Published online Mar 18, 2024. doi: 10.5500/wjt.v14.i1.88833
Peer-review started: October 11, 2023
First decision: November 21, 2023
Revised: December 21, 2023
Accepted: January 22, 2024
Article in press: January 22, 2024
Published online: March 18, 2024
Processing time: 156 Days and 0.5 Hours
Liver transplantation (LT) for hepatocellular carcinoma (HCC) has been widely researched and is well established worldwide. The cornerstone of this treatment lies in the various criteria formulated by expert consensus and experience. The variations among the criteria are staggering, and the short- and long-term out
To study the differences in the current practices of LT for HCC at different centers in India and discuss their clinical implications in the future.
We conducted a survey of major centers in India that performed LT in December 2022. A total of 23 responses were received. The centers were classified as high- and low-volume, and the current trend of care for patients und
Of the 23 centers, 35% were high volume center (> 500 Liver transplants) while 52% were high-volume centers that performed more than 50 transplants/year. Approximately 39% of centers had performed > 50 LT for HCC while the percent distribution for HCC in LT patients was 5%–15% in approximately 73% of the patients. Barring a few, most centers were divided equally between University of California, San Francisco (UCSF) and center-specific criteria when choosing patients with HCC for LT, and most (65%) did not have separate transplant criteria for deceased donor LT and living donor LT (LDLT). Most centers (56%) preferred surgical resection over LT for a Child A cirrhosis patient with a resectable 4 cm HCC lesion. Positron-emission tomography-computed tomography (CT) was the modality of choice for metastatic workup in the majority of centers (74%). Downstaging was the preferred option for over 90% of the centers and included transarterial chemoembolization, transarterial radioembolization, stereotactic body radiotherapy and atezolizumab/bevacizumab with varied indications. The alpha-fetoprotein (AFP) cut-off was used by 74% of centers to decide on transplantation as well as to downstage tumors, even if they met the criteria. The criteria for successful downstaging varied, but most centers conformed to the UCSF or their center-specific criteria for LT, along with the AFP cutoff values. The wait time for LT from down
The current predicted 5-year survival rate of HCC patients in India is less than 15%. The aim of transplantation is to achieve at least a 60% 5-year disease free survival rate, which will provide relief to the prediction of an HCC surge over the next 20 years. The current worldwide criteria (Milan/UCSF) may have a higher 5-year survival (> 70%); however, the majority of patients still do not fit these criteria and are dependent on other suboptimal modes of treatment, with much lower survival rates. To make predictions for 2040, we must prepare to arm ourselves with less stringent selection criteria to widen the pool of patients who may undergo transplantation and have a chance of a better outcome. With more advanced technology and better donor outcomes, LDLT will provide a cutting edge in the fight against liver cancer over the next two decades.
Core Tip: The current predicted 5-year survival rate of hepatocellular carcinoma (HCC) patients in India is less than 15%. The aim of transplantation is to achieve at least a 60% 5-year disease free survival which will truly provide a relief to the predictions of HCC surge over the next 20 years. The current worldwide criteria (Milan/University of California, San Francisco) may have a higher 5-year survival (> 70%) but the majority of patients still do not fit these criteria and are dependent on other sub-optimal modes of treatment with much lower survival rates. In order to face predictions for 2040, we must prepare to arm ourselves with less stringent selection criteria to widen the pool of patients who may avail transplant and have a chance at a better outcome.
- Citation: Pahari H, Raj A, Sawant A, Ahire DS, Rathod R, Rathi C, Sankalecha T, Palnitkar S, Raut V. Liver transplantation for hepatocellular carcinoma in India: Are we ready for 2040? World J Transplant 2024; 14(1): 88833
- URL: https://www.wjgnet.com/2220-3230/full/v14/i1/88833.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i1.88833
Hepatocellular carcinoma (HCC) comprises for approximately 75%–80% of all liver cancer types in most countries[1]. HCC is the sixth most common cancer worldwide, comprising approximately 5% of the total cancer incidence, and causes approximately six deaths per 100000 people annually[2,3]. In 2020, liver cancer was the third most common cause of cancer-related deaths worldwide (830000)[4]. There is a lack of statistical data from India, with the number of deaths estimated to be approximately 6.8 per 100000 people, with a total of approximately 14000 deaths annually in 2010[5,6].
The burden of HCC has been increasing worldwide, and India is no exception[7,8]. Asian countries have reported the highest global liver cancer incidence (73%) and liver cancer deaths in 2020[9]. Between 1978 and 2012, there was a steady increase in the number of HCC cases in India[10,11]. In the United States, a recent study predicted a continued increase in HCC rates through 2030[12]. At present, India contributes to approximately 18% of the incidence and 4% of the mortality. By 2040, the global burden of new cases and deaths from liver cancer may increase by up to 55% (an estimated 1.3 million cases and 1.4 million deaths)[13,14]. However, India still has a low 5-year survival rate for HCC (< 15%) despite the advancement of curative and palliative treatment options over the last two decades[15,16].
Liver transplantation (LT) for HCC in patients with cirrhosis has been widely researched and is now well established worldwide[17-19]. The cornerstone of this treatment lies in the various criteria formulated by expert consensus and experience over the years. The Milan criteria was established by Mazzaferro et al[20] in 1996 to improve the outcomes of LT for HCC in the initial aftermath of low survival and high recurrence rates[20]. Subsequent studies by Yao et al[21] and Mazzaferro et al[22] indicated the restrictive nature of these criteria, and slightly more liberal criteria, called the University of California, San Francisco (UCSF) criteria, were introduced in 2001[21,22]. These mainly included the number and size of HCC nodules, vascular invasion, and extrahepatic spread. Since then, several other criteria have been introduced, each with its own justification and outcomes. The variations among the criteria are staggering, and the short- and long-term outcomes are controversial[19,23,24]. Another factor is the evolution of living donor LT (LDLT) as a treatment option, which has led us to accept less stringent guidelines for LT in patients with HCC, as it does not affect the LT waitlist. However, the survival of HCC-LT recipients outside the standard criteria must be comparable to that of the expanded criteria to mitigate the additional risks to live donors. The incorporation of tumor markers into downstaging protocols has also contributed to improved outcomes and overall survival rates. We aimed to study the differences in the current practices of LT for HCC at different centers in India and discuss their clinical implications in the future.
We created an electronic survey form using Google Docs. It included several multiple-choice and short-answer questions to elaborate on specific choices or topics. Data were collected regarding the name of each center, their overall experience, and their LT practices with respect to HCC. In total, 54 questions were included (Supplementary Figure 1). The survey was reviewed and acknowledged as exempt from the Institutional Review Board at Medicover Hospitals, Navi Mumbai.
The survey was conducted in 42 transplant centers in India. Each center communicated via a transplant surgeon or physician. Responses were obtained over a 3-month period between January 2023 and April 2023. No incentives or honorariums were provided for completing the survey. Participation in the study was voluntary. Any duplicate or doubtful responses were clarified by the concerned center, and only one complete response was included in the final assessment. Eventually, 23 responses were received, which were tabulated and analyzed using standard software.
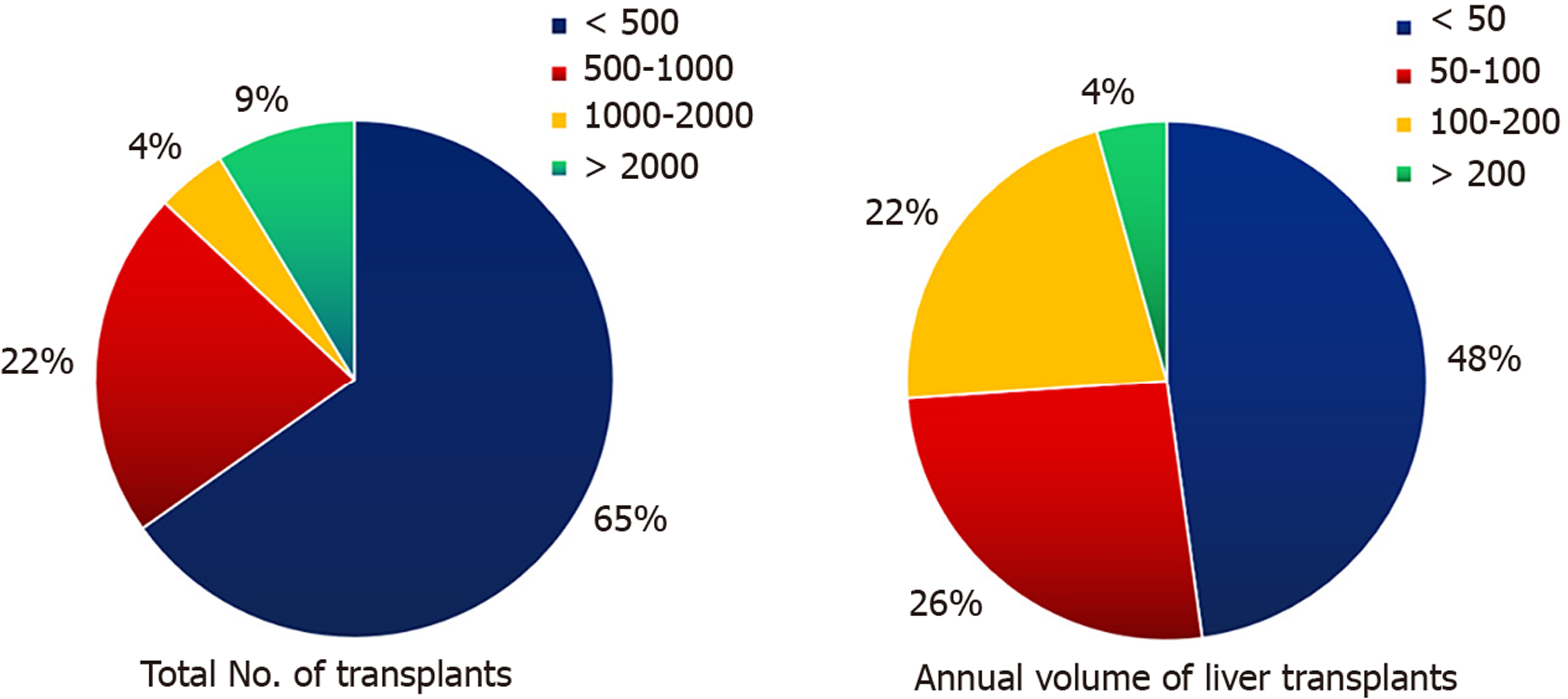
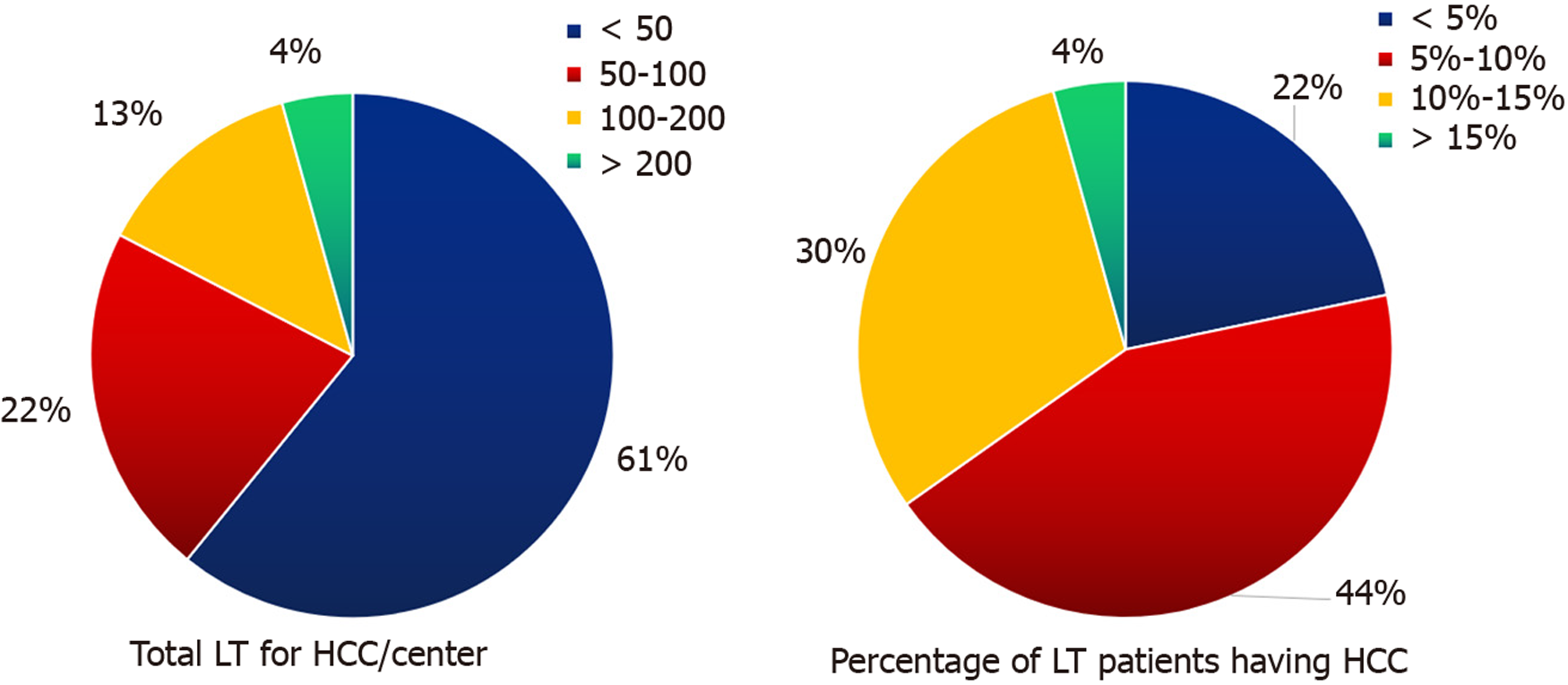
Overall, 23 of 41 (56%) transplant centers across India responded voluntarily to our survey. Almost all centers perform LDLT rather than deceased donor LT (DDLT). High-volume centers were defined as those that had performed more than 50 Liver transplants/year in the last 3 years, whereas low-volume centers were defined as those that had performed less than 50 Liver transplants/year in the last 3 years. Centers with more than 500 Liver transplants were referred to as experienced centers for discussion. Among the 23 centers, eight centers (34.8%) were identified as experienced LT centers, with two centers performing more than 2000 Liver transplants to date. More than 50% (12/23) of the centers were high-volume centers (Figure 1). Approximately 39% (nine centers) of the centers had performed over 50 cases of liver tran
Among the centers, the majority (17/23) responded that HCC was present in 5%–15% of LT recipients (Figure 2). Only one center followed the Milan criteria for LT, whereas the remaining centers were equally divided (11 each) between the UCSF and center-specific criteria for the eligibility of patients with HCC for LT. Apart from one center, all other centers (21/22 responses; 95%) replied that the percentage of patients with HCC within the Milan criteria undergoing LT was < 5%. Thirteen out of 23 centers (56.5%) preferred surgical resection in a 43 year-old Child A cirrhosis patient with a 4 cm solitary HCC and good performance status over LT directly. Nine centers specified the criteria for liver transplant in patients with HCC. The different center-specific criteria at the time of transplantation (either primary or after down
| No. of centers | Center-specific criteria | |||
| Size/No. of tumor | Invasion | Extrahepatic | AFP/markers | |
| 4 | Any size/any No. | No macrovascular | No | Any |
| 2 | Any size/any No. | No macrovascular | No | < 1000 |
| 1 | Encapsulated, any size, < 10 | No macrovascular | No | < 400 |
| 1 | Within UCSF size/No. | Vp1-vp3 invasion | No | < 400 |
| 1 | Any size/any No. | Vp1-vp2 invasion | No | Any |
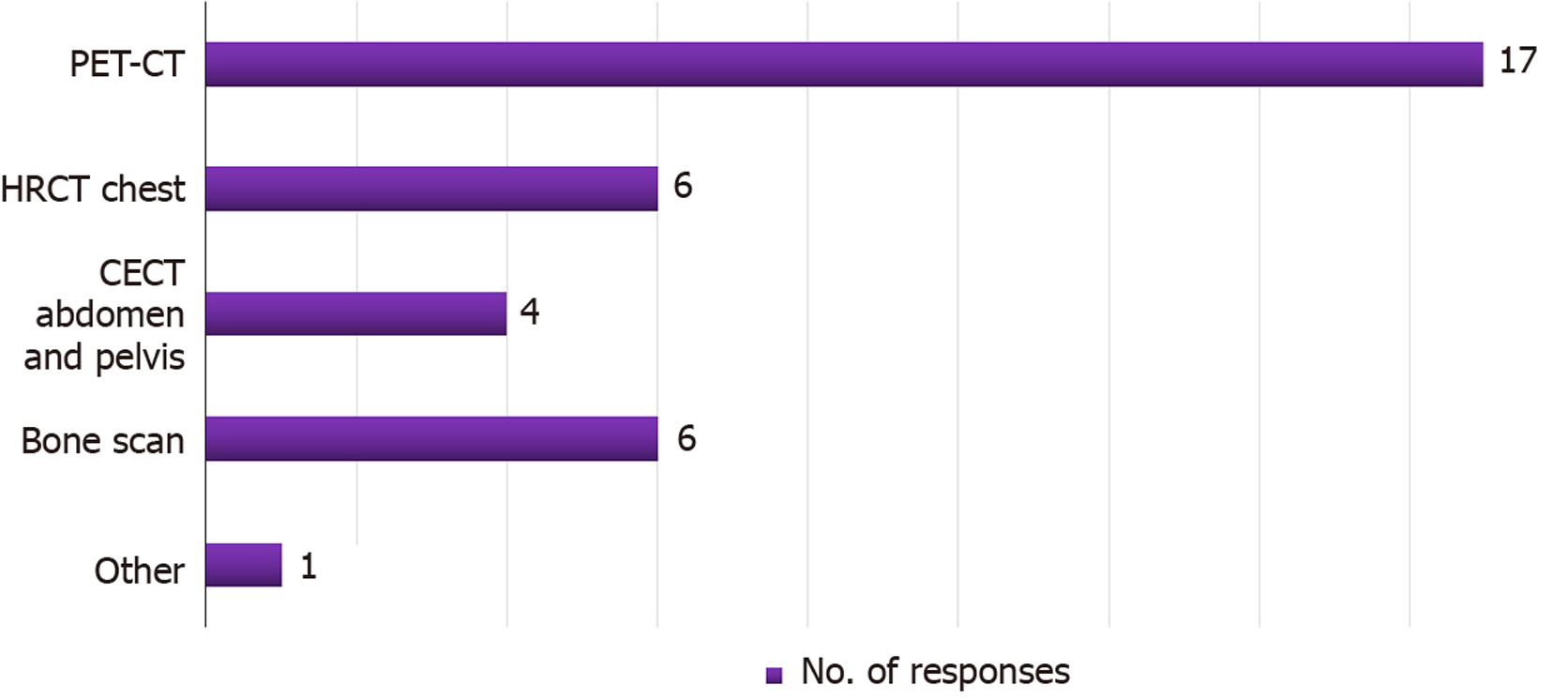
The majority of centers (17/23; 74%) preferred positron-emission tomography (PET)-computed tomography (CT) as their modality of choice for metastatic work-up in HCC patients with chronic liver disease (CLD) planned for LT. The remaining centers (26%) opted for a combination of contrast-enhanced CT (CECT) of the abdomen and pelvis, chest CT, and bone scan (Figure 3). Approximately 65% of the centers did not have different criteria for LDLT and DDLT with respect to HCC–CLD patients. Six of the eight centers that had different criteria explained that they would list patients only under the UCSF criteria for DDLT, while they would opt for center-specific criteria to proceed with LDLT. One center mentioned that downstaged portal vein tumor thrombus (PVTT) with transarterial radioembolization (TARE) or stereotactic body radiotherapy (SBRT) would not be a candidate for DDLT at their center but would be a candidate for LDLT.
Most of the centers (21/23; 91%) used downstaging as a bridge to LT when the center-specific criteria were not fulfilled, but there was no absolute contraindication to LT. Of them, 18 centers (overall 18/23; 78%) would consider branch PVTT for downstaging prior to transplantation. Transarterial chemoembolization (TACE), TARE, and SBRT are common modalities used to downstage tumors for various indications. The indications for TACE, TARE, and/or SBRT as downstaging tools received eight responses, as outlined in Table 2. TARE was preferred over TACE in the presence of PVTT (12 responses), large or multiple tumors (six responses), and in all cases, when financially feasible (three responses), with some overlap in the responses. TACE was preferred mostly for large tumors without PVTT, in cases of financial restrictions, and when TARE was unavailable in some centers. The use of atezolizumab/bevacizumab combination in HCC patients awaiting transplantation was advocated by six centers, of which five would use it universally and one would use it when TACE/TARE was not feasible. Six other centers responded that they had no experience using atezolizumab or bevacizumab as part of the downstaging protocol.
| Modality | TACE | TARE | SBRT |
| Indications (No. Of centers preferred) | HCC patients on waitlist[12] | PVTT[12] | Vp1-3 PVTT[12] |
| > Milan[4] | Large/multiple HCC[6] | Vp2 PVTT[2] | |
| > UCSF[2] | All affordable cases[3] | TACE/TARE not possible[4] | |
| Large tumor size[13] | Exophytic HCC[1] | ||
| Awaiting donor fitness/logistical delay in transplant[2] | Diaphragm involved or local infiltration[1] | ||
| High AFP[5] | Presence of shunt[1] | ||
| Absence of PVTT[2] | Not preferred[3] | ||
| TARE unaffordable/unavailable[4] |
Alpha-fetoprotein (AFP) was used as a marker for downstaging at most centers (17/23; 74%). The cut-off AFP value for transplant was 1000 ng/mL in most (10/17; 59%) centers, 400 ng/mL in four centers, and 2000, 500, and 200 ng/mL in one center each. All 17 centers considered AFP as a criterion for downstaging based on their set cut-off levels. Sixteen centers (70%) used protein induced by vitamin K absence or antagonist II (PIVKA-II) as a biomarker for HCC surve
This survey covered a wide range of transplant centers across India, with an overall experience of over 8000 Liver transplants. Based on these results, we derived an idea of the distinct practices around the country regarding HCC leading to LT and its subsequent follow-up. Despite certain clear-cut agreements, many corresponding answers have highlighted gray areas where judgments and opinions differ and are of utmost importance in different settings.
