Published online Sep 18, 2024. doi: 10.5500/wjt.v14.i3.91637
Revised: June 14, 2024
Accepted: July 2, 2024
Published online: September 18, 2024
Processing time: 212 Days and 0.9 Hours
Heart transplantation (HT), the treatment choice of advanced heart failure pa
Core Tip: Heart transplantation is proven effective in increasing the survival and functional status of the recipients, but compared to normal controls, their functional status is lower. Exercise is shown to improve exercise capacity and its cessation causes the loss of its benefits. Cardiac denervation and immunosuppressive agents used in heart transplantation recipients result in cardiovascular, pulmonary, exercise capacity, psychological, and quality of life problems. Functional improvement is mainly due to musculoskeletal and cardiovascular adaptations. The greatest improvement in exercise capacity was found in recipients given supervised high-intensity training. Quality of life improvement resulted from the improvement of exercise capacity and symptoms.
- Citation: Nazir A. Exercise as a modality to improve heart transplantation-related functional impairments: An article review. World J Transplant 2024; 14(3): 91637
- URL: https://www.wjgnet.com/2220-3230/full/v14/i3/91637.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i3.91637
Heart transplantation (HT) is the treatment choice of advanced heart failure (HF) patients in those for whom the re
The goal of HT is to restore an active lifestyle and improve the quality of life (QoL). In the first year, 45% of recipients are readmitted to the hospital, and after one year 20%-25% of recipients need yearly hospitalization for diagnosis[3]. The benefits of HT on functional status are enabling most patients to return to normal life, and prevent limitations in daily activity by reducing symptoms and improving exercise capacity[4]. Although the benefits of HT are widely proven, compared to healthy controls, exercise capacity and physical performance of HT recipients were lower. The same result was shown in the QoL, especially in work performance[5-9].
Cardiac rehabilitation (CR) is a multidisciplinary approach recommended for HT patients, both before and after surgery. Exercise given in CR has proven benefits in improving patients’ functional status, reducing the severity of cardiac allograft vasculopathy (CAV), reducing risk and complications of other cardiovascular diseases, and decreasing rehospitalization and even death[10]. A decreased risk of readmission one year after discharge is considered a benefit of CR. However, the participation rate among HT recipients in the United States is only approximately 50%[11].
HT recipients have abnormal physiological responses to both acute and regular exercise due to pathological changes in the cardiovascular and musculoskeletal systems. The underlying HF and hospital admission after the surgery contributed to these responses[7,12]. The abnormal physiological responses to exercise are associated with chronotropic incompetence that decreases heart rate (HR) response to exercise and peak HR post HT[13]. Studies regarding the physiological effects of exercise in HT recipients have shown that exercise capacity as measured with peak oxygen uptake (VO2 peak) and muscle strength increased after completion of the program. Despite that, there is also evidence that the benefits of exercise will decrease or be lost after cessation of exercise. These findings should become a consideration to continue exercise to maintain long-term effects[14-16]. Extensive literature has described exercise in HT recipients and this review seeks to describe exercise from a physical and rehabilitation medicine point of view by describing functional disorders in HT recipients and how exercise can improve their condition.
HT is indicated to HF patients with cardiogenic shock with a low probability of recovery, NYHA classification of class IIIb or IV, severe functional limitations, coronary artery disease with refractory angina with Canadian Cardiovascular Score class II or IV despite optimal therapy, localized cardiac tumors with a low probability of metastasis, and recurrent life-threatening ventricular arrhythmias despite optimal therapy[2]. Left ventricular ejection fraction (LVEF) < 35%, acute myocarditis, restrictive cardiomyopathy or severe symptomatic hypertrophic, congenital heart disease with no compli
Long-term complications of HT include CAV, rejection, arrhythmias, infection, and side effects of immunosuppressive agents such as malignancy, nephrotoxicity, and drug-drug interaction[1,2,17]. Progressive graft vasculopathy and peripheral vasculopathy are associated with increased cardiovascular risk and death in HT recipients. These pathologies are associated with endothelial dysfunction due to a reduction in arterial compliance that is caused by deterioration of peripheral vasodilatation, deposition of collagen, decreased elastin, and sympathetic hyperactivity[18]. Transplant rejection is also considered as the major cause of death, and the use of immunosuppressive agents to prevent organ rejection results in further worse complications[1,4]. About 30% of deaths within the first year after transplantation occur due to infection[4]. CPET is suggested to be performed during the first annual screening of HT recipients because it can identify patients at high risk of developing advanced CAV[19].
Cardiovascular alterations in HT recipients are chronotropic incompetence, impaired diastolic function, high resting HR, reduced maximum cardiac output (CO), endothelial dysfunction, increased sympathetic activation, and increased peak exercise systemic vascular resistance[7]. A reduced HR response and reduction of peak HR after transplantation resulted in lower VO2max in HT recipients than in normal controls. This was associated with chronotropic incompetence due to cardiac denervation[13]. Cardiac denervation results in a higher normal resting HR and a delayed and blunted increase during exercise[20].
At supine rest, HT recipients have a high total peripheral resistance and blood pressure, while, during an orthostatic challenge, HT recipients are found to have preserved total peripheral resistance and blood pressure responses. In a standing position, HT recipients are unable to increase their HR and cardiac output further, causing attenuation of the cardiac output resulting in symptoms and a cold hand[21]. HR recovery in HT recipients is impaired. A study found that HR recovery at the first minute and the second minute was 7 beats and 14 beats, respectively. This is equivalent to fourfold lower than normal individuals with 30 beats and 52 beats in the first minute and the second minute, respectively[15].
HT recipients have a 50% elevation of systemic vascular resistance (SVR) resulting from impaired endothelial-depen
HF is associated with a restrictive pattern of pulmonary disorders presented as a decrease in pulmonary function[22]. Severe chronic lung disease in HT patients increases HT complications and decreases functional capacity and survival rate after transplantation. Pulmonary dysfunction due to immunosuppressive agents results in increased pulmonary infection risk. Lower pulmonary function was also associated with prolonged ventilator use[2]. As per response to exercise, HF causes an excessive ventilatory response to exercise due to an increase in wasted ventilation[13].
A study found that HT surgery results in an improvement of forced expiration volume in one second and forced vital capacity. However, respiratory muscle strength remained below the normal predicted value as presented by maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), and peak cough flow (PCF)[22]. Another study found that HT increased pulmonary function to normal or near normal values, but transfer factors and transfer coefficient remained low. The presence of pulmonary edema before HT was considered as a cause[13]. Pulmonary function changes after HT are decreased lung diffusion capacity for carbon monoxide and abnormal gas exchange, which further limit exercise capacity, leading to exercise-induced hypoxemia and exercise intolerance[8].
Exercise capacity is defined as the maximum amount of physical exertion that can be sustained by an individual, which reflects the cardiovascular system function. Exercise intolerance, on the other hand, is defined as the inability to perform physical activities without symptoms of dyspnea and/or fatigue due to impaired exercise capacity, which is the main impairment found in patients with HF. Exercise capacity can be measured by CPET which is the gold standard measure
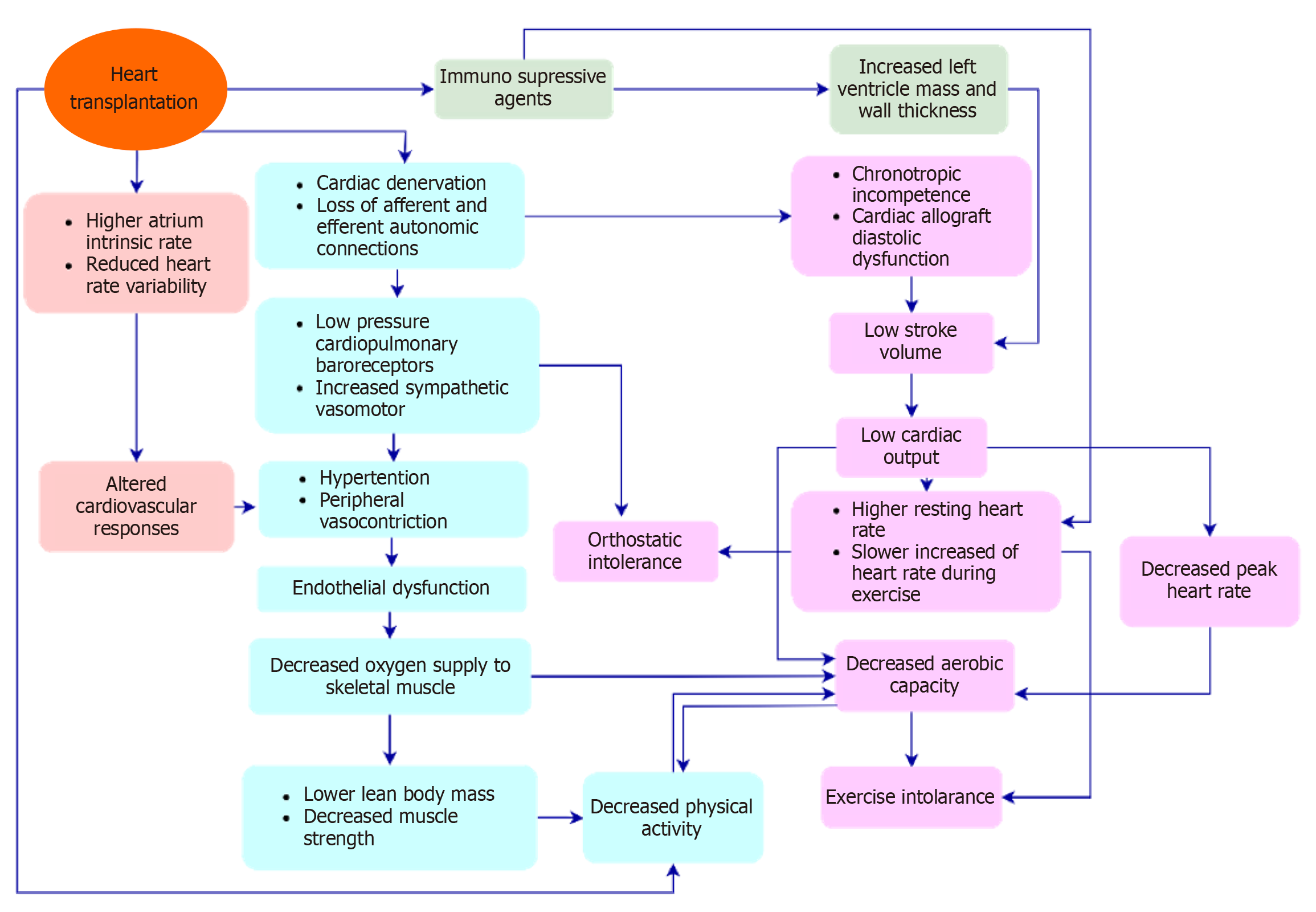
HT recipients have a below-predicted VO2 peak due to several factors. A decrease in exercise capacity in HT recipients is schematically described in Figure 1[7,20,21]. Cardiovascular and skeletal muscle abnormalities result in lower exercise capacity in HT recipients[7,17,20]. Surgical excision in HT results in the denervation of parasympathetic and sympathetic nerve fibers in the heart. Cardiac denervation is responsible for decreased CO, exercise capacity, nocturnal decline in blood pressure, a slower increase in HR during exercise, and higher resting HR early post-HT. Cardiac denervation decreases the catecholamine stores in the myocardium and as a result, the heart relies on non-cardiac circulating ca
Total body and leg mass and muscle strength were associated with decreased exercise capacity[20,22]. Decreased skeletal muscle function contribute to exercise intolerance because the majority of oxygen consumption during exercise occurs in the exercising muscles. Maximal arteriovenous oxygen difference was lower in HT recipients than in normal age-matched healthy controls[20].
