Published online Jun 18, 2024. doi: 10.5500/wjt.v14.i2.92528
Revised: February 19, 2024
Accepted: April 28, 2024
Published online: June 18, 2024
Processing time: 137 Days and 9.9 Hours
Portal vein arterialization (PVA) has been used in liver transplantation (LT) to maximize oxygen delivery when arterial circulation is compromised or has been used as an alternative reperfusion technique for complex portal vein thrombosis (PVT). The effect of PVA on portal perfusion and primary graft dysfunction (PGD) has not been assessed.
To examine the outcomes of patients who required PVA in correlation with their LT procedure.
All patients receiving PVA and LT at the Fundacion Santa Fe de Bogota between 2011 and 2022 were analyzed. To account for the time-sensitive effects of graft perfusion, patients were classified into two groups: prereperfusion (pre-PVA), if the arterioportal anastomosis was performed before graft revascularization, and postreperfusion (post-PVA), if PVA was performed afterward. The pre-PVA rationale contemplated poor portal hemodynamics, severe vascular steal, or PVT. Post-PVA was considered if graft hypoperfusion became evident. Conservative interventions were attempted before PVA.
A total of 25 cases were identified: 15 before and 10 after graft reperfusion. Pre-PVA patients were more affected by diabetes, decompensated cirrhosis, impaired portal vein (PV) hemodynamics, and PVT. PGD was less common after pre-PVA (20.0% vs 60.0%) (P = 0.041). Those who developed PGD had a smaller increase in PV velocity (25.00 cm/s vs 73.42 cm/s) (P = 0.036) and flow (1.31 L/min vs 3.34 L/min) (P = 0.136) after arterialization. Nine patients required PVA closure (median time: 62 d). Pre-PVA and non-PGD cases had better survival rates than their counterparts (56.09 months vs 22.77 months and 54.15 months vs 31.91 months, respectively).
This is the largest report presenting PVA in LT. Results suggest that pre-PVA provides better graft perfusion than post-PVA. Graft hyperperfusion could play a protective role against PGD.
Core Tip: Guaranteeing adequate graft perfusion is essential to obtain optimal outcomes after liver transplantation (LT). This retrospective single-center study analyzed 25 cases of portal vein arterialization (PVA) for portal flow optimization in LT. To account for the time-sensitive effect, cases were classified into two groups: prereperfusion (pre-PVA) if the arterioportal anastomosis was performed before graft revascularization and postreperfusion (post-PVA) if PVA was performed afterward. We found that pre-PVA yields better results than post-PVA and that hyperperfusion could play a protective role against graft dysfunction. Currently, this is the largest case series studying PVA during LT.
- Citation: Cortes-Mejia NA, Bejarano-Ramirez DF, Guerra-Londono JJ, Trivino-Alvarez DR, Tabares-Mesa R, Vera-Torres A. Portal vein arterialization in 25 liver transplant recipients: A Latin American single-center experience. World J Transplant 2024; 14(2): 92528
- URL: https://www.wjgnet.com/2220-3230/full/v14/i2/92528.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i2.92528
Portal vein arterialization (PVA) is a scarcely reported procedure in hepatobiliary and liver transplantation (LT) surgeries. It is considered a salvage intervention in critical scenarios where portal or arterial circulation has been compromised. PVA can artificially provide portal circulation with an arterial input to maximize blood flow and oxygen delivery to hepatic cells[1,2]. During both LT and hepatobiliary surgeries, it has been described as an alternative to guarantee biliary perfusion and prevent acute hepatic failure when the hepatic artery is injured, thrombosed or needs to be resected to obtain negative tumor margins[1,2]. In LT, it has been proposed as a bridge therapy for retransplantation when graft loss is unavoidable[1].
Other authors have used PVA to manage complex portal vein thrombosis (PVT) during LT when alternative methods for portal vein (PV) reconstruction are unsuccessful or unfeasible (e.g., anastomosing the donor’s PV to spontaneous port
Previous reports have not assessed the potential role of PVA in portal flow optimization. Adequate perioperative graft perfusion is critical to reduce the risk of graft dysfunction in LT recipients[4,5]. Primary graft dysfunction (PGD), also known as early allograft dysfunction, is one of the most concerning complications after LT. PGD develops within the first days after graft reperfusion, negatively affecting graft and patient survival[4,6]. It is considered a consequence of graft intolerance in the process of procurement, transportation, and reimplantation[5]. When other interventions are unsu
Here, we present our experience using PVA in LT as an alternative to optimizing graft perfusion, particularly when other interventions have failed to provide adequate portal blood flow in fragile recipients or risky graft conditions[1,3,8-12].
LT surgeries performed at the Fundacion Santa Fe de Bogota between January 2011 and December 2022 were retro
To assess the impact of delivering high-oxygen and high-pressure blood flow before or after graft reperfusion, we classified patients into two groups. Prereperfusion PVA (pre-PVA) was defined as an arterioportal anastomosis before graft revascularization, whereas postreperfusion PVA (post-PVA) was performed after routine portal flow was reestablished, either during the initial procedure or during a second surgical intervention.
Each case was reviewed separately. Patients considered for pre-PVA included those with very low pretransplant portal velocity (PVEL) or flow (PFLOW), those with severe vascular steal phenomenon secondary to SPSS, or those with PVT. Before considering arterialization, portal flow was optimized by surgically ligating accessible SPSS or attempting portal thrombectomy/thromboendovenectomy, if applicable. If the portal flow was considered to remain insufficient, the surgeon proceeded with pre-PVA.
Post-PVA was considered after restoring portal flow if signs of graft hypoperfusion were evident (e.g., mottling, pale appearance, and uneven graft perfusion) or if PVEL or PFLOW remained suboptimal following more conservative interventions, such as SPSS ligation.
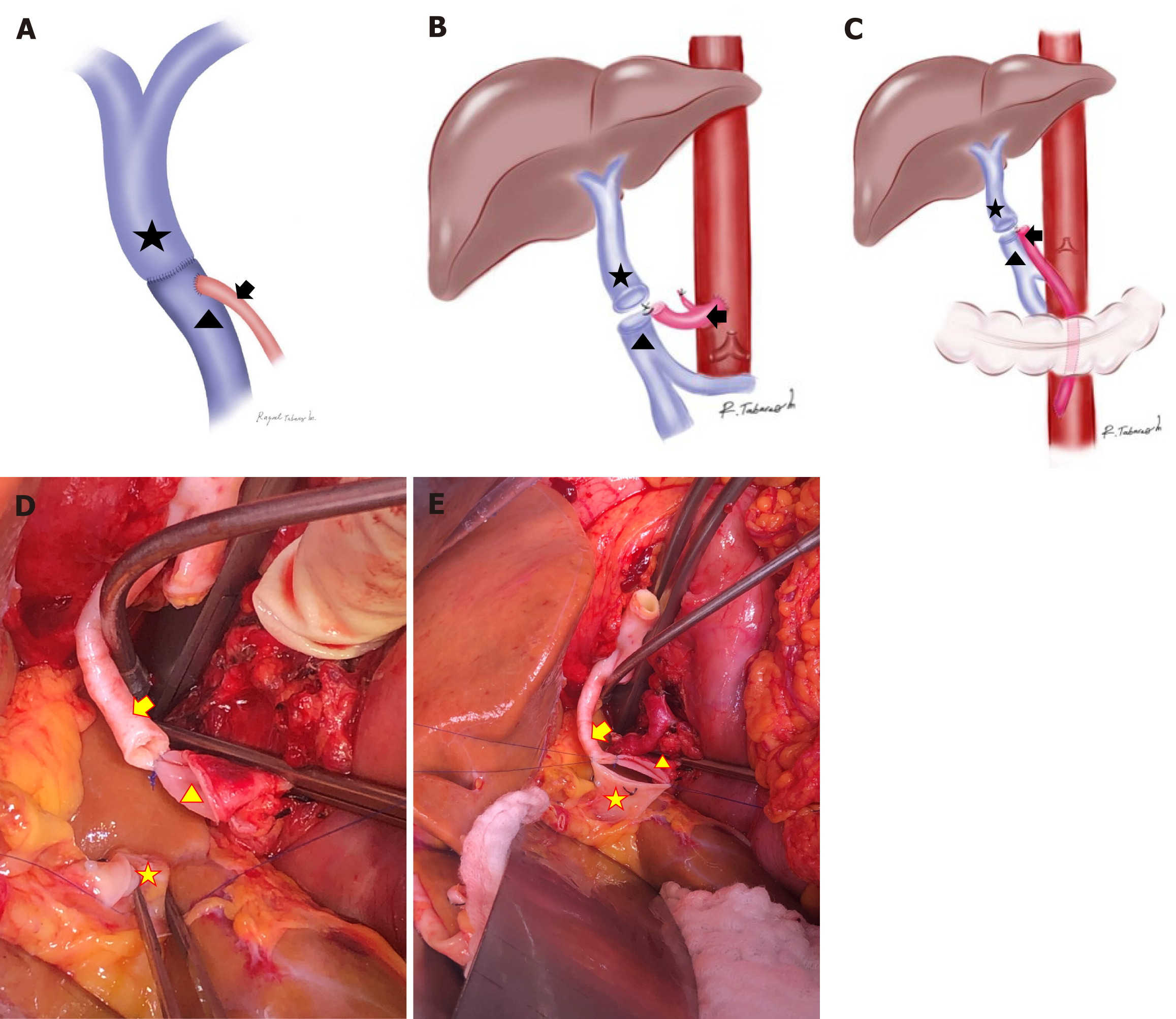
Arterialization was performed either by repositioning the distal end of the recipient’s visceral artery (e.g., accessory mesenteric artery or splenic artery) or by anastomosing an allogenic iliac artery graft directly to the aorta. No synthetic vascular grafts were used. For pre-PVA, the arterial vessel was first attached to the recipient’s PV, and then, a terminolateral anastomosis with the graft’s PV was created (Figure 1A-E). The same technique was used for post-PVA.
Recipient characteristics included liver disease etiology and severity assessed using the Child–Pugh (CP) and model for end stage liver disease (MELD) scores. Hepatic encephalopathy (HE) and ascites were graded using the West Heaven and International Ascites Club Scales, respectively. SPSS were identified using CT or MR imaging. Doppler ultrasound was used to estimate PVEL and PFLOW at the hepatic hilum. PVT was graded on the basis of the intraoperative findings as described by Yerdel et al[13]. Available donor characteristics were recorded. Surgical variables, including surgical times and blood products, were also collected.
Transplant-specific 30-d complications and all PVA-related complications were collected. The primary outcome of the study was the development of PGD. It was defined as an AST > 2000 IU/L within the second and seventh day post-LT, and prothrombin time > 16 s (INR > 1.5) or bilirubin > 10 mg/dL on the seventh day post-LT[4,6].
The US OPTN 9.1 policy for organ allocation and the Colombian national organ transplantation policy define PNF as an AST > 3000 UI/L and at least one of the following: arterial pH ≤ 7.30, venous pH ≤ 7.25, or lactate ≥ 4.0 mmol/L[14,15].
Categorical variables were presented as absolute values (%) and compared using Pearson’s chi-square or Fisher’s exact tests. Continuous variables were assessed for normality using the Shapiro–Wilk test, described as the mean (min–max; SD) or median (95%CI; IQR), and compared using Student’s T or Mann–Whitney’s U tests. Kaplan–Meier’s method was used for survival analyses, and comparison between groups was performed using the logarithmic range test. Statistical significance was set at P-value < 0,05. IBM-SPSS v20® software was used for statistical analysis.
A total of 409 patients underwent LT during the study period. Twenty-five of them received PVA. The mean follow-up period was 76.6 (12.23–150.57) months for arterialized recipients. Fifteen patients required pre-PVA, and 10 required post-PVA.
The basic demographic and pretransplant clinical characteristics are summarized in Table 1. Autoimmune diseases were the main cause of liver disease, followed by alcoholic cirrhosis and NASH. No significant etiological differences were recorded between Pre-PVA and Post-PVA groups. All patients with previous PNF included in the study met the criteria for pre-PVA at their second transplant. PNF was explained by refractory graft hypoperfusion.
| Basic characteristics | Pre-reperfusion PVA | % | Post-reperfusion PVA | % | Overall | % | P value |
| n | 15 | 60.0 | 10 | 40.0 | 25 | 100.0 | |
| Sex (male) | 8 | 53.3 | 5 | 50.0 | 13 | 52.0 | 0.870 |
| Age (yr) | 54.06 [27.0-71.0; 12.1]1 | 53.20 [10.0-78.0; 20.3]1 | 53.72 [10.0-78.0; 15.5]1 | 0.895 | |||
| Body composition | 0.708 | ||||||
| Underweight | 1 | 6.7 | 1 | 10.0 | 2 | 8.0 | |
| Normal | 6 | 40.0 | 6 | 60.0 | 12 | 48.0 | |
| Overweight | 6 | 40.0 | 2 | 20.0 | 8 | 32.0 | |
| Obesity | 2 | 13.3 | 1 | 10.0 | 3 | 12.0 | |
| Liver disease | |||||||
| Etiology | 0.459 | ||||||
| Congenital biliary atresia | 0 | 0.0 | 1 | 10.0 | 1 | 4.0 | |
| Non-alcoholic steatohepatitis | 3 | 20.0 | 2 | 20.0 | 5 | 20.0 | |
| Alcoholic | 3 | 20.0 | 2 | 20.0 | 5 | 20.0 | |
| Autoimmunity2 | 4 | 26.7 | 3 | 30.0 | 7 | 28.0 | |
| Hepatitis C | 2 | 13.3 | 0 | 0.0 | 2 | 8.0 | |
| Hepatocellular carcinoma | 0 | 0.0 | 1 | 10.0 | 1 | 4.0 | |
| Hemochromatosis | 1 | 6.7 | 1 | 10.0 | 2 | 8.0 | |
| Cryptogenic | 1 | 6.7 | 0 | 0.0 | 1 | 4.0 | |
| Secondary biliary cirrhosis | 1 | 6.7 | 0 | 0.0 | 1 | 4.0 | |
| Prior primary non-functioning | 3 | 20.0 | 0 | 0.0 | 3 | 12.0 | 0.132 |
| CHILD Pugh Score B/C | 15 | 100.0 | 7 | 70.0 | 22 | 88.0 | 0.024 |
| MELD Score ≥ 20 points | 4 | 26.7 | 5 | 50.0 | 9 | 36.0 | 0.234 |
| Primary hepatic neoplasia | 1 | 6.7 | 5 | 50.0 | 6 | 24.0 | 0.013 |
| Hepatic encephalopathy | 7 | 46.7 | 6 | 60.0 | 13 | 52.0 | 0.513 |
| Ascites | 13 | 86.7 | 6 | 60.0 | 19 | 76.0 | 0.126 |
| Hypertensive gastropathy | 7 | 46.7 | 6 | 60.0 | 13 | 52.0 | 0.513 |
| Upper gastrointestinal bleeding | 8 | 53.3 | 2 | 20.0 | 10 | 40.0 | 0.096 |
| Hepatorenal syndrome | 2 | 13.3 | 1 | 10.0 | 3 | 12.0 | 0.802 |
| Comorbidities | |||||||
| Type 2 diabetes mellitus | 7 | 46.67 | 0 | 0.0 | 7 | 28.0 | 0.011 |
| Hyperlipidemia | 1 | 6.7 | 3 | 30.0 | 4 | 16.0 | 0.119 |
| Arterial hypertension | 4 | 26.7 | 2 | 20.0 | 6 | 24.0 | 0.702 |
| Cardiac failure | 1 | 6.7 | 1 | 10.0 | 2 | 8.0 | 0.840 |
| Hemodynamic support | 2 | 13.3 | 1 | 10.0 | 3 | 12.0 | 0.840 |
| Chronic renal insufficiency | 1 | 6.7 | 0 | 0.0 | 1 | 4.0 | 0.535 |
| Acute kidney injury | 3 | 20.0 | 1 | 10.0 | 4 | 16.0 | 0.535 |
| Malnourishment | 3 | 20.0 | 5 | 50.0 | 8 | 32.0 | 0.124 |
| Decreased bone mineral density | 7 | 46.7 | 5 | 50.0 | 12 | 48.0 | 0.427 |
| Pulmonary disease/impairment | 9 | 60.0 | 0 | 0.0 | 9 | 36.0 | 0.095 |
| Hypothyroidism | 4 | 26.7 | 1 | 10.0 | 5 | 20.0 | 0.381 |
Pre-PVA patients had a less favorable CP classification (B or C) (100.0% vs 70.0%, P = 0.024) but not the MELD score (≥ 20) (26.7% vs 50.0%, P = 0.234). Liver disease complications were homogeneously distributed in both groups. Diabetes mellitus was common in those who required pre-PVA (46.7% vs 0.0%) (P = 0.011). A total of 16 of 25 patients had PVT at the time of surgery; 3 were identified intraoperatively. PVT was also more frequent in pre-PVA patients (P = 0.009) (Table 2). Grade II and III PVT predominated in the cohort, whereas only one patient presented with grade IV PVT. The latter received pre-PVA (Table 2). NASH (25.0% vs 11.1%) and alcoholic cirrhosis (31.3% vs 0.0%) were frequent in recipients presenting with PVT (P = 0.041). Decompensated liver disease (CP B/C) was common in those with PVT (100.0% vs 66.7%) (P = 0.037) (Supplementary Table 1). All recipients with diabetes had PVT, and 43.8% of those with PVT had diabetes (P = 0.027) (Supplementary Table 1). All patients who required retransplantation had PVT before the first transplant. No recipient characteristic was associated with the development of PGD.
| Pre-reperfusion PVA | % | Post-reperfusion PVA | % | Overall | % | P value | |
| n | 15 | 60.0 | 10 | 40.0 | 25 | 100.0 | |
| Collateral portal circulation | 13 | 87.0 | 7 | 70.0 | 20 | 80.0 | 0.358 |
| Splenomegaly | 12 | 80.0 | 6 | 60.0 | 18 | 72.0 | 0.378 |
| Perigastric/periesophagic varices | 7 | 47.0 | 5 | 50.0 | 12 | 48.0 | 1.000 |
| Umbilical vein recanalization | 6 | 40.0 | 2 | 20.0 | 8 | 32.0 | 0.402 |
| Paracaval portosystemic shunt | 1 | 7.0 | 0 | 0.0 | 1 | 4.0 | 1.000 |
| Epiploic shunts | 2 | 13.0 | 1 | 10.0 | 3 | 12.0 | 1.000 |
| Mesenteric varices | 3 | 20.0 | 0 | 0.0 | 3 | 12.0 | 0.250 |
| Splenorenal shunts | 3 | 20.0 | 3 | 30.0 | 6 | 24.0 | 0.653 |
| Portal vein thrombosis2 | 13 | 86.6 | 3 | 30.0 | 16 | 64.0 | 0.009 |
| Grade I | 3 | 20.0 | 0 | 0.0 | 3 | 12.0 | |
| Grade II | 5 | 33.3 | 3 | 30.0 | 8 | 32.0 | |
| Grade III | 4 | 26.7 | 0 | 0.0 | 4 | 15.0 | |
| Grade IV | 1 | 7.7 | 0 | 0.0 | 1 | 4.0 | |
| Preoperative doppler ultrasound | |||||||
| Portal vein velocity (cm/s)1 | 11.60 [8.30-18.86; 10.8] | 16.00 [13.10-23.20; 7.6] | 14.00 [11.7-19.0; 10.5] | 0.048 | |||
| Portal vein flow (L/min)1 | 0.59 [0.40-0.70; 0.23] | 0.68 [0.40-0.94; 0.80] | 0.59 [0.47-0.73; 0.56] | 0.367 |
Table 2 shows how portosystemic circulation was established and its repercussions on preoperative portal hemodynamics. Although the distribution of these collaterals was relatively homogeneous among groups, a more intense vascular steal phenomenon was evidenced in pre-PVA patients, as demonstrated by the decreased mean PVEL (11.60 cm/s vs 16.00 cm/s) (P = 0.048) and PFLOW (0.59 L/min vs 0.68 L/min) (P = 0.367).
Limited data on donor characteristics could be collected. The mean donor age was 34.00 years (16.0–56.0; 14.5), and most patients were males (64.00%). Here, 80.00% had a normal BMI. The most common cause of brain death was traumatic brain injury (64.00%). No donor characteristic was related to PGD, but PNF was less frequent in grafts from male donors (40.00% vs 70.00%) (P = 0.034).
Intraoperative LT variables were presented according to PVA timing (Table 3), SPSS ligation, preoperative PVT, and PGD development (Supplementary Table 2). The pre-PVA group had longer median transplant surgical times (8.60 h vs 5.60 h) (P = 0.004). Other surgical variables were similar in the two groups. Of the 20 cases presenting with severe portosystemic circulation, 10 (50.0%) had accessible SPSS and all of them were surgically ligated. A tendency to longer WIT was found in those who had PVT or SPSS ligation, although these differences were not statistically significant (P = 0.777 and P = 0.276, respectively). A total of 9 of 16 (53,6%) patients with PVT underwent mechanical thrombectomy/thromboendovenectomy before PVA. PVT and the need for SPSS ligation did not affect other intraoperative variables. No surgical variable was associated with progression to PGD. No hepatic outflow issues were identified.
| Intraoperative events | Pre-reperfusion PVA | % | Post-reperfusion PVA | % | Overall | % | P value |
| n | 15 | 60.0 | 10 | 40.0 | 25 | 100.0 | |
| Surgical time (h) | 8.60 [7.7-10.90; 3.9]2 | 5.6 [4.2-8.82; 2.8]2 | 7.2 [6.8-9.5; 4.30]2 | 0.004 | |||
| Arterialization technique | 0.130 | ||||||
| Supraceliac graft | 7 | 47.0 | 1 | 10.0 | 8 | 32.0 | |
| Infrarenal graft | 5 | 33.0 | 7 | 70.0 | 12 | 48.0 | |
| ARHA | 1 | 7.0 | 1 | 10.0 | 2 | 8.0 | |
| Splenic artery | 0 | 0.0 | 1 | 10.0 | 1 | 4.0 | |
| AMA | 2 | 13.0 | 0 | 0.0 | 2 | 8.0 | |
| Thrombectomy3 | 6 | 40.0 | 3 | 30.0 | 9 | 36.0 | 0.691 |
| CIT (h) | 9.87 [4.0-13.8; 2.4]2 | 8.81 [4.9-13.7; 2.7]2 | 9.4 [4.0-13.8; 1.5]2 | 0.322 | |||
| CIT > 10 h | 6 | 40.0 | 3 | 30.0 | 9 | 36.0 | 0.691 |
| WIT (min) | 43.33 [22.0-8.0; 13.8]2 | 40.50 [24.0-65.0; 12.6]2 | 42.2 [22.0-78.0; 12.8]2 | 0.6 | |||
| WIT > 45 (min) | 5 | 3 | 30.0 | 8 | 32.0 | 1 | |
| PRBCs (Units) | 12.00 [6.7-18.0; 13.0]1 | 4.50 [1.8-12.8; 1]1 | 10.0 [6.4-14.2; 12.0]1 | 0.160 | |||
| Plasma (Units) | 18.00 [12.2-3.1;15.0]1 | 12.00 [8.9-16.5; 8.3]1 | 12.0 [12.2-22.1; 13.0]1 | 0.414 | |||
| Cryoprecipitates (Units) | 22.00 [14.0-3.6;1]1 | 20.00 [7.0-21.4; 20.3]1 | 20.0 [13.5-3.0; 17.5]1 | 0.103 | |||
| Platelets (Units) | 12.00 [4.6-4.1; 24.0]1 | 9.0 [0.0-30.7; 17.0]1 | 12.0 [7.6-3.1; 27.0]1 | 0.311 | |||
| PVA after LT | 0 | 0.0 | 4 | 40.0 | 4 | 16.0 | 0.170 |
Table 4 and Supplementary Table 3 depict the postoperative variables and complications according to PVA timing and preoperative PVT and SPSS ligation, respectively. They were homogeneously distributed in all categories, except for HE and AKI, which were more common in those who underwent SPSS ligation (70.0% vs 20.0%) (P = 0.037) and (60.0% vs 20.0%) (P = 0.041), respectively.
| Postoperative events | Pre-reperfusion PVA | % | Post-reperfusion PVA | % | Overall | % | P value |
| n | 15 | 60.0 | 10 | 40.0 | 25 | 100.0 | |
| ICU LOS (d)1 | 3.00 [0.0-15.1; 4.0] | 3.50 [1.6-4.7; 4.2] | 3.00 [1.0-10.6; 4.0] | 0.935 | |||
| LOS (d)1 | 10.00 [0.0-48.0; 17.0] | 10.50 [5.5-17.8; 11.7] | 10.00 [3.8-33.0; 13.5] | 0.849 | |||
| Hemoperitoneum | 1 | 6.7 | 1 | 10.0 | 2 | 8.0 | 1.000 |
| Bowel resection | 3 | 20.0 | 0 | 0.0 | 3 | 12.0 | 0.250 |
| Upper gastrointestinal bleeding | 3 | 20.0 | 3 | 30.0 | 6 | 24.0 | 0.653 |
| Infection | 7 | 46.7 | 7 | 70.0 | 14 | 56.0 | 0.414 |
| Ascites | 3 | 20.0 | 3 | 30.0 | 6 | 24.0 | 0.653 |
| Pleural effusion | 3 | 20.0 | 2 | 20.0 | 5 | 20.0 | 1.000 |
| Pulmonary complications | 6 | 40.0 | 2 | 20.0 | 8 | 32.0 | 0.402 |
| Encephalopathy | 4 | 26.7 | 6 | 60.0 | 10 | 40.0 | 0.122 |
| Postoperative atrial fibrillation | 1 | 6.7 | 1 | 10.0 | 2 | 8.0 | 1.000 |
| Congestive cardiac failure | 1 | 6.7 | 1 | 10.0 | 2 | 8.0 | 1.000 |
| Acute kidney injury | 7 | 46.7 | 6 | 60.0 | 13 | 52.0 | 0.688 |
| Hemodialysis requirement | 3 | 20.0 | 3 | 30.0 | 6 | 24.0 | 0.653 |
| Reintervention | 4 | 26.7 | 3 | 30.0 | 7 | 28.0 | 1.000 |
| Postoperative PVA closure | 6 | 40.0 | 3 | 30.0 | 9 | 36.0 | 0.691 |
| PGD | 3 | 20.0 | 6 | 60.0 | 9 | 36.0 | 0.041 |
| PNF | 1 | 6.7 | 4 | 40.0 | 5 | 20.0 | 0.121 |
| Retransplantation | 1 | 6.7 | 2 | 20.0 | 3 | 12.0 | 0.543 |
| 30-d mortality | 4 | 26.7 | 5 | 50.0 | 9 | 36.0 | 0.397 |
The overall PGD incidence was 36.0% (9 cases). It was less common in the pre-PVA group (20.0% vs 60.0%) (P = 0.041). Six patients in the post-PVA group developed PGD, and four of them progressed to PNF. In contrast, three patients in the pre-PVA group developed PGD, but only one reached PNF. Three of five PNF cases underwent retransplantation.
Patients with PVT had less PGD (18.8% vs 66.7%) (P = 0.031) and PNF (6.3% vs 44.4%) (P = 0.040), but those whose SPSS were ligated developed PGD more often (60.0% vs 20.0%) (P = 0.041).
Nine patients required PVA closure (eight endovascularly and one surgically) secondary to: ascites (two cases), gastroesophageal varices (four cases), upper gastrointestinal bleeding (UGIB) (two cases), hypertensive gastropathy (one case), and right-heart overload (two cases). No patient developed HAT after surgery. The median time for PVA closure after LT was 62 d (28.37–236.52).
Low pre-LT median PVEL (11.60 cm/s vs 16.00 cm/s) (P = 0.048) was associated with pre-PVA. PFLOW (0.59 L/min vs 0.68 L/min) (P = 0.367) was also decreased, although the difference was not statistically significant (Table 2).
A comparison between preoperative and postoperative PVEL/PFLOW according to the presence of PVT, SPSS, and PGD is found in Table 5. PVT was associated with slower preoperative PVEL (11.80 vs 17.00 cm/s) (P = 0.037) and PFLOW (0.55 L/min vs 0.87 L/min) (P = 0.187), whereas SPSS mainly caused decreased preoperative PFLOW (0.51 L/min vs 0.90 L/min) (P = 0.002). PVA led to a median increase of 52.70 cm/s in PVEL (P < 0.001) and 3.21 L/min in PFLOW (P < 0.001). Notably, those who developed PGD had a smaller increase in PVEL after arterialization (25.00 cm/s vs 73.42 cm/s) (P = 0.036) and PFLOW (1.31 L/min vs 3.34 L/min) (P = 0.136) compared with those who did not develop PGD.
| Doppler ultrasound parameter | Variable | Preoperative | Postoperative | Delta (post-preoperative) |
| Portal vein velocity (cm/s) | Overall | 14.00 [11.7-19.0; 10.45] | 71.00 [58.4-102.8; 68] | 52.70 [41.24-89.23; 73.50] |
| No PVT | 17.00 [13.25-25.98; 12.5] | 88.00 [60.68-116.50; 45] | 36.80 [13.89-80.699; 79.10] | |
| PVT | 11.80 [8.58-17.50; 8.48] | 48.00 [43.09-109.25; 55.91] | 60.35 [41.21-109.44; 81.40] | |
| P value1 | 0.037 | 0.169 | 0.276 | |
| No SPSS | 14.00 [7.31-23.40; 12.8] | 84.50 [61.62-121.41; 78.4] | 75.20 [46.13-109.15; 77.00] | |
| SPSS | 13.50 [11.02-19.82; 9.95] | 67.00 [37.51-106.15; 19.16] | 25.50 [5.32-87.93; 64.60] | |
| P value1 | 0.338 | 0.115 | 0.080 | |
| No PGD | 11.80 [8.52-18.63; 8.98] | 85.82 [64.73-127.48; 80.55] | 73.42 [48.78-115.95; 86.00] | |
| PGD | 15.00 [12.53-24.20; 8.6] | 46.00 [31.05-75.25; 49.25] | 25.00 [9.78-59.78; 65.50] | |
| P value1 | 0.890 | 0.570 | 0.037 | |
| Portal vein flow (L/min) | Overall | 0.59 [0.47-0.73; 0.56] | 3.6 [2.69-4.99; 4.55] | 3.21 [2.09-4.40; 4.24] |
| No PVT | 0.87 [0.41-1.02; 0.82] | 2.68 [3.29-6.04; 3.05] | 1.52 [0.65-4.17; 3.22] | |
| PVT | 0.55 [0.40-0.67; 0.47] | 2.05 [1.68-5.07; 4.4] | 3.35 [2.10-5.31; 4.76] | |
| P value1 | 0.187 | 0.065 | 0.419 | |
| No SPSS | 0.9 [0.73-1.34; 0.32] | 2.68 [2.41-5.40; 4.17] | 1.77 [1.64-4.63; 3.88] | |
| SPSS | 0.51 [0.38-0.66; 0.5] | 3.97 [1.58-5.30; 8.65] | 3.26 [1.19-5.59; 5.50] | |
| P value1 | 0.002 | 0.723 | 0.849 | |
| No PGD | 0.75 [0.50-0.82; 0.36] | 3.95 [2.96-6.07; 4.73] | 3.34 [2.29-5.42; 4.54] | |
| PGD | 0.30 [0.24-0.74; 0.51] | 2.20 [0.93-4.33; 3.1] | 1.31 [0.41-3.88; 3.09] | |
| P value1 | 0.677 | 0.169 | 0.136 |
Pre-PVA patients tended to have better mean survival compared with those with post-PVA (56.09 months vs 22.77 months) (P = 0.256). As expected, recipients who developed PGD had worse survival than those who did not (31.91 months vs 54.15 months) (P = 0.108).
Nine patients died within the first month of transplantation (Table 5). Seven deaths were related to PGD. Of these, five occurred in the post-PVA group and two in the pre-PVA group. The other two early deaths occurred in the pre-PVA group but were unrelated to PGD. One was attributed to complications after an intraoperative cardiac arrest, and the other to renal vein thrombosis during a liver–kidney transplant. Notably, 30-d mortality was increased in those with PVT (P = 0.031), as shown in Supplementary Table 3. No patient died secondary to PVA-related complications.
Late deaths were secondary to a lymphoproliferative disease at 6 and 12 months in 2 cases, mesenteric venous thrombosis after 7 months, COVID-19 at 12 months, sepsis after 3 years, and chronic allograft rejection after 5 years caused death in the other case.
PVA is a controversial procedure that has been described as a last-resort intervention in various scenarios in the setting of LT. Although limited by its retrospective nature, this study is currently the largest cohort presenting PVA during LT. This novel approach was proposed for patients at a higher risk of graft dysfunction.
Physiopathologically, PGD is driven by ischemia–reperfusion injury (IRI). IRI begins at procurement when hepatic circulation ceases, creating a hypoxic environment that triggers cell damage and death[16]. This is further exacerbated during reperfusion when reactive oxygen and nitrogen species are produced, and in combination with a hostile inflammatory environment, it impairs graft adaptation to its new host[17,18]. Although some degree of IRI is admissible during any transplantation procedure, severe and sustained ischemia favors the development of PGD[17]. Several donor, graft, and recipient factors predispose to the development of PGD, and despite the tremendous efforts made by the tran
Multiple diagnostic criteria have been proposed for PGD. They vary according to surrogated biochemical or functional abnormalities that assess the severity of hepatocellular damage and the loss of liver synthetic and clearance capacities[5]. During the last decade, dynamic PGD scores have been validated to predict the risk of 3-month graft loss[7,19,20]. In our setting, a practical definition was used to allow efficient decision-making[4,6].
The overall incidence of PGD in arterialized patients was 36.0%. Despite including only high-risk patients in this cohort, our results fit within the expected 5.2%–36.3% incidence of PGD reported by other groups[4]. No recipient characteristic was related to the development of PGD. Whether liver disease severity is a risk factor for this complication remains controversial as LT is expected to resolve PHT and vascular steal over time. Although some authors suggest that MELD ≥ 20 might be predictive of PGD[21], our data do not support this correlation[22,23]. Although preoperative severity scores were not used as a criterion for PVA, those who received pre-PVA were more often decompensated according to the CP score but not to the MELD score.
Pre-PVA recipients had less PGD than post-PVA recipients (20.0% vs 60.0%; P = 0.041). Five patients progressed to PNF: One patient in the pre-PVA and four in the post-PVA group. Although we used post-PVA as a last resource to prevent PGD, our results suggest that it was not as effective as pre-PVA. This could be partially explained by the fact that before graft reperfusion, ischemia has already depleted the graft’s energy stock, and even if some oxygen is delivered through portal circulation, it takes several minutes for these molecules to become readily available[24]. Providing an insufficient blood supply to the graft would prolong warm ischemia. In this setting, post-PVA may cause a massive entry of oxygen to persistently ischemic mitochondria, resulting in an outburst of reactive oxygen and nitrogen species[25]. This reaction causes direct hepatocyte damage and intense danger-associated molecular pattern production and enhances the inherent sterile inflammatory response caused by the transplantation process[26].
Our hypothesis is further supported by a rat LT model in which arterialization at the moment of graft reperfusion resulted in an adequate flow of highly oxygenated blood that rapidly restored the energy stocks depleted during ischemia[27]. This may result in less reperfusion injury and inflammation, similar to the principles of normothermic perfusion machines[28]. In conclusion, timing is critical: If PVA is considered for optimizing portal flow, it should be performed before graft reperfusion; Post-PVA may not revert the IRI of a hypoperfused graft and perhaps may even aggravate it[29].
When compared with post-PVA, preoperative PVEL and PFLOW were significantly lower in pre-PVA patients. SPSS and PVT were associated with this reduction. Interestingly, patients who developed PGD reached acceptable PVEL and PFLOW after transplantation, challenging the idea that suboptimal portal hemodynamics are necessary for PGD development. Although combining LT with PVA demonstrated a significant increase in both PVEL and PFLOW compared with preoperative values in both groups, patients who reached higher values were less likely to develop PVA. This suggests that hyperperfusion may play a protective role against PGD.
Notably, patients with PVT also demonstrated a reduction in PGD (18.8% vs 66.7%; P = 0.031), whereas those who received SPSS ligation developed PGD more often (60.0% vs 20.0%; P = 0.041). This can be explained by the predominant inclusion of patients with PVT and unmanageable SPSS in the pre-PVA group. As previously described[30], we found that diabetes mellitus was common in patients with PVT and those who received pre-PVA. Because patients with diabetes are predisposed to have PVT or impaired portal hemodynamics, this condition may reinforce the decision to arterialize the PV.
Unfortunately, literature on PVA is scarce. It is limited to case reports or small case series with heterogeneous characteristics. Therefore, it is impossible to contrast our findings and create clinical recommendations because this is the first time that the effects of PVA on graft perfusion have been described. A recent systematic review reported 57 PVA per
A major concern for arterializing the PV in LT recipients relies on the theoretical risk of inducing PHT. Portal circulation is a low-pressure low-oxygen system without valves or flow regulatory mechanisms[3,12]. PVA is a nonp
Significant complications in our cohort included infection, AKI, HE, ventilatory impairment, and ascites. UGIB, bowel ischemia/obstruction, congestive cardiac failure, paroxysmic atrial fibrillation, and intrabdominal bleeding were less common. PVA timing did not affect complication distribution. Interestingly, AKI was more frequent when splenorenal shunts were ligated, although it was not associated with this procedure in previous publications[34,35]. These complications were similar to those reported in previous studies where PVA was used for complex PVT management or HAT: AKI[8,36], ascites[1,10,37], UGIB[37], right-sided heart failure[10], intra-abdominal bleeding, encephalopathy, and PVA thrombosis[1]. Intrahepatic abscess[29] and cholangitis[1] have been described, but they are more likely to be related to HAT than to PVA. Some studies have reported aneurysmatic dilation of the PV[26,38], biliary stenosis[39], hepatomegaly[10,37], or acute hepatic failure[11], but none of these were reported in our cohort.
Some authors recommend that PVA should be closed at the onset of elevated portal pressures or even prophylactically when successful liver perfusion is achieved[2]. Because arterioportal shunts can be easily closed endovascularly, we consider PVA a safe procedure as it can be left in place until signs of PHT or volume overload become evident. Moreover, in some cases, PVA does not cause any complications, so it can be placed indefinitely without obvious side effects. At our center, only 9 of 25 patients required closure of the PVA. This was performed using minimally invasive techniques, except for one case that was ligated prophylactically in another unrelated intervention. PVA was closed secondary to PHT in seven cases and to right-heart overload in two cases. PHT presented as gastroesophageal varices in four cases (two resulting in UGIB), refractory ascites in two cases, and one case of hypertensive gastropathy. Other authors also reported PVA closure by interventional radiology or surgical ligation[10,37,38]. Careful patient selection with adequate cardiac pretransplant assessment is mandatory if PVA is considered before LT.
The long-term consequences of PVA have not been studied. A murine model did not find significant histological disruption: only mild nonspecific inflammation and occasional hepatocyte fat droplets were found in arterialized rat livers. A normal Ki-67 index indicated usual mitotic rates. However, periportal and perisinusoidal collagen deposition has been found at 4 wk after PVA[40]. By contrast, other studies found that PVA was associated with increased apoptosis, portal obliterans vasculopathy, and upregulated procollagen I RNA after 3 months[12,29]. Nevertheless, fibrosis was not found on biopsy after 3 years in a human recipient who required PVA at the time of LT[10].
Studies have found that PGD increases the 6-month mortality rate and 6-month graft loss rate by 10-fold (RR = 10.7, 95%CI: 3.6–31.9) (P < 0.001) and 7-fold (RR = 7.4, 95%CI: 3.4–16.3) (P < 0.0001), respectively[6]. In our case, those who did not develop PGD had better mean survival at 5 years than those who did (54.15 months vs 31.91 months). These survival rates match those found when comparing the pre-PVA and post-PVA groups (56.09 months vs 22.77 months). We hypothesize that if PGD decreases survival and planned PVA can prevent it, pre-PVA may improve survival in patients at risk.
The first 6 months were critical for mortality. Those who survived this period remained alive thereafter. Most importantly, the main cause of death in the post-PVA group was PGD, whereas most deaths in the pre-PVA group were not caused by PGD. No mortality was associated with PVA complications. Notably, PVT was a crucial determinant of 30-d mortality, a finding previously described by Englesbe et al[41], who found that PVT had a 1.32 hazard ratio for post-transplant mortality (P = 0.02). In conclusion, if early mortality is prevented, long-term survival can be achieved. We emphasize that as no other studies have prevented PGD using this technique, no direct comparison can be conducted. However, if the survival rates of those who required LT-related PVA are compared, we found that 3- or 10-year survival can be achieved[9-11,42,43]. In these reports, the earliest mortality was reported by Zhang et al[8] after a pulmonary infection 2 months after LT. Transplanted patients who received PVA for HAT in Bhangui’s series achieved a 1-year survival of 71.0%[1].
Regarding our PVA technique, our team used an interposition donor iliac artery graft in 20 cases and ancillary splanchnic arterial branches in 5 patients. Grafted vessels were preferred to reduce the risk of bleeding or ischemia in the territories supplied by other recipient’s arteries[31], as reported by other authors[10,36-38]. Our approach is in contrast with other reports. For example, Bhangui et al[1] used only ancillary vessels or a prosthetic graft. Similarly, other authors have used branches of the celiac trunk or the superior mesenteric artery[8,9,37,39], as well as direct arterioportal anas
PVA continues to be a viable alternative approach for obtaining adequate graft perfusion during LT. Our investigation revealed that arterialization preceding graft reperfusion (pre-PVA) may confer superior advantages compared to doing so after revascularization. Although some researchers have cited concerns regarding portal hypertension as a significant drawback to this technique, our findings suggest that graft hyperperfusion may effectively mitigate graft dysfunction. Regrettably, our observations did not associate with an optimal survival rate in the pre-PVA cohort. Nonetheless, it is noteworthy that mortality within this group was attributed to external factors unrelated to graft dysfunction or PVA-related complications, underscoring the potential suitability of this technique pending resolution of these external factors.
Our study was limited by its retrospective design, which limited access to specific data, such as donor-related variables. In addition, incomplete data further hindered our capacity to perform a comprehensive comparison between arterialized and nonarterialized LT recipients. Despite the inherent statistical limitations of our modest sample size, it is crucial to highlight that this cohort currently represents the largest series investigating the utilization of PVA in LT. Consequently, it offers invaluable insights into the intricate dynamics surrounding PVA application in LT. Future studies must investigate the suitability and efficacy of this intervention in patients at risk before definitive recommendations can be made.
| 1. | Bhangui P, Salloum C, Lim C, Andreani P, Ariche A, Adam R, Castaing D, Kerba T, Azoulay D. Portal vein arterialization: a salvage procedure for a totally de-arterialized liver. The Paul Brousse Hospital experience. HPB (Oxford). 2014;16:723-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Majlesara A, Ghamarnejad O, Khajeh E, Golriz M, Gharabaghi N, Hoffmann K, Chang DH, Büchler MW, Mehrabi A. Portal vein arterialization as a salvage procedure in hepatopancreatobiliary surgery: a systematic review. Can J Surg. 2021;64:E173-E182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Bhangui P, Lim C, Levesque E, Salloum C, Lahat E, Feray C, Azoulay D. Novel classification of non-malignant portal vein thrombosis: A guide to surgical decision-making during liver transplantation. J Hepatol. 2019;71:1038-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 4. | Derbisz K, Nylec M, Chrząszcz P, Wrońska W, Kunsdorf-Wnuk A, Wystrychowski W, Król R. Recipient-Related Preoperative and Intraoperative Risk Factors for Primary Graft Dysfunction After Orthotopic Liver Transplantation. Transplant Proc. 2018;50:2018-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Chen XB, Xu MQ. Primary graft dysfunction after liver transplantation. Hepatobiliary Pancreat Dis Int. 2014;13:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 874] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 7. | Pareja E, Cortes M, Hervás D, Mir J, Valdivieso A, Castell JV, Lahoz A. A score model for the continuous grading of early allograft dysfunction severity. Liver Transpl. 2015;21:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Zhang K, Jiang Y, Lv LZ, Cai QC, Yang F, Hu HZ, Zhang XJ. Portal vein arterialization technique for liver transplantation patients. World J Gastroenterol. 2014;20:12359-12362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Paloyo S, Nishida S, Fan J, Tekin A, Selvaggi G, Levi D, Tzakis A. Portal vein arterialization using an accessory right hepatic artery in liver transplantation. Liver Transpl. 2013;19:773-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Settmacher U, Stange B, Schaser KD, Puhl G, Glanemann M, Steinmüller T, Heise M, Neuhaus P. Primary permanent arterialization of the portal vein in liver transplantation. Transpl Int. 2003;16:430-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Ott R, Böhner C, Müller S, Aigner T, Bussenius-Kammerer M, Yedibela S, Kissler H, Hohenberger W, Reck T, Müller V. Outcome of patients with pre-existing portal vein thrombosis undergoing arterialization of the portal vein during liver transplantation. Transpl Int. 2003;16:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Schleimer K, Stippel DL, Kasper HU, Prenzel K, Gaudig C, Tawadros S, Hoelscher AH, Beckurts KT. Portal vein arterialization increases hepatocellular apoptosis and inhibits liver regeneration. J Surg Res. 2008;149:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Yerdel MA, Gunson B, Mirza D, Karayalçin K, Olliff S, Buckels J, Mayer D, McMaster P, Pirenne J. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation. 2000;69:1873-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 525] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 14. | Salud INd. Comision de Higado 10a Version - Documento Tecnico Nacional. Ministerio de Salud y de la Proteccion Social; 2019. |
| 15. | Latt NL, Niazi M, Pyrsopoulos NT. Liver transplant allocation policies and outcomes in United States: A comprehensive review. World J Methodol. 2022;12:32-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (2)] |
| 16. | Ronca V, Wootton G, Milani C, Cain O. The Immunological Basis of Liver Allograft Rejection. Front Immunol. 2020;11:2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 17. | Abu-Amara M, Yang SY, Tapuria N, Fuller B, Davidson B, Seifalian A. Liver ischemia/reperfusion injury: processes in inflammatory networks--a review. Liver Transpl. 2010;16:1016-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 18. | Rampes S, Ma D. Hepatic ischemia-reperfusion injury in liver transplant setting: mechanisms and protective strategies. J Biomed Res. 2019;33:221-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Avolio AW, Franco A, Schlegel A, Lai Q, Meli S, Burra P, Patrono D, Ravaioli M, Bassi D, Ferla F, Pagano D, Violi P, Camagni S, Dondossola D, Montalti R, Alrawashdeh W, Vitale A, Teofili L, Spoletini G, Magistri P, Bongini M, Rossi M, Mazzaferro V, Di Benedetto F, Hammond J, Vivarelli M, Agnes S, Colledan M, Carraro A, Cescon M, De Carlis L, Caccamo L, Gruttadauria S, Muiesan P, Cillo U, Romagnoli R, De Simone P. Development and Validation of a Comprehensive Model to Estimate Early Allograft Failure Among Patients Requiring Early Liver Retransplant. JAMA Surg. 2020;155:e204095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 20. | Agopian VG, Harlander-Locke MP, Markovic D, Dumronggittigule W, Xia V, Kaldas FM, Zarrinpar A, Yersiz H, Farmer DG, Hiatt JR, Busuttil RW. Evaluation of Early Allograft Function Using the Liver Graft Assessment Following Transplantation Risk Score Model. JAMA Surg. 2018;153:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 21. | Briceño J, Ciria R, de la Mata M, Rufián S, López-Cillero P. Prediction of graft dysfunction based on extended criteria donors in the model for end-stage liver disease score era. Transplantation. 2010;90:530-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (35)] |
| 22. | Silberhumer GR, Pokorny H, Hetz H, Herkner H, Rasoul-Rockenschaub S, Soliman T, Wekerle T, Berlakovich GA, Steininger R, Muehlbacher F. Combination of extended donor criteria and changes in the Model for End-Stage Liver Disease score predict patient survival and primary dysfunction in liver transplantation: a retrospective analysis. Transplantation. 2007;83:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Ijtsma AJ, van der Hilst CS, de Boer MT, de Jong KP, Peeters PM, Porte RJ, Slooff MJ. The clinical relevance of the anhepatic phase during liver transplantation. Liver Transpl. 2009;15:1050-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1759] [Cited by in RCA: 2098] [Article Influence: 190.7] [Reference Citation Analysis (0)] |
| 25. | Dutkowski P, Clavien PA. Uploading cellular batteries: Caring for mitochondria is key. Liver Transpl. 2018;24:462-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Zarbock A, Eroglu A, Erturk E, Ince C, Westphal M. Ischemia-reperfusion injury and anesthesia. Biomed Res Int. 2014;2014:980318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Shimizu Y, Miyazaki M, Shimizu H, Ito H, Nakagawa K, Ambiru S, Yoshidome H, Nakajima N. Beneficial effects of arterialization of the portal vein on extended hepatectomy. Br J Surg. 2000;87:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Schlegel A, Kalisvaart M, Scalera I, Laing RW, Mergental H, Mirza DF, Perera T, Isaac J, Dutkowski P, Muiesan P. The UK DCD Risk Score: A new proposal to define futility in donation-after-circulatory-death liver transplantation. J Hepatol. 2018;68:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 29. | Müller V, Brummer D, Kissler H, Yedibela S, Bauer M, Erhardt W, Henke J, Amann K, Tannapfel A, Hohenberger W, Ott R. Effects of portal vein arterialization on regeneration and morphology in liver transplantation: investigations using the rat model. Transplantation. 2004;78:1159-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Faccia M, Santopaolo F, Gasbarrini A, Pompili M, Zocco MA, Ponziani FR. Risk factors for portal vein thrombosis or venous thromboembolism in a large cohort of hospitalized cirrhotic patients. Intern Emerg Med. 2022;17:1327-1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Molmenti EP, Santibañes Md, Santibañes Ed. Liver Transplantation: Operative Techniques and Medical Management. New York, NY: McGraw Hill; 2021. |
| 32. | Vaidya S, Dighe M, Bhargava P, Dick AA. Chronic hepatic artery occlusion with collateral formation: imaging findings and outcomes. Transplant Proc. 2011;43:1770-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Young AL, Prasad KR, Adair R, Abu Hilal M, Guthrie JA, Lodge JP. Portal vein arterialization as a salvage procedure during left hepatic trisectionectomy for hilar cholangiocarcinoma. J Am Coll Surg. 2008;207:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Gomez Gavara C, Bhangui P, Salloum C, Osseis M, Esposito F, Moussallem T, Lahat E, Fuentes L, Compagnon P, Ngongang N, Lim C, Azoulay D. Ligation versus no ligation of spontaneous portosystemic shunts during liver transplantation: Audit of a prospective series of 66 consecutive patients. Liver Transpl. 2018;24:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Chen G, Li Q, Zhang Z, Xie B, Luo J, Si Z, Li J. Hemodynamic alterations with large spontaneous splenorenal shunt ligation during adult deceased donor liver transplantation. Front Surg. 2022;9:916327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 36. | Erhard J, Lange R, Giebler R, Rauen U, de Groot H, Eigler FW. Arterialization of the portal vein in orthotopic and auxiliary liver transplantation. A report of three cases. Transplantation. 1995;60:877-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Charco R, Margarit C, López-Talavera JC, Hidalgo E, Castells L, Allende H, Segarra A, Moreíras M, Bilbao I. Outcome and hepatic hemodynamics in liver transplant patients with portal vein arterialization. Am J Transplant. 2001;1:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Neelamekam TK, Geoghegan JG, Curry M, Hegarty JE, Traynor O, McEntee GP. Delayed correction of portal hypertension after portal vein conduit arterialization in liver transplantation. Transplantation. 1997;63:1029-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Nivatvongs S, Sirijindakul B, Nontasoot B. Portal vein arterialization for liver transplantation with extensive portomesenteric vein thrombosis: a case report. Transplant Proc. 2004;36:2267-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Müller V, Ott R, Tannapfel A, Hohenberger W, Reck T. Arterialization of the portal vein in liver transplantation: a new microsurgical model in the rat. Transplantation. 2001;71:977-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Englesbe MJ, Schaubel DE, Cai S, Guidinger MK, Merion RM. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl. 2010;16:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 42. | Bonnet S, Sauvanet A, Bruno O, Sommacale D, Francoz C, Dondero F, Durand F, Belghiti J. Long-term survival after portal vein arterialization for portal vein thrombosis in orthotopic liver transplantation. Gastroenterol Clin Biol. 2010;34:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Cheng Y, Chen Y, Jiang Y, Zhang X. Ten-year survival after portal vein arterialization in liver transplantation. Ann Palliat Med. 2019;8:790-792. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 44. | Northup PG, Garcia-Pagan JC, Garcia-Tsao G, Intagliata NM, Superina RA, Roberts LN, Lisman T, Valla DC. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients With Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:366-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 375] [Article Influence: 93.8] [Reference Citation Analysis (1)] |