Published online Jun 18, 2024. doi: 10.5500/wjt.v14.i2.93567
Revised: May 5, 2024
Accepted: May 20, 2024
Published online: June 18, 2024
Processing time: 105 Days and 10.6 Hours
Transplant recipients commonly harbor multidrug-resistant organisms (MDROs), as a result of frequent hospital admissions and increased exposure to antimicrobials and invasive procedures.
To investigate the impact of patient demographic and clinical characteristics on MDRO acquisition, as well as the impact of MDRO acquisition on intensive care unit (ICU) and hospital length of stay, and on ICU mortality and 1-year mortality post heart transplantation.
This retrospective cohort study analyzed 98 consecutive heart transplant patients over a ten-year period (2013-2022) in a single transplantation center. Data was collected regarding MDROs commonly encountered in critical care.
Among the 98 transplanted patients (70% male), about a third (32%) acquired or already harbored MDROs upon transplantation (MDRO group), while two thirds did not (MDRO-free group). The prevalent MDROs were Acinetobacter baumannii (14%), Pseudomonas aeruginosa (12%) and Klebsiella pneumoniae (11%). Compared to MDRO-free patients, the MDRO group was characterized by higher body mass index (P = 0.002), higher rates of renal failure (P = 0.017), primary graft dysfunction (10% vs 4.5%, P = 0.001), surgical re-exploration (34% vs 14%, P = 0.017), mechanical circulatory support (47% vs 26% P = 0.037) and renal replacement therapy (28% vs 9%, P = 0.014), as well as longer extracorporeal circulation time (median 210 vs 161 min, P = 0.003). The median length of stay was longer in the MDRO group, namely ICU stay was 16 vs 9 d in the MDRO-free group (P = 0.001), and hospital stay was 38 vs 28 d (P = 0.006), while 1-year mortality was higher (28% vs 7.6%, log-rank-χ2: 7.34).
Following heart transplantation, a predominance of Gram-negative MDROs was noted. MDRO acquisition was associated with higher complication rates, prolonged ICU and total hospital stay, and higher post-transplantation mortality.
Core Tip: We evaluated multidrug-resistant organisms (MDROs) in heart transplantation and their impact on patient outcome. Carbapenem-resistant Gram-negative bacteria predominated, in line with the epidemiologic pattern in south-eastern Europe. Among comorbidities, renal failure and higher body mass index were shown to be important risk factors pre-transplantation. Surgical and medical complications were shown to be predictive of MDRO acquisition, while no association was shown for the type of cardiomyopathy, for the mode of admission [from home, ward or intensive care unit (ICU)] or for previous cardiac surgery. MDRO presence was associated with longer ICU and hospital length of stay, and higher ICU-mortality and 1-year mortality.
- Citation: Hatzianastasiou S, Vlachos P, Stravopodis G, Elaiopoulos D, Koukousli A, Papaparaskevas J, Chamogeorgakis T, Papadopoulos K, Soulele T, Chilidou D, Kolovou K, Gkouziouta A, Bonios M, Adamopoulos S, Dimopoulos S. Incidence, risk factors and clinical outcome of multidrug-resistant organisms after heart transplantation. World J Transplant 2024; 14(2): 93567
- URL: https://www.wjgnet.com/2220-3230/full/v14/i2/93567.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i2.93567
In the south-eastern European countries, a higher prevalence of multidrug-resistant organisms (MDROs) is annually being reported in comparison to the northwest of Europe, pertaining mainly to multidrug-resistant (MDR) Gram-negative organisms[1]. High MDRO prevalence is strongly correlated with nosocomial infections[2]. Previous publications suggest an increased mortality in transplanted patients with MDRO colonization or bacteremia[3,4]. In concert with the patient safety guidelines of the World Health Organization, MDRO infections represent the occurrence of avoidable harm: Therefore MDRO prevalence must be recorded and its effects on patients measured[5].
There is a gap of knowledge on the impact of MDRO acquisition on heart transplant recipients in intensive care unit (ICU) environments with a high burden of gram-negative pathogens displaying advanced resistance patterns, for which limited treatment options are available.
We investigated whether MDRO presence negatively affects heart transplantation outcomes and constitutes a valid concern for patient safety, with an emphasis on the Gram-negative MDR pathogens, which are endemic in ICU environments in south-eastern Europe.
This is a retrospective 10-year study conducted at the Onassis Cardiac Surgery Center, a referral hospital for cardiomyopathies and heart failure. The hospital hosts the national heart transplantation program in Greece. The post-surgical care of heart transplant patients takes place in the 16-bed cardiac surgery ICU. The study protocol received approval from the hospital scientific and ethics committee (P.E.E 787/16.10.2023).
As per ICU microbiological surveillance protocol, colonization cultures were obtained from transplanted patients daily during the first week post transplantation, followed by three times per week during ICU stay. Nasal, pharyngeal and rectal swabs, bronchial secretions and urine samples were taken. Blood cultures were drawn as per clinical indication, i.e. following the occurrence of signs consistent with an inflammatory response or circulatory compromise. A specific pathogen isolated from sequential cultures from the same patient was analyzed as a single occurrence, given that recurrent identification of the same pathogen in the ICU is generally ascribed to pathogen tolerance and persistence mechanisms, rather than reinfection[6,7].
The MDROs reviewed were those frequently encountered in ICU care, which are currently under active monitoring (epidemiological surveillance), as per national and European guidelines[8]. These include carbapenem-resistant organisms, namely Acinetobacter baumannii, Klebsiella pneumoniae and Pseudomonas aeruginosa isolates, as well as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus sp (VRE).
The study comprised all 98 consecutive patients who underwent heart transplantation in our unit between 1/1/2013 and 31/12/2022 (10 years). Group comparison was carried out according to MDRO isolation from patient samples, i.e. patients were separated into two groups as per MDRO presence or MDRO absence.
The variables reviewed were: (1) Patient demographic characteristics; (2) Clinical pre-transplantation characteristics: Cause of cardiac failure, presence of ventricular assist device (VAD), comorbidities and acute physiologic derangement e.g. continuous renal replacement treatment (CRRT); (3) Route of admission at transplantation: Ambulant patient/admission from home; inotrope-dependent patient/admission from hospital ward; mechanical circulatory support patient/admission from cardiac ICU; (4) Operating procedure data: Duration of general anesthesia, duration of extracorporeal circulation, aorta closure time, transfusion requirements; (5) Post-transplantation clinical data: Days of mechanical ventilation, use of nitric oxide (NO) as a marker for refractory post-transplantation hypoxemia, days of CRRT, major medical and surgical complications; and (6) Microbiology data: Type of MDRO, MDRO presence upon transplantation or acquisition during ICU stay, donor-acquired MDRO. Outcome measures were ICU length of stay and total hospital length of stay post-transplantation, as well as ICU mortality and early (30-d) and late (1-year) mortality.