The underlying HF causes pre-transplantation skeletal muscle dysfunction, which results in decreased muscle strength[7]. Skeletal muscle dysfunction presents as fiber atrophy and contractile dysfunction. The mechanisms that contribute to skeletal muscle atrophy in HF are dysregulation of myokine expression, inability to activate pro-hypertrophic pathway or anabolic resistance, impaired mitochondrial dynamics in regulating energy metabolism, and impairment of satellite cells responsible for muscle growth. Aging and physical inactivity in HF patients also contribute to skeletal muscle dys
In HT recipients, muscle atrophy is caused mainly by adverse immunosuppressive agent effects and postoperative restrictions[22]. Musculoskeletal changes in HT recipients are decreased total body mass, reduced type 1 oxidative fiber, decreased oxidative enzyme capacity, decreased capillary/fiber ratio, decreased peak exercise arteriovenous oxygen di
A study found decreased skeletal muscle oxidative and beta-oxidative enzyme activities and glycolytic enzyme activity 2 mo after HT compared with those before transplantation. At one year, no restoration of skeletal muscle or oxidative or beta-oxidative enzyme activities occurred, while decreased oxidative muscle fibers, increased glycolytic enzyme activity, and increased glycolytic fatigable fibers were found. This finding suggests that skeletal muscle dysfunction persists within one year after HT[29].
In HT candidates, several factors cause a decrease in QoL, such as the burden of symptoms, the disabling consequences of the treatment protocol, and HF itself which will affect daily lives[30]. Specifically, psychological and social dysfunctions, persistent congestion, activation of neurohormonal response and inflammation, skeletal muscle dysfunction, decreased kidney function, right ventricular dysfunction, severely compromised hemodynamic state, and cachexia were found to be associated with decreased QoL. QoL decreased as NYHA classification worsened and exercise tolerance contributed to a marked decrease in QoL[31].
QoL is a parameter used to evaluate the long-term result of HT. Many HT recipients reported functional impairments in several aspects of QoL at one year after surgery. In addition to disorder in work performance, eating, social interaction, ambulation, home management, and recreation were also reported. There was only approximately a quarter of HT recipients who returned to work within one year and more than half (59%) among them reported problems that affected their work performance. Factors that contributed to this issue were the presence of symptoms, neurological problems, stress, depression, older age, female sex, and lower LVEF[7-9]. Hospital and intensive care unit admission also affect QoL domains. One study found that patients requiring extracorporeal membrane oxygenation pre- or post-HT had impaired physical function and leg complications at intensive care unit discharge[32]. Compared to advanced HF patients, HT recipients demonstrated higher scores in all QoL domains. The improvement of symptoms of dyspnea and exercise intolerance, a decrease in psychological problems, and an improvement in functional ability limitations are considered the reasons[33].
Exercise-based CR is recommended both before (pre-habilitation) and after HT. The reasons for this recommendation are: (1) HT recipients have a poor exercise capacity and deconditioning syndrome; (2) inactivity before and after HT causes deconditioning, which impairs the healing process and causes skeletal muscle dysfunction; and (3) the use of corticosteroid medications causing skeletal muscle dysfunction[4,10,34]. Exercise-based CR is shown to decrease the rehospitalization rate among HT recipients[4]. In addition, participation in exercise-based CR for ≥ 23 sessions was proven bene
Exercise in HT recipients is given as a part of CR that encompasses pre-rehabilitation to phase III CR. Exercise in pre-rehabilitation periods aims at reducing HT-related complications such as intensive care unit-acquired weakness and cardiac cachexia in addition to maintaining a higher fitness level[7,35]. Phase I CR is given to prevent the deleterious effects of prolonged bed rest with early mobilization. Early mobilization can be started soon after extubating. The program given includes kinesiotherapy and the prevention of respiratory infection. In-bed exercise such as passive range of motion can be given initially and progress to seated exercise, standing, and walking. Exercise with cycle ergometry, starting with resistance-free and slow-pace treadmill walking, can be given after in-bed exercise is tolerated by the patients. Regular exercise program given in phase II CR may begin early after HT (from the second or third week) and be given for 6-8 wk. Phase III CR is an intervention that aims to maintain the benefits of exercise given in phase II and promote life-long healthy behavior. Exercise is provided in the form of a home exercise program with activities similar to early phase II CR[7,36].
Resistance exercise is given along with aerobic exercise to counteract the effect of immunosuppressive agents on skeletal muscles[37]. Aerobic exercise recommendation for HT recipients can be given with a frequency of 3–5 d per week, with an intensity of 80%-90% of maximum HR or 60% to 70% of VO2 peak. The duration of initial exercise is 20-60 min. Exercise at the beginning should be supervised, which then continues to non-supervised exercise[38]. Other com
Prescription of exercise must be tailored to the patients based on their recovery rate and current exercise capacity. Exercise programs should include aerobic and strengthening exercises for major muscle groups. Exercise dose (frequency, intensity, and duration) should progress gradually[10]. High-intensity interval training (HIIT) is the most common type of exercise given to HT recipients and given as a hospital-based program (Table 1).
