS, de Faria Neto O, Ferreira TCA, Amorim CEN, Stone WJ, Prestes J, Garcia AMC, Urtado CB. Effects of Combined Resistance Plus Aerobic Training on Body Composition, Muscle Strength, Aerobic Capacity, and Renal Function in Kidney Transplantation Subjects. J Strength Cond Res. 2021;35:3243-3250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Roi GS, Mosconi G, Totti V, Angelini ML, Brugin E, Sarto P, Merlo L, Sgarzi S, Stancari M, Todeschini P, La Manna G, Ermolao A, Tripi F, Andreoli L, Sella G, Anedda A, Stefani L, Galanti G, Di Michele R, Merni F, Trerotola M, Storani D, Nanni Costa A. Renal function and physical fitness after 12-mo supervised training in kidney transplant recipients. World J Transplant. 2018;8:13-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Totti V, Tamè M, Burra P, Mosconi G, Roi GS, Sella G, Ermolao A, Ferrarese A, Sgarzi S, Savino G, Parodi G, Poggioli G, Ricchiuti A, Di Michele R, Trerotola M, Nanni Costa A. Physical Condition, Glycemia, Liver Function, and Quality of Life in Liver Transplant Recipients After a 12-Month Supervised Exercise Program. Transplant Proc. 2019;51:2952-2957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Kastelz A, Fernhall B, Wang E, Tzvetanov I, Spaggiari M, Shetty A, Gallon L, Hachaj G, Kaplan B, Benedetti E. Personalized physical rehabilitation program and employment in kidney transplant recipients: a randomized trial. Transpl Int. 2021;34:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Chan W, Chin SH, Whittaker AC, Jones D, Kaur O, Bosch JA, Borrows R. The Associations of Muscle Strength, Muscle Mass, and Adiposity With Clinical Outcomes and Quality of Life in Prevalent Kidney Transplant Recipients. J Ren Nutr. 2019;29:536-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 47. | Pisano A, Mallamaci F, D'Arrigo G, Bolignano D, Wuerzner G, Ortiz A, Burnier M, Kanaan N, Sarafidis P, Persu A, Ferro CJ, Loutradis C, Boletis IN, London G, Halimi JM, Sautenet B, Rossignol P, Vogt L, Zoccali C. Blood pressure monitoring in kidney transplantation: a systematic review on hypertension and target organ damage. Nephrol Dial Transplant. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Rebelo RNS, Rodrigues CIS. Arterial hypertension in kidney transplantation: huge importance, but few answers. J Bras Nefrol. 2023;45:84-94. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 49. | Antoun J, Brown DJ, Clarkson BG, Shepherd AI, Sangala NC, Lewis RJ, McNarry MA, Mackintosh KA, Corbett J, Saynor ZL. Experiences of adults living with a kidney transplant-Effects on physical activity, physical function, and quality of life: A descriptive phenomenological study. J Ren Care. 2023;49:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 50. | Pinto-Ramirez J, Garcia-Lopez A, Salcedo-Herrera S, Patino-Jaramillo N, Garcia-Lopez J, Barbosa-Salinas J, Riveros-Enriquez S, Hernandez-Herrera G, Giron-Luque F. Risk factors for graft loss and death among kidney transplant recipients: A competing risk analysis. PLoS One. 2022;17:e0269990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |