Published online Sep 18, 2024. doi: 10.5500/wjt.v14.i3.96225
Revised: June 6, 2024
Accepted: July 4, 2024
Published online: September 18, 2024
Processing time: 92 Days and 3.3 Hours
Tuberculosis (TB) is the leading cause of infectious mortality and morbidity in the world, second only to coronavirus disease 2019. Patients with chronic kidney disease and kidney transplant recipients are at a higher risk of developing TB than the general population. Active TB is difficult to diagnose in this population due to close mimics. All transplant candidates should be screened for latent TB infection and given TB prophylaxis. Patients who develop active TB pre- or post-trans
Core Tip: The management of tuberculosis (TB) in transplant candidates and recipients is evolving rapidly. The use of new techniques for detecting TB infection (TBI) and the adoption of TBI screening by developing countries is bringing the world closer to the World Health Organization's goal of ending the TB epidemic by 2030. Literature regarding the acceptance of kidney transplant candidates and donors after intensive phase antitubercular therapy (ATT) is now available. Fluoroquinolone-based nonri
- Citation: Prasad P, Sharma S, Mohanasundaram S, Agarwal A, Verma H. Tuberculosis in kidney transplant candidates and recipients. World J Transplant 2024; 14(3): 96225
- URL: https://www.wjgnet.com/2220-3230/full/v14/i3/96225.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i3.96225
Tuberculosis (TB), which is a curable and preventable disease, is the second most common infectious cause of mortality after coronavirus disease 2019 (COVID-19). It affects close to 10 million people per year[1].
Despite the diagnosis of TB often being a diagnostic dilemma in kidney disease patients, kidney transplant candidates (KTC) and kidney transplant recipients (KTR) have a 3.62- and 11.35 times higher risk of developing TB, respectively, compared to the general population[2]. They also have a higher rate of mortality due to TB. Treatment of TB also poses unique challenges in these patients due to renal dose modifications, drug interactions, and nephrotoxicity of anti
The incidence of TB in patients with chronic kidney disease (CKD) ranges between 60-19, 270 per 100000 population in various countries (highest incidence in the African region and lowest in the Americas), the pooled incidence being 3718 per 100000[3]. In general, extrapulmonary TB is more common than pulmonary TB in this population[2,3]. Amongst patients with CKD, those on dialysis, who are conventionally considered transplant candidates, are at a higher risk of developing TB as compared to earlier stages of CKD. Patients on hemodialysis have a higher incidence than those on peritoneal dialysis (5611/100000 vs 3533/100000 respectively)[3].
TB incidence is said to be 7-27 times higher than the general population in solid organ transplant recipients[4]. KTR have a 4.59 times higher risk of developing TB compared to the general population[5]. The incidence of TB in KTR was 2700/100000 population in a pooled systemic analysis[3] from across the world with a range of 340-14680/100000[6,7].
The primary transmission route of Mycobacterium tuberculosis (M. tuberculosis) is through aerosols, with the lungs being the primary site of host-pathogen interaction. The innate immune system tends to clear the M. tuberculosis bacilli immediately through phagocytosis. However, there is a possibility of the following four distinct outcomes because of complex host-pathogen interplay[8]: (1) Immediate clearance of bacilli; (2) Chronic or latent infection; (3) Rapidly progressive TB; or (4) Reactivation after a prolonged period.
If the bacilli are not removed immediately, granulomas are formed, where inflammatory cells and cytokines come together and generate a localized response, known as the "Ghon's complex". It includes organ parenchymal involvement along with regional adenopathy. Effective cell-mediated immunity usually develops in 4-6 weeks and halts further infection progression[8].
When the host cannot produce a sufficient cell-mediated immune response, the infection spreads and destroys the tissue. Arterial erosion promotes hematogenous spread, which results in disseminated TB that eventually affects multiple organs.
In immunocompromised states, there may be a reactivation of M. tuberculosis CKD, specifically kidney failure, is one such condition where reactivation of previous infection is the most common cause of TB. Earlier, this reactivation was typically limited to a single organ, the most common site being the upper lobe of the lung[8]. However, now extrapulmonary TB is seen to be more common in these patients. Extrapulmonary involvement can affect various other organs and appear with a myriad of clinical symptoms. Almost every organ being involved has been described, including the musculoskeletal system, gastrointestinal tract, liver, skin, orbit, genitourinary tract, lymph nodes, pericardium, larynx, kidneys, and adrenal glands[8,9].
Because of the immunosuppression, the natural history of TB infection is more complex in transplant patients. In developing countries, reactivation from previously acquired infections is more common than re-infection[8,9]. With a median time of onset of 9 months, most active TB cases are recognized during the first year post-transplantation[10-13]. Also, although pulmonary TB is the most common presentation in KTR, they are more likely to develop extrapulmonary TB compared to the general population[12,14,15].