| Ref. | Exercise intervention | Exercise protocol | |||||
| Frequency | Intensity and time | Type | Warm-up | Cool-down | Program duration | ||
| Torto et al[53], 2022 | Single-leg vs double-leg HIIT | 3 sessions per week | Two kinds of HIIT, done alternately. 4-min exercise bouts at high intensity alternated by 3-min rest at low intensity, 4 sets, 12 bouts. 2-min exercise bouts at high intensity alternated by 2-min rest at low intensity, 6 sets, 12 bouts | Ergocycle, pedaling frequency 60-75 RPM | 5 min | 5 min | 8 wk |
| Rustad et al[40], 2014 | HIIT vs usual exercise | 3 sessions per week | 4-min exercise bouts at 85%-95% of HR peak alternated by 3-min rest equal to Borg scale of 11-13, 4 bouts | Treadmill uphill walking or running | 10 min | 3 episodes of 8-wk exercise | |
| Dall et al[15], 2014 | HIIT vs MICT | 3 sessions per week | HIIT: 4-, 2-, and 1-min bouts of exercise at > 80% of VO2 peak alternated by a 2-min rest at 60% of VO2 peak, 32 min | Ergocycle | 10 min | 10 min | 12 wk |
| MICT; 60%-70% of VO2 peak, 45 min | |||||||
| Yardley et al[14], 2017 | HIIT vs usual care | 3 sessions per week | 4-min exercise bouts at 85%–95% of HR max alternated by 3-min active recovery periods equal to Borg scale of 11–13, 4 bouts | Treadmill or ergocycle | 10 min | 10 min | 12 mo |
| Nytrøen et al[51], 2012 | HIIT vs usual care | 3 sessions per week | 4-min exercise bouts at 85%–95% of HR max alternated by 3-min active recovery periods equal to Borg scale of 11–13, 4 bouts | Treadmill | 10 min | 3 episodes of 8-wk exercise | |
| Hermann et al[43], 2011 | HIIT vs no exercise | 3 sessions per week | Initial HIIT 4-min, 2-min, 30-s exercise bouts at 80%, 85%, and 90% of VO2 peak consecutively, equal to Borg scale of 18–19, followed by half a minute of recovery period. After HIIT, 10-min staircase running with an intensity of 80% of VO2 peak. Total time: 42 min | Bicycle and staircase running | 10 min | 10 min | 8 wk |
| Nytrøen et al[16], 2012 | HIIT vs MICT and no exercise | 3 sessions per week | HIIT: Exercise bouts at 85%-95% of peak effort | Treadmill | 9 mo | ||
| MICT: Exercise bouts at 60%-80% of peak effort | |||||||
| Haykowsky et al[41], 2009 | Aerobic and strengthening exercises | Aerobic: 5 sessions per week during the first 8 wk. 3 sessions per week during the final 4 wk | Aerobic: During the first 8 wk: Exercise at HR equal to 60%-80% of VO2 peak for 30-45 min During the final 4 wk: Exercise at HR equal to 80% of VO2 peak for 45 min | Ergocycle | 12 wk | ||
| Strengthening: 2 sessions per week | Strengthening: 50% of maximal strength, 1-2 sets, 10-15 repetitions | Chest press, arm curls, latissimus dorsi pulldown, and leg press | |||||
| Karapolat et al[42], 2008 | Flexibility, aerobic, strengthening, breathing, and relaxation exercises | 3 sessions per week, 1.5 h exercise per session | Flexibility: Stretching and range of motion exercise | Applied to the trunk, upper extremities, and lower extremities joints | 8 wk | ||
| Aerobic: Exercise at 60%-70% of the VO2 peak equal to a Borg scale of 13-15, 30 min | Treadmill or ergocycle | ||||||
| Strengthening: Lightweight of 250-500 g, 2 wk after performing aerobic exercise | Abdominal, upper extremities, and lower extremities muscle groups | ||||||
| Pursed-lip breathing, synchronization of thoracic and abdominal movement, and expiratory abdominal augmentation | |||||||
| Jacobson’s progressive muscle relaxation | |||||||
| Hsu et al[52], 2011 | Hospital-based-aerobic exercise | 3 sessions per week | Exercise at 50%-80% of VO2 peak, 25-30 min | Combination of ergocycle and treadmill | 10 min | 10 min | 12 wk |
Data regarding the efficacy and safety of exercise in HT recipients, especially data on cardiovascular mortality, hospitalization rates, and side effects, are limited[5]. One study reported no adverse event related to exercise during the follow-up period[15]. A case study reported no complications or life-threatening events during CR that was given long-term with the HIIT protocol[22]. A systematic review and meta-analysis found that none of the included studies reported either cardiovascular or all-cause mortality. An adverse event of myocardial infarction in the usual care group was reported in one study that caused participants to be withdrawn from the study. No considerable adverse event was reported in the remaining studies[39].
Previous studies found that heart function parameters such as systolic and diastolic functions, left ventricular systolic function, and chronotropic variables were not significantly improved after exercise[40-42]. One study found an improve
In terms of improvement in HR recovery, a study found that HIIT resulted in a modest improvement. HIIT improved heightened reaction and shortened reaction time of the sinus node, leading to a faster HR recovery and higher HR peak, which is considered as a marked decrease of vagal activity. The HR recovery is also considered as an independent and powerful predictor of death. Improvement in HR recovery also occurred in the moderate intensity continuous training (MICT) group, but the improvement was marked in the HIIT group[15]. The impact of exercise on chronotropic function is due to an increase in chronotropic response and partial improvement in autonomic imbalance secondary to prevention of overactivation of the sympathetic system and improvement in HR variability, baroreflex sensitivity, and HR recovery[42]. Studies that examined vascular function in HT recipients were limited. Flow-mediated dilatation (FMD) was found to increase significantly in the HIIT group[43]. However, endothelial-independent and -dependent vasodilatation was unchanged in the group given a combination of aerobic and resistance exercises and no exercise[41].
Studies regarding pulmonary function in HT recipients are limited. Breathing exercises given to HT recipients were found to improve pulmonary function test parameters[44]. Another study that gave a combination of both HIIT and MICT aerobic and resistance exercises found no difference between groups on pulmonary function changes from baseline to 3-year follow-up. However, no data on changes in pulmonary function are available[45]. A previous case report found that aerobic exercise improved MIP, MEP, and PCF. The mechanism by which pulmonary function improves is increasing respiratory muscle strength, thoracic mobility, and the balance between chest and lung elasticity[22].
The physiological mechanisms of exercise capacity improvement are suggested to be due to the improvement of pe
The VO2 peak in HT recipients is 40%-50% lower than that of normal healthy subjects. VO2 peak is a powerful pro
A previous study found that VO2 peak increased significantly in HT recipients who attended CR, with an average increase in VO2 peak of 10.2% compared to those who did not[49]. One study gave a 3-mo CR and found an increase in VO2 peak of 10.5 mL/min/kg compared to baseline up to 1 year after HT[22].
The variation of increased exercise capacity value depends on the exercise intensity, baseline exercise capacity, and HT procedure including surgical techniques, drugs, and postoperative management[22]. HIIT was found superior to MICT in improving VO2 peak (Table 1). Improvement of VO2 peak was greater in the group receiving HIIT compared to MICT in the follow-up period of 12 wk[15]. The superiority of HIIT was assumed due to long exercise duration and high-intensity exercise bouts, as well as higher HR reserve and peak HR obtained with HIIT[50]. In addition to a greater improvement in VO2 peak, HIIT was superior to MICT due to greater improvement in peak heart rate and FMD. However, impro
The increase in VO2 peak with exercise in HT recipients may be due to cardiac changes such as reinnervation induced by exercise and skeletal muscle changes. HT recipients with reinnervation have greater exercise capacity than those without. Karapolat et al[42] found that the CR program for 8 wk provided a 19% increase in VO2 peak. In their study, the hospital exercise group had a better increase in VO2 peak than the home exercise group. Lack of improvement after exercise in the home-exercise group was suggested to be due to improper technique in conducting exercises.