The selection criteria for HCC in LT have always been debated. From the early days of the Milan criteria to UCSF and, more recently, the Expanded Selection Criteria, it has been well established that cancer-free survival is dependent largely on extrahepatic spread and the level of vascular invasion, as compared to that on the size and number of tumors[20,21,25]. There is increasing evidence that outcomes outside the age-old criteria, such as the Milan criteria, are near-equivalent or at least good, as shown in Table 3[21,25-33]. In a country like India, where the burden of cirrhosis patients is huge and most patients are from the lower socioeconomic status, it is most usual for HCC to present in a late-stage with a back
| Criteria name (yr) | Size of tumor (cm) | No. of tumors | Additional criteria | Overall 5-year survival |
| Milan criteria (1996) | ≤ 5; ≤ 3 | 1; 3 | None | 75% |
| UCSF criteria (2001) | 6.5; ≤ 4.5 (total ≤ 8) | 1; 3 | None | 75.2% |
| Up-to-7 criteria (2001) | Size (cm) + No. ≤ 7 | None | ||
| Navarro criteria (2001) | ≤ 6; ≤ 5 | 1; 3 | None | 79% |
| Tokyo criteria (2007) | ≤ 5 | ≤ 5 | None | 75% |
| Asan criteria (2008) | ≤ 5 | ≤ 6 | None | 82% |
| Hangzhou criteria (2008) | < 8 (total) | Any No. | AFP < 400 ng/mL | 72% |
| Chang Gung criteria (2008) | ≤ 6.5; ≤ 4.5 | 1; ≤ 3 | None | 90% |
| Hong Kong criteria (2008) | ≤ 6.5; ≤ 4.5 | 1; ≤ 3 | None | 66% |
| Kyushu criteria (2009) | ≤ 5 | Any No. | PIVKA-II < 300 mAU/mL | 83% |
| Kyoto criteria (2010) | ≤ 5 | ≤ 10 | PIVKA-II < 400 mAU/mL | 87% |
| Toronto criteria (2011) | Any Size | Any No. | Poorly differentiated HCC excluded | 72% |
| Japanese National Expanded criteria (2019) | ≤ 5 | ≤ 5 | AFP < 500 ng/mL | 75.8% |
In our survey, 5%-15% of patients undergoing LT in India were diagnosed with HCC annually. Of these, only 5% belonged to the Milan category. Since the advent of the Milan criteria, advancements in radiological techniques have made it possible to achieve extremely accurate staging. LDLT, with a high degree of donor safety, has mitigated organ availability issues. Hence, the expansion of recipient criteria has become possible with LDLT, even with slightly inferior outcomes compared to those in Milan[37]. In our opinion, any treatment that offers at least a chance of 60% 5-year disease-free survival should be acceptable and offered to a patient and their donor for LDLT and should not be outrightly rejected[38].
Regarding the listing of patients with HCC–CLD, there has been considerable debate on whether the same criteria used for LDLT are applicable for DDLT. More recently, expanded criteria have been shown to have comparable outcomes, and this dilemma has intensified. In general, DDLT listing has been reserved for those patients who have a similar 5 year survival as compared to non HCC patients (e.g., Milan or UCSF criteria)[37,38]. This reservation is due to the potential impact of this listing on other patients on the liver waitlist. It has also been suggested that DDLT listings should be subject to regional listing criteria for patients with HCC, whereas LDLT can be pursued with more liberal center-specific criteria, providing a full disclosure of risks and outcome benefits[37]. Our survey sheds light on the fact that up to 65% of centers preferred to use the same criteria for LDLT and DDLT listing. Of the eight experienced centers, three opted for separate listing criteria, while five opted for the same criteria.
The current diagnostic tools for HCC include ultrasonography, CT, magnetic resonance imaging (MRI), and biopsy[39]. Biopsy confirmation is usually not required for a diagnosis[40]. Triple CT or MRI is the best imaging modality to diagnose HCC in patients with CLD. Current literature on the best imaging method for the evaluation of HCC metastasis is scarce. CT is the most accurate technique; however, it has limitations with respect to bone lesions, small vascular tumors, and difficulty in distinguishing between scarring and metastases[41-43]. The 18-Fluoro-deoxy-glucose-PET-CT has become increasingly established for the evaluation and treatment of metastatic HCC, with an average sensitivity of 60%–80% in most studies[44-46]. Other programs use a combination of dynamic CECT or MRI, chest CT and bone scintigraphy[47]. In our survey, 74% of centers chose PET-CT, whereas the remaining opted for the latter as a metastatic work-up prior to transplantation. AFP is considered an important biomarker for the diagnosis, treatment, and follow-up of patients with HCC before and after treatment[48]. It has also been implicated in the development and progression of HCC along with drug resistance in HCC cells[49]. However, only 60%–70% of HCC cases show elevated AFP levels, while 30%–40% of patients have normal values[50,51]. Newer biomarkers and models such as lens culinaris agglutinin-reactive fraction of AFP, des-carboxy-prothrombin, and GALAD scores (gender, age, AFP-L3, AFP, and DCP) are being increasingly used by various centers around the world[52,53]. In our study, AFP was universally followed, whereas PIVKA II was followed up in nearly 70% of the centers.
In our survey, more than 90% of the centers considered downstaging of HCC either as a bridge to transplantation or to fit the respective listing criteria or center-specific criteria for LDLT. The various indications mentioned by the surveyed participants, along with their corresponding modalities, are listed in Table 3. TACE and TARE were the most popular choices depending on availability and feasibility, whereas SBRT was mostly reserved for branch PVTT. A recent meta-analysis found that down-staged HCC–CLD patients who were initially beyond the listing criteria and who underwent transplantation had much better 3- and 5-year survival rates than non-transplanted patients[54]. They also noted that patients with downstaged HCC–CLD did not have inferior outcomes to transplant recipients who met the listing criteria[54]. Although the current European Association for the Study of the Liver and American Association for the Study of Liver Diseases guidelines suggest LT for downstaging to the Milan criteria, while the United Network for Organ Sharing (UNOS) adopted the UCSF criteria, the Indian perspective is different from the point of view of its socio-economics, advanced stage at diagnosis, and overall poor 5-year survival[55-57]. Mazzaferro et al[58] demonstrated that patients with downstaged HCC–CLD (to Milan) had a 77% 5-year overall survival rate compared to that of 31% with conventional anticancer therapies[58]. In this survey, TARE was preferred in many centers when available and affordable, especially in the presence of PVTT or multifocal HCC. An international systematic review of TARE as a downstaging tool before LT in 178 patients concluded that TARE is safer and better than TACE, with a 79% success rate[59]. Radunz et al[60] performed TARE downstaging in 40 pre-transplant patients and demonstrated an 87% tumor response (both complete and partial)[60]. However, another comparative meta-analysis indicated that TACE may have a better overall outcome than TARE when indicated with an approximately 60% tumor response[61-63]. Soin et al[64] demonstrated that after successful downstaging of PVTT (Vp1-3), a 5-year overall survival rate of 57% was obtained, which was comparable to that of patients without PVTT (65%)[64]. Regardless of the preference, downstaging with TACE or TARE is widely used throughout the country, with comparable results to those within the respective criteria for LDLT or DDLT.