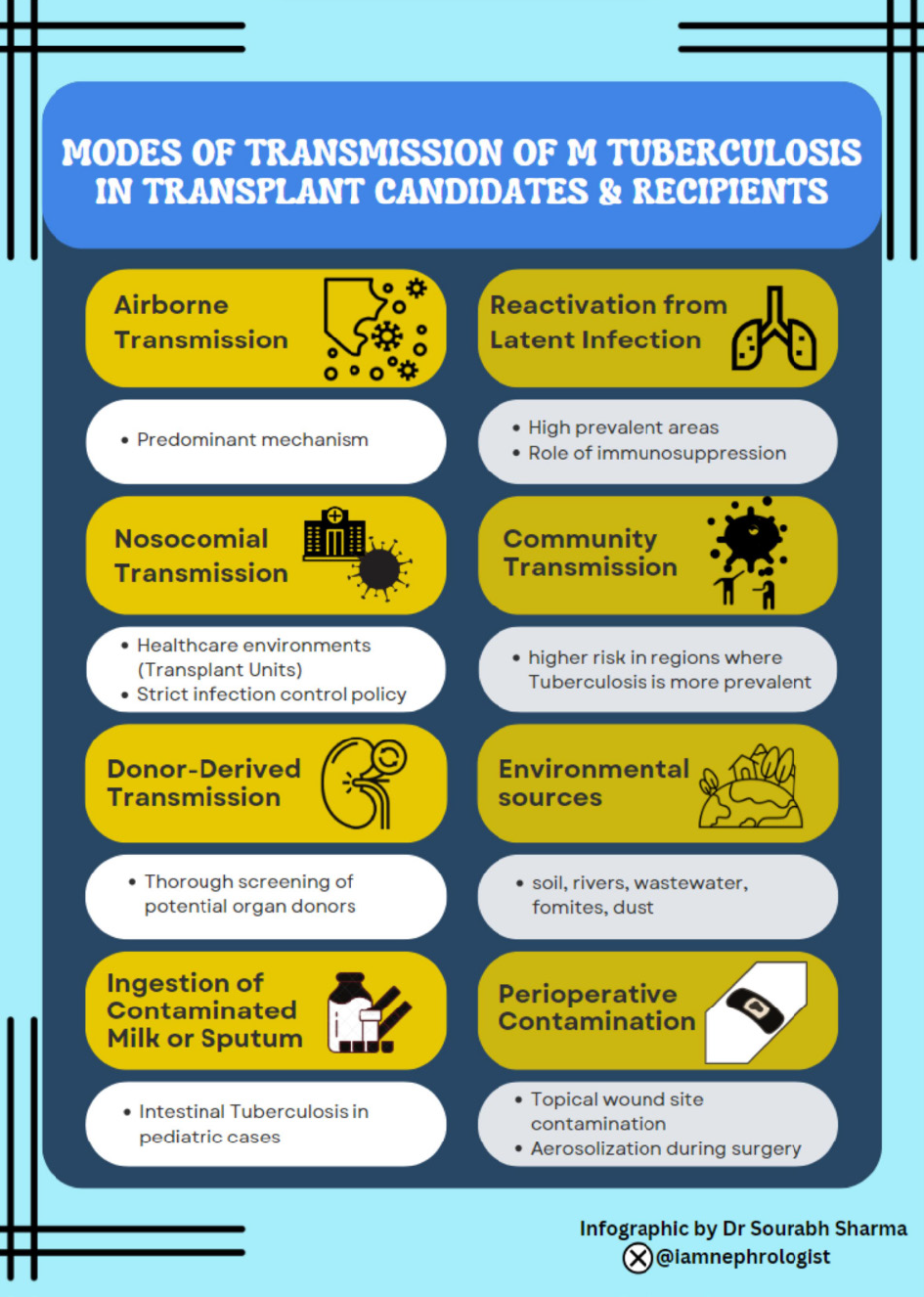
For primary prevention, early diagnosis, and prompt treatment, understanding the various modes of transmission of TB is crucial. The various modes of transmission among transplant candidates and recipients are illustrated in Figure 1 and enlisted below[16,17]: (1) Airborne transmission: Aerosol transmission remains the predominant mechanism, particularly in enclosed and congested environments; (2) Reactivation from latent infection: In areas where TB is highly prevalent, reactivation of latent TB is a frequent mechanism of transmission; (3) Nosocomial transmission: The possibility of nosocomial transmission is a worry in healthcare environments. Strict infection control procedures are necessary in transplant units, where immunocompromised patients are concentrated, to stop TB from spreading among recipients; (4) Donor-derived transmission: Rarely, transmission can occur directly from the donor organ. Thorough screening of potential organ donors is essential to avoid unintentionally spreading TB during transplant procedures; and (5) Unusual routes of transmission: Environmental sources have been reported to host viable and infectious TB for long periods. These sources include soil, rivers, wastewater, fomites, dust, and even cadavers. There have been reports of TB transmission through topical wound site contamination, aerosolization during surgery, and intake of water tainted with sanatorium effluent. Also, the incidence of pediatric cases due to intestinal TB is showing an increasing trend, probably due to the ingestion of contaminated milk or sputum[16].
The probability that an individual with TB will transmit M. tuberculosis to others is determined by many factors, including the number and rate of infectious droplet production and virulence of the disease of the original host who transmits the infection[18]. Environmental factors include duration and extent of contact. Better air circulation and increased ultraviolet (UV) light exposure in the space of contact decrease the chances of transmission. Host factors include the type of induction and maintenance immunosuppression among transplant patients[18].
The various risk factors for TB can be stratified as those related to transplant candidates and those related to recipients (Table 1)[9].
| Risk factors in transplant candidates | Risk factors in transplant recipients |
| Presence of comorbidities: Diabetes mellitus, COPD | Donor-derived tuberculosis |
| Smoking | Co-existing infections: COVID-19, HIV, Nocardiosis, Pneumocystis jirovecii, CMV |
| Transplant candidate age: Young and Elderly | Induction: Anti-T-lymphocyte antibodies or OKT3 |
| Immunomodulatory states: Chronic HCV infection, CMV infection | Maintenance immunosuppression: Antiproliferative agents (MMF); Prednisolone-Azathioprine; immunosuppression; Everolimus |
| Chronic liver disease | Graft rejection: Intensification of immunosuppression |
| Dialysis vintage | Genetic factors: HLA A68(28)/A69(28) locus (in Indian sub-population) |
| Previous history of tuberculosis or TBI: Positive tuberculin test or chest X-ray; suggestive of previous infection | — |
| Family history of tuberculosis | — |
| Other co-existing infections: COVID19, HIV | — |
A history of previous TB exposure (positive tuberculin test or residual lesions in chest X-ray) is the foremost risk factor in transplant candidates. Post-transplantation TB is known to be associated with the recipient's age, dialysis vintage, various comorbidities like diabetes mellitus, chronic obstructive pulmonary disease, chronic liver disease, various immunomodulatory conditions like Hepatitis C virus or cytomegalovirus (CMV) infection, and the degree of immu
It would seem logical to consider other factors- such as smoking, malnourishment, or co-existence of other infections like COVID-19[16], CMV, mycoses, and human immunodeficiency virus (HIV) infection- that are linked to an elevated risk of TB in the general population as significant risk factors in transplant patients. More research is needed on genetic risk factors, such as the human leukocyte antigen A68 (28)/A69(28) locus, which in the Indian population appears to predispose to post-transplantation TB[26].
