Published online Sep 18, 2024. doi: 10.5500/wjt.v14.i3.94914
Revised: May 13, 2024
Accepted: May 30, 2024
Published online: September 18, 2024
Processing time: 125 Days and 7.5 Hours
Liver transplantation (LT) is a potentially curative therapy for patients with hepatocellular carcinoma (HCC). HCC-recurrence following LT is associated with reduced survival. There is increasing interest in chemoprophylaxis to improve HCC-related outcomes post-LT.
To investigate whether there is any benefit for the use of drugs with proposed chemoprophylactic properties against HCC, and patient outcomes following LT.
This was a retrospective study of adult patients who received Deceased Donor LT for HCC from 2005-2022, from a single Australian centre. Drug use was defined as statin, aspirin or metformin therapy for ≥ 29 days, within 24 months post-LT. A cox proportional-hazards model with time-dependent covariates was used for survival analysis. Outcome measures were the composite-endpoint of HCC-recurrence and all-cause mortality, HCC-recurrence and HCC-related mortality. Sensitivity analysis was performed to account for immortality time bias and statin dosing.
Three hundred and five patients were included in this study, with 253 (82.95%) males with a median age of 58.90 years. Aetiologies of liver disease were 150 (49.18%) hepatitis C, 73 (23.93%) hepatitis B (HBV) and 33 (10.82%) non-alcoholic fatty liver disease (NAFLD). 56 (18.36%) took statins, 51 (16.72%) aspirin and 50 (16.39%) metformin. During a median follow-up time of 59.90 months, 34 (11.15%) developed HCC-recurrence, 48 (15.74%) died, 17 (5.57%) from HCC-related mortality. Statin, aspirin or metformin use was not associated with statistically significant differences in the composite endpoint of HCC-recurrence or all-cause mortality [hazard ratio (HR): 1.16, 95%CI: 0.58-2.30; HR: 1.21, 95%CI: 0.28-5.27; HR: 0.61, 95%CI: 0.27-1.36], HCC-recurrence (HR: 0.52, 95%CI: 0.20-1.35; HR: 0.51, 95%CI: 0.14-1.93; HR 1.00, 95%CI: 0.37-2.72), or HCC-related mortality (HR: 0.32, 95%CI: 0.033-3.09; HR: 0.71, 95%CI: 0.14-3.73; HR: 1.57, 95%CI: 0.61-4.04) respectively. Statin dosing was not associated with statistically significant differences in HCC-related outcomes.
Statin, metformin or aspirin use was not associated with improved HCC-related outcomes post-LT, in a largely historical cohort of Australian patients with a low proportion of NAFLD. Further prospective, multicentre studies are required to clarify any potential benefit of these drugs to improve HCC-related outcomes.
Core Tip: In this single centre, retrospective study of adult patients who received liver transplantation (LT) for Hepatocellular carcinoma in Australia, statin, aspirin or metformin use after LT was not associated with a difference in the composite endpoint of hepatocellular carcinoma (HCC)-recurrence and all-cause mortality, HCC-recurrence or HCC-related mortality.
- Citation: Chung W, Wong K, Ravindranayagam N, Tang L, Grace J, Wong D, Con D, Sinclair M, Majumdar A, Kutaiba N, Hui S, Gow P, Muralidharan V, Dobrovic A, Testro A. Statin, aspirin and metformin use and risk of hepatocellular carcinoma related outcomes following liver transplantation: A retrospective study. World J Transplant 2024; 14(3): 94914
- URL: https://www.wjgnet.com/2220-3230/full/v14/i3/94914.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i3.94914
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide and the second for premature cancer death[1]. While the highest incidence of HCC occurs in Asia and Africa, HCC remains a significant contributor to the burden of morbidity and mortality in Europe and the United States[2].
For selected patients with HCC, liver transplantation (LT) may be considered for those within Milan criteria[3], defined as having a single tumour ≤ 5 cm or 2-3 tumours ≤ 3 cm. Following LT, HCC-recurrence occurs in 6%-18% of patients, with the majority (60%) of recurrences occurring within 24 months. In recent years, expanded listing criteria such as Metroticket 2.0[4] and University of California San Francisco (UCSF) criteria[5] have allowed patients with higher burden of disease to receive LT with comparable 5 years survival outcomes.
Early HCC-recurrence (within 24 months post-LT) is thought to occur due to peri-operative engraftment of microscopic or undetected macroscopic metastases in a target organ, and from intraoperative release of circulating tumour cells[6]. Late recurrence has been postulated to be due to activation of residual latent or indolent cancer cells. De novo HCC development in the allograft is less common but is associated with recurrent Hepatitis B virus (HBV) post-LT[7]. HCC-recurrence is associated with poor survival and lack of effective therapies[8].
Chemoprevention has been studied as a potential strategy to reduce HCC recurrence following surgery. It is thought that statins, through HMG-CoA reductase inhibition and its pleotropic effects, may have tumour suppressive benefits through inhibition of the mevalonate cascade, which plays a role in inducing the intrinsic apoptosis pathway in tumour cells[9]. A recent meta-analysis found an association with statin use and reduced HCC-recurrence in patients who underwent liver surgery[10]. There are conflicting evidence to support the use of statins to reduce HCC-recurrence following LT, with retrospective studies that demonstrate a dose dependent relationship between statin use and reduction of HCC-recurrence[11,12] and HCC-related mortality[11], however other studies did not find a benefit for HCC-recurrence[13,14].
Aspirin is a non-steroidal anti-inflammatory drug which inhibits Cyclooxygenase (COX). Population association studies have shown reduced HCC incidence in a dose dependent manner[15], apparent after 5 or more years of use[16]. Its role in HCC prevention following LT is less clear.
Metformin is a biguanide used for treatment of Type 2 Diabetes Mellitus (T2DM). Its use has been associated with a decrease in breast, colon, pancreatic, endometrial and lung cancer[17] and is supported by pre-clinical models of HCC[18]. Clinical evidence, however, for metformin’s role for chemoprophylaxis for HCC are limited[19], with negative studies[20] including following hepatic resection[21].
Limited data are available for HCC chemoprophylaxis in an Australian post-LT setting. In this study, we aim to investigate for any potential benefit for statins, aspirin and metformin use for HCC-related outcomes.