SBRT is less frequently used but has been established as a safe alternative to conventional bridging therapies such as radiofrequency ablation (RFA), TACE, and TARE[64-67]. Patients with contraindications to TACE, especially those with PVTT, may receive SBRT[68]. Compared to other forms of treatment for PVTT like 3D-chemoradiation therapy, hepatic artery infusion chemotherapy, and molecular targeted drugs for HCC, SBRT offers a higher biologically effective dose in a shorter duration[69]. Retrospective studies of SBRT as a downstaging tool have indicated a good response and overall 5-year survival post-LT. In India, most centers select SBRT when TACE/TARE is not feasible or in the presence of branch PVTT (Vp1-2). However, the use of AFP in downstaging protocols remains controversial. There is no consensus among centers around the globe regarding the incorporation of biological (tumor markers, such as AFP) and morphological features for downstaging prior to transplantation. When adopting the UCSF criteria, the UNOS also suggested that a significant drop in AFP (< 500 ng/mL) along with stable disease at 6 months would be acceptable for DDLT listing[21,57]. Other studies have proposed various cutoffs for initial listing and downstaging endpoints ranging from < 100 to < 1000 ng/mL, while a few criteria have no cutoff and would accept any AFP if morphological variables were acceptable[25,30,48,70]. In our study, the majority of centers used 1000 ng/mL as a cut-off for AFP either at primary listing or after downstaging to proceed with LT. It is universally agreed that higher AFP levels impact the risk of recurrence and have worse outcomes than lower AFP levels. Finally, a combination of atezolizumab and bevacizumab was used by six centers as a bridge to transplantation. Several worldwide reports have suggested successful downstaging of advanced HCC with combination immunotherapy[71-72]. There is significant concern regarding the safety of using immunotherapy in patients with HCC who may later undergo liver transplant, especially given the risk of immune-related adverse events. In the IMBrave 150 trial, grade 3 to 4 toxicities were reported in 38% of patients receiving combination therapy with atezolizumab and bevacizumab[73]. In our study, many other centers did not use it because of a lack of experience, controversial nature or affordability issues.
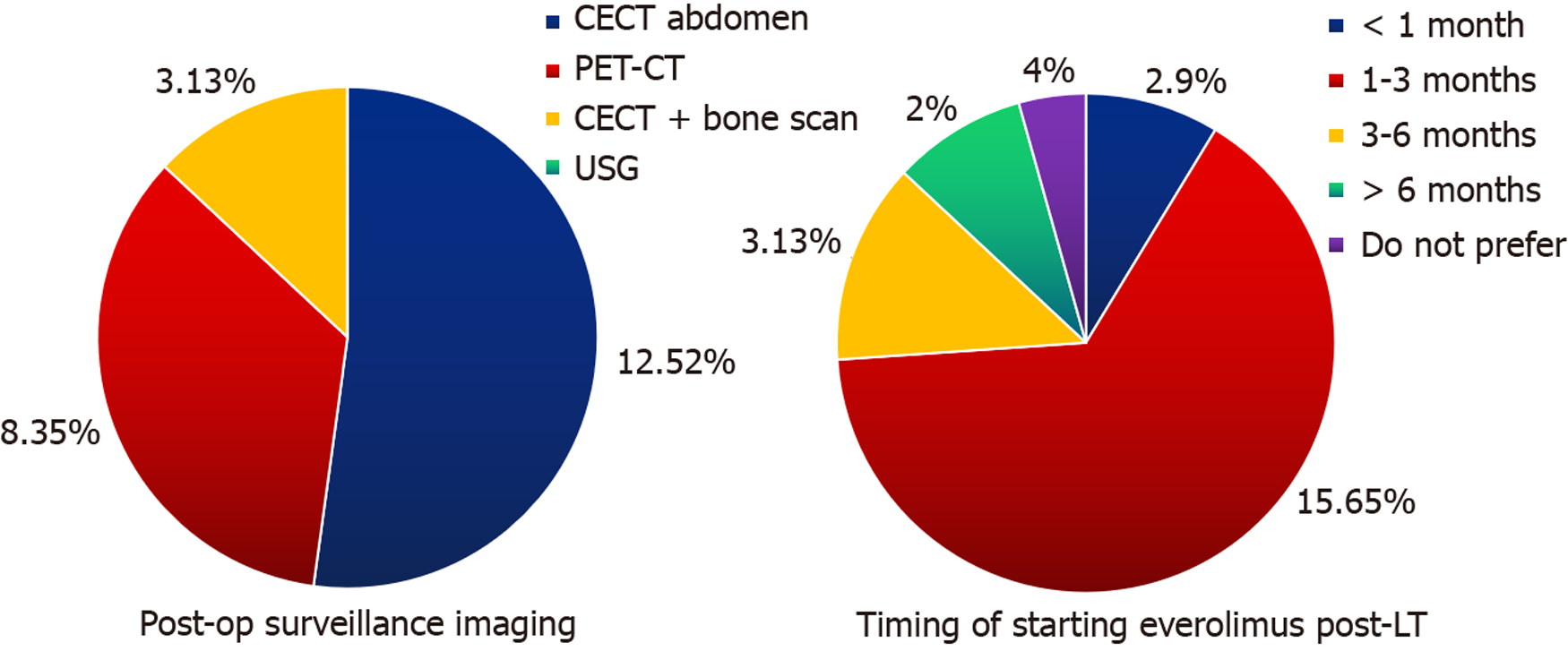
The downstaging criteria for most centers were similar to their respective criteria for LT. The overall goal of downstaging is to give the opportunity for higher survival through LT to patients with HCC–CLD who would otherwise not fall into the LT criteria. Clavien et al[37] recommended that downstaging should only be performed when the 5-year survival rate after LT is comparable to those that fit the criteria without downstaging[37]. Our survey provided varying opinions on this aspect. Morphological and biological tumor responses were the main aspects, while the non-progression of tumors was also an important factor to consider. The modified Response Evaluation Criteria in Solid Tumors was also used by several centers[74,75]. Notably, all transplant centers waited at least 4 wk, with nearly 70% preferring to wait 6 wk after successful downstaging to ensure disease stability.
There is no international consensus on the post-transplant surveillance of HCC patients. The National Comprehensive Cancer Network guidelines suggest imaging and AFP every 3–6 months initially, followed annually thereafter[76]. We have a similar protocol for HCC surveillance after LT. Patients with hepatitis B usually continue antiviral therapy. In this survey, more than 50% respondents opted for CECT abdomen alone as their imaging of choice, while the remaining picked PET-CT or CECT abdomen with bone scintigraphy. Many pre-transplant factors are implicated in the risk of HCC recurrence, such as the number and size of nodules, vascular invasion, AFP level, neutrophil-to-lymphocyte ratio, bridging therapy prior to transplantation, presence of metabolic syndrome, viral infections, and time to transplant[77]. In the post-transplant period, immunosuppression with calcineurin inhibitors at higher levels has been implicated in recurrence but has not yet been established[78]. However, it is well established that most HCC recurrences occur within 2 years post-LT[79-81]. Regardless of the type of imaging or cause of recurrence, early diagnosis and treatment by RFA or resection offer the only hope for long-term survival. The use of mammalian target of rapamycin inhibitors in post-transplant period is not routinely recommended according to International Liver Transplant Society guidelines[78]. However, in the current context, everolimus was routinely used by 22 of the 23 centers listed in this study.