TB infection (TBI) (earlier called "Latent TB") is defined by the World Health Organization (WHO) as a state of persistent immune response to stimulation by M. tuberculosis antigens with no evidence of clinically manifested active TB disease[27]. The term “Latent TB” was discarded because TBI cannot always be considered dormant. In high-income countries, the baseline risk of reactivation of latent TBI varies between 6 and 20 per 100000 persons per year[28]. Patients on immunosuppression- especially solid organ transplant recipients and patients on dialysis are recognized to be at high risk for reactivation of TBI into active TB. The incidence of TBI in patients with CKD varies between 25.3% to 46%, and that in solid organ transplant recipients varies from 9-20%[4]. However, a recent study from China demonstrated a higher prevalence of interferon-gamma release assay (IGRA) positivity in transplant recipients vs candidates (20% vs 10%). It also showed a higher rate of IGRA conversion from negative to positive in recipients vs candidates (20% vs 5.5%) over a 2-year follow-up[29]. The risk of developing active TB from a TBI is higher in patients treated with anti-rejection therapy[28].
The RESITRA (Spanish Network of Infection in Transplantation) cohort found a relative risk of 4.3 for symptomatic TB[9,10] when the pure protein derivative (PPD) test was positive rather than negative. Of note is that this study was conducted in a low-prevalence country. Therefore, only 19% of patients in this study had a positive PPD pre-transplantation, and only 0.9% developed TB post-transplantation[10].
The WHO recommends three tests for screening for TBI. These include the tuberculin skin test (TST) and two types of IGRA, QuantiFERON-TB, Gold In-Tube (Cellestis, Australia) and T-SPOT (Oxford Immunotec, United Kingdom)[27]. It is recommended to use these for TBI screening in those with a high risk of developing active TB, including high-risk groups like organ transplant candidates and recipients in both high and lower-middle-income countries (LMIC). However, they should not be used for diagnosing active TB in LMIC. For detecting active TB, the sensitivity of IGRA ranges between 73% to 83% and specificity from 49% to 58%. Hence, almost 25% of patients with culture-confirmed TB in LMIC may be negative for IGRA, precluding it as a test for diagnosing active TB[27].
TST may yield unreliable results in patients receiving immunosuppressive medications, or it may be inaccurate in those with advanced chronic renal disease. When identifying TBI in immunocompromised patients, IGRAs have a high specificity as they are more specific to M. tuberculosis antigens[28]. However, the sensitivity and specificity of IGRAs and TST in the screening of LTBI in recipients of renal transplants are poorly studied. Additionally, there is a lack of valid and accurate reference standards for IGRA and TST in the immunosuppressed population. False positives may lead to unnecessary testing and delay in transplantation in candidates, and false negatives may lead to a missed diagnosis and reactivation of TB in the post-transplant period[29].
Apart from TST, new skin-based tests using early secretory antigen 6kDa and culture filtrate protein-10 have been developed, including Cy-Tb (Serum Institute of India, India), Diaskintest® (Generium, Russian Federation), and C-TST (formerly known as ESAT6-CFP10 test, Anhui Zhifei Longcom, China) which are endorsed by the WHO for further analysis on their performance. Although studies are ongoing to confirm this, they may have similar specificity as IGRA and more reliability than TST[27].
A review of guidelines for TBI screening in high-risk settings, including organ transplantation (solid organ + stem cell) from various countries, highlighted that most guidelines recommend screening for TBI in patients starting immunosuppression or were highly likely to start immune suppression[30]. Most guidelines include a two-step algorithm using both TST and IGRA, where IGRA is performed only for TST-negative cases, whereas others recommend IGRA alone. Some guidelines published prior to 2011 recommend TST alone for TBI screening. The two-step algorithm may give a false positive TST in patients who have received BCG vaccination but is seen to improve the TBI detection rates overall[30]. Annual repetition of IGRA testing has also been suggested by some guidelines in patients receiving tumor necrosis factor inhibitor therapy[31] but not in transplantation. IGRA has been recommended in patients with skin disease in whom performing TST may be difficult. In areas with high endemicity, the cost-benefit ratio of screening for TBI and the use of IGRA/TST for screening has been called into question[32]. A guideline from South Asia, for example, recommends that TBI screening in endemic countries should be done in those with a history of TB in close contact or with a history of inadequately treated TB but does not recommend large-scale screening for all donors and candidates[32].
TST positivity is variably defined as positive at a 5 mm cutoff[33-35] or between 6-20 mm[36] cutoff in different guidelines. Some guidelines recommend repeat TST testing at 8-12 weeks when initially negative[37].
Given that TB may have a direct impact on allograft function, it is critical to take all necessary precautions to prevent TBI in this specific patient category. Prior to transplantation, every attempt should be taken to identify and treat active TB and TBI in transplant candidates. Once TBI is detected, active TB must be ruled out before starting therapy for TBI.
The following options are recommended for the treatment of TBI by WHO in 2020 regardless of HIV status[38]: either 6 months or 9 months of daily isoniazid; a 3-month regimen of daily isoniazid plus rifampicin[39,40]; or a 3-month regimen of weekly rifapentine plus isoniazid[41,42]. A 1-month regimen of daily rifapentine plus isoniazid[43] or 4 months of daily rifampicin alone[44] may also be offered as alternatives. Alternative regimens such as ethambutol plus fluoroquinolone (levofloxacin or moxifloxacin) may be considered for those who do not tolerate standard regimens[35].