Exercise has been proven to improve vascular endothelial function in addition to VO2 peak in chronic HF patients and those with impaired vascular endothelial function. Exercise also improves VO2 peak via increased bioavailability of nitric oxide, suppression of oxidative stress, and induction of vasodilation[7].
Muscle strength and muscular exercise capacity were the common parameters used in previous studies. When compared to MICT, the HIIT group demonstrated a higher maximum muscle strength at 1-year follow-up, but improvement in muscular exercise capacity was not significant in either group. This result was similar in the HIIT group compared to the no-exercise group[16]. In contrast to this result, another study found that quadriceps and hamstring muscle capacity significantly improved by 15% and 19% in the exercise group and control group, respectively, while, in the control group, no change was found[51]. A study on muscular endurance found that muscular endurance at 1-year and 3-year follow-ups was higher in the HIIT group compared to the MICT group[45]. Regarding body composition changes, Haykowsky et al[41] found that leg lean mass was significantly higher in the group given a combination of aerobic and resistance exercises compared to no exercise. Muscle exercise capacity and improvement in lean body mass were considered to have a role in increased exercise capacity[20]. The changes in skeletal muscle morphology and oxidative enzyme capacity after aerobic and resistance exercises increased oxygen utilization by the exercising muscles, resulting in greater improvement in exercise capacity[41].
Previous studies found varying results regarding QoL (Table 2). In studies that found significant changes in QoL after the intervention or during the follow-up periods, improvement of QoL was suggested to be due to increased exercise capacity, decreased symptoms as a result of pulmonary function and physical capacity improvement, improvement in anxiety and depression, and a relatively good physical fitness at the beginning of the study[14,44,45,52]. One study found no significant changes in QoL sum scores, but, in the general health sub-score, there was a significant between-group difference. Participants in the exercise group had a higher subjectively measured general health score[51]. A previous review stated that aerobic exercise in the form of HIIT is proven as an effective means to improve exercise capacity, resulting in improvement in the QoL and a reduction in anxiety and depression[47]. However, one systematic review and meta-analysis did not find a consistent effect of exercise-based CR on QoL. The beneficial effect of HIIT on short- and long-term anxiety is well-established in this study[39].
| Ref. | Aim(s) | Subjects | Outcomes | Results |
| Torto et al[53], 2022 | To compare the effect of SL HIIT and DL HIIT on pulmonary VO2 and HR kinetics in three groups of transplanted patients | 33 subjects underwent heart, kidney, and liver transplantation | Pulmonary VO2; HR kinetic; Exercise capacity | During moderate-intensity exercise: SL and DL were effective in improving pulmonary VO2 and HR kinetics; No difference between SL and DL in pulmonary VO2 and HR kinetics; During heavy–intensity exercise, SL was as effective as DL in improving exercise capacity |
| Rustad et al[40], 2014 | To investigate the effect of HIIT on exercise capacity and cardiac function | 52 subjects, 1-8 years after heart transplantation randomized equally to the exercise group (EG) and control group (CG) | Exercise capacity; Cardiac function (systolic and diastolic) was determined by echocardiography | HIIT effectively improved exercise capacity; No clinically important improvement in systolic and diastolic functions with HIIT |
| Dall et al[15], 2014 | To compare the effect of HIIT on VO2 peak, BP, HR rest, HR peak, HR recovery, HR reserve, and workload during exercise test | 17 adult stable HT recipients (≥ 12 mo after HT) were randomized into HIIT and CON | Primary outcome: VO2 peak; Secondary outcomes: BP, HR rest, HR peak, HR recovery, and HR reserve during exercise testing | The effect of HIIT on VO2 peak was superior to CON; Improved HR reserve and HR peak were only found in the HIIT group; Improved HR recovery in both groups; A marked loss of effects after 5 mo |
| Yardley et al[14], 2017 | To evaluate the continuity of HIIT and maintenance of exercise benefits on physical capacity long term after the intervention ended | 41 stable heart transplant recipients who underwent 12-mo HIIT were followed until 5 years | Physical activity; Physical capacity; Exercise variables; Muscular exercise capacity; Body composition and metabolic profile; HRQoL; Depression and anxiety | Both groups maintained moderate physical activity levels after 5 years; Both groups demonstrated equal aerobic performance and daily activities after 5 years; HIIT was associated with a significant increase in VO2 peak after 1 year and a smaller decline in VO2 peak after 5 years; No difference between the two groups at 5-year follow-up in decreased VO2 peak; There was a non-clinically significant increase in VE/VCO2 slope in the HIIT group after 5 years; No difference in muscular exercise capacity between the two groups after 5 years; HRQoL score was good and prevalence of depression was low in both groups after 5 years; Long-term anxiety symptoms was reduced in the HIIT group |
| Nytrøen et al[51], 2012 | To prove that HIIT would improve VO2 peak with a higher percent of predicted than previously shown in most studies; To investigate possible peripheral and central mechanisms behind an increase in VO2 peak | 52 stable heart transplant recipients were randomized into HIIT and control groups | VO2 peak; Muscle strength and muscular exercise capacity; Body composition; HRQoL | VO2 peak was significantly improved in the HIIT group and no changes in the control group. A predicted VO2 peak level of 89% was achieved and higher than previous studies; Muscular exercise capacity was significantly improved; No significant difference between groups in changes in body composition at the follow-up; No significant changes in HRQoL sum scores in both groups at the follow-up, but the general health sub-score was significantly different between groups |