HCC is one of the leading causes of cancer-related deaths worldwide, with an annual global mortality rate of more than 800000[4]. An increase of up to 55% in the global burden of HCC is expected by 2040 (an estimated 1.3 million cases and 1.4 million deaths)[12-14]. LT offers hope to patients with HCC–CLD without extrahepatic disease for a better chance of survival[15-19]. It has already been established as the best treatment option for patients, with the highest survival rate. However, for long, LT was not considered an option for patients with HCC–CLD. This was followed by an era in which stringent criteria for sufficiently good outcomes were used to justify the use of deceased donor livers for other patients on waitlists[20,21,37]. Over the years, this has been accepted as the benchmark for new and upcoming guidelines and their corresponding results. The use of living donor grafts has mitigated the concern of the use of deceased donor livers for HCC patients; however, it has raised issues over overall survival rates compared to the risk of living liver donation. The benchmark of survival is highly debatable, but in a country like India, where the non-transplant survival of HCC–CLD patients is extremely low, any chance of a 5-year success beyond 50% warrants sufficient discussion[37,64]. Markov models and other recent downstaging studies suggest that a 5-year survival rate of 60% is worth the minimal risk of living donations and deceased donor candidacy[38]. However, other guidelines have suggested deceased donor candidacy at outcomes comparable to those of patients with CLD without HCC, whereas LDLT can be pursued with lower outcomes in the setting of full disclosure of risks and benefits[37].
Based on our survey, we summarize the following trends across liver transplant programs in India:
(1) Approximately 10% of CLD patients in India undergoing LT are diagnosed with HCC; however, only 5% of these patients fall within Milan criteria;
(2) Most centers follow the expanded center-specific criteria for LDLT, with comparable outcomes to those who fall within the Milan criteria. However, further validation is required through national collaborations and multicenter studies;
(3) PET-CT is the most preferred modality of metastatic work-up in HCC–CLD patients. AFP is the biological marker of choice; however, many centers opt for PIVKA-II surveillance;
(4) All centers opted for downstaging as a bridge to LT or to fit center-specific criteria if no extrahepatic metastasis or major vascular invasion was present. TACE, TARE, and SBRT are the therapies of choice with varying indications, whereas atezolizumab/bevacizumab combination immunotherapy is infrequently used. Downstaging was confirmed using both morphological and biological markers according to either international or center-specific guidelines;
And (5) Post-transplant surveillance was mostly guided by CECT abdomen and tumor markers, while some centers opted for PET-CT or CECT and bone scintigraphy. Despite the lack of concrete evidence, almost all centers started administering everolimus in the post-transplant period for HCC–LT patients.
The current predicted 5-year survival rate of HCC patients in India is less than 15%. The aim of transplantation is to achieve at least a 60% 5-year disease free survival rate, which will provide relief to the prediction of an HCC surge over the next 20 years. The current worldwide criteria (Milan/UCSF) may have a higher 5-year survival (> 70%); however, the majority of patients still do not fit these criteria and are dependent on other suboptimal modes of treatment, with much lower survival rates. To make predictions for 2040, we must prepare to arm ourselves with less stringent selection criteria to widen the pool of patients who may undergo transplantation and have a chance of a better outcome. With more advanced technology and better donor outcomes, LDLT will provide a cutting edge in the fight against liver cancer over the next two decades.
Hepatocellular carcinoma (HCC) with chronic liver disease (CLD) is an indication for liver transplantation (LT). However, the overall survival for this condition is low in India, especially due to late presentation.
The various criteria that are established worldwide may lead to comparable outcomes compared to non-HCC patients, but significantly limit the number of patients that can avail this treatment option.
The aim of our study was to establish the current trends and give our opinion as to how to improve the donor pool or increase the access of patients to this life saving treatment option by relaxing stringent criteria while maintaining at least significant survival benefit.
We conducted a survey to see the current trend of practices in India with regards to HCC-CLD patients undergoing LT.
In this survey, we were able to ascertain trends of practice in HCC-CLD patients with respect to LT. We were also able to identify possible pathways to improve access of LT to these patients and improve the overall survival rates of HCC patients in India to make it comparable to other cancers.
This study shows that majority of patients are still dependent on sub optimal modes of treatment, and less stringent criteria may need to be followed with acceptable outcomes so that we may be able to match the increasing burden on HCC predicted over next 2 decades.
To make predictions for 2040, we must prepare to arm ourselves with less stringent selection criteria to widen the pool of patients who may undergo transplantation and have a chance of a better outcome.
We would like to acknowledge the following hospitals for their invaluable contributions to this survey. In alphabetical order, they are: Aakash Hospital, New Delhi; AIG Hospital, Hyderabad; Amrita Institute, Kochi; Apollo Hospital, Chennai; Apollo Multispecialty Hospital, Kolkata; Aster CMI Hospital, Bengaluru; BGS Gleneagles Global Hospital, Bengaluru; Center for Liver & Biliary Sciences, New Delhi; Deenanath Mangeshkar Hospital, Pune; Gem Hospital, Coimbatore; Gleneagles Global Hospital, Chennai; Global Hospital, Hyderabad; Global Hospital, Mumbai; Indraprastha Apollo Hospital, New Delhi; Jaypee Hospital, Noida; Kokilaben Dhirubhai Ambani Hospital, Mumbai; Medicover Hospitals, Navi Mumbai; MGM Healthcare, Chennai; Narayana Health; New Era Hospital & Research Institute, Nagpur; Sahyadri Hospital, Pune; Sir HN Reliance Foundation Hospital, Mumbai; Zydus Hospital, Ahmedabad.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: International Liver Transplant Society (ILTS); Liver Transplant Society of India (LTSI), 303; Indian Society of Organ Transplantation, LM 1427.
Specialty type: Transplantation
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabbous H, Egypt S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Rumgay H, Ferlay J, de Martel C, Georges D, Ibrahim AS, Zheng R, Wei W, Lemmens VEPP, Soerjomataram I. Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer. 2022;161:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 258] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 2. | World Cancer Research Fund International. Global cancer statistics for the most common cancers in the world. 2020. [cited 10 October 2023]. Available from: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/. |
| 3. | World Health Organization. Cancer – key facts. Feb 3, 2022. [cited 10 October 2023]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer. |
| 4. | Roser M, Ritchie H. Cancers are one of the leading causes of death globally. Are we making progress against cancer? 2019. [cited 10 October 2023]. Available from: https://ourworldindata.org/cancer#cancer-death-rates. |
| 5. | Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Badwe R, Kumar R, Roy S, Suraweera W, Bray F, Mallath M, Singh PK, Sinha DN, Shet AS, Gelband H, Jha P; Million Death Study Collaborators. Cancer mortality in India: a nationally representative survey. Lancet. 2012;379:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 6. | Acharya SK. Epidemiology of hepatocellular carcinoma in India. J Clin Exp Hepatol. 2014;4:S27-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64702] [Article Influence: 16175.5] [Reference Citation Analysis (177)] |
| 8. | Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159:335-349.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1232] [Article Influence: 246.4] [Reference Citation Analysis (0)] |