Rifampicin interacts with calcineurin inhibitors and is associated with high rates of graft dysfunction and rejection[45,46]. Hence, isoniazid monotherapy for 6-9 months may be considered for TBI treatment in transplant recipients[9,33,35]. The efficacy of the 6H (6 months of therapy with isoniazid monotherapy) regimen is 64% in patients with positive TST. Retrospective data analysis of studies from the 1950s and 1960s done in the United States shows that the benefit of isoniazid increases when given for up to 9–10 months, leading to an efficacy of approximately 90%[47]. Hence, the American Society of Transplantation recommends against using a 6H regime in transplant patients[35]. Most of the trials for TBI prevention have been done in non-transplant patients, predominantly in the HIV population, and efficacy data of different regimens in transplant candidates or recipients is sparse[38]. Co-administration with pyridoxine 20-50 mg once daily must be given in all patients receiving isoniazid therapy[30,35]. Close monitoring of liver function tests (2-week intervals for 6 weeks and then monthly) and for symptoms of peripheral neuropathy should be done in all patients[30,35].
Transplant candidates should be treated as early as possible and should ideally complete therapy before tran
Universal isoniazid prophylaxis in KTR has been used in endemic countries to prevent reactivation in the post-transplantation period. A systematic review of universal prophylaxis in transplant candidates demonstrated a lower risk of active TB (relative risk: 0.35, 95%CI: 0.27–0.45, P < 0.01) in the isoniazid treatment group than in those without prophylaxis, without any difference in mortality, acute rejection rates and hepatotoxicity[48]. Further studies are required to know the benefits or harms of treatment of TBI in transplant recipients in endemic countries[32].
TB in transplant recipients is more likely to occur at disseminated sites or be extrapulmonary TB as compared to the general population[12,32]. Disseminated infection is more common with orthoclone muromonab-CD3 or other anti-T cell therapy[12]. Atypical presentations like pyrexia of unknown origin, pyomyositis, cutaneous ulcers, or tenosynovitis may occur. Ninety-one% of patients with disseminated TB and sixty-four% with localized TB present with fever. In pulmonary TB, cavitary changes are infrequent (< 5%), and patients may present with a focal infiltrate, miliary pattern, nodules, pleural effusions, or diffuse interstitial infiltrates[12].
TB in transplant recipients most often occurs in the first 6 months after transplantation, with 95% of cases occurring in the first year after transplantation[10]. It may occur earlier in patients with donor-derived TB (median of 2-3 months post-transplantation)[49,50].
Samples from unusual sites of involvement and even sputum may not have a positive acid-fast bacilli stain, and there may be a delay in the confirmation of diagnosis. Therefore, for an early and prompt diagnosis, quick diagnostic techniques like adenosine deaminase and rapid molecular diagnostic tests (i.e. GeneXpert/RIF or TB polymerase chain reaction-PCR) should be performed[10,32]. Patients who lose significant weight, have pyrexia, and have little sputum should be the subject of a thorough evaluation. For these patients, bronchoalveolar lavage and computed tomography of the chest should be performed[10].
Active TB is a contraindication to transplantation. Any transplant candidate who is detected to have TB during investigations for transplantation should be treated with combination antitubercular therapy (ATT) without delay, and transplantation should be deferred until the treatment of the disease. The optimal duration of treatment for active TB before proceeding with transplantation remains controversial, especially in endemic areas[32]. In South Asia, it is a common practice to accept candidates for transplantation after 8 weeks of intensive phase therapy[32]. A retrospective analysis of data from two centers following this practice found that it was safe with regard to TB reactivation/flare[51,52]. Patient and graft survival was comparable to other KTR[52]. Guidelines, however, recommend that, wherever possible, treatment should be completed in the pretransplant period itself[32].
Organs should not be accepted from living or deceased donors with active TB[35]. Here again, the duration of therapy before transplantation is different in endemic countries vs low-prevalence countries. Whereas completion of therapy is considered mandatory in low prevalence countries, the South Asian guidelines recommend at least 2 months of intensive treatment (4 drugs therapy including rifampicin) with documentation of clinical and radiological response in donor before proceeding for transplantation[32]. In case of an incomplete response in the donor, either clinically or radiologically, this period may be extended, and the recipient should be given isoniazid prophylaxis for 6 months[32]. The recipient should be kept under close clinical observation for evidence of donor-derived TB in the graft or elsewhere.
TB is treated with combination ATT in an intensive bactericidal phase focused on mycobacterial clearance and continuation phase to prevent disease relapse[53]. In patients with reduced glomerular filtration rate, as is the case in KTC and often in recipients, anti-tuberculous drugs should be used with dose modification and interval based on estimated glomerular filtration rate (eGFR) for renally eliminated drugs (Table 2), including ethambutol, pyrazinamide, and aminoglycosides[54,55]. Guidelines recommend the same duration of ATT in CKD as in the general population, i.e. a minimum of 6 months for drug-sensitive pulmonary TB[54,55]. Despite this, in practice, a longer duration of therapy is often given in solid organ transplant recipients and CKD, especially if second-line drugs are used or in the presence of disseminated disease[33,35,56,57].
| Drug | eGFR > 60 | eGFR 30-60 | 10-30 | < 10 | Maintenance hemodialysis |
| Isoniazid1 | 5 mg/kg/day | No dose adjustment | No dose adjustment | No dose adjustment | No dose adjustment |
| Rifampicin1 | 10 mg/kg/day | No dose adjustment | No dose adjustment | No dose adjustment | No dose adjustment |
| Pyrazinamide2 | 25 mg/kg/day | No dose adjustment | 25 mg/kg/dose every 48 h | 25 mg/kg/dose three times/week | 25 mg/kg/dose three times/week |
| Ethambutol3 | 15 mg/kg/day | No dose adjustment is required | 15 mg/kg/day every 48 h | 15 mg/kg/day every 48 h | 15 mg/kg 3 times/week after dialysis sessions |
| Streptomycin | 15 mg/kg/day | 15 mg/kg individual doses with intervals between doses adjusted to achieve undetectable plasma trough levels | |||
| Levofloxacin | 750-1000 mg/day | 750-1000 mg 3 times/week | 750-1000 mg 3 times/week | 750-1000 mg 3 times/week | 750-1000 mg 3 times/week |
| Moxifloxacin4 | 400 mg/day | 400 mg/day | 400 mg/day | 400 mg/day | 400 mg/day |
Pyridoxine should be given to all patients receiving isoniazid to prevent the development of peripheral neuropathy. The dose recommended is 25 mg per day[54], but some authors suggest giving higher doses of up to 200 mg/day in patients with reduced eGFR[53]. High dose pyridoxine itself can cause neuropathy and is speculated to decrease the antitubercular effect of isoniazid[58,59].