This was a single-centre retrospective study of adult patients (aged 18 years or older) with HCC, who received Deceased Donor LT from 2005 to 2022 in the state of Victoria, Australia. Patients were excluded if their first diagnosis of HCC was found on explant, if cholangiocarcinoma or biphenotypic cancer was present, or if their medical records were incomplete. This study was approved by the Austin Health Human Ethics Research Committee (No. HREC/87459/Austin-2022).
Statin, aspirin and metformin use was defined as use for at least 29 days within 2 years following LT. Statin dosage data was also collected. Mammalian target of rapamycin inhibitor (MTORi) use was defined as taking therapy for at least 29 days, at any time following LT. Clinical data pertaining to aetiology of liver disease was collected including HBV, Hepatitis C virus (HCV), non-alcoholic fatty liver disease (NAFLD), or alcohol related liver disease (ALD) status. Pre LT co-morbidities were recorded, including diabetes, hypertension (HT), obesity, stroke, total cholesterol ≥ 4 mmol/L, dates of initiation and duration of pharmacotherapy of statins, aspirin, metformin, Mammalian target of rapamycin (MTOR), statin dosing, sex, age at LT, histological evidence of microvascular invasion, largest tumour size if ≥ 3 cm, prior regional therapy for HCC if ≥ 3 were performed, pre-LT alfa-fetoprotein (AFP) ≥ 100 ng/mL, tumour burden beyond Milan criteria, beyond-up-to-7 criteria, radiological complete remission status prior to LT (based upon mRECIST criteria were possible) were collected.
Prior regional therapies for HCC were defined as Transarterial chemo-embolisation, Transarterial Embolisation, microwave ablation, radiofrequency ablation, percutaneous ethanol ablation, selective internal radiation therapy, stereotactic body radiotherapy, or surgical resection.
Summary statistics of baseline patient characteristics and pharmacotherapy is presented, and the Mann-Whitney U test (XLstat, Lumivero) was used to compare groups of patients with HCC-recurrence and without.
Survival analysis was performed using COX proportional hazards with time-dependent covariates (Survival package for R, version 3.5-5). Outcomes measures were the composite endpoint of HCC-recurrence or all-cause mortality, HCC-recurrence, and HCC-related mortality. Patients were censored as of February 2022 if an event did not occur. Variables that had a P value ≤ 0.10 in the univariate analysis were subsequently included in the multivariate analysis. Inclusion of a priori variables known to be associated with HCC-recurrence following LT included sex, age at LT, microvascular invasion, pre-LT AFP ≥ 100 ng/mL and pre-LT tumour burden[22]. To avoid co-linearity bias, either beyond Milan or beyond up-to-7 criteria status was included as a priori in the multivariate analysis, depending on which had the lowest P value in the univariate analysis. Status of statin, aspirin, metformin and MTOR inhibitor use were included as a priori in the multivariate analysis. A separate multivariate analysis was performed for the composite variable of statins, aspirin or metformin use.
Kaplan-Meier analysis was performed for statins, aspirin, metformin, MTORi use against the composite endpoint of HCC-recurrence and all-cause mortality, HCC-recurrence, HCC-related mortality. Univariate analysis was performed using the log-rank test.
Sensitivity analysis was performed to account for immortal time bias by removing those with events within 3, 6, 12, 24 months with regards to statin, aspirin and metformin use. Patients who received ≥ 30 defined daily doses (cDDD) of statins, within 3, 6, 12, 24 months were analysed with univariate and multivariate analysis, to account for immortal time bias. Further sensitivity analysis of patients with post-LT obesity and weight [body mass index (BMI) ≥ 25] was performed, against the outcome of HCC-related mortality.
Three hundred and five patients were included in this analysis, with 253 (82.95%) males, 73 (23.93%) HBV, 150 (49.18%) HCV, 86 (28.20%) ALD, 33 (10.82%) NAFLD as the aetiology of liver disease with a median follow-up time of 59.90 months (Table 1). Ninety-six (31.48%) patients had pre-existing diabetes, 73 (23.93%) HT, 6 (1.97%) stroke and 103 (33.77%) obesity. Fifty-six (18.36%) took statins, 50 (16.39%) took metformin and 51 (16.72%) took aspirin for at least 29 days within 2 years of LT (Table 2).
| n | % | |
| Patients | 305 | |
| Sex (Male) | 253 | 82.95 |
| Age at LT [median (Q1-3)] | 58.90 (53.8-63) | |
| Aetiology of liver disease | ||
| HBV | 73 | 23.93 |
| HCV | 150 | 49.18 |
| Alcohol related liver disease | 86 | 28.20 |
| NAFLD | 33 | 10.82 |
| Co-morbidities prior to LT | ||
| Diabetes | 96 | 31.48 |
| Hypertension | 73 | 23.93 |
| Stroke | 6 | 1.97 |
| Obesity (BMI > 30) | 103 | 33.77 |
| Total cholesterol ≥ 4 mmol/L | 253 | 82.95 |
| AFP ≥ 100 ng/mL | 26 | 8.52 |
| Regional therapy ≥ 3 | 140 | 45.90 |
| Largest diameter of HCC ≥ 3 cm | 97 | 31.80 |
| Histology | ||
| Microvascular invasion | 40 | 13.11 |
| Beyond Milan criteria | 90 | 29.51 |
| Beyond Up-to-7 criteria | 52 | 17.05 |
| Complete response at LT | 107 | 35.08 |
| Follow up time [median months (Q1-Q3)] | 59.90 (27.93-99.61) | |
| HCC-recurrence (overall) | 34 | 11.15 |
| HCC-recurrence after 24 months | 16 | 5.25 |
| All-cause mortality | 48 | 15.74 |
| Composite endpoint of HCC-recurrence or all-cause mortality | 65 | 21.31 |
| HCC-related mortality | 17 | 5.57 |
| n | % | |
| Statin use | 56 | 18.36 |
| Aspirin use | 51 | 16.72 |
| Metformin use | 50 | 16.39 |
| Statins, aspirin or metformin use | 126 | 41.31 |
| MTORi use | 85 | 27.87 |
Thirty-four (11.15%) patients developed HCC-recurrence, of whom 16 (5.25%) recurred after 24 months. Forty-eight (15.74%) died from any cause during follow up with 17 (5.57%) from HCC-related mortality. 65 (21.31%) reached the composite of HCC-recurrence or all-cause mortality.
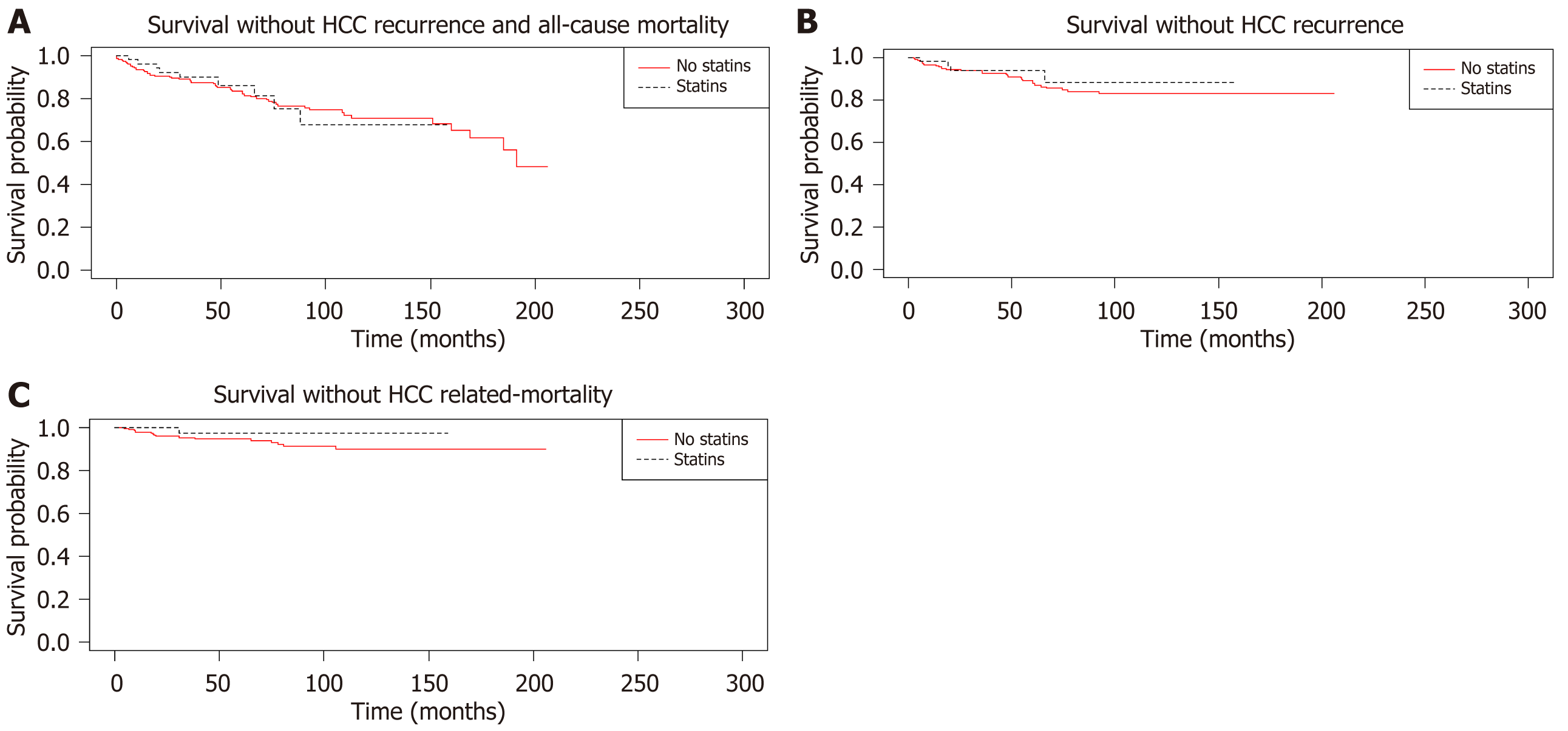
A comparison between patients showed that patients who had HCC-recurrence were more likely to have a higher AFP ≥ 100 ng/mL (26.47% vs 6.27%, P < 0.01), microvascular invasion (38.24% vs 9.96%, P < 0.01), all-cause mortality (52.94% vs 11.07%, P < 0.01), pre-LT regional therapy ≥ 3 (64.71% vs 43.54%, P = 0.02), pre-LT largest diameter of HCC ≥ 3 cm (55.88% vs 28.78%, P < 0.01), complete response prior to LT (17.65% vs 37.27%, P = 0.024), statin, aspirin or metformin use (58.82% vs 39.11%, P = 0.028), all-cause mortality within 2 years (32.35% vs 4.06%, P < 0.01), beyond up-to-7 criteria (41.18% vs 14.02%, P < 0.01) and were more likely to be taking aspirin (44.12% vs 13.28%, P < 0.01). Patients who did not have HCC-recurrence were more likely to have a longer median follow up time (65.99 vs 30.46 months, P < 0.01; Table 3). Kaplan-Meier curves and their P values are shown in Figure 1.
| Characteristics | HCC-recurrence | No HCC-recurrence | P value |
| Total | 34 | 277 | |
| Sex (male) | 30 (88.24) | 223 (82.29) | 0.39 |
| Age at LT [median (Q1-Q3)] | 58.83 (53.85-62.35) | 58.9 (53.9-63.1) | 0.79 |
| Aetiology of liver disease | |||
| HBV | 12 (35.29) | 61 (22.51) | 0.1 |
| HCV | 20 (58.82) | 130 (47.97) | 0.23 |
| Alcohol related liver disease | 5 (14.71) | 81 (29.89) | 0.064 |
| NAFLD | 3 (8.82) | 30 (11.07) | 0.69 |
| Pre LT co-morbidities | |||
| Diabetes | 9 (26.47) | 87 (32.10) | 0.51 |
| Hypertension | 7 (20.59) | 66 (24.35) | 0.63 |
| Stroke | 2 (5.88) | 5 (1.85) | 0.67 |
| Ischaemic heart disease | 0 (0) | 7 (2.58) | 0.35 |
| Obesity (BMI > 30) | 12 (35.29) | 91 (33.58) | 0.84 |
| Total cholesterol ≥ 4 mmol/L [median (Q1-Q3)] | 26 (76.47) | 227 (83.76) | 0.29 |
| Pre-LT AFP ≥ 100 ng/mL | 9 (26.47) | 17 (6.27) | < 0.01 |
| Pre-LT regional therapy ≥ 3 | 22 (64.71) | 118 (43.54) | 0.02 |
| Pre-LT largest diameter of HCC ≥ 3 cm | 19 (55.88) | 78 (28.78) | < 0.01 |
| Histology: Microvascular invasion | 13 (38.24) | 27 (9.96) | < 0.01 |
| All-cause mortality | 18 (52.84) | 30 (11.07) | < 0.01 |
| Death within 2 years | 11 (32.35) | 11 (4.06) | < 0.01 |
| Beyond Milan criteria | 20 (58.82) | 70 (25.83) | < 0.01 |
| Beyond up-to-7 criteria | 14 (41.18) | 38 (14.03) | < 0.01 |
| Complete response prior to LT | 6 (17.65) | 101 (37.27) | 0.024 |
| Chemoprophylaxis | |||
| Statins | 4 (11.76) | 52 (19.19) | 0.29 |
| Aspirin | 15 (44.12) | 36 (13.28) | < 0.01 |
| Metformin | 6 (17.65) | 44 (16.24) | 0.84 |
| Statins, metformin or aspirin use | 20 (58.82) | 106 (39.11) | 0.028 |
| MTORi use | 10 (29.41) | 75 (27.68) | 0.83 |
| Follow up time [median months (Q1-Q3)] | 30.46 (8.97-58.87) | 65.99 (30.97-104.70) | < 0.01 |
For the composite endpoint of HCC-recurrence and all-cause mortality, univariate analysis for statins [hazard ratio (HR) 1.20, 95%CI: 0.59-2.41], aspirin (HR 1.01, 95%CI: 0.22-4.61) or metformin (HR 0.68, 95%CI: 0.31-1.50) and multivariate analysis (HR 1.16, 95%CI: 0.58-2.30, HR 1.21, 95%CI: 0.28-5.27, HR 0.61, 95%CI: 0.27-1.36 respectively) did not show statistically significant differences (Table 4). The composite variable of statins, aspirin or metformin use did not show a difference on univariate (HR 1.10, 95%CI: 0.60-2.01) or multivariate (HR 1.06, 95%CI: 0.56-1.91) analysis. Tumour burden beyond up-to-7 criteria was associated with a higher risk of the composite endpoint (HR 2.25, 95%CI: 1.23-4.10) and multivariate analysis (HR 1.68, 95%CI: 0.94-2.99).
| Univariate analysis | P value | Multivariate analysis | P value | |
| Variables | ||||
| Sex | 1.94 (95%CI: 0.79-4.76) | 0.15 | 2.02 (95%CI: 0.82-4.96) | 0.12 |
| Age at LT | 1.03 (95%CI: 1.00-1.06) | 0.10 | 1.02 (95%CI: 0.98-1.05) | 0.33 |
| Aetiology of liver disease | ||||
| HBV | 1.33 (95%CI: 0.72-2.44) | 0.37 | ||
| HCV | 1.21 (95%CI: 0.70-2.10) | 0.50 | ||
| NAFLD | 0.97 (95%CI: 0.39-2.41) | 0.95 | ||
| Statin use | 1.20 (95%CI: 0.59-2.41) | 0.62 | 1.16 (95%CI: 0.58-2.30) | 0.67 |
| Aspirin use | 1.01 (95%CI: 0.22-4.61) | 0.99 | 1.21 (95%CI: 0.28-5.27) | 0.80 |
| Metformin use | 0.68 (95%CI: 0.31-1.50) | 0.34 | 0.61 (95%CI: 0.27-1.36) | 0.23 |
| Statins, aspirin or metformin use1 | 1.10 (95%CI: 0.60-2.01) | 0.76 | 1.06 (95%CI: 0.56-1.91) | 0.85 |
| MTORi use | 1.14 (95%CI: 0.59-2.21) | 0.70 | 0.81 (95%CI: 0.43-1.53) | 0.51 |
| Pre LT co-morbidities | ||||
| Diabetes | 1.08 (95%CI: 0.61-1.93) | 0.79 | ||
| Stroke | 0.98 (95%CI: 0.14-7.02) | 0.99 | ||
| Hypertension | 0.68 (95%CI: 0.35-1.33) | 0.26 | ||
| Obesity | 0.68 (95%CI: 0.37-1.26) | 0.22 | ||
| Ischaemic heart disease | 2.08 (95%CI: 0.52-8.22) | 0.27 | ||
| Total cholesterol mmol/L | 0.58 (95%CI: 0.30-1.12) | 0.11 | ||
| Other variables | ||||
| Microvascular invasion | 3.54 (95%CI: 1.97-6.34) | < 0.01 | 2.82 (95%CI: 1.51-5.27) | < 0.01 |
| Pre-LT AFP ≥ 100 ng/mL pre-LT | 3.04 (95%CI: 1.53-6.02) | < 0.01 | 2.73 (95%CI: 1.38-5.40) | < 0.01 |
| Pre-LT Regional treatments ≥ 3 | 1.26 (95%CI: 0.73-2.18) | 0.41 | ||
| Pre-LT size of largest lesion ≥ 3 cm | 1.62 (95%CI: 0.93-2.83) | 0.092 | 1.50 (95%CI: 0.86-2.61) | 0.16 |
| Beyond Milan | 2.06 (95%CI: 1.19-3.56) | 0.01 | ||
| Beyond up-to-7 | 2.25 (95%CI: 1.23-4.10) | < 0.01 | 1.68 (95%CI: 0.94-2.99) | 0.077 |
| Complete response at LT | 0.77 (95%CI: 0.43-1.38) | 0.38 |
On univariate analysis, AFP ≥ 100 ng/mL (HR 3.04, 95%CI: 1.53-6.02), and microvascular invasion (HR 3.54, 95%CI: 1.97-6.34) were associated with an increased risk of the composite endpoint, as with multivariate analysis (HR 2.73, 95%CI: 1.38-5.40, HR 2.82, 95%CI: 1.51-5.27) respectively.
For the endpoint of HCC-recurrence, no difference was seen on univariate analysis for statin therapy (HR 0.52, 95%CI: 0.22-1.26), aspirin (HR 0.32, 95%CI: 0.07-1.43), metformin (HR 0.90, 95%CI: 0.33-2.46), or multivariate analysis (HR 0.52, 95%CI: 0.20-1.35, HR 0.51, 95%CI: 0.14-1.93, HR 1.00, 95%CI: 0.37-2.72) respectively (Table 5). For the composite variable of statins, aspirin or metformin use, no difference was seen on univariate (HR 0.61, 95%CI: 0.27-1.37) or multivariate (HR 0.64, 95%CI: 0.27-1.53) analysis. Microvascular invasion (HR 5.34, 95%CI: 2.54-11.21), AFP ≥ 100 ng/mL (HR 4.20, 95% 1.86-9.51), tumour burden beyond up-to-7 criteria (HR 4.59, 95%CI: 2.22-9.49) were associated with an increased risk of HCC-recurrence on univariate, and multivariate analysis (HR 4.04, 95%CI: 1.81-9.04, HR 3.14, 95%CI: 1.33-7.41 and HR 2.82, 95%CI: 1.35-5.89 respectively).
| Variables | Univariate analysis | Multivariate analysis | ||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Sex | 1.88 (95%CI: 0.63-5.60) | 0.26 | 2.27 (95%CI: 0.64-8.02) | 0.22 |
| Age at LT | 1.02 (95%CI: 0.98-1.07) | 0.84 | 1.01 (95%CI: 0.96-1.05) | 0.81 |
| Aetiology of liver disease | ||||
| HBV | 2.09 (95%CI: 0.98-4.45) | 0.06 | 1.98 (95%CI: 0.92-4.27) | 0.081 |
| HCV | 1.68 (95%CI: 0.80-3.49) | 0.17 | ||
| NAFLD | 0.50 (95%CI: 0.14-1.78) | 0.28 | ||
| Statin use | 0.52 (95%CI: 0.22-1.26) | 0.40 | 0.52 (95%CI: 0.20-1.35) | 0.18 |
| Aspirin use | 0.32 (95%CI: 0.07-1.43) | 0.14 | 0.51 (95%CI: 0.14-1.93) | 0.32 |
| Metformin use | 0.90 (95%CI: 0.33-2.46) | 0.84 | 1.0 (95%CI: 0.37-2.72) | 1.00 |
| Statin, aspirin or metformin use1 | 0.61 (95%CI: 0.27-1.37) | 0.45 | 0.64 (95% 0.27-1.53) | 0.31 |
| MTORi use | 0.97 (95%CI: 0.49-1.93) | 0.94 | 0.68 (95%CI: 0.41-1.13) | 0.14 |
| Pre LT co-morbidities | ||||
| Diabetes | 0.67 (95%CI: 0.30-1.51) | 0.33 | ||
| Stroke | 1.88 (95%CI: 0.26-13.50) | 0.53 | ||
| Hypertension | 0.67 (95%CI: 0.26-1.73) | 0.41 | ||
| Obesity | 0.96 (95%CI: 0.45-2.08) | 0.92 | ||
| Ischaemic heart disease | N/A | N/A | ||
| Total cholesterol mmol/L | 0.59 (95%CI: 0.25-1.42) | 0.24 | ||
| Other variables | ||||
| Microvascular invasion | 5.34 (95%CI: 2.54-11.21) | < 0.01 | 4.04 (95%CI: 1.81-9.04) | < 0.01 |
| AFP > 100 ng/mL pre-LT | 4.20 (95%CI: 1.86-9.51) | < 0.01 | 3.14 (95%CI: 1.33-7.41) | < 0.01 |
| Pre-LT regional treatments ≥ 3 | 2.27 (95%CI: 1.07-4.84) | 0.033 | 1.46 (95%CI: 0.60-3.52) | 0.40 |
| Pre-LT size of largest lesion ≥ 3 cm | 3 (95%CI: 1.43-6.31) | < 0.01 | 2.21 (0.96-5.06) | 0.062 |
| Beyond Milan | 4.31 (95%CI: 2.09-8.89) | < 0.01 | ||
| Beyond up-to-7 | 4.59 (95%CI: 2.22-9.49) | < 0.01 | 2.82 (95%CI: 1.35-5.89) | < 0.01 |
| Complete response at LT | 0.36 (95%CI: 0.14-0.92)) | 0.034 | 0.66 (95%CI: 0.24-1.81) | 0.42 |
For the endpoint of HCC-related mortality, statin (HR 0.46, 95%CI: 0.059-3.53), aspirin (HR 0.50, 95%CI: 0.10-2.40), metformin use (HR 1.27, 95%CI: 0.35-4.49) were not associated with any differences in outcome, nor on multivariate analysis (HR 0.32, 95%CI: 0.033-3.09, HR 0.71, 95%CI: 0.13-3.73, HR 1.57, 95%CI: 0.61-4.04) respectively (Table 6). For the composite variable of statin, aspirin or metformin use, no differences were seen on univariate (HR 0.67, 95%CI: 0.21-2.08) or multivariate (HR 0.61, 95%CI: 0.18-2.08) analysis. Microvascular invasion (HR 7.52, 95%CI: 2.22-25.47), AFP ≥ 100 ng/mL (HR 2.83, 95%CI: 0.85-9.44), beyond-up-to-7 (HR 5.29, 95%CI: 1.55-18.13) were associated with increased HCC-related mortality on univariate analysis, but only microvascular invasion (HR 8.05, 95%CI: 2.63-24.68) was statistically significant on multivariate analysis. Obesity was found to be associated with reduced HCC-related mortality on univariate (HR 0.35, 95%CI: 0.10-1.15) and multivariate (HR 0.23, 95%CI: 0.06-0.85) analysis.
| Variables | Univariate analysis | Multivariate analysis | ||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Sex | 0.87 (95%CI: 0.20-3.84) | 0.86 | 0.90 (95%CI: 0.23-3.56) | 0.88 |
| Age at LT | 1.02 (95%CI: 0.93-1.12) | 0.68 | 1.05 (95%CI: 0.94-1.17) | 0.36 |
| Aetiology of liver disease | ||||
| HBV | 1.19 (95%CI: 0.34-4.21) | 0.78 | ||
| HCV | 1.41 (95%CI: 0.41-4.21) | 0.57 | ||
| NAFLD | 0.80 (95%CI: 0.16-3.90) | 0.78 | ||
| Statin use | 0.46 (95%CI: 0.059-3.53) | 0.45 | 0.32 (95%CI: 0.033-3.09) | 0.32 |
| Aspirin use | 0.50 (95%CI: 0.10-2.40) | 0.39 | 0.71 (95%CI: 0.14-3.73) | 0.69 |
| Metformin use | 1.27 (95%CI: 0.36-4.49) | 0.71 | 1.57 (95%CI: 0.61-4.04) | 0.35 |
| Statins, aspirin or metformin use1 | 0.67 (95%CI: 0.21-2.08) | 0.48 | 0.61 (95%CI: 0.18-2.08) | 0.43 |
| MTORi use | 0.96 (95%CI: 0.35-2.65) | 0.93 | 0.63 (95%CI: 0.26-1.49) | 0.29 |
| Pre LT co-morbidities | ||||
| Diabetes | 0.62 (95%CI: 0.11-3.51) | 0.59 | ||
| Stroke | N/A | |||
| Hypertension | 0.29 (95%CI: 0.06-1.47) | 0.14 | ||
| Obesity | 0.35 (95%CI: 0.10-1.15) | 0.08 | 0.23 (95%CI: 0.06-0.85) | 0.027 |
| Ischaemic heart disease | N/A | N/A | ||
| Total cholesterol mmol/L | 0.77 (95%CI: 0.17-3.45) | 0.74 | ||
| Other variables | ||||
| Microvascular invasion | 7.52 (95%CI: 2.22-25.47) | < 0.01 | 8.05 (95%CI: 2.63-24.68) | < 0.01 |
| AFP > 100 ng/mL immediately pre-LT | 2.83 (95%CI: 0.85-9.44) | 0.09 | 3.11 (95%CI: 0.70-13.76) | 0.14 |
| Pre-LT regional treatments ≥ 3 | 1.58 (95%CI: 0.45-5.52) | 0.48 | ||
| Pre-LT size of largest lesion ≥ 3 cm | 2.19 (95%CI: 0.60-7.96) | 0.23 | ||
| Beyond Milan | 2.72 (95%CI: 0.80-9.30) | 0.11 | ||
| Beyond up-to-7 | 5.29 (95%CI: 1.55-18.13) | < 0.01 | 4.14 (95%CI: 1.09-15.81) | 0.038 |
| Complete response at LT | 0.32 (95%CI: 0.055-1.88) | 0.21 | ||
Sensitivity analysis was performed to account for immortal time bias and statin dosing. No difference in outcomes were seen in univariate or multivariate analysis for patients taking statins, aspirin or metformin (Supplementary Tables 1-5). Sensitivity analysis for pre-LT overweight (HR 1.01, 95%CI: 0.43-2.35) and post-LT obesity status (HR 0.66, 95%CI: 0.29-1.51) was not associated with statistically significant differences with HCC-related mortality (Supplementary Table 6). Statin dosing (with ≥ 30 cDDD as a cutoff) was not associated with a difference in HCC-related outcomes (Supplementary Table 7).
Due to the poor prognosis of those who develop HCC-recurrence post-LT, there is increasing interest in the potential role of chemoprophylactic drugs to improve patient outcomes. The current body of evidence for post-LT chemoprevention is heterogenous, and mostly consists of retrospective studies, predominantly from East Asia. To date this is the only study that aims to investigate for potential benefit of statins, aspirin, metformin either separately or in combination following LT, in an Australian cohort.
Statins are widely used to modify cardiovascular risk following LT through its lipid lowering and pleotropic benefits, especially anti-inflammatory activity on plaque stabilisation. There are conflicting data for the use of statins to reduce the risk of HCC-recurrence following LT, with one retrospective South Korean study demonstrating lower rates of HCC-recurrence in patients who took statins[12], and another showing reduced HCC-recurrence, all-cause mortality and HCC-related mortality in a dose dependent manner[11]. In this study, even after accounting for drug dosage on sensitivity analysis, statin use was not associated with improved HCC-related outcomes post-LT. These results are consistent with a retrospective South Korean study in patients who received Living Donor LT (LDLT), which did not demonstrate any benefit of statins to reduce HCC-recurrence[13]. A European observational study did not demonstrate any reduction in HCC-recurrence for those taking statins, but did show reduced rates of post operative complications and improved survival[14]. Clearly there are regional differences in LT practice, with LDLT far more prevalent in Asia and the United States than Australia[23] as well as differences in LT listing criteria with regards to tumour burden, making comparisons between these studies difficult.
Aspirin is widely used in the setting of primary and secondary prevention of cardiovascular disease. A recent meta-analysis has demonstrated that aspirin use is associated with a 30% risk reduction of HCC incidence across cohort studies but was associated with an increased risk of major bleeding[15]. In this study, aspirin use was not associated any differences in HCC-related outcomes. These results are in keeping with a recent propensity matched retrospective study from South Korea which failed to find a benefit for antiplatelet use following LT, to prevent HCC-recurrence or HCC-related mortality[24].
The benefits of metformin use for chemoprophylaxis is less clear. A systematic review has shown that metformin use was associated with improved survival in diabetic patients with liver cancer[25]. A retrospective Chinese study found that a combination of sirolimus and metformin reduced incidence of HCC-recurrence in patients who received LT for HBV associated HCC, compared to those who did not take either medication, but did not find a difference with those who were taking sirolimus without metformin[19]. A retrospective Taiwanese study showed no benefit of metformin use to reduce HCC-recurrence in 857 patients who received a primary resection for HCC[21]. A retrospective Korean study did not find a benefit for progression free survival and overall survival in patients with recurrent HCC, receiving sorafenib therapy in combination with metformin, following hepatic resection or LT[20]. This study did not demonstrate any association with metformin with improved HCC-related outcomes post-LT.
Early clinical data suggests that obesity and the metabolic syndrome were associated with increased tumour recurrence and worse clinical outcomes including worse survival, microvascular invasion[26]. Mechanisms postulated to explain these findings include induction of a pro-oncogenic state with upregulated Vascular Endothelial Growth Factor, re
As expected, this study also demonstrates that AFP ≥ 100 ng/mL was associated with the composite endpoint of HCC-recurrence and all-cause mortality, and HCC-recurrence in the univariate analyses. Higher AFP levels are well known to be associated with micro and macrovascular invasion[29], metastatic disease, HCC-recurrence following treatment and worse outcomes. Microvascular invasion was associated higher rates of the composite endpoint, HCC-recurrence alone and HCC-related mortality in this study, which is consistent with the published literature.
Calcineurin inhibitors (CNI), especially tacrolimus and ciclosporin are the standard of care for induction and maintenance of immunosuppression following LT. CNIs, however may increase risk of post-LT hepato-carcinogenesis[30]. MTORi have been shown to reduce the rate of HCC-recurrence post-LT in several retrospective studies[31]. A prospective randomised open-label study showed that MTORi based regimens improved HCC-recurrence free survival and overall survival in the first 3 to 5 years, but not beyond 5 years[32]. Therefore while MTORi use did not demonstrate statistically significant benefit for improved HCC-related outcomes, it was nonetheless included in the multivariate analysis to account for any potential confounding.
Strengths of this study included the availability of a well-maintained LT patient database, which was retrospectively analysed. Multivariate analysis was performed to account for potential confounders in the form of cardiovascular risk factors, which are particularly relevant when investigating chemoprophylactic benefits of drugs classes which are best known for their metabolic benefits. Statistical analysis with COX proportional hazards with time-dependent covariates allowed survival analysis of drug exposure over time, allowing analysis despite discontinuation and resumption of drug. Another strength of this study is the inclusion of statin dosing to account for any potential dose dependent effects as well as sensitivity analyses to account for immortal time bias, inherent to survival analyses.
Limitations of this study are that this was a single centre study with small numbers, unlikely to be sufficiently powered to optimally demonstrate a benefit for drug therapy on post-LT HCC-related outcomes. This study focused on an Australian cohort, which may not accurately reflect global trends or practice variations in LT or HCC management in international centres. As this study examined a largely historic patient population, there was a large proportion of patients with HCV and a low proportion of patients with NAFLD. This may limit generalisability of this study in the current era, due to widespread availability of effective antiviral therapy for HCV and an ever-increasing proportion of patients with NAFLD who require LT[33]. More research is required to understand the role of chemoprophylaxis in patients with NAFLD and HCC, both on risk of tumour related outcomes as well as post-LT cardiovascular risk modification.
Another limitation is that the type and dosage of most drugs, were not analysed for this study. Analysis on the type of statin used, whether lipophilic or hydrophilic may help further clarify any potential effect on HCC-related outcomes. With regards to anti-platelet agents, more granular data on the effects of mono- or combination therapy with other agents of the GPIIb/IIIa class such as clopidogrel or ticagrelor, may give further insights into their potential benefits, if present.
Going forward, a prospective, multi-centre study in a larger patient cohort, taking into consideration drug types and dosages is required to further understand the effect of chemoprophylaxis on HCC-related outcomes post-LT.
Statin, metformin and aspirin use was not associated with improvement of HCC-related outcomes post-LT in a largely historic Australian cohort, with a low prevalence of NAFLD. Further prospective, multicentre studies are required to clarify any potential benefit of these drugs, especially with regards to drug dosage and type, in patients with NAFLD.
| 1. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1017] [Article Influence: 339.0] [Reference Citation Analysis (0)] |
| 2. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5115] [Article Influence: 465.0] [Reference Citation Analysis (0)] |
| 3. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5299] [Article Influence: 182.7] [Reference Citation Analysis (0)] |
| 4. | Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, Bongini M, Cucchetti A. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 470] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 5. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1691] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 6. | Toso C, Mentha G, Majno P. Liver transplantation for hepatocellular carcinoma: five steps to prevent recurrence. Am J Transplant. 2011;11:2031-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Chou HS, Cheng CH, Hung HC, Lee JC, Wang YC, Wu TH, Lee CF, Wu TJ, Chan KM, Lee WC. Significance of Hepatitis B Recurrence in Liver Transplantation Recipients. Biomed Res Int. 2020;2020:2489526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 8. | Rajendran L, Ivanics T, Claasen MP, Muaddi H, Sapisochin G. The management of post-transplantation recurrence of hepatocellular carcinoma. Clin Mol Hepatol. 2022;28:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Alizadeh J, Zeki AA, Mirzaei N, Tewary S, Rezaei Moghadam A, Glogowska A, Nagakannan P, Eftekharpour E, Wiechec E, Gordon JW, Xu FY, Field JT, Yoneda KY, Kenyon NJ, Hashemi M, Hatch GM, Hombach-Klonisch S, Klonisch T, Ghavami S. Mevalonate Cascade Inhibition by Simvastatin Induces the Intrinsic Apoptosis Pathway via Depletion of Isoprenoids in Tumor Cells. Sci Rep. 2017;7:44841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Khajeh E, Moghadam AD, Eslami P, Ali-Hasan-Al-Saegh S, Ramouz A, Shafiei S, Ghamarnejad O, Dezfouli SA, Rupp C, Springfeld C, Carvalho C, Probst P, Mousavizadeh SM, Mehrabi A. Statin use is associated with the reduction in hepatocellular carcinoma recurrence after liver surgery. BMC Cancer. 2022;22:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Lee HL, Lee SW, Jang JW, Bae SH, Choi JY, Yoon SK, Choi HJ, Na GH, You YK, Park IY, Kim DG. Anticancer Effect of Statins in Patients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Liver Transpl. 2022;28:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Cho Y, Kim MS, Nam CM, Kang ES. Statin Use is Associated with Decreased Hepatocellular Carcinoma Recurrence in Liver Transplant Patients. Sci Rep. 2019;9:1467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Lee O, Rhu J, Choi GS, Kim JM, Kim K, Joh JW. Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver Transplantation. Ann Transplant. 2022;27:e935604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Becchetti C, Dirchwolf M, Schropp J, Magini G, Müllhaupt B, Immer F, Dufour JF, Banz V, Berzigotti A, Bosch J; Swiss Transplant Cohort Study. Use of statins after liver transplantation is associated with improved survival: results of a nationwide study. Aliment Pharmacol Ther. 2022;56:1194-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Abdelmalak J, Tan N, Con D, Eslick G, Majeed A, Kemp W, Roberts SK. The Effect of Aspirin Use on Incident Hepatocellular Carcinoma-An Updated Systematic Review and Meta-Analysis. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Simon TG, Ma Y, Ludvigsson JF, Chong DQ, Giovannucci EL, Fuchs CS, Meyerhardt JA, Corey KE, Chung RT, Zhang X, Chan AT. Association Between Aspirin Use and Risk of Hepatocellular Carcinoma. JAMA Oncol. 2018;4:1683-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 17. | Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 2017;60:1639-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 217] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 18. | Cauchy F, Mebarki M, Leporq B, Laouirem S, Albuquerque M, Lambert S, Bourgoin P, Soubrane O, Van Beers BE, Faivre S, Bedossa P, Paradis V. Strong antineoplastic effects of metformin in preclinical models of liver carcinogenesis. Clin Sci (Lond). 2017;131:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Shen C, Peng C, Shen B, Zhu Z, Xu N, Li T, Xie J. Sirolimus and metformin synergistically inhibit hepatocellular carcinoma cell proliferation and improve long-term survival in patients with HCC related to hepatitis B virus induced cirrhosis after liver transplantation. Oncotarget. 2016;7:62647-62656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Chung YK, Hwang S, Song GW, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, Jung DH, Park GC, Ryoo BY, Lee SG. Absence of antitumor effects of metformin in sorafenib-treated patients with hepatocellular carcinoma recurrence after hepatic resection and liver transplantation. Ann Hepatobiliary Pancreat Surg. 2018;22:297-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Cho WR, Wang CC, Tsai MY, Chou CK, Liu YW, Wu YJ, Lin MT, Chen KD, Chuang CH, Huang PY, Hu TH, Tsai MC. Impact of metformin use on the recurrence of hepatocellular carcinoma after initial liver resection in diabetic patients. PLoS One. 2021;16:e0247231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1566] [Article Influence: 92.1] [Reference Citation Analysis (1)] |
| 23. | Terrault NA, Francoz C, Berenguer M, Charlton M, Heimbach J. Liver Transplantation 2023: Status Report, Current and Future Challenges. Clin Gastroenterol Hepatol. 2023;21:2150-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 124] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 24. | Choi MC, Min EK, Lee JG, Joo DJ, Kim MS, Kim DG. Antiplatelet Drugs on the Recurrence of Hepatocellular Carcinoma after Liver Transplantation. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Ma SJ, Zheng YX, Zhou PC, Xiao YN, Tan HZ. Metformin use improves survival of diabetic liver cancer patients: systematic review and meta-analysis. Oncotarget. 2016;7:66202-66211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Siegel AB, Lim EA, Wang S, Brubaker W, Rodriguez RD, Goyal A, Jacobson JS, Hershman DL, Verna EC, Zaretsky J, Halazun K, Dove L, Brown RS Jr, Neugut AI, Kato T, Remotti H, Coppleson YJ, Emond JC. Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation. Transplantation. 2012;94:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Mathur A, Franco ES, Leone JP, Osman-Mohamed H, Rojas H, Kemmer N, Neff GW, Rosemurgy AS, Alsina AE. Obesity portends increased morbidity and earlier recurrence following liver transplantation for hepatocellular carcinoma. HPB (Oxford). 2013;15:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | El-Domiaty N, Saliba F, Karam V, Sobesky R, Ibrahim W, Vibert E, Pittau G, Amer K, Saeed MA, Shawky JA, Cherqui D, Adam R, Samuel D. Impact of body mass index on hepatocellular carcinoma recurrence after liver transplantation through long-term follow-up. Hepatobiliary Surg Nutr. 2021;10:598-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | McHugh PP, Gilbert J, Vera S, Koch A, Ranjan D, Gedaly R. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB (Oxford). 2010;12:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Vivarelli M, Cucchetti A, La Barba G, Ravaioli M, Del Gaudio M, Lauro A, Grazi GL, Pinna AD. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg. 2008;248:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 31. | Fan GH, Zhang CZ, Gao FQ, Wei XY, Ling SB, Wang K, Wang JG, Zheng SS, Nikfarjam M, Xu X. A mixed blessing for liver transplantation patients - Rapamycin. Hepatobiliary Pancreat Dis Int. 2023;22:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Geissler EK, Schnitzbauer AA, Zülke C, Lamby PE, Proneth A, Duvoux C, Burra P, Jauch KW, Rentsch M, Ganten TM, Schmidt J, Settmacher U, Heise M, Rossi G, Cillo U, Kneteman N, Adam R, van Hoek B, Bachellier P, Wolf P, Rostaing L, Bechstein WO, Rizell M, Powell J, Hidalgo E, Gugenheim J, Wolters H, Brockmann J, Roy A, Mutzbauer I, Schlitt A, Beckebaum S, Graeb C, Nadalin S, Valente U, Turrión VS, Jamieson N, Scholz T, Colledan M, Fändrich F, Becker T, Söderdahl G, Chazouillères O, Mäkisalo H, Pageaux GP, Steininger R, Soliman T, de Jong KP, Pirenne J, Margreiter R, Pratschke J, Pinna AD, Hauss J, Schreiber S, Strasser S, Klempnauer J, Troisi RI, Bhoori S, Lerut J, Bilbao I, Klein CG, Königsrainer A, Mirza DF, Otto G, Mazzaferro V, Neuhaus P, Schlitt HJ. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016;100:116-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 348] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 33. | Pais R, Barritt AS 4th, Calmus Y, Scatton O, Runge T, Lebray P, Poynard T, Ratziu V, Conti F. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016;65:1245-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 323] [Article Influence: 35.9] [Reference Citation Analysis (1)] |