SPSS v.25 software was employed for statistical analysis. Categorical values are given as absolute numbers and percentages (relative frequency). The Kolmogorov-Smirnoff test was used to check whether continuous variables conformed to a normal distribution. Normally distributed continuous variables, namely weight, height and body mass index (BMI), are given as mean values with SD. Continuous variables non-normally distributed are given as median with percentiles (25-75th). Demographic and comorbidity data prior to transplantation were explored through descriptive statistics. The Chi-square test was used for the analysis of categorical variables. The Mann-Whitney test was used for the comparison of means of continuous variables with a non-normal distribution, while the t-test was used for normally distributed continuous variables. The Kaplan-Meier curve was used for 1-year post-transplantation survival analysis.
The population of heart transplant recipients consisted of 98 Caucasian patients, of whom 69 were male (70.4%) (Table 1). The median age was 49.5 years (range: 15-66 years). The mean patient BMI was 25.1 (range: 15.1-33); six patients fell within the cachexia range (BMI < 18.5), and seven patients within the obesity range (BMI ≥ 30). When comparing patients with or without MDRO presence, a statistically significant association between higher body weight/BMI and MRDO presence was found (P = 0.001), while age was not shown to have an impact.
| Heart transplant recipients | Total (n = 98) | MDRO presence (n = 32) | MDRO-free (n = 66) | P value |
| Demographics | ||||
| Age (yr) | 50 (37-56) | 51 (41-56) | 45.5 (35.5-56) | 0.48 |
| Sex (male) | 69 (70.4) | 25 (78.1) | 44 (66.7) | 0.24 |
| Height (cm), SD | 172 (9) | 174 (8) | 172 (9) | 0.25 |
| Body weight (kg), SD | 75 (15) | 81 (11) | 72 (16) | 0.002 |
| ΒΜΙ (kg/m2), SD | 25.1 (3.8) | 26.9 (3) | 24.3 (3.9) | 0.001 |
| Heart failure etiology | ||||
| Non-ischemic cardiomyopathy | 75 (76.5) | 23 (71.9) | 52 (78.8) | 0.45 |
| Ischemic cardiomyopathy | 22 (22.4) | 8 (25) | 14 (21.2) | 0.67 |
| Comorbidities | ||||
| Diabetes mellitus | 21 (21.4) | 8 (25) | 13 (19.7) | 0.54 |
| CRF (eGFR ≤ 60 mL/min/m2) | 13 (13.3) | 8 (25) | 5 (7.6) | 0.017 |
| Vasculopathy | 40 (40.8) | 17 (53.1) | 23 (34.8) | 0.08 |
| COPD | 5 (5.1) | 2 (6.3) | 3 (4.5) | 0.72 |
| Previous cardiac surgery | 18 (18.4) | 24 (75) | 38 (57.6) | 0.09 |
| Smoking history | 55 (56.7) | 21 (65.6) | 34 (52.3) | 0.21 |
| Status at transplantation | ||||
| VAD | 54 (55.1) | 22 (68.8) | 32 (48.5) | 0.06 |
| Admitted from home | 65 (66.3) | 20 (62.5) | 45 (68.2) | 0.10 |
| Admitted from ward | 15 (15.3) | 5 (15.6) | 10 (15.2) | 0.95 |
| Admitted from ICU | 18 (18.4) | 7 (21.9) | 11 (16.7) | 0.53 |
| Operating room | ||||
| General anesthesia (hours) | 7 (6-8) | 6.5 (6-8) | 7 (6-8) | 0.84 |
| Extracorporeal circulation (min) | 168 (144-229) | 210 (146-271) | 161 (141-193) | 0.003 |
| Aorta closure (min) | 115 (77-198) | 115 (85-130) | 115 (67-211) | 0.94 |
| Transfusion (RBC units) | 4 (2-10) | 9.5 (4-13) | 4 (2-7) | 0.001 |
| Post transplantation | ||||
| Primary graft dysfunction | 13 (13.3) | 10 (31) | 3 (4.5) | 0.001 |
| Surgical re-exploration | 20 (20.4) | 11 (34) | 9 (13.6) | 0.017 |
| Ventilator days | 2 (1-5) | 5 (3-3.5) | 1.3 (1-3) | 0.001 |
| NO for refractory hypoxemia | 25 (25.5) | 14 (43.8) | 11 (16.7) | 0.04 |
| CRRT for renal failure | 15 (15.3) | 9 (28) | 6 (9) | 0.014 |
| Post Tx mechanical circulatory support | 32 (32.7) | 15 (46.9) | 17 (25.8) | 0.037 |
| ATG treatment (days) | 5 (4-7) | 6 (5-8) | 5 (4-6) | 0.08 |
| Outcomes | ||||
| ICU stay, days | 10 (7-17) | 15.5 (10-26) | 9 (7-12) | 0.001 |
| Post transplantation total hospital stay, days | 30 (24-41) | 38 (25-62) | 28 (21-39) | 0.006 |
| Early mortality (30 d) | 9 (1) | 6 (18.8) | 3 (4.5) | 0.02 |
| Late mortality (1-year) | 14 (14.3) | 9 (28.1) | 5 (7.6) | 0.006 |
| Died in ICU | 12 (12.2) | 8 (25) | 4 (6) | 0.003 |
The reason for transplantation was non-ischemic cardiomyopathy in 76 patients (77.5%) and ischemic cardiomyopathy in 22 patients (22.4%). Of the former, 60 cases (61%) pertained to dilated cardiomyopathy, 10 cases (10.2%) to hypertrophic obstructive cardiomyopathy and 6 (6.1%) to other non-ischemic cardiomyopathies. Previous chemotherapy for hematologic neoplasia, mostly lymphoma, was the underlying cause for dilated cardiomyopathy in 9 patients (9.2%).
Over half of the patients (54/98 or 55%) were supported by a VAD on transplantation, equally divided between left ventricular and biventricular support (left ventricular assist device and bi-VAD respectively). Apart from these patients, an additional number of 8 patients had undergone cardiac surgery before transplantation, namely coronary artery bypass or valvular surgery. Therefore, the total number of patients with a history of cardiac surgery added up to 62 patients (63.3%).
Most patients were ambulant on admission (admitted from home: 65/98, 66.3%), of whom 16 were supported by a VAD (16.3% of total transplantations, 24.6% of home admissions). Upon transplantation, 33 patients were already hospitalized (33.7%). Of these, 15 were admitted from a cardiology ward (15.3% of total patients, 45% of hospitalized recipients), while 18 were admitted from the cardiac ICU (18.4% of total patients, 54.5% of hospitalized recipients). The patients admitted from a hospital ward had a median pre-transplantation hospitalization of 16 d (range: 2-520 d), while 9/15 (60%) were supported by a VAD. The patients admitted from a cardiac ICU had a median pre-transplantation ICU stay of 47 d (26-429), while 2/18 (11%) had a VAD in place, 14/18 (77.8%) were on circulatory support by intra-aortic balloon pump (IABP) and 1/98 was on Extracorporeal Membrane Oxygenation (ECMO) support and mechanically ventilated.
The type of cardiomyopathy, the presence of VAD or the mode of admission (home/ward/ICU) were not shown to differ between patients with MDROs and MDRO-free patients.
Pertaining to comorbidities, peripheral vasculopathy was the most prevalent condition (40.8%), followed by diabetes mellitus (21.4%) and chronic renal failure (13.3%). An additional 10% of patients had arterial hypertension without vasculopathy. Chronic obstructive pulmonary disease and sleep apnea was present in 5.1%. Nearly a quarter of patients (24.5%) had suffered a cerebrovascular accident prior to transplantation. The prevalence of dyslipidemia was 23.4%, while 56% of patients had a smoking history.
Renal failure was the only comorbidity shown to be related to MDRO presence in the recipient.
The median duration of general anesthesia was 7 h (range: 4-18), the median duration of extracorporeal circulation was 168 min (range: 99-413) and median aorta closure time was 115 min (range: 36-283). The median number of packed-red-cell units transfused was 4 (range: 0-41).
Extracorporeal circulation time was shown to differ across comparison groups, namely longer duration was associated with a greater MDRO prevalence in heart recipients. Similarly, larger transfusion requirements, a marker of surgical complications, were associated with a greater MDRO prevalence. No difference was shown for general anesthesia duration or mean aorta closure time.
Primary graft dysfunction occurred in 13 patients (13.3%). Mechanical circulatory support was required in 32 patients (32.7%). Of the latter, 26/32 (81%) were supported by IABP (median duration: 4 d, range: 1-39 d), of whom 7/26 (26.9%) died in ICU. ECMO support was needed for 11/32 patients (34%, median duration: 13.5 d, range: 1-26 d), of whom 8/11 (72.7%) died in ICU.
Major surgical complications occurred in 32 patients (32.7%). Surgical re-exploration was needed in 21/32 cases (65.6%), mainly due to hemorrhage (16/21, 76.2%) or tamponade (5/21, 23.8%). Delayed sternal closure was necessary in 18 patients (median open chest duration: 2 d, range: 1-20). Sternal wound debridement was carried out in 18/32 patients (18.4%), of whom 12 were open chest cases.
Major medical complications included acute renal failure requiring CRRT in 15 patients (median duration: 13 d, range: 1-33), major thromboembolism in 6 patients (6.1%) and multiple organ failure in 11 patients (11.2%). Septic shock occurred in 7 patients, of whom 5 had MDRO bacteremia.
Ventilator support exceeded 48 h post-surgery in 48/98 patients (49%), while re-intubation was necessary in 16/98 patients (16.3%). Post-transplantation refractory hypoxemia requiring treatment with inhaled NO was noted in 25/98 patients (25.5%). Median post-transplantation sedation was 40 h (range: 5-650). Median chest tube days were 7.5 (range: 6-39). Tracheostomy was performed on 8 patients (median duration: 12.5 d, range: 3-83).
Induction anti-thymocyte globulin (ATG) was used in all but 4 patients (96%, median duration: 6 d, range: 1-13).
Ventilator days, sedation hours and NO administration for refractory hypoxemia, right ventricle support were all associated with MDRO presence in the recipient. This was also true for primary graft dysfunction, post-transplantation mechanical circulatory support and any major surgical or medical complication, such as surgical re-exploration and renal replacement (CRRT). No impact was shown for the administration of ATG or its duration.
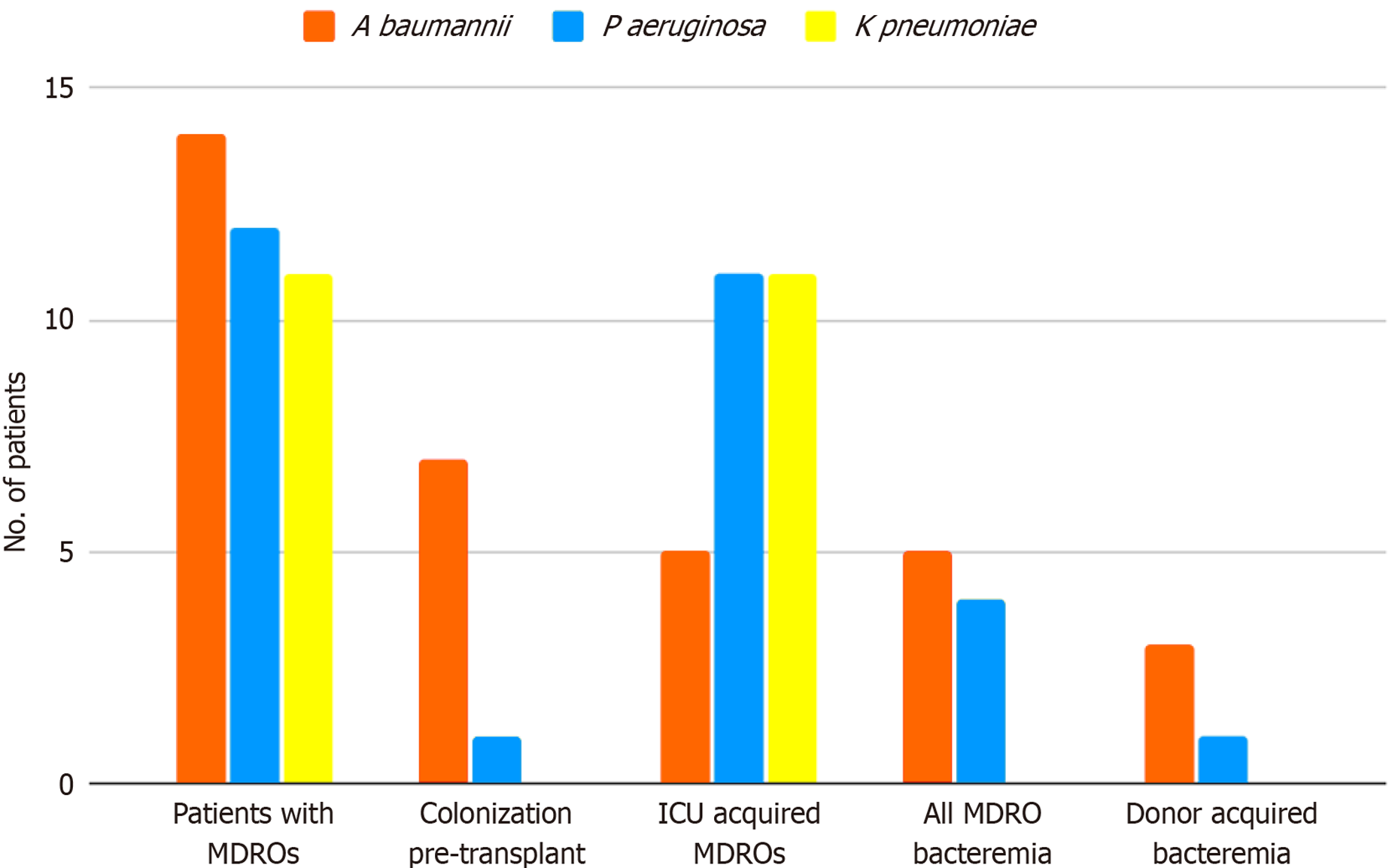
MDRO presence pertained almost exclusively to Gram-negative carbapenemase producing pathogens (94,8% of MDRO presence and 100% of MDRO bacteremias, Figure 1). Carbapenem-resistant Acinetobacter baumannii was the prevalent MDRO (36% of MDROs isolated), found in 14 patients (14.3% of all transplanted patients). Half of those already harbored Acinetobacter upon transplantation, five more acquired the organism in the ICU (median length of ICU stay before MDRO acquisition: 11 d, range: 8-33 d), while two acquired the pathogen from a donor subsequently found to have been bacteremic (blood cultures had been drawn from the donor upon organ procurement).
Carbapenem-resistant Pseudomonas aeruginosa followed in frequency (30.8% of MDROs isolated), found in 12 recipients (12.2%). Of those, 10 patients acquired the organism in ICU (median length of ICU stay for acquisition: 20 d, range: 2-31 d), one patient was already a carrier upon transplantation, while one acquired the pathogen from the donor.
Carbapenem-resistant Klebsiella pneumoniae (28.2% of MDROs isolated) was found in 11 patients (11.2%). All patients acquired the organism in ICU (median length of ICU stay for acquisition: 8 d, range: 3-40 d).
Overall, regarding the timeline of Gram-negative pathogen acquisition within the ICU, Klebsiella ICU acquisition generally preceded Acinetobacter acquisition, while Pseudomonas acquisition was a delayed event (median acquisition day: 8th for Klebsiella, 11th for Acinetobacter and 20th for Pseudomonas).
Two patients were found to harbor MRSA, acquired on days 2 and 5 of ICU hospitalization respectively. No VRE cases were recorded.
Gram-negative MDRO bacteremia occurred in 11 patients (11.2%): 5 were caused by A. baumannii, 4 by P. aeruginosa and 2 by K. pneumoniae. Four out of eleven bacteremic patients had received a graft from a donor subsequently found to be bacteremic on the day of procurement (3 cases of A. baumannii, 1 case of P. aeruginosa).
However, not all recipients of bacteremic donors developed bacteremia post transplantation: Four additional patients having received a heart from a donor with MDRO bacteremia (2 cases of A. baumannii and 2 cases of K. pneumoniae) did not develop bacteremia during the first month post transplantation. In total, eight patients received a heart from a MDRO bacteremic donor; half of these developed bacteremia by that pathogen. The characteristics and outcomes of these patients are summarized in Table 2. Nο bacteremia was recorded by gram-positive MDROs.
| Patient | 1 | 2 | 3 | 4 |
| Diagnosis | DCM | DCM | DCM | DCM |
| Age (yr) | 21 | 53 | 58 | 61 |
| Sex | M | F | M | M |
| Device pre-transplant | BiVAD | IABP | LVAD | LVAD |
| Comorbidities | - | DM | - | CRF, vasculopathy |
| Admitted from (days of stay pre-transplant) | Ward (3 d) | Cardiac ICU (123 d) | Home | Ward (36 d) |
| Recipient MDRO colonization pre-transplant | No | No | No | No |
| MDRO donor bacteremia | Acinetobacter baumannii | Acinetobacter baumannii | Acinetobacter baumannii | Pseudomonas aeruginosa |
| Post-transplant complications | Re-exploration for hemorrhage | IABP (3 d) | IABP (9 d), re-exploration for hemorrhage | - |
| Septic shock | No | No | Yes | No |
| Post-transplant ICU stay | 10 d | 8 d | 27 d (death) | 11 d |
| Post-transplant total hospital stay | 35 d | 50 d | 27 d (death) | 34 d |
| Outcome (1 yr) | Fully functional at home | Fully functional at home | Death (day 27) due to thromboembolism/MOF | Partially dependent at home, frequent readmissions |
Median ICU stay for all patients was 10 d (range: 3-22), while the median total hospital stay post transplantation was 30 d (range: 6-198). In total, 11 patients died in ICU, of whom 9 during the first 30 d (median survival: 26 d, range: 6-60 d). One male patient died in the operating room. Two more patients died within one year, following a long course of frequent readmissions with poor functional status (survival: 190 and 337 d respectively). Early mortality (30 d), ICU mortality and late mortality (1-year) were higher in the MDRO group (1-year mortality: 28% vs 7.6%, log-rank-χ2 = 7.34, Figure 2).
MDRO presence occurred frequently in heart transplant recipients and was associated with surgical or medical complications post-transplantation. MDRO presence had a negative impact on patient outcomes, with a longer ICU and hospital stay, as well as an increased early and late mortality rate.
In patients with Acinetobacter, the organism was already present upon transplantation in about half of the cases, while Pseudomonas and Klebsiella were ICU acquired. Pre-transplantation hospitalization was not shown to be a predictor of MDRO presence upon transplantation, i.e. hospitalization at the time of transplantation did not seem to impact MDRO prevalence, as compared to patients admitted from home. However, most advanced cardiac failure patients are intermittently admitted in hospital, a fact which may account for this finding[9].
Among comorbidities, peripheral vasculopathy prevailed, but only renal failure and higher BMI was significantly higher in MDRO patients.
Bacteremia occurred in about a third of patients with MDRO presence. Moreover, bacteremia cases (36%) were donor acquired. This is a remarkably high percentage, which could be attributed to a delay in decision making about brain death declaration and organ procurement, resulting in donor increased length of stay in the ICU, a MDRO burdened environment[10].
ICUs harbor a distinct pathogen ecosystem arising through ongoing antimicrobial selection pressure[11,12]. As compared to other acute hospital settings, the ICU endemicity pattern comprises MDROs, which carry genes entailing evolving resistance to advanced antimicrobial agents[13]. In referral hospitals and specialty ICUs, patient previous inter-hospital transfers are an additional source of MDR pathogen importation[14].
In Europe, a geographical north-to-south and west-to-east gradient of bacterial resistance exists, with higher rates observed in the southeast: The selected bacteria surveyed in this study are the most prevalent microorganisms in ICU environments in south-eastern Europe, where carbapenem-resistant gram-negative pathogens prevail[15]. These pathogens are accordingly deemed relevant for hospital infection surveillance purposes in Greece, in consonance with the National Action Plan for Antimicrobial Resistance. The Action Plan draws on the antimicrobial resistance reporting protocol issued by the European Center for Disease Control (ECDC)[8].
Acinetobacter baumannii, the prevailing MDRO in our study, is characterized by the capacity to acquire and harbor a battery of determinants of antimicrobial resistance and environmental persistence[16]. In low-prevalence ICU environments, e.g. in the north and west of Europe, Acinetobacter outbreaks are usually monoclonal and can be traced to an index transmission event; in contrast, high prevalence ICU environments, similar to those of south-eastern Europe, are marked by a diversity of polyclonal isolates and entail an increased complexity regarding the pathways of transmission[17]. In this setting, accelerated evolution of resistance via plasmid transfer between different isolates has been documented[18]. In a similar manner, mobile genetic elements, including plasmids or phages, play a critical role in Klebsiella ICU clusters, via horizontal transmission between polyclonal strains[19].
Heart transplant candidates constitute a heterogenous patient group. Considering the most frequent underlying diagnoses, patients with dilated cardiomyopathy in our cohort were younger, while patients with ischemic cardiomyopathy were middle-aged. Both in this cohort and as a general rule, the latter group is relatively more burdened by comorbidities, such as hypertension, diabetes, peripheral vasculopathy and renal disease[20]. Patients re-transplanted for graft vasculopathy represent a small, but challenging subgroup[21].
Candidates for heart transplantation share numerous risk factors for MDRO acquisition pre-transplant. Notably, heart failure is a leading cause of hospitalization, while asymptomatic colonization of patients with MDRO is a recognized consequence of frequent admissions[22]. Further, a subgroup of heart transplantation candidates (about a third of patients in our study) is confined to the inpatient setting in a state of “dependent stability”, i.e. in need of continuous inotrope infusion (15.3%) and/or support with an IABP (14.3%). A small group of patients pertains to a hyper-acute state, such as ECMO support (one patient in our study).
A feature particular to heart transplantation candidates is the presence of VAD (55% in our study). VAD at the time of transplantation confers an increased risk of MDRO colonization, usually at the driveline entry point (5.5% of patients in our study)[23]. VAD entry point infection occasionally spreads across the driveline (mediastinitis)[24,25]. However, active VAD-related bacterial infection in the transplant recipient does not constitute a contraindication to transplantation[26]. Given that heart transplantation is never a scheduled procedure, bacteremia secondary to active device infection cannot always be accurately excluded at the time of transplantation. Further, weight-gain in patients on VAD is not infrequent. Among VAD patients subsequently transplanted, increased BMI was associated with post-transplantation complications[27].
Regarding the timing of MDRO acquisition, MDRO pathogens in our study were mainly acquired post-transplantation in the ICU, rather than already present at transplantation. Patients in the ICU become colonized via cross-contamination between patients, via direct or indirect contact due to environmental MDRO persistence, or via acquisition of resistant strains in the patient’s gut or skin following prolonged antimicrobial exposure[28,29].
Indeed, newly transplanted patients are commonly being treated with long courses of antimicrobials, even in the absence of complications or clinical indications of infection[30]. To address the use of antimicrobials out of proportion to infection prevalence, current guidelines recommend the discontinuation of prophylactic antimicrobials 24-48 h post transplantation[31]. Nevertheless, the familiarity of common practice prevails over best available evidence; it is notably difficult to de-implement customary treatments and practices devoid of an evidence-based foundation[32]. This effect is further enhanced by the fluctuating post-surgical physiological state of transplanted patients, generating uncertainty about whether antimicrobials may be safely discontinued or even de-escalated.
Infection control strategies and antimicrobial stewardship need to be constantly promoted in the ICU, to improve patient outcomes. However, even when relevant policies are in place, high workload may act as a hindrance to guideline implementation[33]. Our cardiac surgery ICU has a 100% occupancy, while the average nurse-to-patient ratio is 0.7, becoming 1 for transplanted patients. The steadily high bed occupancy rate entails an increased work volume per nurse, a possible impediment to optimal infection prevention practices[34].
In the ICU, bacteremia arising from vascular catheter colonization is an important cause of patient destabilization[35]. MDRO bacteremia, in particular, is a recognized cause of sepsis in the ICU[36,37]. Sepsis-related mortality remains high in ICUs, although increasing attention is being given to the prompt recognition and control of septic episodes, and despite the availability of incrementally advanced and precise diagnostics[38].
Due to concerns for post-transplantation bacteremia, MDRO presence in the donor or the recipient was formerly listed as a contraindication for transplantation[39,40]. Indeed, MDRO bacteremias are mostly encountered in the early post-transplantation period, when transplanted patients recover from major surgery, while being treated with high dose immunosuppression[41]. In our unit, immunosuppressive treatment comprises ATG, corticosteroids, mycophenolate and tacrolimus.
Further, an increased propensity for bacterial translocation to the blood through the gut mucosa is noted in heart transplant patients, given that hemodynamic instability is common in the early post-transplantation period, even in the absence of graft dysfunction[42].
All post-transplantation bacteremia episodes in our study were caused by Gram-negative MDROs. Globally, the epidemiology of bacteremia in transplanted patients has shifted from Gram-positive to Gram-negative pathogens, matched with a rising emergence of resistant strains[43].
In this study MDRO acquisition preceded bacteremia episodes, a finding in accordance with other studies[44,45], although MDRO bacteremia without previous colonization has been previously reported[35]. Due to the limited number of post transplantation bacteremia episodes, the study was underpowered to support a valid analysis of the impact of MDRO bacteremia on patient outcomes.
Whether the presence of MDROs in the ICU has a measurable impact on patient ICU mortality remains an unsettled question. Different studies report opposing results, both positive[43-45] and negative[46]. When matched immunocompetent patients serve as a control group, solid organ transplantation patients generally experience more bacteremia episodes during hospitalization, but mortality does not seem to be greater[47]. Therefore, while MDRO colonization was previously listed as a contra-indication for transplantation, presently, donor or recipient MRDO colonization no longer constitutes an exclusion criterion per se for organ procurement and allocation[48,49]. Rather, comprehensive recipient evaluation and effective interinstitutional communication channels are recommended, so that information about MDRO presence can be rapidly communicated and managed at the time of transplantation[50,51].
In our study, immediate post-transplantation mortality (within 10 days) was due to primary graft dysfunction and surgical complications. Indeed, immediate post transplantation mortality is generally not attributed to microbial causes[52]. However, ICU mortality, 30-d mortality and 1-year mortality were higher in patients harboring MDROs. Therefore, while heart transplant recipients with MDROs are not more likely to die when compared with matched non-transplanted ICU patients, they have a higher mortality rate when compared with MDRO-free transplant recipients. It is unclear whether this association represents a direct causal relation, given that MDRO presence may be either a consequence of medical and surgical complications of the transplantation procedure, or a marker of prolonged poor status, rather than a direct cause of death[53].
A longer ICU stay and total hospital stay were clearly correlated with MDRO presence. Given the detrimental impact of prolonged ICU stay on patient functional status and hospital resource allocation, it becomes clear that infection control practices need to be constantly revisited, so that patient MDRO colonization can be prevented. Teamwork is essential for infection prevention effectiveness, i.e. nurses daily inspecting indwelling devices (venous, arterial and urinary catheters, tracheal tubes etc.), physicians reassessing the need for such devices, and cleaning staff implementing surface disinfection. Antimicrobial stewardship support is decisive for discontinuation of antimicrobials as appropriate[54]. Best efforts for adequate ICU staffing and continuing staff education and support must be made by the hospital administration[55].
Strengths and limitations of the study, and future directions: Although this was a retrospective study, which constitutes a limitation per se, all transplanted patients were consecutively included in the study, and data completeness was 100% for all the variables studied. Despite the relatively low sample size, the study demonstrated a statistically significant association between MDRO presence and patient outcomes. However, the study was underpowered to support a valid analysis of infrequent events, such as receiving a heart from a donor subsequently proven to have been bacteremic at the time of organ procurement. In the future, these parameters need to be re-evaluated by pooling data from several transplantation centers.
Although the study was conducted in a single transplantation center, the pattern of resistance observed is representative of the prevailing resistance pattern in south-eastern Europe (high prevalence of carbapenemase producing MDROs/high level of resistance). Given the geographical epidemiological differences, the results may not be generalizable for institutions in the north-west of Europe, where the pathogen resistance pattern is characterized by a predominance of Gram-positive MDROs or by the prevalence of ESBL-producing Gram-negative MDROs with lower level of resistance[56].
Finally, in this study, bacterial isolation and identification was based on classic microbiological detection methods. In the future, novel sequencing techniques may provide more information on MDRO clonal spread and virulence characteristics. Different patient outcomes may be influenced by dissimilar virulence capacity among distinct isolates of the same pathogen[57,58]. Therefore, molecular pathogen data may clarify phenomena, such as the persistence of colonizing MDRO strains and their interaction with the transplanted host, as well as the way these phenomena determine tran
This single center retrospective study showed that heart transplant recipients had a high incidence of MRDO presence with a clear predominance of Gram-negative carbapenemase-producing pathogens, a pattern characteristic of south-eastern Europe. MDROs were mainly ICU acquired during the early post-transplantation period, rather than already present at transplantation. Higher BMI and pre-existing renal failure were shown to be risk factors pre-transplant, while medical and surgical complications upon transplantation were shown to be risk factors of MDRO acquisition post-transplant. MDRO acquisition was associated with prolonged ICU and total hospital stay, as well as early and late post transplantation mortality.
| 1. | European Centre for Disease Prevention and Control; World Health Organization (WHO). Antimicrobial Resistance Surveillance in Europe 2023: 2021 Data. 2023;. [DOI] [Full Text] |
| 2. | Wang X, Liu J, Li A. Incidence and risk factors for subsequent infections among rectal carriers with carbapenem-resistant Klebsiella pneumoniae: a systematic review and meta-analysis. J Hosp Infect. 2024;145:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Wu D, Chen C, Liu T, Jia Y, Wan Q, Peng J. Epidemiology, Susceptibility, and Risk Factors Associated with Mortality in Carbapenem-Resistant Gram-Negative Bacterial Infections Among Abdominal Solid Organ Transplant Recipients: A Retrospective Cohort Study. Infect Dis Ther. 2021;10:559-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Gao S, Huang X, Zhou X, Dai X, Han J, Chen Y, Qiao H, Li Y, Zhou Y, Wang T, He H, Liu Q, Tang S. A comprehensive evaluation of risk factors for mortality, infection and colonization associated with CRGNB in adult solid organ transplant recipients: a systematic review and meta-analysis. Ann Med. 2024;56:2314236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 5. | WHO. Global Patient Safety Action Plan 2021-2030 Towards eliminating avoidable harm in health care. 2021. Available from: https://iris.who.int/bitstream/handle/10665/343477/9789240032705-eng.pdf?sequence=1. |
| 6. | Cavallo I, Oliva A, Pages R, Sivori F, Truglio M, Fabrizio G, Pasqua M, Pimpinelli F, Di Domenico EG. Acinetobacter baumannii in the critically ill: complex infections get complicated. Front Microbiol. 2023;14:1196774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 7. | Algammal A, Hetta HF, Mabrok M, Behzadi P. Editorial: Emerging multidrug-resistant bacterial pathogens "superbugs": A rising public health threat. Front Microbiol. 2023;14:1135614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 93] [Reference Citation Analysis (0)] |
| 8. | ECDC. Antimicrobial resistance (AMR) Reporting Protocol 2023. https://www.ecdc.europa.eu/sites/default/files/documents/EARS-Net-reporting-protocol-2023_1.pdf. |
| 9. | Clark KAA, Reinhardt SW, Chouairi F, Miller PE, Kay B, Fuery M, Guha A, Ahmad T, Desai NR. Trends in Heart Failure Hospitalizations in the US from 2008 to 2018. J Card Fail. 2022;28:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 10. | Johnston-Webber C, Prionas A, Wharton G, Streit S, Mah J, Boletis I, Mossialos E, Papalois V. The National Organ Donation and Transplantation Program in Greece: Gap Analysis and Recommendations for Change. Transpl Int. 2023;36:11013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Jiang Y, Ding Y, Wei Y, Jian C, Liu J, Zeng Z. Carbapenem-resistant Acinetobacter baumannii: A challenge in the intensive care unit. Front Microbiol. 2022;13:1045206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 12. | Doughty EL, Liu H, Moran RA, Hua X, Ba X, Guo F, Chen X, Zhang L, Holmes M, van Schaik W, McNally A, Yu Y. Endemicity and diversification of carbapenem-resistant Acinetobacter baumannii in an intensive care unit. Lancet Reg Health West Pac. 2023;37:100780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Binsker U, Käsbohrer A, Hammerl JA. Global colistin use: a review of the emergence of resistant Enterobacterales and the impact on their genetic basis. FEMS Microbiol Rev. 2022;46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 14. | Piotrowska MJ, Sakowski K, Lonc A, Tahir H, Kretzschmar ME. Impact of inter-hospital transfers on the prevalence of resistant pathogens in a hospital-community system. Epidemics. 2020;33:100408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | ECDC. Healthcare-associated infections acquired in intensive care units - Annual Epidemiological Report for 2020. 2024. Available from: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-acquired-intensive-care-units-annual. |
| 16. | Ababneh Q, Abulaila S, Jaradat Z. Isolation of extensively drug resistant Acinetobacter baumannii from environmental surfaces inside intensive care units. Am J Infect Control. 2022;50:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Swaminathan M, Sharma S, Poliansky Blash S, Patel G, Banach DB, Phillips M, LaBombardi V, Anderson KF, Kitchel B, Srinivasan A, Calfee DP. Prevalence and risk factors for acquisition of carbapenem-resistant Enterobacteriaceae in the setting of endemicity. Infect Control Hosp Epidemiol. 2013;34:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Protonotariou E, Meletis G, Pilalas D, Mantzana P, Tychala A, Kotzamanidis C, Papadopoulou D, Papadopoulos T, Polemis M, Metallidis S, Skoura L. Polyclonal Endemicity of Carbapenemase-Producing Klebsiella pneumoniae in ICUs of a Greek Tertiary Care Hospital. Antibiotics (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Zhao H, He Z, Li Y, Sun B. Epidemiology of carbapenem-resistant Klebsiella pneumoniae ST15 of producing KPC-2, SHV-106 and CTX-M-15 in Anhui, China. BMC Microbiol. 2022;22:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 20. | DeFilippis EM, Khush KK, Farr MA, Fiedler A, Kilic A, Givertz MM. Evolving Characteristics of Heart Transplantation Donors and Recipients: JACC Focus Seminar. J Am Coll Cardiol. 2022;79:1108-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Batra J, DeFilippis EM, Clerkin K, Bae D, Oh KT, Lotan D, Topkara VK, Lee SH, Latif F, Colombo P, Yuzefpolskaya M, Raikhelkar J, Majure DT, Sayer G, Uriel N. A change of heart: Characteristics and outcomes of multiple cardiac retransplant recipients. Clin Transplant. 2024;38:e15214. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Wilson GM, Suda KJ, Fitzpatrick MA, Bartle B, Pfeiffer CD, Jones M, Rubin MA, Perencevich E, Evans M, Evans CT; QUERI CARRIAGE Program. Risk Factors Associated With Carbapenemase-Producing Carbapenem-Resistant Enterobacteriaceae Positive Cultures in a Cohort of US Veterans. Clin Infect Dis. 2021;73:1370-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Krzelj K, Petricevic M, Gasparovic H, Biocina B, McGiffin D. Ventricular Assist Device Driveline Infections: A Systematic Review. Thorac Cardiovasc Surg. 2022;70:493-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Parikh A, Halista M, Raymond S, Feinman J, Mancini D, Mitter S, Barghash M, Trivieri M, Contreras J, Taimur S, Roldan J, Murphy J, Pawale A, Anyanwu A, Moss N, Lala A, Pinney S. Relation of Left Ventricular Assist Device Infections With Cardiac Transplant Outcomes. Am J Cardiol. 2021;160:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Lambadaris M, Vishram-Nielsen JKK, Amadio JM, Husain S, Rao V, Billia F, Alba AC. Association between continuous-flow left ventricular assist device infections requiring long-term antibiotic use and post-heart transplant morbidity and mortality. J Card Surg. 2022;37:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Esquer Garrigos Z, Castillo Almeida NE, Gurram P, Vijayvargiya P, Corsini Campioli CG, Stulak JM, Rizza SA, Baddour LM, Rizwan Sohail M. Management and Outcome of Left Ventricular Assist Device Infections in Patients Undergoing Cardiac Transplantation. Open Forum Infect Dis. 2020;7:ofaa303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Whitbread JJ, Etchill EW, Giuliano KA, Suarez-Pierre A, Lawton JS, Hsu S, Sharma K, Choi CW, Higgins RSD, Kilic A. Posttransplant Long-Term Outcomes for Patients with Ventricular Assist Devices on the Heart Transplant Waitlist. ASAIO J. 2022;68:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Cruz-López F, Martínez-Meléndez A, Garza-González E. How Does Hospital Microbiota Contribute to Healthcare-Associated Infections? Microorganisms. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 29. | Shamalov L, Heath M, Lynch E, Green DA, Gomez-Simmonds A, Freedberg DE. Timing and clinical risk factors for early acquisition of gut pathogen colonization with multidrug resistant organisms in the intensive care unit. Gut Pathog. 2024;16:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Mowrer C, Lyden E, Matthews S, Abbas A, Bergman S, Alexander BT, Van Schooneveld TC, Stohs EJ. Beta-lactam allergies, surgical site infections, and prophylaxis in solid organ transplant recipients at a single center: A retrospective cohort study. Transpl Infect Dis. 2022;24:e13907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Abbo LM, Grossi PA; AST ID Community of Practice. Surgical site infections: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 32. | Correa VC, Lugo-Agudelo LH, Aguirre-Acevedo DC, Contreras JAP, Borrero AMP, Patiño-Lugo DF, Valencia DAC. Individual, health system, and contextual barriers and facilitators for the implementation of clinical practice guidelines: a systematic metareview. Health Res Policy Syst. 2020;18:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 33. | Dodek PM, Cheung EO, Burns KEA, Martin CM, Archambault PM, Lauzier F, Sarti AJ, Mehta S, Fox-Robichaud AE, Seely AJE, Parshuram C, Garros D, Withington DE, Cook DJ, Piquette D, Carnevale FA, Boyd JG, Downar J, Kutsogiannis DJ, Chassé M, Fontela P, Fowler RA, Bagshaw S, Dhanani S, Murthy S, Gehrke P, Fujii T. Moral Distress and Other Wellness Measures in Canadian Critical Care Physicians. Ann Am Thorac Soc. 2021;18:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Rae PJL, Pearce S, Greaves PJ, Dall'Ora C, Griffiths P, Endacott R. Outcomes sensitive to critical care nurse staffing levels: A systematic review. Intensive Crit Care Nurs. 2021;67:103110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Florescu DF, Kalil AC. Survival Outcome of Sepsis in Recipients of Solid Organ Transplant. Semin Respir Crit Care Med. 2021;42:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Yin M, Tambyah PA, Perencevich EN. Infection, Antibiotics, and Patient Outcomes in the Intensive Care Unit. JAMA. 2020;323:1451-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Neofytos D, Stampf S, Hoessly LD, D'Asaro M, Tang GN, Boggian K, Hirzel C, Khanna N, Manuel O, Mueller NJ, Van Delden C; Swiss Transplant Cohort Study. Bacteremia During the First Year After Solid Organ Transplantation: An Epidemiological Update. Open Forum Infect Dis. 2023;10:ofad247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Chiotos K, Tamma PD, Gerber JS. Antibiotic stewardship in the intensive care unit: Challenges and opportunities. Infect Control Hosp Epidemiol. 2019;40:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Dorschner P, McElroy LM, Ison MG. Nosocomial infections within the first month of solid organ transplantation. Transpl Infect Dis. 2014;16:171-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Cervera C, van Delden C, Gavaldà J, Welte T, Akova M, Carratalà J; ESCMID Study Group for Infections in Compromised Hosts. Multidrug-resistant bacteria in solid organ transplant recipients. Clin Microbiol Infect. 2014;20 Suppl 7:49-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 41. | Adelman MW, Connor AA, Hsu E, Saharia A, Mobley CM, Victor DW 3rd, Hobeika MJ, Lin J, Grimes KA, Ramos E, Pedroza C, Brombosz EW, Ghobrial RM, Arias CA. Bloodstream infections after solid organ transplantation: clinical epidemiology and antimicrobial resistance (2016-21). JAC Antimicrob Resist. 2024;6:dlad158. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Kędziora A, Piątek J, Hymczak H, Wasilewski G, Guzik B, Drwiła R, Kapelak B, Sobczyk D, Konstanty-Kalandyk J, Wierzbicki K. Early postoperative hemodynamic instability after heart transplantation - incidence and metabolic indicators. BMC Anesthesiol. 2021;21:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Huh K, Chung DR, Ha YE, Ko JH, Kim SH, Kim MJ, Huh HJ, Lee NY, Cho SY, Kang CI, Peck KR, Song JH; Korean Antimicrobial Resistance Surveillance Network (KARS-Net) Investigators. Impact of Difficult-to-Treat Resistance in Gram-negative Bacteremia on Mortality: Retrospective Analysis of Nationwide Surveillance Data. Clin Infect Dis. 2020;71:e487-e496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 44. | Zhou R, Fang X, Zhang J, Zheng X, Shangguan S, Chen S, Shen Y, Liu Z, Li J, Zhang R, Shen J, Walsh TR, Wang Y. Impact of carbapenem resistance on mortality in patients infected with Enterobacteriaceae: a systematic review and meta-analysis. BMJ Open. 2021;11:e054971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 45. | Falcone M, Tiseo G, Carbonara S, Marino A, Di Caprio G, Carretta A, Mularoni A, Mariani MF, Maraolo AE, Scotto R, Dalfino L, Corbo L, Macera M, Medaglia AA, d'Errico ML, Gioè C, Sgroi C, Del Vecchio RF, Ceccarelli G, Albanese A, Buscemi C, Talamanca S, Raponi G, Foti G, De Stefano G, Franco A, Iacobello C, Corrao S, Morana U, Pieralli F, Gentile I, Santantonio T, Cascio A, Coppola N, Cacopardo B, Farcomeni A, Venditti M, Menichetti F; Advancing knowLedge on Antimicrobial Resistant Infections Collaboration Network (ALARICO Network). Mortality Attributable to Bloodstream Infections Caused by Different Carbapenem-Resistant Gram-Negative Bacilli: Results From a Nationwide Study in Italy (ALARICO Network). Clin Infect Dis. 2023;76:2059-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 79] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 46. | Babiker A, Clarke LG, Saul M, Gealey JA, Clancy CJ, Nguyen MH, Shields RK. Changing Epidemiology and Decreased Mortality Associated With Carbapenem-resistant Gram-negative Bacteria, 2000-2017. Clin Infect Dis. 2021;73:e4521-e4530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 47. | Eichenberger EM, Ruffin F, Dagher M, Lerebours R, Jung SH, Sharma-Kuinkel B, Macintyre AN, Thaden JT, Sinclair M, Hale L, Kohler C, Palmer SM, Alexander BD, Fowler VG Jr, Maskarinec SA. Bacteremia in solid organ transplant recipients as compared to immunocompetent patients: Acute phase cytokines and outcomes in a prospective, matched cohort study. Am J Transplant. 2021;21:2113-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Pouch SM, Patel G; AST Infectious Diseases Community of Practice. Multidrug-resistant Gram-negative bacterial infections in solid organ transplant recipients-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 49. | Huprikar S, Casner L, Pouch S, Pinheiro Freire M, Madan R, Kwak E, Satlin M, Hartman P, Pisney L, Henrique Mourão P, La Hoz R, Patel G. Prior Infection or Colonization with Carbapenem-Resistant Enterobacteriaceae Is Not an Absolute Contraindication for Solid Organ Transplantation. ATC Abstracts. 2024. https://atcmeetingabstracts.com/abstract/prior-infection-or-colonization-with-carbapenem-resistant-enterobacteriaceae-is-not-an-absolute-contraindication-for-solid-organ-transplantation/. |
| 50. | Fischer SA. Is This Organ Donor Safe?: Donor-Derived Infections in Solid Organ Transplantation. Surg Clin North Am. 2019;99:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Mularoni A, Cona A, Campanella M, Barbera F, Medaglia AA, Cervo A, Cuscino N, Di Mento G, Graziano E, El Jalbout JD, Alduino R, Tuzzolino F, Monaco F, Cascio A, Peghin M, Gruttadauria S, Bertani A, Conaldi PG, Mikulska M, Grossi PA. Donor-derived carbapenem-resistant gram-negative bacterial infections in solid organ transplant recipients: Active surveillance enhances recipient safety. Am J Transplant. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 52. | Hsich EM, Blackstone EH, Thuita LW, McNamara DM, Rogers JG, Yancy CW, Goldberg LR, Valapour M, Xu G, Ishwaran H. Heart Transplantation: An In-Depth Survival Analysis. JACC Heart Fail. 2020;8:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 53. | Macdonald PS, Gorrie N, Brennan X, Aili SR, De Silva R, Jha SR, Fritis-Lamora R, Montgomery E, Wilhelm K, Pierce R, Lam F, Schnegg B, Hayward C, Jabbour A, Kotlyar E, Muthiah K, Keogh AM, Granger E, Connellan M, Watson A, Iyer A, Jansz PC. The impact of frailty on mortality after heart transplantation. J Heart Lung Transplant. 2021;40:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 54. | Graziano E, Peghin M, Grossi PA. Perioperative antibiotic stewardship in the organ transplant setting. Transpl Infect Dis. 2022;24:e13895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 55. | Wilcox ME, Harrison DA, Patel A, Rowan KM. Higher ICU Capacity Strain Is Associated With Increased Acute Mortality in Closed ICUs. Crit Care Med. 2020;48:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 56. | Anesi JA, Lautenbach E, Tamma PD, Thom KA, Blumberg EA, Alby K, Bilker WB, Werzen A, Tolomeo P, Omorogbe J, Pineles L, Han JH. Risk Factors for Extended-Spectrum β-lactamase-Producing Enterobacterales Bloodstream Infection Among Solid-Organ Transplant Recipients. Clin Infect Dis. 2021;72:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 57. | Bautista-Cerón A, Monroy-Pérez E, García-Cortés LR, Rojas-Jiménez EA, Vaca-Paniagua F, Paniagua-Contreras GL. Hypervirulence and Multiresistance to Antibiotics in Klebsiella pneumoniae Strains Isolated from Patients with Hospital- and Community-Acquired Infections in a Mexican Medical Center. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 58. | Shelenkov A, Mikhaylova Y, Yanushevich Y, Samoilov A, Petrova L, Fomina V, Gusarov V, Zamyatin M, Shagin D, Akimkin V. Molecular Typing, Characterization of Antimicrobial Resistance, Virulence Profiling and Analysis of Whole-Genome Sequence of Clinical Klebsiella pneumoniae Isolates. Antibiotics (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (1)] |