Drug interactions are an essential factor to consider before starting ATT in transplant recipients. Rifampicin, a first-line agent in ATT, is a potent CYP3A4 inducer. It also induces uridine diphosphate glucuronosyltransferases, glutathione S-transferases, and monoamine oxidases, which are involved in drug metabolism[60]. Both calcineurin inhibitors (tacrolimus, cyclosporine) and mammalian target of rapamycin inhibitor levels may decrease with rifampicin therapy[61,62]. It also decreases levels of mycophenolic acid (two-fold reduction in dose-corrected exposure) and prednisolone[60,63,64]. Rifampicin-based ATT has been associated with an increased risk of rejections, graft dysfunction, and allograft failure[45,48,65] and may require an increase in dose up to 3.8 times the baseline dose[66]. Up to a 6-fold increase in sirolimus levels may be needed to maintain trough levels with rifampicin[62]. In general, the level should be titrated in individual patients with close monitoring of trough levels after initiation of rifampicin. Although rifabutin is a less potent inducer than rifampicin, an increase in tacrolimus dose up to 1.5 times the baseline dose when initiated in transplant recipients[67,68] may still be required. The rejection rates were higher in the rifampicin arm vs the rifabutin arm in one retrospective study of liver transplant recipients, but this did not achieve statistical significance[68].
To mitigate the risk of graft dysfunction and graft loss post-ATT, fluoroquinolone-based nonrifamycin ATT has been used post-transplantation with promising results[56,65,67]. The newer generation fluoroquinolones (moxifloxacin, gatifloxacin, and levofloxacin) have better efficacy compared to older agents (ciprofloxacin, ofloxacin) and hence should be preferred agents for transplant recipients with TB[69,70]. Also, it has been shown that fluoroquinolones, especially ofloxacin and ciprofloxacin, raise cyclosporine levels in the blood, although this effect is less prominent with newer agents like levofloxacin[71,72].
TB is associated with a high morbidity and significant reduction in patient and graft survival[14,15]. Mortality and morbidity are higher in patients with disseminated disease[15]. In a case series, 85% of patients post kidney trans
Effective prevention and management of TB in transplant candidates and recipients depend critically on knowledge of the transmission mechanisms. A sophisticated strategy is needed to reduce the risk in situations including donor-derived cases, airborne transmission, reactivation, and other factors. The cornerstones of preventive interventions include donor evaluation, pre-transplant screening, and stringent infection control protocols. In settings with a high risk of TB transmission, to protect transplant candidates, donors, and recipients, the use of germicidal UV disinfection systems and ventilation systems, including natural ventilation, mechanical ventilation, and use of high-efficiency particulate air filters should be considered[27].
Although TB is a significant cause of morbidity and mortality in transplant recipients and candidates, the current available TB vaccine (Bacillus Calmette–Guérin or BCG vaccine) is not recommended in this population since it is a live vaccine. Disseminated BCGosis or ulcerative necrotic lesions at the local site of injection have been reported even in patients with primary immunodeficiency disorder[74].
New candidate vaccines in the pipeline include whole-cell vaccines, adjuvanted proteins, and recombinant subunit vector vaccines. An investigational TB vaccine candidate (M72/AS01E) has recently shown 50% efficacy in preventing the reactivation of TBI over 3 years in non-HIV patients[75]. This trial did not mention the percentage of patients with CKD or on immunosuppression. Data regarding the safety of this vaccine in preventing reactivation in the post-transplant period is not available.
Ending the TB epidemic by 2030 is one of the United Nations' sustainable development goals. The WHO has also termed the era of 2023-2027 as the “FIND.TREAT.ALL#EndTB" era. Research priorities in the field of TB include developing a vaccine to reduce the risk of infection, a vaccine or new drug treatment to cut the risk of TB disease in people already infected, rapid diagnostic tests (point-of-care) for accurate detection of TB disease and shorter treatments for TB disease[76].
TB is an important opportunistic infection to look out for in KTC and recipients. It may present with atypical features in this population, leading to delayed diagnosis. Treatment of TBI and active TB disease is imperative to prevent patient morbidity and mortality. Combination ATT should be given in all patients diagnosed with TB based on eGFR. New vaccines are needed to decrease the risk of TB in the post-transplant period.