| Hermann et al[43], 2011 | To investigate the long-term effect of HIIT on VO2 peak, FMD, BP, inflammation markers, and natriuretic peptide in HT recipients | 30 HT recipients at 12 mo of transplantation were randomized into exercise and control groups | VO2 peak; FMD; BP; Inflammation markers; Natriuretic peptide | VO2 peak and FMD increased significantly in the HIIT group compared to the control group; No correlation was noted between BP reduction and improvement in FMD in the HIIT group; CRP was significantly decreased in the HIIT group while there was no change in the control group; TNF-alpha and IL-6 concentrations were unchanged in both groups; No significant decrease in pro-BNP and a significant decrease in pro-ANP in the HIIT group; No change in the natriuretic peptide concentration in the control group |
| Nytrøen et al[16], 2012 | To report the effect of HITT vs MICT or no exercise among young HT recipients | 28 young subjects (< 40 years) from the previous two studies | The primary outcome was VO2 peak; Secondary outcomes were maximum muscle strength and muscular endurance | HIIT vs MICT: Increased VO2 peak and maximum muscle strength were higher in HIIT than MICT after 1 year; No significant difference between the two groups in muscular exercise capacity |
| HIIT vs no exercise: Increased VO2 peak and maximum muscle strength were higher in HIIT compared to the no exercise group; No significant difference between the two groups in muscular exercise capacity | ||||
| Haykowsky et al[41], 2009 | To investigate the effect of supervised aerobic exercise combined with strength training (SET) vs control with no training (NT) on VO2 peak, peripheral vascular function, LV systolic function, maximum strength, and lean mass in stable HT recipients | 43 stable HT recipients at 0.5 years or more post-surgery were randomized into two groups | Primary outcome: VO2 peak; Secondary outcomes: Brachial artery endothelial function, LV systolic function, maximum strength, and lean mass | VO2 peak and peak power output were higher in SET than in NT; LV systolic function was not different after intervention in both groups; Endothelial-independent or -dependent vasodilation was unchanged in both groups; Chest- and leg-press maximum strength was significantly increased after SET and no change in arm curl strength or latissimus dorsi pulldown; The lean mass of the leg was significantly higher after SET than NT |
| Karapolat et al[42], 2008 | To explore the effects of home-based (Group 1) and hospital-based exercise (Group 2) on chronotropic variables and exercise capacity in HT recipients | 42 subjects, randomized into two groups | Exercise capacity; Metabolic function; Chronotropic variables (chronotropic response and HR recovery) | Group 1: A significant difference in post-exercise VO2 peak and metabolic function; The difference between HR reserve pre- and post-exercise was significant; No significant differences in other chronotropic variables |
| Group 2: No significant change in all outcomes after the exercise | ||||
| Karapolat et al[44], 2013 | To investigate the effects of CR on pulmonary functions, exercise capacity, HRQoL, and psychological state of HF, HT, or LVAD patients | 46, 40, and 11 subjects diagnosed with end-stage HF, HT, and LVAD, respectively | Exercise capacity (VO2 peak); Pulmonary function (PFT); HRQoL (SF-36); Psychological state (BDI) and State-Trait Anxiety Inventory (STAI) | Pre- and post-exercise VO2 peak, pulmonary function test (FEV 1% and FVC%), SF-36, and depressive symptoms were significantly different in all three groups, but the ratio of FEV 1 to FVC was not significantly different; No significant differences in VO2 peak, PFT, SF-36, or BDI scores among the three groups; STAI scores in intergroup and intragroup assessments of the three groups were not significantly different |
| Rolid et al[45], 2020 | To compare effects of HIIT and MICT on biomarkers, pulmonary function, heart function, VO2 peak, muscle strength, daily PA, symptoms of anxiety and depression, and HRQoL after 1 year and 3 years | 78 HT recipients completed 1-year follow-up and 65 subjects completed 3-year follow-up | Biomarkers; Pulmonary function; Heart function; VO2 peak; Muscle strength; Daily PA; Symptoms of depression and anxiety; HRQoL | Changes in biomarker, pulmonary function, heart function, VO2 peak, daily PA, and mental and physical summary scores were not significantly different between groups from baseline to 3 years; No differences between the two groups from 1 year to 3 years; Both groups had a stable exercise capacity with a small decline in VO2 peak; HIIT group showed a higher change in VO2 peak from baseline to 1 year; Muscle endurance improved significantly from baseline to 1 year and remained significantly higher at 3 years in both groups. An improvement was higher in the HIIT group; The median value of HRQoL in physical and mental components was > 50 in both groups. Physical component scores were changed in both groups, while mental component scores remained high and stable after 3 years. Symptoms of anxiety were low in both groups and no between-group difference from baseline to the 3-year follow-up |
| Hsu et al[52], 2011 | To investigate the effect of the phase 2 CR program on exercise capacity and HRQoL; To test the hypothesis (the peak physical capacity achieved after training is not a major determinant of HRQoL) | 45 clinically stable HT recipients and 34 CABG patients who completed a phase II CR | VO2 peak; HRQoL | An early CR program improved VO2 peak and HRQoL significantly; Improvement of HRQoL was greater in the HT group compared to CABG; The relative increase in physical capacity is the major determinant of HRQoL |
Several factors related to cardiac denervation and the use of immunosuppressive agents in HT recipients result in func
The author would like to thank Padjadjaran University for database facilitation.
| 1. | Alraies MC, Eckman P. Adult heart transplant: indications and outcomes. J Thorac Dis. 2014;6:1120-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 2. | Casscells SW. Chondromalacia of the patella. J Pediatr Orthop. 1982;2:560-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Stehlik J, Kobashigawa J, Hunt SA, Reichenspurner H, Kirklin JK. Honoring 50 Years of Clinical Heart Transplantation in Circulation: In-Depth State-of-the-Art Review. Circulation. 2018;137:71-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 4. | Wilhelm MJ. Long-term outcome following heart transplantation: current perspective. J Thorac Dis. 2015;7:549-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 5. | Anderson L, Nguyen TT, Dall CH, Burgess L, Bridges C, Taylor RS. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst Rev. 2017;4:CD012264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Grupper A, Gewirtz H, Kushwaha S. Reinnervation post-heart transplantation. Eur Heart J. 2018;39:1799-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Kourek C, Karatzanos E, Nanas S, Karabinis A, Dimopoulos S. Exercise training in heart transplantation. World J Transplant. 2021;11:466-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | McCartney SL, Patel C, Del Rio JM. Long-term outcomes and management of the heart transplant recipient. Best Pract Res Clin Anaesthesiol. 2017;31:237-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Carvalho WDN, Alves Maria GDS, Gonçalves KC, Miranda AL, Moreira MDCV. Health-Related Quality of Life of Heart Transplant Recipients Living in a Developing Country. Transplant Proc. 2021;53:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 10. | Squires RW, Bonikowske AR. Cardiac rehabilitation for heart transplant patients: Considerations for exercise training. Prog Cardiovasc Dis. 2022;70:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 11. | Bachmann JM, Shah AS, Duncan MS, Greevy RA Jr, Graves AJ, Ni S, Ooi HH, Wang TJ, Thomas RJ, Whooley MA, Freiberg MS. Cardiac rehabilitation and readmissions after heart transplantation. J Heart Lung Transplant. 2018;37:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Squires RW. Cardiac transplant and exercise cardiac rehabilitation. Heart Fail Rev. 2023;28:1267-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Carter R, Al-Rawas OA, Stevenson A, Mcdonagh T, Stevenson RD. Exercise responses following heart transplantation: 5 year follow-up. Scott Med J. 2006;51:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Yardley M, Gullestad L, Bendz B, Bjørkelund E, Rolid K, Arora S, Nytrøen K. Long-term effects of high-intensity interval training in heart transplant recipients: A 5-year follow-up study of a randomized controlled trial. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Dall CH, Snoer M, Christensen S, Monk-Hansen T, Frederiksen M, Gustafsson F, Langberg H, Prescott E. Effect of high-intensity training versus moderate training on peak oxygen uptake and chronotropic response in heart transplant recipients: a randomized crossover trial. Am J Transplant. 2014;14:2391-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Nytrøen K, Rolid K, Yardley M, Gullestad L. Effect of high-intensity interval training in young heart transplant recipients: results from two randomized controlled trials. BMC Sports Sci Med Rehabil. 2020;12:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Kim IC, Youn JC, Kobashigawa JA. The Past, Present and Future of Heart Transplantation. Korean Circ J. 2018;48:565-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 18. | de Souza JAF, Araújo BTS, de Lima GHC, Dornelas de Andrade A, Campos SL, de Aguiar MIR, Carneiro RMD, Brandão DC. Effect of exercise on endothelial function in heart transplant recipients: systematic review and meta-analysis. Heart Fail Rev. 2020;25:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Yu MD, Liebo MJ, Lundgren S, Salim AM, Joyce C, Zolty R, Moulton MJ, Um JY, Lowes BD, Raichlin E. Impaired Exercise Tolerance Early After Heart Transplantation Is Associated With Development of Cardiac Allograft Vasculopathy. Transplantation. 2020;104:2196-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Tucker WJ, Beaudry RI, Samuel TJ, Nelson MD, Halle M, Baggish AL, Haykowsky MJ. Performance Limitations in Heart Transplant Recipients. Exerc Sport Sci Rev. 2018;46:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Nygaard S, Christensen AH, Rolid K, Nytrøen K, Gullestad L, Fiane A, Thaulow E, Døhlen G, Godang K, Saul JP, Wyller VBB. Autonomic cardiovascular control changes in recent heart transplant recipients lead to physiological limitations in response to orthostatic challenge and isometric exercise. Eur J Appl Physiol. 2019;119:2225-2236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Choi HE, Kim C, Park SH. One-year follow-up of heart transplant recipient with cardiac rehabilitation: A case report. Medicine (Baltimore). 2020;99:e19874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, Garten R, Rodriguez-Miguelez P, Guazzi M, Lavie CJ, Abbate A. Exercise Intolerance in Patients With Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2209-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 24. | Lewis GD, Docherty KF, Voors AA, Cohen-Solal A, Metra M, Whellan DJ, Ezekowitz JA, Ponikowski P, Böhm M, Teerlink JR, Heitner SB, Kupfer S, Malik FI, Meng L, Felker GM. Developments in Exercise Capacity Assessment in Heart Failure Clinical Trials and the Rationale for the Design of METEORIC-HF. Circ Heart Fail. 2022;15:e008970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Hebisz P, Jastrzębska AD, Hebisz R. Real Assessment of Maximum Oxygen Uptake as a Verification After an Incremental Test Versus Without a Test. Front Physiol. 2021;12:739745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Malhotra R, Bakken K, D'Elia E, Lewis GD. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016;4:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 27. | Gallagher H, Hendrickse PW, Pereira MG, Bowen TS. Skeletal muscle atrophy, regeneration, and dysfunction in heart failure: Impact of exercise training. J Sport Health Sci. 2023;12:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Bekfani T, Bekhite Elsaied M, Derlien S, Nisser J, Westermann M, Nietzsche S, Hamadanchi A, Fröb E, Westphal J, Haase D, Kretzschmar T, Schlattmann P, Smolenski UC, Lichtenauer M, Wernly B, Jirak P, Lehmann G, Möbius-Winkler S, Schulze PC. Skeletal Muscle Function, Structure, and Metabolism in Patients With Heart Failure With Reduced Ejection Fraction and Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2020;13:e007198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | Pierce GL, Magyari PM, Aranda JM Jr, Edwards DG, Hamlin SA, Hill JA, Braith RW. Effect of heart transplantation on skeletal muscle metabolic enzyme reserve and fiber type in end-stage heart failure patients. Clin Transplant. 2007;21:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 30. | Jaarsma T, Johansson P, Agren S, Strömberg A. Quality of life and symptoms of depression in advanced heart failure patients and their partners. Curr Opin Support Palliat Care. 2010;4:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Nieminen MS, Dickstein K, Fonseca C, Serrano JM, Parissis J, Fedele F, Wikström G, Agostoni P, Atar S, Baholli L, Brito D, Colet JC, Édes I, Gómez Mesa JE, Gorjup V, Garza EH, González Juanatey JR, Karanovic N, Karavidas A, Katsytadze I, Kivikko M, Matskeplishvili S, Merkely B, Morandi F, Novoa A, Oliva F, Ostadal P, Pereira-Barretto A, Pollesello P, Rudiger A, Schwinger RH, Wieser M, Yavelov I, Zymliński R. The patient perspective: Quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol. 2015;191:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 32. | Hayes K, Holland AE, Pellegrino VA, Leet AS, Fuller LM, Hodgson CL. Physical function after extracorporeal membrane oxygenation in patients pre or post heart transplantation - An observational study. Heart Lung. 2016;45:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Carvalho WDN, Maria GDSA, Gonçalves KC, Miranda AL, Moreira MDCV. Comparison of Quality of Life Between Patients with Advanced Heart Failure and Heart Transplant Recipients. Braz J Cardiovasc Surg. 2021;36:623-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 34. | Uithoven KE, Smith JR, Medina-Inojosa JR, Squires RW, Olson TP. The Role of Cardiac Rehabilitation in Reducing Major Adverse Cardiac Events in Heart Transplant Patients. J Card Fail. 2020;26:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Velleca A, Shullo MA, Dhital K, Azeka E, Colvin M, DePasquale E, Farrero M, García-Guereta L, Jamero G, Khush K, Lavee J, Pouch S, Patel J, Michaud CJ, Shullo MA, Schubert S, Angelini A, Carlos L, Mirabet S, Patel J, Pham M, Urschel S, Kim KH, Miyamoto S, Chih S, Daly K, Grossi P, Jennings DL, Kim IC, Lim HS, Miller T, Potena L, Velleca A, Eisen H, Bellumkonda L, Danziger-Isakov L, Dobbels F, Harkess M, Kim D, Lyster H, Peled Y, Reinhardt Z. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2023;42:e1-e141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 268] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 36. | Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther. 2012;2:38-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 37. | Braith RW, Magyari PM, Pierce GL, Edwards DG, Hill JA, White LJ, Aranda JM Jr. Effect of resistance exercise on skeletal muscle myopathy in heart transplant recipients. Am J Cardiol. 2005;95:1192-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Guimarães GV, Ribeiro F, Arthuso FZ, Castro RE, Cornelissen V, Ciolac EG. Contemporary review of exercise in heart transplant recipients. Transplant Rev (Orlando). 2021;35:100597. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Costa R, Moreira E, Silva Cardoso J, Azevedo LF, Ribeiro JA, Pinto R. Effectiveness of Exercise-Based Cardiac Rehabilitation for Heart Transplant Recipients: A Systematic Review and Meta-Analysis. Health Serv Insights. 2023;16:11786329231161482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Rustad LA, Nytrøen K, Amundsen BH, Gullestad L, Aakhus S. One year of high-intensity interval training improves exercise capacity, but not left ventricular function in stable heart transplant recipients: a randomised controlled trial. Eur J Prev Cardiol. 2014;21:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Haykowsky M, Taylor D, Kim D, Tymchak W. Exercise training improves aerobic capacity and skeletal muscle function in heart transplant recipients. Am J Transplant. 2009;9:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Karapolat H, Eyigor S, Zoghi M, Yagdi T, Nalbantgil S, Durmaz B, Ozbaran M. Effects of cardiac rehabilitation program on exercise capacity and chronotropic variables in patients with orthotopic heart transplant. Clin Res Cardiol. 2008;97:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Hermann TS, Dall CH, Christensen SB, Goetze JP, Prescott E, Gustafsson F. Effect of high intensity exercise on peak oxygen uptake and endothelial function in long-term heart transplant recipients. Am J Transplant. 2011;11:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Karapolat H, Engin C, Eroglu M, Yagdi T, Zoghi M, Nalbantgil S, Durmaz B, Kirazlı Y, Ozbaran M. Efficacy of the cardiac rehabilitation program in patients with end-stage heart failure, heart transplant patients, and left ventricular assist device recipients. Transplant Proc. 2013;45:3381-3385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Rolid K, Andreassen AK, Yardley M, Gude E, Bjørkelund E, Authen AR, Grov I, Broch K, Gullestad L, Nytrøen K. Long-term effects of high-intensity training vs moderate intensity training in heart transplant recipients: A 3-year follow-up study of the randomized-controlled HITTS study. Am J Transplant. 2020;20:3538-3549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Turri-Silva N, Santos FV, Rodrigues WCC, Freire JS, Cahalin LC, Verboven K, Quaglioti Durigan JL, Hansen D, Cipriano G Jr. Impact of Exercise Modalities on Peripheral and Central Components of Cardiorespiratory Capacity in Heart Transplantation Patients: A Systematic Review and Meta-Analysis. Medicina (Kaunas). 2021;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 47. | Masarone D, Melillo E, Petraio A, Valente F, Gravino R, Verrengia M, Pacileo G. Exercise-based rehabilitation strategies in heart transplant recipients: Focus on high-intensity interval training. Clin Transplant. 2021;35:e14143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Dunlay SM, Allison TG, Pereira NL. Changes in cardiopulmonary exercise testing parameters following continuous flow left ventricular assist device implantation and heart transplantation. J Card Fail. 2014;20:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Didsbury M, McGee RG, Tong A, Craig JC, Chapman JR, Chadban S, Wong G. Exercise training in solid organ transplant recipients: a systematic review and meta-analysis. Transplantation. 2013;95:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 50. | Conceição LSR, Gois CO, Fernandes RES, Martins-Filho PRS, Gomes M Neto, Neves VR, Carvalho VO. Effect of High-Intensity Interval Training on Aerobic Capacity and Heart Rate Control of Heart Transplant Recipients: a Systematic Review with Meta-Analysis. Braz J Cardiovasc Surg. 2021;36:86-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Nytrøen K, Rustad LA, Aukrust P, Ueland T, Hallén J, Holm I, Rolid K, Lekva T, Fiane AE, Amlie JP, Aakhus S, Gullestad L. High-intensity interval training improves peak oxygen uptake and muscular exercise capacity in heart transplant recipients. Am J Transplant. 2012;12:3134-3142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 52. | Hsu CJ, Chen SY, Su S, Yang MC, Lan C, Chou NK, Hsu RB, Lai JS, Wang SS. The effect of early cardiac rehabilitation on health-related quality of life among heart transplant recipients and patients with coronary artery bypass graft surgery. Transplant Proc. 2011;43:2714-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Torto AD, Capelli C, Peressutti R, Di Silvestre A, Livi U, Nalli C, Sponga S, Amici G, Baccarani U, Lazzer S. The Effect of Endurance Training on Pulmonary V˙O(2) Kinetics in Solid Organs Transplanted Recipients. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |