Published online Jun 18, 2024. doi: 10.5500/wjt.v14.i2.90554
Revised: February 7, 2024
Accepted: April 3, 2024
Published online: June 18, 2024
Processing time: 178 Days and 9.3 Hours
Mesenchymal stem cells (MSCs) have tantalized regenerative medicine with their therapeutic potential, yet a cloud of controversies looms over their clinical tran
Core Tip: Controversies surrounding mesenchymal stem cell (MSC) transplantation demand nuanced evaluation. Autologous vs allogeneic choices, long-term efficacy, and safety remain hot topics. Understanding the mechanisms of homing, integration, and paracrine signaling is vital for predictable outcomes. Standardization, regulatory clarity, and cost considerations require urgent attention. Combining MSCs with other therapies offers a promising horizon. Ethical, legal, and publication quality concerns persist. Rigorous research, informed patient selection and personalized strategies are paramount. In this dynamic field, our review underscores the need for clarity, transparency, and evidence-based practice to harness the transformative potential of MSCs effectively.
- Citation: Velikova T, Dekova T, Miteva DG. Controversies regarding transplantation of mesenchymal stem cells. World J Transplant 2024; 14(2): 90554
- URL: https://www.wjgnet.com/2220-3230/full/v14/i2/90554.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i2.90554
Mesenchymal stem cells (MSCs), once hailed as regenerative medicine's promising panacea, have ushered in a new era of therapeutic possibilities. With their multipotent nature and immunomodulatory capabilities, MSCs have attracted widespread attention for their potential to treat various diseases and injuries. However, amid the optimism, a series of controversies has emerged, casting a critical spotlight on the clinical translation of MSC transplantation[1].
First and foremost, the fundamental properties of MSCs are based on their unique characteristics and therapeutic potential. Many surface markers define MSCs and intricate to their immunomodulatory properties. This groundwork sets the stage for a critical analysis of their applications, focusing on autologous vs allogeneic transplantation, efficacy concerns, and safety considerations[2].
One of the central controversies we address revolves around the comparison between using a patient's MSCs and those from an allogeneic source[3]. While autologous MSCs may seem the logical choice, they raise questions concerning cell quality and availability. Conversely, allogeneic sources offer scalability and accessibility but pose potential immunogenicity risks. Balancing these considerations is pivotal to the field's progression.
Furthermore, we examine the efficacy and long-term outcomes associated with MSC transplantation, emphasizing the need for rigorous clinical evidence. The clinical community grapples with variances in therapeutic outcomes, raising uncertainties about the consistency and durability of MSC-based therapies. Safety concerns, including the potential for tumorigenesis and adverse effects, are also addressed, highlighting the imperative to navigate these issues in the clinic[4].
We delve into the intricate processes of homing, integration, and paracrine signaling to unravel the mechanisms and uncertainties surrounding MSC transplantation. Understanding these mechanisms is crucial for optimizing therapeutic outcomes and addressing unresolved questions. We also scrutinize the balance between MSC differentiation and immunomodulation, a central dilemma in harnessing their therapeutic potential[5].
In summary, the controversies surrounding MSC transplantation underscore the need for a nuanced understanding of their properties, applications, and limitations. By navigating these controversies with precision and objectivity, we aspire to contribute to the informed dialogue that shapes the future of MSC-based regenerative medicine. As we look toward the future, we discuss emerging trends and research directions, including the potential for personalized medicine, innovative delivery methods, and integrating MSC therapies with other treatment modalities.
This review extends its purview to the clinical domain, analyzing ongoing clinical trials and the regulatory landscape. Promising trials offer glimpses of hope, while regulatory challenges underscore the need for standardized guidelines and oversight. We focus on a comprehensive and up-to-date overview of the controversies and debates surrounding the transplantation of MSCs; exploring MSC characteristics, analysing controversial applications, mechanisms and uncertainty, assessing clinical trials and regulatory landscape, and exploring future directions. We tried to offer a balanced and informed perspective on the controversies surrounding MSC transplantation, providing readers with a clear understanding of the complexities, challenges, and potential solutions in this rapidly evolving field. By addressing these objectives, this review paper aims to contribute to the ongoing dialogue and decision-making processes regarding the transplantation of MSCs within the scientific and clinical communities.
A systematic literature search was conducted for this comprehensive review to identify relevant studies and publications. The following databases were used: PubMed/Medline, Scopus, Web of Science, Embase, and Google Scholar. The search strategy employed a combination of Medical Subject Headings terms and free-text keywords. The following template was used to construct search queries: ("Mesenchymal Stem Cells" OR "MSCs") AND ("Transplantation" OR "Transplant" OR "Transplantation Techniques" OR "Cell Transplantation") AND ("Controversies" OR "Debate" OR "Challenges" OR "Issues" OR "Uncertainties" OR "Controversial Applications").
The search queries were adapted to the specific syntax and features of each database to ensure a comprehensive retrieval of relevant articles. Filters were applied to include articles published within the last 15 years to align with the clinical experience and recent developments addressed in this review. The initial search yielded a substantial number of articles, and duplicates were removed to ensure the accuracy of the search results. Titles and abstracts of the remaining articles were screened for relevance to the scope of the review, and full texts of potentially relevant articles were retrieved and assessed for inclusion in the review. This systematic search strategy ensured the comprehensive coverage of controversies regarding the transplantation of MSCs in clinical practice.
MSCs represent a fascinating class of multipotent cells that have garnered significant attention in regenerative medicine and tissue engineering[6]. These remarkable cells can differentiate into various cell types, including osteoblasts, chondrocytes, and adipocytes, making them a potent tool for tissue repair and regeneration. MSCs can be sourced from diverse tissues within the body, with the most commonly utilized sources being bone marrow, adipose tissue, and umbilical cord tissue[7].
Characterization of MSCs is essential, and a set of surface markers and functional properties typically defines them. Commonly expressed surface markers include CD73, CD90, and CD105 while lacking hematopoietic markers like CD45 and CD34[8].
However, MSCs exhibit considerable heterogeneity, varying depending on their tissue of origin and donor-specific factors. Beyond their differentiative capacity, MSCs possess a remarkable immunomodulatory profile, a feature that has led to their investigation as potential immune modulators in various disease contexts. MSCs can suppress the activity of immune cells, such as T cells, B cells, and dendritic cells (DCs), by secretion of anti-inflammatory cytokines like inter
Additionally, MSCs secrete a wide array of bioactive molecules, known as paracrine factors, that contribute to their therapeutic potential. These factors encompass growth factors, chemokines, and extracellular vesicles, which collectively orchestrate tissue repair processes, stimulate angiogenesis, and modulate inflammation[11].
MSCs have gained prominence for their remarkable immunomodulatory properties, making them valuable candidates for various therapeutic applications. These cells exert their immunomodulatory effects through a multifaceted array of mechanisms that help regulate immune responses[12-15].
Here, we delve into some of the most common immunological mechanisms employed by MSCs, with references[15-25] to support their significance in Table 1.
| Properties | Mechanisms | Ref. |
| Suppression of T-cell proliferation | MSCs are adept at inhibiting the proliferation and activation of T lymphocytes, a vital component of the adaptive immune response. This effect is mediated by releasing soluble factors such as IDO and PGE2, which create an immunosuppressive microenvironment | [15,16] |
| Induction of Tregs | MSCs promote the generation and expansion of regulatory T cells, or Tregs, which play a crucial role in immune tolerance and suppressing excessive immune reactions. This induction of Tregs is partly attributed to interactions between PD-L1 on MSCs and PD-1 on T cells | [17-19] |
| Modulation of DCs | MSCs influence the maturation and function of DCs, pivotal antigen-presenting cells in the immune system. They inhibit the expression of co-stimulatory molecules on DCs and reduce their ability to activate T cells, thereby tempering immune responses | [20] |
| Reduction of inflammatory cytokines | MSCs secrete anti-inflammatory cytokines like IL-10, TGF-β, and HGF, while simultaneously dampening the production of pro-inflammatory cytokines, including IFN-γ | [21,22] |
| Promotion of macrophage polarization | MSCs can skew macrophages towards an anti-inflammatory, tissue-healing M2 phenotype, fostering a regenerative environment and mitigating tissue damage | [23] |
| Immune cell anergy | MSCs can induce a state of anergy in T cells, rendering them functionally inactive and refractory to activation signals. This effect is conducive to immune tolerance and reduced autoimmune responses | [24] |
| Exosome-mediated communication | MSCs release immunomodulatory exosomes that carry bioactive molecules, including microRNAs and proteins, capable of regulating immune cell behavior and suppressing inflammation | [25] |
MSCs are adept at inhibiting the proliferation and activation of T lymphocytes, a vital component of the adaptive immune response. This effect is mediated by releasing soluble factors such as indoleamine 2,3-dioxygenase and prostaglandin E2, which create an immunosuppressive microenvironment[15,16].
Induction of regulatory T cells: MSCs promote the generation and expansion of regulatory T cells, or regulatory T cells (Tregs), which play a crucial role in immune tolerance and suppressing excessive immune reactions. This induction of Tregs is partly attributed to interactions between programmed death-ligand 1 on MSCs and programmed cell death protein 1 on T cells[17-19].
Modulation of DCs: MSCs influence the maturation and function of DCs, pivotal antigen-presenting cells in the immune system. They inhibit the expression of co-stimulatory molecules on DCs and reduce their ability to activate T cells, thereby tempering immune responses[20].
MSCs secrete anti-inflammatory cytokines like IL-10, TGF-β, and hepatocyte growth factor, while simultaneously dampening the production of pro-inflammatory cytokines, including tumor necrosis factor-alpha and interferon-gamma[21,22].
MSCs can skew macrophages towards an anti-inflammatory, tissue-healing M2 phenotype, fostering a regenerative environment and mitigating tissue damage[23].
MSCs can induce a state of anergy in T cells, rendering them functionally inactive and refractory to activation signals. This effect is conducive to immune tolerance and reduced autoimmune responses[24].
MSCs release immunomodulatory exosomes that carry bioactive molecules, including microRNAs and proteins, capable of regulating immune cell behavior and suppressing inflammation[25].
These immunological mechanisms collectively contribute to the immunomodulatory potential of MSCs, rendering them pivotal in managing autoimmune disorders, inflammatory diseases, and conditions marked by aberrant immune responses. As research advances, a deeper understanding of these mechanisms holds promise for enhancing the thera
This extensive repertoire of immunomodulatory and trophic effects highlights MSCs as promising agents in regenerative medicine, with ongoing research exploring their applications in diverse clinical scenarios, ranging from musculoskeletal disorders and cardiovascular diseases to neurological conditions and graft-versus-host disease (GVHD). Despite the controversies and challenges surrounding their clinical use, MSCs remain at the forefront of therapeutic innovation, continuously revealing new facets of their biology and therapeutic potential that hold immense promise for the future of personalized medicine and regenerative therapies[26,27].
MSCs have garnered immense interest in regenerative medicine due to their remarkable clinical potential. These versatile cells hold promise in various therapeutic applications, offering new avenues for treating multiple diseases and injuries. Here, we explore the clinical potential of MSCs in 400 words, with references supporting their diverse applications (Table 2)[28-37].
| Application | Effects | Ref. |
| Musculoskeletal disorders | MSCs have shown great promise in treating musculoskeletal conditions, including osteoarthritis, rheumatoid arthritis, and bone fractures. Their ability to differentiate into bone and cartilage cells and their anti-inflammatory properties make them valuable for tissue repair and regeneration | [28] |
| Cardiovascular diseases | MSCs exhibit cardio-protective effects and can enhance cardiac repair following myocardial infarction. Clinical trials have explored their potential for improving heart function, reducing scar formation, and stimulating angiogenesis | [29] |
| Neurological disorders | MSCs hold potential for treating neurodegenerative conditions such as Parkinson's disease, Alzheimer's disease, and spinal cord injuries. They promote neuroprotection, neural differentiation, and the secretion of neurotrophic factors, fostering neural tissue repair | [30] |
| Autoimmune disorders | In autoimmune diseases like multiple sclerosis and systemic lupus erythematosus, MSCs' immunomodulatory properties help suppress aberrant immune responses and reduce disease severity. They promote tolerance and reduce inflammation | [31,32] |
| GVHD | MSCs have demonstrated effectiveness in managing GVHD, a potentially fatal complication of hematopoietic stem cell transplantation. They modulate immune reactions, aiding in GVHD prevention and treatment | [33] |
| IBD | MSCs are under investigation for their role in managing Crohn's disease and ulcerative colitis. They promote mucosal healing, reduce inflammation, and regulate the immune system within the gut | [34] |
| Diabetes | MSCs hold the potential for treating type 1 diabetes by promoting pancreatic beta-cell regeneration and modulating the autoimmune response that leads to beta-cell destruction | [35] |
| Wound healing and dermatological conditions | MSCs facilitate wound healing by enhancing tissue regeneration and reducing scar formation. They are explored for treating skin conditions like chronic ulcers and epidermolysis bullosa | [36] |
| Lung disorders | In conditions like COPD and idiopathic pulmonary fibrosis, MSCs can mitigate inflammation, promote lung tissue repair, and enhance pulmonary function | [37] |
MSCs have shown great promise in treating musculoskeletal conditions, including osteoarthritis, rheumatoid arthritis, and bone fractures. Their ability to differentiate into bone and cartilage cells and their anti-inflammatory properties make them valuable for tissue repair and regeneration[28].
MSCs exhibit cardio-protective effects and can enhance cardiac repair following myocardial infarction. Clinical trials have explored their potential for improving heart function, reducing scar formation, and stimulating angiogenesis[29].
MSCs hold potential for treating neurodegenerative conditions such as Parkinson's disease, Alzheimer's disease, and spinal cord injuries. They promote neuroprotection, neural differentiation, and the secretion of neurotrophic factors, fostering neural tissue repair[30].
In autoimmune diseases like multiple sclerosis and systemic lupus erythematosus, MSCs' immunomodulatory properties help suppress aberrant immune responses and reduce disease severity. They promote tolerance and reduce inflammation[31,32].
MSCs have demonstrated effectiveness in managing GVHD, a potentially fatal complication of hematopoietic stem cell transplantation. They modulate immune reactions, aiding in GVHD prevention and treatment[33].
MSCs are under investigation for their role in managing Crohn's disease and ulcerative colitis. They promote mucosal healing, reduce inflammation, and regulate the immune system within the gut[34].
MSCs hold the potential for treating type 1 diabetes by promoting pancreatic beta-cell regeneration and modulating the autoimmune response that leads to beta-cell destruction[35].
MSCs facilitate wound healing by enhancing tissue regeneration and reducing scar formation. They are explored for treating skin conditions like chronic ulcers and epidermolysis bullosa[36].
In conditions like chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis, MSCs can mitigate inflammation, promote lung tissue repair, and enhance pulmonary function[37].
Ongoing clinical trials are investigating the use of MSCs in various conditions, including solid organ transplantation, kidney diseases, and hematological disorders. Personalized medicine approaches, combinatorial therapies, and innovative delivery methods are also being explored to optimize MSC-based treatments[38-41].
While MSC-based therapies hold significant promise, challenges remain, including standardization of protocols, safety concerns, and regulatory frameworks. Nonetheless, their clinical potential continues to expand, offering hope for improved outcomes and novel treatments across a spectrum of medical conditions.
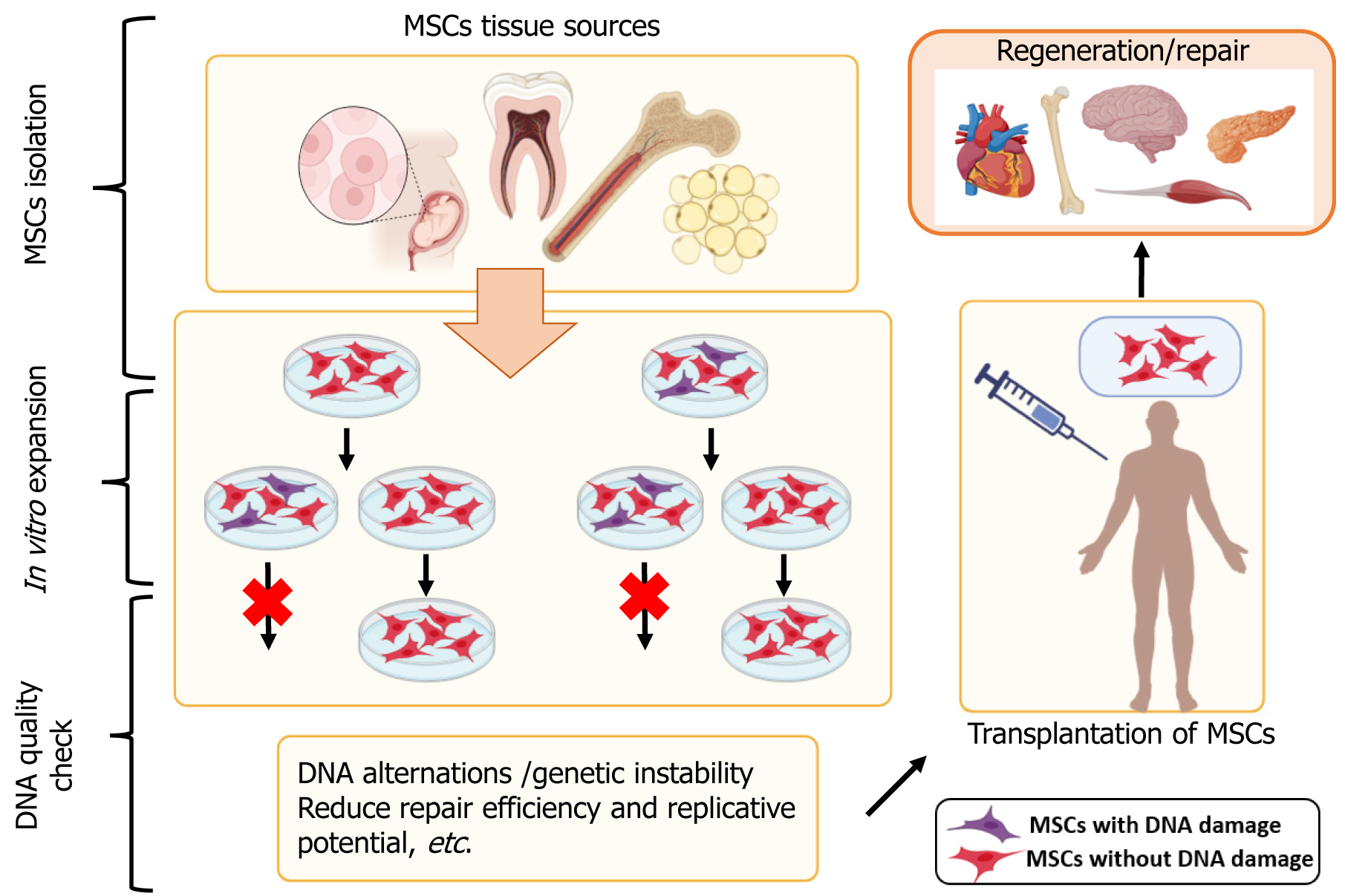
Some controversies regarding MSC use in regenerative medicine are related to their immunomodulatory properties, sources, safety and efficacy concerns, etc. In Figure 1, we present some of the MSC sources and the potential of MSC to be used in regenerative medicine.
Autologous vs allogeneic msc transplantation: The choice between using a patient's MSCs (autologous) or MSCs from a donor (allogeneic) raises debates. Autologous MSCs may reduce the risk of immune rejection but can be limited in quantity and may have reduced quality, while allogeneic MSCs offer scalability but carry potential immunogenicity risks. Allogeneic MSCs can provide several advantages such as donor selection, various sources, low immunogenicity, and off-the-shelf availability; however, autologous MSCs require a few weeks for isolation, in-vitro expansion and release, and patient-derived autologous MSCs may underlie systemic diseases[3].
Efficacy and long-term outcomes: Variability in therapeutic outcomes and long-term durability of MSC-based therapies is a contention. Robust clinical evidence is needed to establish the efficacy and assess the longevity of these treatments. A systematic review and meta-analysis by Xie et al[42] on human MSC therapy's clinical efficacy and safety for degenerative disc diseases revealed that MSC could significantly improve patients' clinical outcomes. However, the optimal dosage, frequency, time, route of administration, and suitable stages of the diseases should be elucidated.
Safety concerns: Safety concerns include the potential for tumorigenicity, immunogenic reactions, and adverse effects. Ensuring the safety of MSC transplantation is essential for its widespread clinical adoption.
Wang et al[43] discussed the safety of MSC therapy over the past 15 years in their meta-analysis. A total of 62 randomized clinical trials with 3546 patients treated with intravenous or local implantation against placebo or no treatment were included. The participants were diagnosed with about 20 different types of illnesses. In conclusion, when compared to alternative placebo modalities, MSC delivery proved safe in a variety of demographics, where the most common side effects were transient fever, administration site adverse effects, constipation, fatigue and insomnia.
The mechanisms by which MSCs home to target tissues and integrate into host environments are not fully understood. This raises questions about the predictability and control of MSC behavior in vivo. Ullah et al[44] reviewed the molecular mechanisms underlying MSC homing based on a multistep model involving: (1) Initial tethering by selectins; (2) activation by cytokines; (3) arrest by integrins; (4) diapedesis or transmigration using matrix remodelers; and (5) extravascular migration toward chemokine gradients. Unfortunately, MSC homing is inefficient, with only a small percentage of cells reaching the target tissue following systemic administration. This attrition represents a significant bottleneck in realizing the full therapeutic potential of MSC-based therapies. Therefore, a variety of strategies have been employed in the hopes of improving this process.
The balance between the paracrine signaling effects of MSCs and their potential to differentiate into specific cell types is a critical dilemma. Deciphering the optimal mode of action for various clinical applications remains a challenge[45,46].
The lack of standardized protocols for MSC isolation, expansion, and characterization hampers comparability across studies and poses challenges for regulatory approval and clinical translation.
Throughout the past few decades, adult bone marrow has been the usual source of MSCs; however, this process is highly invasive, and the quality and quantity of isolated cells are significantly influenced by patient age, medication, and related comorbidities. As a result, there is a debate on the convenience of allogeneic over autologous treatments despite potential drawbacks like host rejection. This move to the allogeneic setting necessitates high MSC production to ensure the availability of sufficient cell numbers for transplantation. Searching for alternative tissue sources of highly proliferative MSC cultures with low levels of senescence occurrence is one of the biggest obstacles currently facing the scaling up of therapeutic use.
García-Muñoz and Vives[47] revealed the primary techniques for isolating MSCs from bone marrow, adipose tissue, and Wharton's jelly of the umbilical cord here; and compared their attributes from a bioprocess perspective, addressing both quality and regulatory considerations.
The regulatory landscape for MSC therapies varies between countries and regions. Establishing consistent and clear regulatory guidelines is essential for safe and effective clinical implementation.
Notwithstanding the differences in the field, MSC shareholders are united by a single objective: To employ MSCs as a therapeutic modality to enhance the quality of life for individuals afflicted with a disease whose current standard of care is inadequate or ineffective. Currently, no MSC therapy approved by the Food and Drug Administration is available for purchase in the United States. However, several MSC products have received regulatory approval in other nations[48].
The commercialization of MSC therapies has led to concerns about affordability, accessibility, and equitable distribution, potentially limiting access to these treatments for some patients.
A systematic review protocol by Pettitt et al[49] outlined the factors affecting the commercialization of cell-based therapeutics and the assessment of these factors. The translation of cellular-based therapeutics from "bench to bedside" remains challenging, and the number of industry products available for widespread clinical use is still relatively low, despite a notable increase in basic science activity within the cell therapy arena, a growing portfolio of cell therapy trials, and promising investment.
Identifying the most suitable patient populations for MSC-based therapies and developing personalized treatment approaches is a complex issue with significant implications for clinical practice[50].
It was shown that patient selection and stratification are crucial for better outcomes in MSC-based therapies. Soto-Gordoa et al[51] showed that a thorough, patient-centered, integrated care intervention was linked to a lower risk of hospital admission among patients who were prioritized to receive it but not among patients who were not, according to a before-and-after study utilizing propensity score matching.
Integrating MSC transplantation with other therapeutic modalities, such as drugs or gene therapies, poses challenges in timing, dosing, and potential interactions, raising questions about optimal combination strategies[52,53].
Ethical dilemmas include the use of fetal-derived MSCs and concerns about informed consent, especially in cases where unproven treatments are offered to vulnerable patient populations.
Volarevic et al[54] and other investigators highlighted the ethical challenges surrounding the research of human embryonic stem cells (hESCs), highlighting that the destruction of a human embryo is a significant factor that may have hindered the development of hESC-based clinical therapies. This problem has been resolved with the previous development of induced pluripotent stem cells (iPSCs), but there are still issues with current perspectives regarding the clinical translation of iPSCs. One major ethical issue is the unlimited differentiation potential of iPSCs, which can be used in human reproductive cloning, posing a risk of resulting in genetically altered human embryos and human-animal chimeras. On the other hand, there are significant safety issues with undesired differentiation and malignant trans
Variability in the quality of published studies and potential reporting bias in clinical trials can make it challenging to assess the true efficacy and safety of MSC-based interventions.
These controversies underline the complexity and ongoing debates surrounding the clinical application of MSCs, highlighting the need for rigorous research, standardized practices, and transparent regulatory frameworks to address these challenges[43,57,58].
The predominant applications of MSCs are in autoimmune, musculoskeletal, and vascular diseases, regenerative medicine, wounds, injuries, etc. These applications for different clinical conditions require continuous monitoring for their safety. The most important question for risk assessment is related to the genetic stability of MSCs, which can alter during in vitro manipulations[59,60]. There are over 1000 clinical trials on their safety and effectiveness, but the results of their use as immunomodulatory agents have yet to provide a clear answer. In most trials, only a few patients show sufficient or poor therapeutic response; therefore, it can still be said that they are unstable and controversial for clinical practice.
Genome stability is an essential characteristic of any species to preserve and accurately transmit genetic material from generation to generation. This is associated with correct replication, repair of replication errors or damaged DNA, and proper cell cycle progression. Genomic instability, in turn, is associated with a high rate of mutation occurrence because of direct and indirect mutagens, external factors, and epigenetic changes in the genome. Various methods can be used to analyze genetic instability-karyotyping[61], fluorescence in situ hybridization, array-wide comparative genomic hybridization, microsatellite analysis, in vitro micronucleus assay, spectral karyotyping, RNA sequencing, and others[62-66]. Each gives a very informative analysis and shows if there are genetic alternations.
Genome evaluation is most important for MSC stability, and all DNA alterations, even smaller ones, should be assessed. MSCs senesce with vast cultivation in vitro, so they lose their proliferation and differentiation potential[67]. Such culture expansion can generate genetic and epigenetic instability, including different chromosomal changes, which pose a risk to MSC therapy[59].
Much research has shown that MSCs get changes in both autosomes and sex chromosomes[64,68-72] and other anomalies[73-75], while other studies have reported unchanged chromosomes in MSC cultures of different tissues, such as humanfetal dermis[76], adipose[77], human adult mesenchymal stromal cells[78-82]. Depending on various factors such as culture times, conditions, and sources, these changes can occur in early or late passages with varying frequency[73].
In 2011, a study described a 4% incidence of aneuploidy in large numbers of MSC preparations[62]. A few years later, Kim et al[83] observed cytogenetic changes in different MSC preparations. They estimated the genetic stability of MSCs on 68 preparations with varying origins of tissue and found variable aneuploidy clonal proportions of 1%-20% with overwhelming X, 16, 17, and 18 chromosomes. In 2017, a study revealed that MSCs show genomic instability during ex vivo expansion, with the most remarkable change associated with single nucleotide variations that appear at later passages[66]. They performed whole genome sequencing of two peripheral blood-derived MSC lines and described that 90% and 70% of all such variations were observed at passage 9 (for MSC1) and passage 7/9 (for MSC2), respectively.
Roselli et al[64] also assessed the genomic stability of MSCs of chorionic villus by different methods. They found abnormal clones after passage 10, which showed no growth advantage, no signs of transformation, and stable microsatellites. This suggests that if the cells in MSC culture become senescent, the mismatch repair system is efficient, and it is doubtful to form tumors in patients[64,84]. А study reported alternations in the gene expression from passage[59]. This suggests using cells up to passage four and analyzing cells from higher culture passages.
Different DNA alternations are still subject to elucidation because the genomic instability (chromosomal changes) is characteristic of cancer, transformation and/or aging[73,84-86]. Through these changes, the tumorigenicity and impaired biological activity of MSCs can be predicted.
MSCs are potent tools in regenerative medicine because of their potential to self-renew, multipotency, and trophic and immunosuppressive properties[87]. The various genetic alternations/aberrations reported in MCS cultures should be analyzed in detail because the gaps in our understanding of MSC applications have to become more precise. Therefore, monitoring the genetic profiles during culture passages is vital because possible modifications and genetic changes can potentially affect the therapeutic efficiency of cell therapy.
MSC therapy has entered a dynamic phase of exploration and innovation. As researchers and clinicians gain a deeper understanding of MSCs' capabilities and limitations, new frontiers emerge. In this section, we delve into three promising areas that represent the future directions of MSC therapy: Personalized medicine, advanced delivery methods, and combining therapies. These evolving trends hold significant potential to enhance the effectiveness and applicability of MSC-based treatments across various medical conditions.
Personalized medicine, a paradigm that tailors medical treatment to the specific characteristics of each patient, is becoming increasingly relevant in the field of MSC therapy. The unique genetic makeup, medical history, and disease profile of each necessitate a more precise and patient-centered approach to treatment. Personalized medicine can be applied to MSC therapy in several ways (Table 3).
| Aspect | MSC treatment | |
| Personalized medicine | Patient-specific MSCs | One of the critical strategies in personalized medicine involves using patient-derived MSCs. These autologous MSCs are obtained from the patient's own tissues, such as bone marrow or adipose tissue. Using a patient's cells minimizes the risk of immune rejection, and treatment can be tailored to the individual's needs |
| Genomic and molecular profiling | Advances in genomics and molecular profiling techniques enable the identification of specific markers or genetic characteristics that influence a patient's response to MSC therapy. This information can guide treatment decisions, allowing for the selection of the most appropriate MSC source and optimization of the therapeutic regimen | |
| Disease-specific approaches | Tailoring MSC therapies to the unique features of a particular disease is another aspect of personalized medicine. For example, MSCs can be engineered in cancer therapy to deliver anti-tumor agents or enhance immune responses, depending on the patient's cancer type and stage | |
| Dosage and timing optimization | Personalized medicine also extends to optimizing the dosage and timing of MSC treatments. Factors such as the severity of the condition, the patient's age, and comorbidities can all influence the treatment protocol, ensuring the best possible outcomes | |
| Advanced delivery methods | Microencapsulation and biomaterials | Microencapsulation involves encapsulating MSCs within biocompatible materials or hydrogels. This protective environment shields MSCs from immune responses while providing a sustained release of therapeutic factors. This approach is encouraging for conditions like diabetes, where encapsulated MSCs can help regulate blood sugar levels |
| Intravenous infusion techniques | Intravenous delivery of MSCs is a common method, but refinements in infusion techniques are being explored to maximize cell retention and tissue homing. Pre-conditioning or priming MSCs before infusion can enhance their migratory properties and tissue-specific targeting | |
| Nanoparticle-based carriers | Nanoparticles can serve as carriers for MSCs, protecting them during transit and improving their ability to reach target sites. These carriers can be loaded with therapeutic or imaging agents for tracking and treatment monitoring | |
| Direct injection and endoscopic delivery | For localized conditions, such as osteoarthritis or IBD, direct injection of MSCs into the affected area or endoscopic delivery methods are being refined to target tissues and minimize invasiveness precisely | |
| Exosome-mediated delivery | MSC-derived exosomes, tiny vesicles containing bioactive molecules, offer a cell-free approach to therapy. Exosomes can be isolated and administered to mediate therapeutic effects, making them a promising alternative to whole-cell therapy | |
| Combining therapy | Immunomodulatory Combinations | Combining MSC therapy with immunomodulatory agents or immune checkpoint inhibitors can potentiate the immunosuppressive effects of MSCs, particularly in the context of autoimmune diseases or organ transplantation |
| Gene editing and engineering | Genetic modification of MSCs allows for the precise manipulation of their properties. Engineered MSCs can be equipped with therapeutic genes or targeted for specific functions, such as enhancing tissue regeneration or tumor suppression | |
| Drug delivery systems | MSCs can serve as drug delivery vehicles, transporting therapeutic compounds directly to diseased tissues. This approach is particularly relevant in cancer therapy, where MSCs can deliver anti-cancer drugs to tumor sites | |
| Stem cell combinations | Combining different types of stem cells, such as iPSCs or neural stem cells, with MSCs can offer multi-pronged approaches to conditions like spinal cord injuries or neurodegenerative diseases | |
| Adjunct therapies | MSC transplantation can complement traditional treatments, such as surgery or radiation therapy. For example, MSCs can be used alongside surgical procedures in bone repair to accelerate healing and improve outcomes | |
Patient-specific MSCs: One of the critical strategies in personalized medicine involves using patient-derived MSCs. These autologous MSCs are obtained from the patient's own tissues, such as bone marrow or adipose tissue. Using a patient's cells minimizes the risk of immune rejection, and treatment can be tailored to the individual's needs.
Genomic and molecular profiling: Advances in genomics and molecular profiling techniques enable the identification of specific markers or genetic characteristics that influence a patient's response to MSC therapy. This information can guide treatment decisions, allowing for the selection of the most appropriate MSC source and optimization of the therapeutic regimen.
Disease-specific approaches: Tailoring MSC therapies to the unique features of a particular disease is another aspect of personalized medicine. For example, MSCs can be engineered in cancer therapy to deliver anti-tumor agents or enhance immune responses, depending on the patient's cancer type and stage.
Dosage and timing optimization: Personalized medicine also extends to optimizing the dosage and timing of MSC treatments. Factors such as the severity of the condition, the patient's age, and comorbidities can all influence the treatment protocol, ensuring the best possible outcomes.
Microencapsulation and biomaterials: Microencapsulation involves encapsulating MSCs within biocompatible materials or hydrogels. This protective environment shields MSCs from immune responses while providing a sustained release of therapeutic factors. This approach is encouraging for conditions like diabetes, where encapsulated MSCs can help regulate blood sugar levels.
Intravenous infusion techniques: Intravenous delivery of MSCs is a common method, but refinements in infusion techniques are being explored to maximize cell retention and tissue homing. Pre-conditioning or priming MSCs before infusion can enhance their migratory properties and tissue-specific targeting.
Nanoparticle-based carriers: Nanoparticles can serve as carriers for MSCs, protecting them during transit and improving their ability to reach target sites. These carriers can be loaded with therapeutic or imaging agents for tracking and treatment monitoring.
Direct injection and endoscopic delivery: For localized conditions, such as osteoarthritis or inflammatory bowel disease (IBD), direct injection of MSCs into the affected area or endoscopic delivery methods are being refined to target tissues and minimize invasiveness precisely.
Exosome-mediated delivery: MSC-derived exosomes, tiny vesicles containing bioactive molecules, offer a cell-free approach to therapy. Exosomes can be isolated and administered to mediate therapeutic effects, making them a promising alternative to whole-cell therapy.
Immunomodulatory combinations: Combining MSC therapy with immunomodulatory agents or immune checkpoint inhibitors can potentiate the immunosuppressive effects of MSCs, particularly in the context of autoimmune diseases or organ transplantation.
Gene editing and engineering: Genetic modification of MSCs allows for the precise manipulation of their properties. Engineered MSCs can be equipped with therapeutic genes or targeted for specific functions, such as enhancing tissue regeneration or tumor suppression.
Drug delivery systems: MSCs can serve as drug delivery vehicles, transporting therapeutic compounds directly to diseased tissues. This approach is particularly relevant in cancer therapy, where MSCs can deliver anti-cancer drugs to tumor sites.
Stem cell combinations: Combining different types of stem cells, such as iPSCs or neural stem cells, with MSCs can offer multi-pronged approaches to conditions like spinal cord injuries or neurodegenerative diseases.
Adjunct therapies: MSC transplantation can complement traditional treatments, such as surgery or radiation therapy. For example, MSCs can be used alongside surgical procedures in bone repair to accelerate healing and improve outcomes.
Applying personalized medicine principles to MSC therapy holds great promise for improving treatment efficacy while minimizing potential side effects. However, it also presents cost, logistics, and regulatory challenges. As research in this area continues, we expect to see more tailored and effective MSC-based therapies for various diseases.
Effective delivery of MSCs to target tissues or organs is critical to successful therapy. Advanced delivery methods aim to enhance MSC administration's precision, efficiency, and safety. Several innovative approaches are being explored: Advanced delivery methods improve the therapeutic potential of MSCs and expand the range of conditions that can be effectively treated. These innovations address challenges related to MSC homing, survival, and engraftment, enhancing the clinical feasibility of MSC-based therapies.
The future of MSC therapy lies in harnessing the synergistic potential of combinatorial approaches. By integrating MSC transplantation with other therapeutic modalities, researchers aim to enhance treatment outcomes and address complex medical conditions more comprehensively. Several strategies are being explored: These combinatorial approaches hold immense potential for addressing complex and multifaceted diseases that may not respond optimally to single-modality treatments. However, they also present safety, regulatory approval, and treatment optimization challenges.
While these emerging trends and research directions in MSC therapy offer great promise, they also raise important considerations and challenges.
Safety and regulation: Ensuring the safety of personalized, genetically modified, or engineered MSCs is paramount. Regulatory frameworks need to adapt to accommodate these innovations while safeguarding patient well-being.
Standardization: As MSC therapies become increasingly personalized and complex, standardized protocols and quality control measures are more critical to ensure consistency and reproducibility.
Cost and accessibility: Some advanced delivery methods and combinatorial approaches may be cost-prohibitive or less accessible to specific patient populations. Balancing innovation with affordability is a significant challenge.
Ethical considerations: The genetic engineering of MSCs and their use in certain applications, such as cancer therapy, raise ethical questions that must be addressed in research and clinical practice.
Long-term safety and efficacy: Ensuring the long-term safety and efficacy of personalized, genetically modified, or engineered MSC therapies requires ongoing monitoring and research.
In conclusion, the future of MSC therapy is characterized by personalized medicine, advanced delivery methods, and the synergy of multiple treatment modalities. These emerging trends hold immense potential to revolutionize regenerative medicine and offer new hope to patients with various medical conditions. However, addressing the associated challenges and ethical considerations is essential to unlock the full therapeutic potential of MSCs safely and effectively.
MSC therapy has journeyed far from its early beginnings as an intriguing scientific discovery to a dynamic and rapidly evolving field of regenerative medicine. The discussion of emerging trends and research directions in this paper underscores the transformative potential of MSCs, paving the way for a new era in healthcare. As we conclude this exploration, several vital insights emerge.
First and foremost, personalized medicine is poised to reshape the landscape of MSC therapy. Tailoring treatments to individual patients enhances efficacy and minimizes the risks associated with immune reactions. Patient-specific MSCs, genomic profiling, and disease-specific approaches usher in a new era of precision medicine, offering hope to those facing previously insurmountable medical challenges.
Secondly, advanced delivery methods are revolutionizing how MSCs reach their intended targets. From microencapsulation to nanoparticle carriers, these innovations address longstanding challenges related to cell homing, engraftment, and survival. Such advancements expand the therapeutic reach of MSCs, making treatment more accessible and practical.
Lastly, the future of MSC therapy lies in the power of synergy—combining MSC transplantation with other treatment modalities to tackle complex diseases comprehensively. Immunomodulatory combinations, gene editing, drug delivery systems, and stem cell co-therapies promise to unlock new possibilities for conditions once considered intractable.
However, amidst the promise and potential of MSC therapy, it is essential to acknowledge the challenges. Safety and regulatory concerns, standardization of protocols, cost considerations, and ethical dilemmas require diligent attention. The responsible and ethical advancement of MSC-based treatments necessitates collaboration among scientists, clinicians, policymakers, and regulatory bodies to ensure these therapies reach their full potential while safeguarding patient well-being.
In closing, the future of MSC therapy is marked by a confluence of innovation and responsibility. As researchers and clinicians continue to push the boundaries of what is possible, the transformative potential of MSCs remains boundless. With personalized medicine, advanced delivery methods, and the integration of complementary therapies, we stand at the threshold of a new era in regenerative medicine—where MSCs offer hope and healing to individuals facing diverse medical conditions. By carefully navigating the evolving landscape of MSC therapy, we can transform these possibilities into realities, improving lives and shaping the future of healthcare.
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
| 1. | Kassem M. Mesenchymal stem cells: biological characteristics and potential clinical applications. Cloning Stem Cells. 2004;6:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Margiana R, Markov A, Zekiy AO, Hamza MU, Al-Dabbagh KA, Al-Zubaidi SH, Hameed NM, Ahmad I, Sivaraman R, Kzar HH, Al-Gazally ME, Mustafa YF, Siahmansouri H. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther. 2022;13:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 203] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 3. | Li C, Zhao H, Cheng L, Wang B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021;11:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 4. | Jovic D, Yu Y, Wang D, Wang K, Li H, Xu F, Liu C, Liu J, Luo Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev Rep. 2022;18:1525-1545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 131] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 5. | Jimenez-Puerta GJ, Marchal JA, López-Ruiz E, Gálvez-Martín P. Role of Mesenchymal Stromal Cells as Therapeutic Agents: Potential Mechanisms of Action and Implications in Their Clinical Use. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15203] [Article Influence: 584.7] [Reference Citation Analysis (0)] |
| 7. | Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1330] [Cited by in RCA: 1243] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 8. | Baghaei K, Hashemi SM, Tokhanbigli S, Asadi Rad A, Assadzadeh-Aghdaei H, Sharifian A, Zali MR. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol Hepatol Bed Bench. 2017;10:208-213. [PubMed] |
| 9. | Wilson AJ, Rand E, Webster AJ, Genever PG. Characterisation of mesenchymal stromal cells in clinical trial reports: analysis of published descriptors. Stem Cell Res Ther. 2021;12:360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Bhat S, Viswanathan P, Chandanala S, Prasanna SJ, Seetharam RN. Expansion and characterization of bone marrow derived human mesenchymal stromal cells in serum-free conditions. Sci Rep. 2021;11:3403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 11. | Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, Hua D, Shao C, Shi Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther. 2022;7:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 356] [Article Influence: 118.7] [Reference Citation Analysis (0)] |
| 12. | Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41:653-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 13. | Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012;35:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 14. | Li P, Ou Q, Shi S, Shao C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell Mol Immunol. 2023;20:558-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 108] [Reference Citation Analysis (0)] |
| 15. | Laing AG, Fanelli G, Ramirez-Valdez A, Lechler RI, Lombardi G, Sharpe PT. Mesenchymal stem cells inhibit T-cell function through conserved induction of cellular stress. PLoS One. 2019;14:e0213170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed Res Int. 2014;2014:216806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Naserian S, Shamdani S, Arouche N, Uzan G. Regulatory T cell induction by mesenchymal stem cells depends on the expression of TNFR2 by T cells. Stem Cell Res Ther. 2020;11:534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Azevedo RI, Minskaia E, Fernandes-Platzgummer A, Vieira AIS, da Silva CL, Cabral JMS, Lacerda JF. Mesenchymal stromal cells induce regulatory T cells via epigenetic conversion of human conventional CD4 T cells in vitro. Stem Cells. 2020;38:1007-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, Noël D, Jorgensen C, Figueroa F, Djouad F, Carrión F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 367] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 20. | Darlan DM, Raga A, Muhar AM, Putra A, Alif I. Mesenchymal Stem Cells Suppress Dendritic Cells and Modulate Proinflammatory Milieu Through Interleukin-10 Expression in Peripheral Blood Mononuclear Cells of Human Systemic Lupus Erythematosus. Acta Inform Med. 2023;31:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Salari V, Mengoni F, Del Gallo F, Bertini G, Fabene PF. The Anti-Inflammatory Properties of Mesenchymal Stem Cells in Epilepsy: Possible Treatments and Future Perspectives. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Kaffash Farkhad N, Reihani H, Sedaghat A, Moghadam AA, Moghadam AB, Tavakol-Afshari J. Are mesenchymal stem cells able to manage cytokine storm in COVID-19 patients? A review of recent studies. Regen Ther. 2021;18:152-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Luque-Campos N, Bustamante-Barrientos FA, Pradenas C, García C, Araya MJ, Bohaud C, Contreras-López R, Elizondo-Vega R, Djouad F, Luz-Crawford P, Vega-Letter AM. The Macrophage Response Is Driven by Mesenchymal Stem Cell-Mediated Metabolic Reprogramming. Front Immunol. 2021;12:624746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 391] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 25. | Wu Q, Zhou L, Lv D, Zhu X, Tang H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol. 2019;12:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 26. | Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Intern Med. 2013;28:387-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 27. | Merimi M, El-Majzoub R, Lagneaux L, Moussa Agha D, Bouhtit F, Meuleman N, Fahmi H, Lewalle P, Fayyad-Kazan M, Najar M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front Cell Dev Biol. 2021;9:661532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 28. | Law L, Hunt CL, van Wijnen AJ, Nassr A, Larson AN, Eldrige JS, Mauck WD, Pingree MJ, Yang J, Muir CW, Erwin PJ, Bydon M, Qu W. Office-Based Mesenchymal Stem Cell Therapy for the Treatment of Musculoskeletal Disease: A Systematic Review of Recent Human Studies. Pain Med. 2019;20:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Bagno L, Hatzistergos KE, Balkan W, Hare JM. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol Ther. 2018;26:1610-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 30. | Andrzejewska A, Dabrowska S, Lukomska B, Janowski M. Mesenchymal Stem Cells for Neurological Disorders. Adv Sci (Weinh). 2021;8:2002944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 217] [Article Influence: 54.3] [Reference Citation Analysis (1)] |
| 31. | Jasim SA, Yumashev AV, Abdelbasset WK, Margiana R, Markov A, Suksatan W, Pineda B, Thangavelu L, Ahmadi SH. Shining the light on clinical application of mesenchymal stem cell therapy in autoimmune diseases. Stem Cell Res Ther. 2022;13:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 32. | Rad F, Ghorbani M, Mohammadi Roushandeh A, Habibi Roudkenar M. Mesenchymal stem cell-based therapy for autoimmune diseases: emerging roles of extracellular vesicles. Mol Biol Rep. 2019;46:1533-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Kadri N, Amu S, Iacobaeus E, Boberg E, Le Blanc K. Current perspectives on mesenchymal stromal cell therapy for graft versus host disease. Cell Mol Immunol. 2023;20:613-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 34. | Shi MY, Liu L, Yang FY. Strategies to improve the effect of mesenchymal stem cell therapy on inflammatory bowel disease. World J Stem Cells. 2022;14:684-699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (4)] |
| 35. | Jayasinghe M, Prathiraja O, Perera PB, Jena R, Silva MS, Weerawarna PSH, Singhal M, Kayani AMA, Karnakoti S, Jain S. The Role of Mesenchymal Stem Cells in the Treatment of Type 1 Diabetes. Cureus. 2022;14:e27337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 36. | Hu MS, Borrelli MR, Lorenz HP, Longaker MT, Wan DC. Mesenchymal Stromal Cells and Cutaneous Wound Healing: A Comprehensive Review of the Background, Role, and Therapeutic Potential. Stem Cells Int. 2018;2018:6901983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 37. | Cruz FF, Rocco PRM. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev Respir Med. 2020;14:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 38. | Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 731] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 39. | Thalakiriyawa DS, Jayasooriya PR, Dissanayaka WL. Regenerative Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles. Curr Mol Med. 2022;22:98-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Maldonado VV, Patel NH, Smith EE, Barnes CL, Gustafson MP, Rao RR, Samsonraj RM. Clinical utility of mesenchymal stem/stromal cells in regenerative medicine and cellular therapy. J Biol Eng. 2023;17:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 41. | Foo JB, Looi QH, Chong PP, Hassan NH, Yeo GEC, Ng CY, Koh B, How CW, Lee SH, Law JX. Comparing the Therapeutic Potential of Stem Cells and their Secretory Products in Regenerative Medicine. Stem Cells Int. 2021;2021:2616807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 42. | Xie B, Chen S, Xu Y, Han W, Hu R, Chen M, He R, Ding S. Clinical Efficacy and Safety of Human Mesenchymal Stem Cell Therapy for Degenerative Disc Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Stem Cells Int. 2021;2021:9149315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 43. | Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther. 2021;12:545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 44. | Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 371] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 45. | Musiał-Wysocka A, Kot M, Majka M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019;28:801-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 46. | Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019;2019:9628536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 357] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 47. | García-Muñoz E, Vives J. Towards the standardization of methods of tissue processing for the isolation of mesenchymal stromal cells for clinical use. Cytotechnology. 2021;73:513-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Wright A, Arthaud-Day ML, Weiss ML. Therapeutic Use of Mesenchymal Stromal Cells: The Need for Inclusive Characterization Guidelines to Accommodate All Tissue Sources and Species. Front Cell Dev Biol. 2021;9:632717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 49. | Pettitt D, Arshad Z, Davies B, Smith J, French A, Cole D, Bure K, Dopson S, DiGiusto D, Karp J, Reeve B, Barker R, Holländer G, Brindley D. An assessment of the factors affecting the commercialization of cell-based therapeutics: a systematic review protocol. Syst Rev. 2017;6:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Mastrolia I, Foppiani EM, Murgia A, Candini O, Samarelli AV, Grisendi G, Veronesi E, Horwitz EM, Dominici M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl Med. 2019;8:1135-1148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 51. | Soto-Gordoa M, de Manuel E, Fullaondo A, Merino M, Arrospide A, Igartua JI, Mar J; CareWell Group. Impact of stratification on the effectiveness of a comprehensive patient-centered strategy for multimorbid patients. Health Serv Res. 2019;54:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Wang Z, Zhou C, Yang S. The roles, controversies, and combination therapies of autophagy in lung cancer. Cell Biol Int. 2022;46:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Zhou H, He Y, Xiong W, Jing S, Duan X, Huang Z, Nahal GS, Peng Y, Li M, Zhu Y, Ye Q. MSC based gene delivery methods and strategies improve the therapeutic efficacy of neurological diseases. Bioact Mater. 2023;23:409-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 54. | Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 527] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 55. | Feier AM, Portan D, Manu DR, Kostopoulos V, Kotrotsos A, Strnad G, Dobreanu M, Salcudean A, Bataga T. Primary MSCs for Personalized Medicine: Ethical Challenges, Isolation and Biocompatibility Evaluation of 3D Electrospun and Printed Scaffolds. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Harris AR, Walker MJ, Gilbert F. Ethical and regulatory issues of stem cell-derived 3-dimensional organoid and tissue therapy for personalised regenerative medicine. BMC Med. 2022;20:499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 57. | Chen Y, Zhang Q, Peng W, Liu D, You Y, Liu X, Tang S, Zhang T. Efficacy and safety of mesenchymal stem cells for the treatment of patients infected with COVID-19: a systematic review and meta-analysis protocol. BMJ Open. 2020;10:e042085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Yao W, Dong H, Qi J, Zhang Y, Shi L. Safety and efficacy of mesenchymal stem cells in severe/critical patients with COVID-19: A systematic review and meta-analysis. EClinicalMedicine. 2022;51:101545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Binato R, de Souza Fernandez T, Lazzarotto-Silva C, Du Rocher B, Mencalha A, Pizzatti L, Bouzas LF, Abdelhay E. Stability of human mesenchymal stem cells during in vitro culture: considerations for cell therapy. Cell Prolif. 2013;46:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 60. | Yang YK, Ogando CR, Wang See C, Chang TY, Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 426] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 61. | Gantchev J, Ramchatesingh B, Berman-Rosa M, Sikorski D, Raveendra K, Amar L, Xu HH, Villarreal AM, Ordaz DJG, Litvinov IV. Tools used to assay genomic instability in cancers and cancer meiomitosis. J Cell Commun Signal. 2022;16:159-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Ben-David U, Mayshar Y, Benvenisty N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell. 2011;9:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 63. | Borghesi A, Avanzini MA, Novara F, Mantelli M, Lenta E, Achille V, Cerbo RM, Tzialla C, Longo S, De Silvestri A, Zimmermann LJ, Manzoni P, Zecca M, Spinillo A, Maccario R, Zuffardi O, Stronati M. Genomic alterations in human umbilical cord-derived mesenchymal stromal cells call for stringent quality control before any possible therapeutic approach. Cytotherapy. 2013;15:1362-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Roselli EA, Lazzati S, Iseppon F, Manganini M, Marcato L, Gariboldi MB, Maggi F, Grati FR, Simoni G. Fetal mesenchymal stromal cells from cryopreserved human chorionic villi: cytogenetic and molecular analysis of genome stability in long-term cultures. Cytotherapy. 2013;15:1340-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Borgonovo T, Vaz IM, Senegaglia AC, Rebelatto CL, Brofman PR. Genetic evaluation of mesenchymal stem cells by G-banded karyotyping in a Cell Technology Center. Rev Bras Hematol Hemoter. 2014;36:202-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Kim M, Rhee JK, Choi H, Kwon A, Kim J, Lee GD, Jekarl DW, Lee S, Kim Y, Kim TM. Passage-dependent accumulation of somatic mutations in mesenchymal stromal cells during in vitro culture revealed by whole genome sequencing. Sci Rep. 2017;7:14508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Solchaga LA, Penick KJ, Welter JF. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Methods Mol Biol. 2011;698:253-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 68. | Meng Z, Chen G, Chen J, Yang B, Yu M, Feng L, Jiang Z, Guo W, Tian W. Tumorigenicity analysis of heterogeneous dental stem cells and its self-modification for chromosome instability. Cell Cycle. 2015;14:3396-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Vériter S, André W, Aouassar N, Poirel HA, Lafosse A, Docquier PL, Dufrane D. Human Adipose-Derived Mesenchymal Stem Cells in Cell Therapy: Safety and Feasibility in Different "Hospital Exemption" Clinical Applications. PLoS One. 2015;10:e0139566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 70. | Duarte DM, Cornélio DA, Corado C, Medeiros VK, de Araújo LA, Cavalvanti GB Jr, de Medeiros SR. Chromosomal characterization of cryopreserved mesenchymal stem cells from the human subendothelium umbilical cord vein. Regen Med. 2012;7:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Lucarelli E, Bellotti C, Mantelli M, Avanzini MA, Maccario R, Novara F, Arrigo G, Zuffardi O, Zuntini M, Pandolfi M, Sangiorgi L, Lisini D, Donati D, Duchi S. In vitro biosafety profile evaluation of multipotent mesenchymal stem cells derived from the bone marrow of sarcoma patients. J Transl Med. 2014;12:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Neri S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 73. | Rebuzzini P, Zuccotti M, Redi CA, Garagna S. Chromosomal Abnormalities in Embryonic and Somatic Stem Cells. Cytogenet Genome Res. 2015;147:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Stultz BG, McGinnis K, Thompson EE, Lo Surdo JL, Bauer SR, Hursh DA. Chromosomal stability of mesenchymal stromal cells during in vitro culture. Cytotherapy. 2016;18:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 75. | Weissbein U, Ben-David U, Benvenisty N. Virtual karyotyping reveals greater chromosomal stability in neural cells derived by transdifferentiation than those from stem cells. Cell Stem Cell. 2014;15:687-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Chinnici CM, Amico G, Monti M, Motta S, Casalone R, Petri SL, Spada M, Gridelli B, Conaldi PG. Isolation and characterization of multipotent cells from human fetal dermis. Cell Transplant. 2014;23:1169-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Agostini F, Rossi FM, Aldinucci D, Battiston M, Lombardi E, Zanolin S, Massarut S, Parodi PC, Da Ponte A, Tessitori G, Pivetta B, Durante C, Mazzucato M. Improved GMP compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media. Stem Cell Res Ther. 2018;9:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 78. | Sensebé L, Tarte K, Galipeau J, Krampera M, Martin I, Phinney DG, Shi Y; MSC Committee of the International Society for Cellular Therapy. Limited acquisition of chromosomal aberrations in human adult mesenchymal stromal cells. Cell Stem Cell. 2012;10:9-10; author reply 10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 79. | Hwang SH, Lee W, Park SH, Lee HJ, Lee DC, Lim MH, Back SA, Yun BG, Jeun JH, Lim JY, Kang JM, Kim SW. Evaluation of characteristic of human turbinate derived mesenchymal stem cells cultured in the serum free media. PLoS One. 2017;12:e0186249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Hladik D, Höfig I, Oestreicher U, Beckers J, Matjanovski M, Bao X, Scherthan H, Atkinson MJ, Rosemann M. Long-term culture of mesenchymal stem cells impairs ATM-dependent recognition of DNA breaks and increases genetic instability. Stem Cell Res Ther. 2019;10:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 81. | Zamani H, Karami F, Mehdizadeh M, Baakhlag S, Zamani M. Long Term Culture of Mesenchymal Stem Cells: No Evidence of Chromosomal Instability. Asian Pac J Cancer Bio. 2022;7:349-353. [DOI] [Full Text] |
| 82. | Li J, Huang H, Xu X. Biological characteristics and karyotiping of a new isolation method for human adipose mesenchymal stem cells in vitro. Tissue Cell. 2017;49:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Kim SY, Im K, Park SN, Kwon J, Kim JA, Choi Q, Hwang SM, Han SH, Kwon S, Oh IH, Lee DS. Asymmetric aneuploidy in mesenchymal stromal cells detected by in situ karyotyping and fluorescence in situ hybridization: suggestions for reference values for stem cells. Stem Cells Dev. 2015;24:77-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Neri S, Guidotti S, Lilli NL, Cattini L, Mariani E. Infrapatellar fat pad-derived mesenchymal stromal cells from osteoarthritis patients: In vitro genetic stability and replicative senescence. J Orthop Res. 2017;35:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, Tchirkov A, Rouard H, Henry C, Splingard M, Dulong J, Monnier D, Gourmelon P, Gorin NC, Sensebé L; Société Française de Greffe de Moelle et Thérapie Cellulaire. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115:1549-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 342] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 86. | Ferguson LR, Chen H, Collins AR, Connell M, Damia G, Dasgupta S, Malhotra M, Meeker AK, Amedei A, Amin A, Ashraf SS, Aquilano K, Azmi AS, Bhakta D, Bilsland A, Boosani CS, Chen S, Ciriolo MR, Fujii H, Guha G, Halicka D, Helferich WG, Keith WN, Mohammed SI, Niccolai E, Yang X, Honoki K, Parslow VR, Prakash S, Rezazadeh S, Shackelford RE, Sidransky D, Tran PT, Yang ES, Maxwell CA. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol. 2015;35 Suppl:S5-S24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 87. | Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 664] [Article Influence: 36.9] [Reference Citation Analysis (0)] |