| 1. | WHO. Tuberculosis. https://www.who.int/news-room/fact-sheets/detail/tuberculosis#. |
| 2. | Al-Efraij K, Mota L, Lunny C, Schachter M, Cook V, Johnston J. Risk of active tuberculosis in chronic kidney disease: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19:1493-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Alemu A, Bitew ZW, Diriba G, Seid G, Eshetu K, Chekol MT, Berhe N, Gumi B. Tuberculosis incidence in patients with chronic kidney disease: a systematic review and meta-analysis. Int J Infect Dis. 2022;122:188-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Shu CC, Tsai MK, Lin SW, Wang JY, Yu CJ, Lee CY. Latent Tuberculosis Infection Increases in Kidney Transplantation Recipients Compared With Transplantation Candidates: A Neglected Perspective in Tuberculosis Control. Clin Infect Dis. 2020;71:914-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Lopez de Castilla D, Schluger NW. Tuberculosis following solid organ transplantation. Transpl Infect Dis. 2010;12:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Basiri A, Hosseini-Moghaddam SM, Simforoosh N, Einollahi B, Hosseini M, Foirouzan A, Pourrezagholi F, Nafar M, Zargar MA, Pourmand G, Tara A, Mombeni H, Moradi MR, Afshar AT, Gholamrezaee HR, Bohlouli A, Nezhadgashti H, Akbarzadehpasha A, Ahmad E, Salehipour M, Yazdani M, Nasrollahi A, Oghbaee N, Azad RE, Mohammadi Z, Razzaghi Z. The risk factors and laboratory diagnostics for post renal transplant tuberculosis: a case-control, country-wide study on definitive cases. Transpl Infect Dis. 2008;10:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Vachharajani T, Abreo K, Phadke A, Oza U, Kirpalani A. Diagnosis and treatment of tuberculosis in hemodialysis and renal transplant patients. Am J Nephrol. 2000;20:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Sundaram M, Adhikary SD, John GT, Kekre NS. Tuberculosis in renal transplant recipients. Indian J Urol. 2008;24:396-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Aguado JM, Torre-Cisneros J, Fortún J, Benito N, Meije Y, Doblas A, Muñoz P. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 10. | Torre-Cisneros J, Doblas A, Aguado JM, San Juan R, Blanes M, Montejo M, Cervera C, Len O, Carratala J, Cisneros JM, Bou G, Muñoz P, Ramos A, Gurgui M, Borrell N, Fortún J, Moreno A, Gavalda J; Spanish Network for Research in Infectious Diseases. Tuberculosis after solid-organ transplant: incidence, risk factors, and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis. 2009;48:1657-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Yehia BR, Blumberg EA. Mycobacterium tuberculosis infection in liver transplantation. Liver Transpl. 2010;16:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998;27:1266-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 405] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 13. | Muñoz P, Rodríguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis. 2005;40:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Ou SM, Liu CJ, Teng CJ, Lin YT, Chang YS, Chiang SC, Tzeng CH, Chen TJ. Impact of pulmonary and extrapulmonary tuberculosis infection in kidney transplantation: a nationwide population-based study in Taiwan. Transpl Infect Dis. 2012;14:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Meinerz G, da Silva CK, Goldani JC, Garcia VD, Keitel E. Epidemiology of tuberculosis after kidney transplantation in a developing country. Transpl Infect Dis. 2016;18:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Coleman M, Martinez L, Theron G, Wood R, Marais B. Mycobacterium tuberculosis Transmission in High-Incidence Settings-New Paradigms and Insights. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Aguado JM, Silva JT, Samanta P, Singh N. Tuberculosis and Transplantation. Microbiol Spectr. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Mathema B, Andrews JR, Cohen T, Borgdorff MW, Behr M, Glynn JR, Rustomjee R, Silk BJ, Wood R. Drivers of Tuberculosis Transmission. J Infect Dis. 2017;216:S644-S653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 19. | Basiri A, Moghaddam SM, Simforoosh N, Einollahi B, Hosseini M, Foirouzan A, Pourrezagholi F, Nafar M, Zargar MA, Pourmand G, Tara A, Mombeni H, Moradi MR, Taghizadeh A, Gholamrezaee HR, Bohlouli A, Nezhadgashti H, Amirzadehpasha A, Ahmad E, Salehipour M, Yazdani M, Nasrollahi A, Falaknazi K, Mahdavi MR, Shamsa A, Feizzadeh B, Mojahedi MJ, Oghbaee N, Azad RE, Mohammadi Z. Preliminary report of a nationwide case-control study for identifying risk factors of tuberculosis following renal transplantation. Transplant Proc. 2005;37:3041-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Torres J, Aguado JM, San Juan R, Andrés A, Sierra P, López-Medrano F, Morales JM. Hepatitis C virus, an important risk factor for tuberculosis in immunocompromised: experience with kidney transplantation. Transpl Int. 2008;21:873-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | John GT, Shankar V, Abraham AM, Mukundan U, Thomas PP, Jacob CK. Risk factors for post-transplant tuberculosis. Kidney Int. 2001;60:1148-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Hall CM, Willcox PA, Swanepoel CR, Kahn D, Van Zyl Smit R. Mycobacterial infection in renal transplant recipients. Chest. 1994;106:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Meyers BR, Halpern M, Sheiner P, Mendelson MH, Neibart E, Miller C. Tuberculosis in liver transplant patients. Transplantation. 1994;58:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Müllerova M, Pekárek J, Nouza K, Svejcar J, Trnka L, Matousek V. Immunosuppression and experimental tuberculosis. II. The effects of immunosuppressive agents and antilymphocyte serum on the organ dissemination of mycobacteria and on the development of specific delayed hypersensitivity. Biomedicine. 1974;20:390-397. [PubMed] |
| 25. | Guirao-Arrabal E, Santos F, Redel-Montero J, Vaquero JM, Cantisán S, Vidal E, Torre-Giménez Á, Rivero A, Torre-Cisneros J. Risk of tuberculosis after lung transplantation: the value of pretransplant chest computed tomography and the impact of mTOR inhibitors and azathioprine use. Transpl Infect Dis. 2016;18:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | John GT, Murugesan K, Jeyaseelan L, Pulimood RB, Jacob CK, Shastry JC. HLA phenotypes in Asians developing tuberculosis on dialysis or after renal transplantation. Natl Med J India. 1995;8:144, 146. [PubMed] |
| 27. | WHO. WHO operational handbook on tuberculosis: module 1: prevention: infection prevention and control. 2023. Available from: https://www.who.int/publications/i/item/9789240078154. |
| 28. | Guirao-Arrabal E, Torre-Cisneros J. Tuberculin skin test, Interferon gamma release assays or just chest x-ray to study latent tuberculosis before solid organ transplantation? Transpl Infect Dis. 2018;20:e12920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Sester M, van Leth F, Bruchfeld J, Bumbacea D, Cirillo DM, Dilektasli AG, Domínguez J, Duarte R, Ernst M, Eyuboglu FO, Gerogianni I, Girardi E, Goletti D, Janssens JP, Julander I, Lange B, Latorre I, Losi M, Markova R, Matteelli A, Milburn H, Ravn P, Scholman T, Soccal PM, Straub M, Wagner D, Wolf T, Yalcin A, Lange C; TBNET. Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am J Respir Crit Care Med. 2014;190:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 30. | Hasan T, Au E, Chen S, Tong A, Wong G. Screening and prevention for latent tuberculosis in immunosuppressed patients at risk for tuberculosis: a systematic review of clinical practice guidelines. BMJ Open. 2018;8:e022445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (2)] |
| 31. | Nordgaard-Lassen I, Dahlerup JF, Belard E, Gerstoft J, Kjeldsen J, Kragballe K, Ravn P, Sørensen IJ, Theede K, Tjellesen L; Danish Society for Gastroenterology. Guidelines for screening, prophylaxis and critical information prior to initiating anti-TNF-alpha treatment. Dan Med J. 2012;59:C4480. [PubMed] |
| 32. | Bansal SB, Ramasubramanian V, Prasad N, Saraf N, Soman R, Makharia G, Varughese S, Sahay M, Deswal V, Jeloka T, Gang S, Sharma A, Rupali P, Shah DS, Jha V, Kotton CN. South Asian Transplant Infectious Disease Guidelines for Solid Organ Transplant Candidates, Recipients, and Donors. Transplantation. 2023;107:1910-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Meije Y, Piersimoni C, Torre-Cisneros J, Dilektasli AG, Aguado JM; ESCMID Study Group of Infection in Compromised Hosts. Mycobacterial infections in solid organ transplant recipients. Clin Microbiol Infect. 2014;20 Suppl 7:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.11 Paediatrics (specific problems). Nephrol Dial Transplant. 2002;17 Suppl 4:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Subramanian AK, Morris MI; AST Infectious Diseases Community of Practice. Mycobacterium tuberculosis infections in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 36. | Bumbacea D, Arend SM, Eyuboglu F, Fishman JA, Goletti D, Ison MG, Jones CE, Kampmann B, Kotton CN, Lange C, Ljungman P, Milburn H, Morris MI, Muller E, Muñoz P, Nellore A, Rieder HL, Sester U, Theodoropoulos N, Wagner D, Sester M. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir J. 2012;40:990-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 37. | Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81. [PubMed] |
| 38. | National Library of Medicine. WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554956/. |
| 39. | Spyridis NP, Spyridis PG, Gelesme A, Sypsa V, Valianatou M, Metsou F, Gourgiotis D, Tsolia MN. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. Clin Infect Dis. 2007;45:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 40. | Stagg HR, Zenner D, Harris RJ, Muñoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med. 2014;161:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 41. | Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, Hackman J, Hamilton CD, Menzies D, Kerrigan A, Weis SE, Weiner M, Wing D, Conde MB, Bozeman L, Horsburgh CR Jr, Chaisson RE; TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 630] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 42. | Villarino ME, Scott NA, Weis SE, Weiner M, Conde MB, Jones B, Nachman S, Oliveira R, Moro RN, Shang N, Goldberg SV, Sterling TR; International Maternal Pediatric and Adolescents AIDS Clinical Trials Group; Tuberculosis Trials Consortium. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr. 2015;169:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 43. | Swindells S, Ramchandani R, Gupta A, Benson CA, Leon-Cruz J, Mwelase N, Jean Juste MA, Lama JR, Valencia J, Omoz-Oarhe A, Supparatpinyo K, Masheto G, Mohapi L, da Silva Escada RO, Mawlana S, Banda P, Severe P, Hakim J, Kanyama C, Langat D, Moran L, Andersen J, Fletcher CV, Nuermberger E, Chaisson RE; BRIEF TB/A5279 Study Team. One Month of Rifapentine plus Isoniazid to Prevent HIV-Related Tuberculosis. N Engl J Med. 2019;380:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 44. | Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H, Obeng Baah J, Marks GB, Long R, Hoeppner V, Elwood K, Al-Jahdali H, Gninafon M, Apriani L, Koesoemadinata RC, Kritski A, Rolla V, Bah B, Camara A, Boakye I, Cook VJ, Goldberg H, Valiquette C, Hornby K, Dion MJ, Li PZ, Hill PC, Schwartzman K, Benedetti A. Four Months of Rifampin or Nine Months of Isoniazid for Latent Tuberculosis in Adults. N Engl J Med. 2018;379:440-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 241] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 45. | El-Agroudy AE, El-Baz MA, Ismail AM, Ali-El-Dein B, El-Dein AB, Ghoneim MA. Clinical features and course of Kaposi's sarcoma in Egyptian kidney transplant recipients. Am J Transplant. 2003;3:1595-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Chenhsu RY, Loong CC, Chou MH, Lin MF, Yang WC. Renal allograft dysfunction associated with rifampin-tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis. 1999;3:847-850. [PubMed] |
| 48. | Yuan Z, Chao S, Xu Y, Niu Y. Chemoprophylaxis for the prevention of tuberculosis in kidney transplant recipients: A systematic review and meta-analysis. Front Pharmacol. 2023;14:1022579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Malinis M, La Hoz RM, Vece G, Annambhotla P, Aslam S, Basavaraju SV, Bucio J, Danziger-Isakov L, Florescu DF, Jones JM, Rana M, Wolfe CR, Michaels MG. Donor-derived tuberculosis among solid organ transplant recipients in the United States-2008 to 2018. Transpl Infect Dis. 2022;24:e13800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Boubaker K, Gargah T, Abderrahim E, Abdallah TB, Kheder A. Mycobacterium tuberculosis infection following kidney transplantation. Biomed Res Int. 2013;2013:347103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Prasad P, Bagai S, Prasad V, Grover R, Chhabra G, Khullar D. Kidney transplantation in patients on anti-tubercular therapy: A single centre observational study. Transpl Infect Dis. 2024;26:e14242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 52. | Gadde AB, Jha PK, Bansal SB, Rana A, Jain M, Bansal D, Yadav DK, Mahapatra AK, Sethi SK, Kher V. Renal Transplantation in Patients With Tuberculosis: A Single-center Experience From an Endemic Region. Transplant Direct. 2023;9:e1541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | WHO. Toman's tuberculosis: Case detection, treatment and monitoring: questions and answers. Available from: https://iris.who.int/handle/10665/42701. |
| 54. | Milburn H, Ashman N, Davies P, Doffman S, Drobniewski F, Khoo S, Ormerod P, Ostermann M, Snelson C; British Thoracic Society Standards of Care Committee and Joint Tuberculosis Committee. Guidelines for the prevention and management of Mycobacterium tuberculosis infection and disease in adult patients with chronic kidney disease. Thorax. 2010;65:557-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 55. | Queensland Health. Clinical guidance. Available from: https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/diseases-infection/diseases/tuberculosis/guidance. |
| 56. | Gupta KL, Bagai S, Kumar H, Nayak S, Muthu V, Kumar V, Rathi M, Kohli HS, Sharma A, Ramachandran R. Levofloxacin based non-rifampicin anti-tuberculous therapy: An effective alternative in renal transplant recipients in resource limited setting. Nephrology (Carlton). 2021;26:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 57. | Bedi RS. Management of Tuberculosis in Special Situations. Lung India. 2005;22:138-41. |
| 58. | Muhamad R, Akrivaki A, Papagiannopoulou G, Zavridis P, Zis P. The Role of Vitamin B6 in Peripheral Neuropathy: A Systematic Review. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 59. | McCune R, Deuschle K, McDermott W. The delayed appearance of isoniazid antagonism by pyridoxine in vivo. Am Rev Tuberc. 1957;76:1100-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 60. | Kuypers DR, Verleden G, Naesens M, Vanrenterghem Y. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate-glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Cassidy MJ, Van Zyl-Smit R, Pascoe MD, Swanepoel CR, Jacobson JE. Effect of rifampicin on cyclosporin A blood levels in a renal transplant recipient. Nephron. 1985;41:207-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Ngo BT, Pascoe M, Khan D. Drug interaction between rifampicin and sirolimus in transplant patients. Saudi J Kidney Dis Transpl. 2011;22:112-115. [PubMed] |
| 63. | Annapandian VM, Fleming DH, Mathew BS, John GT. Mycophenolic acid area under the curve recovery time following rifampicin withdrawal. Indian J Nephrol. 2010;20:51-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | McAllister WA, Thompson PJ, Al-Habet SM, Rogers HJ. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Yoon HE, Jeon YJ, Chung HW, Shin SJ, Hwang HS, Lee SJ, Chang YK, Choi BS, Park CW, Kim YS, Kim SY, Yang CW. Safety and efficacy of a quinolone-based regimen for treatment of tuberculosis in renal transplant recipients. Transplant Proc. 2012;44:730-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Hickey MD, Quan DJ, Chin-Hong PV, Roberts JP. Use of rifabutin for the treatment of a latent tuberculosis infection in a patient after solid organ transplantation. Liver Transpl. 2013;19:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Kim OH, Shim TS, Jo KW. Drug-level change and optimal dose adjustment of tacrolimus with the use of rifabutin for treating mycobacterial disease in solid organ transplant recipients. Transpl Infect Dis. 2022;24:e13893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 68. | Wang YC, Salvador NG, Lin CC, Wu CC, Lin TL, Lee WF, Chan YC, Chen CL, Co JS, Encarnacion DD. Comparative analysis of the drug-drug interaction between immunosuppressants, safety and efficacy of rifabutin from rifampicin-based Anti-TB treatment in living donor liver transplant recipients with active tuberculosis. Biomed J. 2021;44:S162-S170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Falzon D, Schünemann HJ, Harausz E, González-Angulo L, Lienhardt C, Jaramillo E, Weyer K. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J. 2017;49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 70. | Chien JY, Chien ST, Chiu WY, Yu CJ, Hsueh PR. Moxifloxacin Improves Treatment Outcomes in Patients with Ofloxacin-Resistant Multidrug-Resistant Tuberculosis. Antimicrob Agents Chemother. 2016;60:4708-4716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 71. | Borrás-Blasco J, Conesa-García V, Navarro-Ruiz A, Marín-Jiménez F, González-Delgado M, Gomez-Corrons A. Ciprofloxacin, but not levofloxacin, affects cyclosporine blood levels in a patient with pure red blood cell aplasia. Am J Med Sci. 2005;330:144-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 72. | Federico S, Carrano R, Capone D, Gentile A, Palmiero G, Basile V. Pharmacokinetic interaction between levofloxacin and ciclosporin or tacrolimus in kidney transplant recipients: ciclosporin, tacrolimus and levofloxacin in renal transplantation. Clin Pharmacokinet. 2006;45:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Costa SD, de Sandes-Freitas TV, Jacinto CN, Martiniano LVM, Amaral YS, Paes FJVN, Sales MLMBO, Esmeraldo RM, Daher EF. Tuberculosis after kidney transplantation is associated with significantly impaired allograft function. Transpl Infect Dis. 2017;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Boisson-Dupuis S, Bustamante J, El-Baghdadi J, Camcioglu Y, Parvaneh N, El Azbaoui S, Agader A, Hassani A, El Hafidi N, Mrani NA, Jouhadi Z, Ailal F, Najib J, Reisli I, Zamani A, Yosunkaya S, Gulle-Girit S, Yildiran A, Cipe FE, Torun SH, Metin A, Atikan BY, Hatipoglu N, Aydogmus C, Kilic SS, Dogu F, Karaca N, Aksu G, Kutukculer N, Keser-Emiroglu M, Somer A, Tanir G, Aytekin C, Adimi P, Mahdaviani SA, Mamishi S, Bousfiha A, Sanal O, Mansouri D, Casanova JL, Abel L. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol Rev. 2015;264:103-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 75. | Tait DR, Hatherill M, Van Der Meeren O, Ginsberg AM, Van Brakel E, Salaun B, Scriba TJ, Akite EJ, Ayles HM, Bollaerts A, Demoitié MA, Diacon A, Evans TG, Gillard P, Hellström E, Innes JC, Lempicki M, Malahleha M, Martinson N, Mesia Vela D, Muyoyeta M, Nduba V, Pascal TG, Tameris M, Thienemann F, Wilkinson RJ, Roman F. Final Analysis of a Trial of M72/AS01(E) Vaccine to Prevent Tuberculosis. N Engl J Med. 2019;381:2429-2439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 360] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 76. | WHO. Global Tuberculosis program. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports. |