| 9. | Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2029-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 194] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 10. | Petrick JL, Florio AA, Znaor A, Ruggieri D, Laversanne M, Alvarez CS, Ferlay J, Valery PC, Bray F, McGlynn KA. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. 2020;147:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 11. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1346] [Article Influence: 336.5] [Reference Citation Analysis (1)] |
| 12. | Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. 2016;34:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 358] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 13. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1072] [Article Influence: 357.3] [Reference Citation Analysis (0)] |
| 14. | Nelson R. Liver Cancer Deaths to Rise by More Than 55% by 2040. Oct 10, 2022 [cited 10 October 2023]. Available from: https://www.medscape.com/viewarticle/982155?form=fpf. |
| 15. | Paul SB, Chalamalasetty SB, Vishnubhatla S, Madan K, Gamanagatti SR, Batra Y, Gupta SD, Panda SK, Acharya SK. Clinical profile, etiology and therapeutic outcome in 324 hepatocellular carcinoma patients at a tertiary care center in India. Oncology. 2009;77:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Koshy A, Devadas K, Panackel C, Philip M, Premaletha N, Zacharias P, Ramachandran TM, Gopalakrishna R, Mukkada RJ, Philips CA, Augustine P, Krishnakumar R, Sebastian B, Chettupuzha AP, Sadasivan S, Thomas GK, Siyad I, Sandesh K, Abhilash VB, Antony R, Kandathil JC, Pratap T, Mahadevan P; Kerala Hepatocellular Carcinoma Study Group. Multi-center prospective survey of hepatocellular carcinoma in Kerala: More than 1,200 cases. Indian J Gastroenterol. 2023;42:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Muhammad H, Tehreem A, Ting PS, Gurakar M, Li SY, Simsek C, Alqahtani SA, Kim AK, Kohli R, Gurakar A. Hepatocellular Carcinoma and the Role of Liver Transplantation: A Review. J Clin Transl Hepatol. 2021;9:738-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Van Kleek EJ, Schwartz JM, Rayhill SC, Rosen HR, Cotler SJ. Liver transplantation for hepatocellular carcinoma: a survey of practices. J Clin Gastroenterol. 2006;40:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Mehta N, Bhangui P, Yao FY, Mazzaferro V, Toso C, Akamatsu N, Durand F, Ijzermans J, Polak W, Zheng S, Roberts JP, Sapisochin G, Hibi T, Kwan NM, Ghobrial M, Soin A. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 20. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5313] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 21. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1696] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 22. | Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, Bhoori S, Lee SG. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 23. | Majno P, Mazzaferro V. Living donor liver transplantation for hepatocellular carcinoma exceeding conventional criteria: questions, answers and demands for a common language. Liver Transpl. 2006;12:896-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Lin CC, Elsarawy AMAA, Chen CL. Living Donor Liver Transplantation for Hepatocellular Carcinoma. InTech. 2017;. [DOI] [Full Text] |
| 25. | Shimamura T, Akamatsu N, Fujiyoshi M, Kawaguchi A, Morita S, Kawasaki S, Uemoto S, Kokudo N, Hasegawa K, Ohdan H, Egawa H, Furukawa H, Todo S; Japanese Liver Transplantation Society. Expanded living-donor liver transplantation criteria for patients with hepatocellular carcinoma based on the Japanese nationwide survey: the 5-5-500 rule - a retrospective study. Transpl Int. 2019;32:356-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 26. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1574] [Article Influence: 92.6] [Reference Citation Analysis (1)] |
| 27. | Herrero JI, Sangro B, Quiroga J, Pardo F, Herraiz M, Cienfuegos JA, Prieto J. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl. 2001;7:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007;25:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 29. | Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, Ko GY, Park KM, Ha TY, Song GW. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 30. | Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 31. | Concejero A, Chen CL, Wang CC, Wang SH, Lin CC, Liu YW, Yang CH, Yong CC, Lin TS, Jawan B, Huang TL, Cheng YF, Eng HL. Living donor liver transplantation for hepatocellular carcinoma: a single-center experience in Taiwan. Transplantation. 2008;85:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Takada Y, Uemoto S. Liver transplantation for hepatocellular carcinoma: the Kyoto experience. J Hepatobiliary Pancreat Sci. 2010;17:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, McGilvray I, Ghanekar A, Selzner M, Greig PD, Grant DR. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011;253:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 34. | ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol. 2016;65:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 35. | Nahon P, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Thabut D, Pilette C, Silvain C, Christidis C, Nguyen-Khac E, Bernard-Chabert B, Zucman D, Di Martino V, Sutton A, Roudot-Thoraval F, Audureau E; ANRS CO12 CirVir Group. Incidence of Hepatocellular Carcinoma After Direct Antiviral Therapy for HCV in Patients With Cirrhosis Included in Surveillance Programs. Gastroenterology. 2018;155:1436-1450.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 36. | Vallet-Pichard A, Correas JM, Dorival C, Zoulim F, Tran A, Bourlière M, Calès P, Guyader D, Bronowicki JP, Larrey D, Hezode C, Loustaud-Ratti V, Gournay J, de Ledinghen V, Asselah T, Ganne N, Metivier S, Chazouillères O, Leroy V, Rosa I, Samuel D, Mathurin P, Cagnot C, Fontaine H, Carrat F, Pol S; AFEF ANRS study group. Absence of impact of direct acting antivirals for hepatitis C virus on recurrent hepatocellular carcinoma tumor growth in the AFEF/ANRS CO22 Hepather cohort. Clin Res Hepatol Gastroenterol. 2021;45:101459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 38. | Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant. 2008;8:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Parra NS, Ross HM, Khan A, Wu M, Goldberg R, Shah L, Mukhtar S, Beiriger J, Gerber A, Halegoua-DeMarzio D. Advancements in the Diagnosis of Hepatocellular Carcinoma. Int J Transl Med. 2023;3:51-65. [DOI] [Full Text] |
| 40. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3245] [Article Influence: 463.6] [Reference Citation Analysis (1)] |
| 41. | Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 515] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 42. | Kim HC, Kim TK, Sung KB, Yoon HK, Kim PN, Ha HK, Kim AY, Kim HJ, Lee MG. CT during hepatic arteriography and portography: an illustrative review. Radiographics. 2002;22:1041-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Borghetti M, Benelli G, Bonardi R, Reduzzi L, Iori M. [Bone metastasis of hepatocarcinoma. Review of the literature, radiologic pictures and personal caseload]. Radiol Med. 1991;82:48-51. [PubMed] |
| 44. | Sugiyama M, Sakahara H, Torizuka T, Kanno T, Nakamura F, Futatsubashi M, Nakamura S. 18F-FDG PET in the detection of extrahepatic metastases from hepatocellular carcinoma. J Gastroenterol. 2004;39:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Chen YK, Hsieh DS, Liao CS, Bai CH, Su CT, Shen YY, Hsieh JF, Liao AC, Kao CH. Utility of FDG-PET for investigating unexplained serum AFP elevation in patients with suspected hepatocellular carcinoma recurrence. Anticancer Res. 2005;25:4719-4725. [PubMed] |
| 46. | Ho CL, Chen S, Yeung DW, Cheng TK. Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma. J Nucl Med. 2007;48:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 47. | Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging. 2013;12:530-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 48. | Li W, Liu K, Chen Y, Zhu M, Li M. Role of Alpha-Fetoprotein in Hepatocellular Carcinoma Drug Resistance. Curr Med Chem. 2021;28:1126-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 49. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3315] [Article Influence: 414.4] [Reference Citation Analysis (1)] |
| 50. | Zheng Y, Zhu M, Li M. Effects of alpha-fetoprotein on the occurrence and progression of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146:2439-2446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 51. | Shahini E, Pasculli G, Solimando AG, Tiribelli C, Cozzolongo R, Giannelli G. Updating the Clinical Application of Blood Biomarkers and Their Algorithms in the Diagnosis and Surveillance of Hepatocellular Carcinoma: A Critical Review. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 52. | Taketomi A, Sanefuji K, Soejima Y, Yoshizumi T, Uhciyama H, Ikegami T, Harada N, Yamashita Y, Sugimachi K, Kayashima H, Iguchi T, Maehara Y. Impact of des-gamma-carboxy prothrombin and tumor size on the recurrence of hepatocellular carcinoma after living donor liver transplantation. Transplantation. 2009;87:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 53. | Lee HA, Lee YR, Lee YS, Jung YK, Kim JH, An H, Yim HJ, Jeen YT, Yeon JE, Byun KS, Seo YS. Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein improves diagnostic accuracy for hepatocellular carcinoma. World J Gastroenterol. 2021;27:4687-4696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Di Martino M, Vitale A, Ferraro D, Maniscalco M, Pisaniello D, Arenga G, Falaschi F, Terrone A, Iacomino A, Galeota Lanza A, Esposito C, Cillo U, Vennarecci G. Downstaging Therapies for Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation: A Systematic Review and Meta-Analysis on Intention-to-Treat Outcomes. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6065] [Article Influence: 866.4] [Reference Citation Analysis (3)] |
| 56. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3031] [Article Influence: 433.0] [Reference Citation Analysis (3)] |
| 57. | Mehta N, Dodge JL, Grab JD, Yao FY. National Experience on Down-Staging of Hepatocellular Carcinoma Before Liver Transplant: Influence of Tumor Burden, Alpha-Fetoprotein, and Wait Time. Hepatology. 2020;71:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 58. | Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M, Romagnoli R, Antonelli B, Vivarelli M, Tisone G, Rossi M, Gruttadauria S, Di Sandro S, De Carlis R, Lucà MG, De Giorgio M, Mirabella S, Belli L, Fagiuoli S, Martini S, Iavarone M, Svegliati Baroni G, Angelico M, Ginanni Corradini S, Volpes R, Mariani L, Regalia E, Flores M, Droz Dit Busset M, Sposito C. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21:947-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 59. | Levi Sandri GB, Ettorre GM, Giannelli V, Colasanti M, Sciuto R, Pizzi G, Cianni R, D'Offizi G, Antonini M, Vennarecci G, Lucatelli P. Trans-arterial radio-embolization: a new chance for patients with hepatocellular cancer to access liver transplantation, a world review. Transl Gastroenterol Hepatol. 2017;2:98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Radunz S, Treckmann J, Baba HA, Best J, Müller S, Theysohn JM, Paul A, Benkö T. Long-Term Outcome After Liver Transplantation for Hepatocellular Carcinoma Following Yttrium-90 Radioembolization Bridging Treatment. Ann Transplant. 2017;22:215-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Facciorusso A, Bellanti F, Villani R, Salvatore V, Muscatiello N, Piscaglia F, Vendemiale G, Serviddio G. Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: A meta-analysis of randomized trials. United European Gastroenterol J. 2017;5:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 62. | Tsochatzis E, Garcovich M, Marelli L, Papastergiou V, Fatourou E, Rodriguez-Peralvarez ML, Germani G, Davies N, Yu D, Luong TV, Dhillon AP, Thorburn D, Patch D, O'Beirne J, Meyer T, Burroughs AK. Transarterial embolization as neo-adjuvant therapy pretransplantation in patients with hepatocellular carcinoma. Liver Int. 2013;33:944-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Hołówko W, Wróblewski T, Wojtaszek M, Grąt M, Kobryń K, Ziarkiewicz-Wróblewska B, Krawczyk M. Transarterial Chemoembolization Prior to Liver Transplantation in Patients with Hepatocellular Carcinoma. Ann Transplant. 2015;20:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Soin AS, Bhangui P, Kataria T, Baijal SS, Piplani T, Gautam D, Choudhary NS, Thiagarajan S, Rastogi A, Saraf N, Saigal S. Experience With LDLT in Patients With Hepatocellular Carcinoma and Portal Vein Tumor Thrombosis Postdownstaging. Transplantation. 2020;104:2334-2345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 65. | Wong TC, Lee VH, Law AL, Pang HH, Lam KO, Lau V, Cui TY, Fong AS, Lee SW, Wong EC, Dai JW, Chan AC, Cheung TT, Fung JY, Yeung RM, Luk MY, Leung TW, Lo CM. Prospective Study of Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma on Waitlist for Liver Transplant. Hepatology. 2021;74:2580-2594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 66. | Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, Russo M, Beecroft R, Ghanekar A, Bhat M, Brierley J, Greig PD, Knox JJ, Dawson LA, Grant DR. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 67. | Moore A, Cohen-Naftaly M, Tobar A, Kundel Y, Benjaminov O, Braun M, Issachar A, Mor E, Sarfaty M, Bragilovski D, Hur RB, Gordon N, Stemmer SM, Allen AM. Stereotactic body radiation therapy (SBRT) for definitive treatment and as a bridge to liver transplantation in early stage inoperable Hepatocellular carcinoma. Radiat Oncol. 2017;12:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 68. | Shui Y, Yu W, Ren X, Guo Y, Xu J, Ma T, Zhang B, Wu J, Li Q, Hu Q, Shen L, Bai X, Liang T, Wei Q. Stereotactic body radiotherapy based treatment for hepatocellular carcinoma with extensive portal vein tumor thrombosis. Radiat Oncol. 2018;13:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 69. | Kimura T, Fujiwara T, Kameoka T, Adachi Y, Kariya S. The Current Role of Stereotactic Body Radiation Therapy (SBRT) in Hepatocellular Carcinoma (HCC). Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 70. | Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J, Dharancy S, Gugenheim J, Bernard PH, Adam R, Radenne S, Muscari F, Conti F, Hardwigsen J, Pageaux GP, Chazouillères O, Salame E, Hilleret MN, Lebray P, Abergel A, Debette-Gratien M, Kluger MD, Mallat A, Azoulay D, Cherqui D; Liver Transplantation French Study Group. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986-94.e3; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 728] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 71. | Giudicelli H, Roux C, Monsel A, Conti F, Scatton O, Allaire M. Successful advanced hepatocellular carcinoma downstaging with atezolizumab-Bevacizumab and radioembolization before liver transplantation. Clin Res Hepatol Gastroenterol. 2023;47:102167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 72. | Ouranos K, Chatziioannou A, Goulis I, Sinakos E. Role of immunotherapy in downsizing hepatocellular carcinoma prior to liver transplantation. World J Transplant. 2022;12:331-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 73. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 963] [Article Influence: 321.0] [Reference Citation Analysis (0)] |
| 74. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3303] [Article Influence: 220.2] [Reference Citation Analysis (36)] |
| 75. | Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72:288-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 437] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 76. | National Comprehensive Cancer Network®. National Comprehensive Cancer Network (NCCN) guidelines. 2020. [cited 10 October 2023]. Available from: https://www.nccn.org/. |
| 77. | Straś WA, Wasiak D, Łągiewska B, Tronina O, Hreńczuk M, Gotlib J, Lisik W, Małkowski P. Recurrence of Hepatocellular Carcinoma After Liver Transplantation: Risk Factors and Predictive Models. Ann Transplant. 2022;27:e934924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (1)] |
| 78. | Berenguer M, Burra P, Ghobrial M, Hibi T, Metselaar H, Sapisochin G, Bhoori S, Kwan Man N, Mas V, Ohira M, Sangro B, van der Laan LJW. Posttransplant Management of Recipients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 79. | Goldaracena N, Mehta N, Scalera I, Sposito C, Atenafu EG, Yao FY, Muiesan P, Mazzaferro V, Sapisochin G. Multicenter validation of a score to predict prognosis after the development of HCC recurrence following liver transplantation. HPB (Oxford). 2019;21:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 81. | Ekpanyapong S, Philips N, Loza BL, Abt P, Furth EE, Tondon R, Khungar V, Olthoff K, Shaked A, Hoteit MA, Reddy KR. Predictors, Presentation, and Treatment Outcomes of Recurrent Hepatocellular Carcinoma After Liver Transplantation: A Large Single Center Experience. J Clin Exp Hepatol. 2020;10:304-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |