Published online Mar 18, 2024. doi: 10.5500/wjt.v14.i1.88734
Peer-review started: October 17, 2023
First decision: November 2, 2023
Revised: November 14, 2023
Accepted: December 18, 2023
Article in press: December 18, 2023
Published online: March 18, 2024
Processing time: 149 Days and 18.7 Hours
Biliary atresia (BA) is the most common indication for pediatric liver trans
To investigate the effect of prior portoenterostomy in infants un
This was a retrospective cohort study of 42 pediatric patients with BA who underwent primary liver transplantation from 2013 to 2023 at a single tertiary center in Brazil. Patients with BA were divided into two groups: Those under
Forty-two patients were included in the study (25 [60%] girls), 23 undergoing liver transplantation without prior portoenterostomy, and 19 undergoing liver transplantation with prior portoenterostomy. Patients with prior portoenterostomy were older (12 vs 8 months; P = 0.02) at the time of liver transplantation and had lower Pediatric End-Stage Liver Disease scores (13.2 vs 21.4; P = 0.01). The majority of the patients (35/42, 83%) underwent living-donor liver transplantation. The group of patients without prior portoenterostomy appeared to have a higher incidence of portal vein thrombosis (39 vs 11%), but this result did not reach statistical significance. Prior portoenterostomy was not a protective factor against portal vein thrombosis in the multivariable analysis after adjusting for age at liver transplantation, graft-to-recipient weight ratio, and use of vascular grafts. Finally, the groups did not significantly differ in terms of post-transplant survival.
In our study, prior portoenterostomy did not significantly affect the outcomes of liver transplantation.
Core Tip: Children with biliary atresia comprise the majority of patients undergoing liver transplantation worldwide. Timely portoenterostomy can postpone or even remove the need for liver transplantation. Current data are not conclusive regarding whether performing a portoenterostomy negatively affects the transplantation procedure. In this study, we compared the outcomes of liver transplantation in patients with biliary atresia with or without prior portoenterostomy in a single center. Our results indicate that it does not affect the outcomes.
- Citation: Utz Melere M, Sanha V, Farina M, da Silva CS, Nader L, Trein C, Lucchese AM, Ferreira C, Kalil AN, Feier FH. Primary liver transplantation vs transplant after Kasai portoenterostomy in children with biliary atresia: A retrospective Brazilian single-center cohort. World J Transplant 2024; 14(1): 88734
- URL: https://www.wjgnet.com/2220-3230/full/v14/i1/88734.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i1.88734
Biliary atresia (BA) is a progressive fibroinflammatory process that leads to obstruction of the biliary tree and cirrhosis if left untreated. It affects people worldwide across ethnicities. BA is the most common cause of pediatric liver-related death and the leading indication for pediatric liver transplantation (LT)[1]. Symptoms are usually present in the 1st weeks of life, with a pattern of obstructive jaundice and abnormal liver function test results. Early diagnosis and portoenterostomy (PE) are essential for adequate bile flow, clearance of jaundice, and normalization of the serum bilirubin concentration.
Kasai PE is the standard initial procedure for BA, followed by LT for patients in whom PE fails or the condition pro
Advances in pediatric LT have improved outcomes. A subset of patients with BA benefit from primary LT without first undergoing PE, especially those who are diagnosed at a later stage[4]. PE before 60 d of life is associated with a higher native liver survival rate than PE after 60 d[5]. However, whether prior PE negatively affects LT outcomes in patients with BA remains unclear[6-11].
Here, we aim to add further data on this issue by comparing the outcomes of children with BA who underwent LT without previous PE with those who underwent PE before LT at our institution.
This was a retrospective, single-center cohort study of patients who underwent LT for BA at Santa Casa de Porto Alegre, Brazil, a tertiary center. Data were extracted from a database of children who underwent LT at our center from 2013 to 2023. Only recipients of primary LT with a diagnosis of BA were selected and divided into two groups: BA without prior PE (no-PE) and BA with prior PE (PE). Demographic and perioperative variables such as sex, age at LT, Pediatric End-Stage Liver Disease (PELD) score, Model for End Stage Liver Disease score, and weight were included in the analysis. Post-LT outcomes, such as vascular and biliary complications, hospital and intensive care unit (ICU) stay, and acute and chronic rejection, were also evaluated. The hospital’s ethics committee approved this study.
ABO blood group compatibility determined recipient and donor selection, and no incompatible blood type transplantations were performed during the study period. The grafts were orthotopically implanted using a “piggyback technique.” The graft’s portal vein was anastomosed in an end-to-end fashion, either to the recipient’s portal vein trunk or by inter
Tacrolimus (FK 506, Prograf) and steroids were used for immunosuppression in the majority of recipients. Basiliximab (Simulect; Novartis, Basel, Switzerland) was used to induce immunosuppression in the majority of the recipients. Doppler ultrasound was routinely performed on the 1st postoperative day, and thereafter, according to the clinician’s dis
Means ± standard deviations and medians (interquartile ranges) were calculated to summarize continuous variables, and the results were compared using the Student’s t-test or the Kruskal-Wallis test as non-parametric test when distributional assumptions were in doubt. Categorical variables are expressed as numbers and percentages. Differences between groups were assessed using the χ2 or Fisher’s exact test, as appropriate. Patient and graft survival analyses were conducted with the Kaplan-Meier product-limit estimator, and patient subgroups were compared using the two-sided log-rank test. Multivariable Cox regression analysis was performed, adjusting for risk factors. Variables with P < 0.1 during univariate analysis and those deemed clinically significant were included in the model. The study was reviewed by our expert biostatistician, Gabriele Dell'Era, MD.
In summary, prior PE did not significantly affect post-LT outcomes in our study. The apparent trend for more portal vein thrombosis (PVT) events in the no-PE group was probably due to the smaller size and younger age of patients in this group. The post-LT survival did not differ between the groups. Larger multicenter studies are required to confirm our results.
LT is primarily indicated for patients with BA in whom initial PE fails or who present with advanced, progressive liver disease at the time of diagnosis. The reported impact of prior PE on LT outcomes differ between studies. A meta-analysis conducted by Wang et al[12] did not reveal statistically significant differences in major outcomes, overall survival, and complications between patients undergoing LT with prior PE and those undergoing LT without prior PE. Subsequent studies have not resolved the question[13-16]. Our study did not reveal in survival between the groups.
Kasai PE is performed in an attempt to salvage the native liver and reestablish biliary flow. It yields 10-year LT-free survival in more than 50% of patients with BA. Although the procedure is effective in most cases, adequate biliary dra
The present study revealed interesting results in the subgroup of patients who underwent LT without prior PE, including a higher incidence of PVT than in the group who had previously undergone PE. In accordance with the literature, patients with BA who underwent LT without prior PE were younger and smaller in this study. This com
Excellent outcomes have been reported with LDLT for BA[20,21]. LDLT is considered the first-choice graft in various centers for children with BA, particularly in Asian countries. In accordance with other high-volume centers in Brazil[7], our cohort was mainly composed of children undergoing LDLT (83%). In contrast to Asian countries, deceased donations are widely accepted in Brazil. However, pediatric and adult donors suitable for graft reduction or splitting are scarce, and LDLT is a safe alternative for enlisted patients[22,23].
The early BA diagnosis and the timing to perform the Kasai procedure also influences the decision to indicate a primary LT for BA. A recent European cohort study in BA patients compared early Kasai, late Kasai and primary LT. As expected, native liver survival in 5-y was under 50% (47% early, 30% late Kasai, and 4% for those without a portoenterostomy). Overall 5-y survival, however, was quite comparable among the same groups (91, 83 and 80%, respectively). This study raises an important question as to whether age alone should limit the indication to perform a Kasai procedure[24].
Lemoine et al[25] documented their cohort of 113 patients with BA who underwent LT. Notably, only 14 individuals (12%) in their study underwent primary LT. By contrast, our findings indicate that 54.7% of BA patients in our report underwent primary LT. This observation could suggest the influence of delayed BA diagnosis, preventing the implementation of the Kasai procedure in developing countries, such as Brazil.
The retrospective nature of the study and relatively small sample are acknowledged as drawbacks. However, survival and post-transplant complication rates in this study were in accordance with those of large transplant centers[19]. Our study might have been underpowered due to the small size of the cohort. The impact of PE on the outcome of LT remains debatable, and center expertise, especially with LDLT, plays an important role in the outcomes of children with BA. Larger, multicenter studies could help in answering this question.
Of the forty-two recipients with BA, twenty-five (60%) were girls. LDLT was the main LT modality (83% of patients). Twenty-three patients were in the no-PE group and nineteen in the PE group. Patients in the no-PE group were significantly younger than those in the PE group (8 vs 12 months; P = 0.02). Patients in the no-PE group had higher PELD scores than those in the PE group (21.4 ± 9.5 vs 13.2 ± 8.9; P = 0.01). The groups did not differ in terms of ischemia times, blood transfusion volume, or hospital and ICU stay (Table 1).
| Parameter | No-PE, n = 23 | PE, n = 19 | P value |
| Sex, female | 13 (56.5) | 12 (63.2) | 0.75 |
| Age at LT, months | 8 (6-10) | 12 (7-23) | 0.02 |
| Weight at LT, kg, median (IQR) | 6.5 (5.7-7.4) | 7 (6.4-13.5) | 0.15 |
| PELD/MELD, mean ± SD | 21.4 ± 9.5 | 13.2 ± 8.9 | 0.01 |
| Living donor | 19 (82.6) | 16 (84.2) | 1 |
| Deceased donor | 4 (17.4) | 3 (15.8) | |
| GRWR, mean ± SD | 4.0 ± 1.3 | 3.7 ± 1.7 | 0.4 |
| RCBT in mL/kg, mean ± SD | 2.4 ± 0.9 | 1.6 ± 1.3 | 0.15 |
| CIT in min, median (IQR) | 81 (61-140) | 105 (73-189) | 0.24 |
| WIT in min, mean ± SD | 39.4 ± 12.5 | 33.8 ± 8.4 | 0.11 |
| Time to extubate in d, median (IQR) | 1 (0-2) | 0 (0-1) | 0.55 |
| ICU stay in d, median (IQR) | 12 (6-17) | 8 (5-14) | 0.4 |
| Hospital stay in d, median (IQR) | 21 (16-37) | 23 (15-30) | 0.25 |
The no-PE group had a seemingly higher incidence of PVT (39% vs 11%; P = 0.07) (Table 2). Although this difference was not statistically significant, we conducted a subgroup analysis on patients with PVT as it might have been clinically significant.
| Parameter | No-PE, n = 23 | PE, n = 19 | P value |
| HAT | 2 (8.7) | 4 (21.1) | 0.38 |
| PVT | 9 (39.1) | 2 (10.5) | 0.07 |
| PVS | 4 (17.4) | 4 (21.1) | 1 |
| Biliary fistula | 9 (39.1) | 6 (31.6) | 0.75 |
| Biliary stricture | 5 (21.7) | 8 (42.1) | 0.19 |
| Reoperation | 7 (30.4) | 2 (10.5) | 0.14 |
| Acute rejection | 5 (21.7) | 3 (15.8) | 0.7 |
| Chronic rejection | 2 (8.7) | 0 | 0.49 |
| EBV infection | 14 (60.9) | 9 (47.4) | 0.5 |
| CMV infection | 19 (82.6) | 13 (68.4) | 0.46 |
The PVT and no-PVT groups did not reach statistically significant difference in terms of age (8 vs 10 months; P = 0.06) or mean GRWR (4.38 + -1.20 vs 3.75 + -1.56; P = 0.08). The use of vascular grafts as substitutes for the portal vein (cryo
| Parameter | No-PVT, n = 31 | PVT, n = 11 | P value |
| Age at LT, months, median (IQR) | 10 (6-15) | 8 (5-8) | 0.06 |
| Weight at LT, kg, median (IQR) | 7 (6.2-10) | 6.4 (5.7-7.3) | 0.2 |
| PE | 17 (54.8) | 2 (18.2) | 0.07 |
| PELD/MELD, mean ± SD | 16.1 ± 10.3 | 22 ± 8 | 0.12 |
| Living donor | 26 (83.9) | 9 (81.8) | 1 |
| Deceased donor | 5 (16.1) | 2 (18.2) | 1 |
| Portal vein graft | 5 (16.1) | 5 (45.5) | 0.09 |
| GRWR, mean ± SD | 3.75 ± 1.56 | 4.38 ± 1.2 | 0.08 |
| RCBT in mL/kg, mean + SD | 1.8 ± 1.3 | 2.5 ± 0.9 | 0.14 |
| CIT in min, median (IQR) | 95 (66-163.5) | 88 (68-127) | 0.8 |
| WIT in min, mean ± SD | 36.1 ± 9.9 | 39 ± 14.1 | 0.7 |
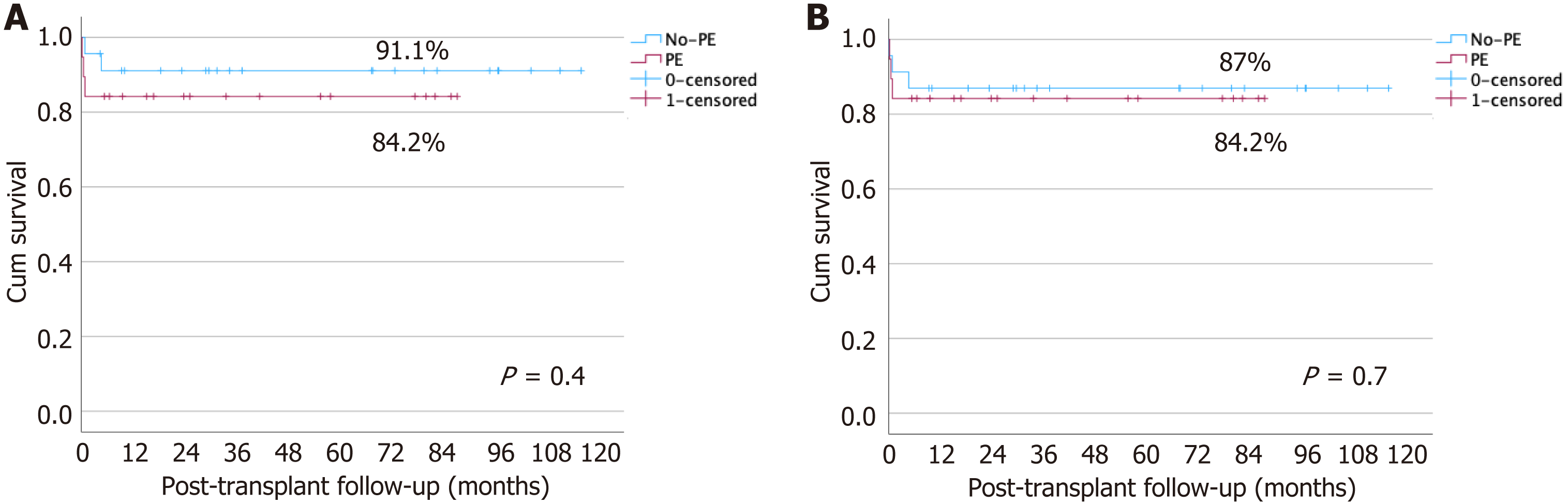
Multivariable Cox regression analysis was performed to evaluate factors associated with PVT. After adjusting for age at LT, GRWR, and vascular grafting, the protective effect of PE was attenuated (Table 4). The 1-year patient and graft survival did not differ between the no-PE and PE groups (91% vs 84%; P = 0.4 and 87% vs 84%; P = 0.7, respectively) (Figure 1).
| Parameter | OR | 95%CI | P value |
| PE | 0.35 | 0.05-2.27 | 0.27 |
| GRWR | 1.03 | 0.52-2.02 | 0.92 |
| Age at LT | 0.84 | 0.63-1.12 | 0.24 |
| Portal vein graft | 2.87 | 0.54-15.1 | 0.21 |
Biliary atresia (BA) is the most common indication for pediatric liver transplantation, although portoenterostomy is usually performed first. However, due to the high failure rate of portoenterostomy, liver transplantation has been advocated as the primary procedure for patients with BA. It is still unclear if a previous portoenterostomy has a negative impact on liver transplantation outcomes.
Is there a negative impact of a prior portoenterostomy on liver transplantation outcomes?
To analyze the post-transplant complications and survival in children with BA with or without a previous portoenterostomy.
This was a retrospective cohort study of 42 pediatric patients with BA who underwent primary liver transplantation from 2013 to 2023 at a single tertiary center in Brazil. Patients with BA were divided into two groups: Those undergoing primary liver transplantation without portoenterostomy and those undergoing liver transplantation with prior portoenterostomy.
In our study, prior portoenterostomy did not significantly affect the outcomes of liver transplantation.
There are no survival differences in patients transplanted with or without a prior portoenterostomy. There is a trend for more portal vein complications in the group of patients transplanted without a portoenterostomy.
Larger studies, also multicenter studies would be important to better address this issue.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ullah K, Pakistan; Bredt LC, Brazil S-Editor: Liu JH L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Schreiber RA, Harpavat S, Hulscher JBF, Wildhaber BE. Biliary Atresia in 2021: Epidemiology, Screening and Public Policy. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Nio M, Ohi R, Miyano T, Saeki M, Shiraki K, Tanaka K; Japanese Biliary Atresia Registry. Five- and 10-year survival rates after surgery for biliary atresia: a report from the Japanese Biliary Atresia Registry. J Pediatr Surg. 2003;38:997-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Shneider BL, Magee JC, Karpen SJ, Rand EB, Narkewicz MR, Bass LM, Schwarz K, Whitington PF, Bezerra JA, Kerkar N. Total Serum Bilirubin within 3 Months of Hepatoportoenterostomy Predicts Short-Term Outcomes in Biliary Atresia. J Pediatr. 2016;170:211-217. [DOI] [Full Text] |
| 4. | Sundaram SS, Mack CL, Feldman AG, Sokol RJ. Biliary atresia: Indications and timing of liver transplantation and optimization of pretransplant care. Liver Transpl. 2017;23:96-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 5. | Yang C, Ke M, Zhou Y, Xu H, Diao M, Li L. Impact of early Kasai portoenterostomy on short-term outcomes of biliary atresia: A systematic review and meta-analysis. Front Surg. 2022;9:924506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 6. | Alexopoulos SP, Merrill M, Kin C, Matsuoka L, Dorey F, Concepcion W, Esquivel C, Bonham A. The impact of hepatic portoenterostomy on liver transplantation for the treatment of biliary atresia: early failure adversely affects outcome. Pediatr Transplant. 2012;16:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Neto JS, Feier FH, Bierrenbach AL, Toscano CM, Fonseca EA, Pugliese R, Candido HL, Benavides MR, Porta G, Chapchap P. Impact of Kasai portoenterostomy on liver transplantation outcomes: A retrospective cohort study of 347 children with biliary atresia. Liver Transpl. 2015;21:922-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Sandler AD, Azarow KS, Superina RA. The impact of a previous Kasai procedure on liver transplantation for biliary atresia. J Pediatr Surg. 1997;32:416-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Meister RK, Esquivel CO, Cox KL, Concepcion W, Berquist W, Nakazato P, deVries PA. The influence of portoenterostomy with stoma on morbidity in pediatric patients with biliary atresia undergoing orthotopic liver transplantation. J Pediatr Surg. 1993;28:387-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Millis JM, Brems JJ, Hiatt JR, Klein AS, Ashizawa T, Ramming KP, Quinones-Baldrich WJ, Busuttil RW. Orthotopic liver transplantation for biliary atresia. Evolution of management. Arch Surg. 1988;123:1237-1239. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Visser BC, Suh I, Hirose S, Rosenthal P, Lee H, Roberts JP, Hirose R. The influence of portoenterostomy on transplantation for biliary atresia. Liver Transpl. 2004;10:1279-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Wang P, Xun P, He K, Cai W. Comparison of liver transplantation outcomes in biliary atresia patients with and without prior portoenterostomy: A meta-analysis. Dig Liver Dis. 2016;48:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | LeeVan E, Matsuoka L, Cao S, Groshen S, Alexopoulos S. Biliary-Enteric Drainage vs Primary Liver Transplant as Initial Treatment for Children With Biliary Atresia. JAMA Surg. 2019;154:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Yoeli D, Choudhury RA, Sundaram SS, Mack CL, Roach JP, Karrer FM, Wachs ME, Adams MA. Primary vs. salvage liver transplantation for biliary atresia: A retrospective cohort study. J Pediatr Surg. 2022;57:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Chan KWE, Lee KH, Wong HYV, Tsui SYB, Mou JWC, Tam YH. Impact of Age of Patient and Experience of Surgeon on the Outcome after Kasai Portoenterostomy: Can We Delay the Surgery? Eur J Pediatr Surg. 2021;31:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Kelley-Quon LI, Shue E, Burke RV, Smith C, Kling K, Mahdi E, Ourshalimian S, Fenlon M, Dellinger M, Shew SB, Lee J, Padilla B, Inge T, Roach J, Marwan AI, Russell KW, Ignacio R, Fialkowski E, Nijagal A, Im C, Azarow KS, Ostlie DJ, Wang K. The need for early Kasai portoenterostomy: a Western Pediatric Surgery Research Consortium study. Pediatr Surg Int. 2022;38:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Chung PHY, Chan EKW, Yeung F, Chan ACY, Mou JWC, Lee KH, Hung JWS, Leung MWY, Tam PKH, Wong KKY. Life long follow up and management strategies of patients living with native livers after Kasai portoenterostomy. Sci Rep. 2021;11:11207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Tam PKH, Chung PHY, St Peter SD, Gayer CP, Ford HR, Tam GCH, Wong KKY, Pakarinen MP, Davenport M. Advances in paediatric gastroenterology. Lancet 2017; 390: 1072-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Neto JS, Fonseca EA, Feier FH, Pugliese R, Candido HL, Benavides MR, Porta G, Miura IK, Danesi VB, Guimaraes T, Porta A, Borges C, Godoy A, Kondo M, Chapchap P. Analysis of factors associated with portal vein thrombosis in pediatric living donor liver transplant recipients. Liver Transpl. 2014;20:1157-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Mizuta K, Sanada Y, Wakiya T, Urahashi T, Umehara M, Egami S, Hishikawa S, Okada N, Kawano Y, Saito T, Hayashida M, Takahashi S, Yoshino H, Shimizu A, Takatsuka Y, Kitamura T, Kita Y, Uno T, Yoshida Y, Hyodo M, Sakuma Y, Fujiwara T, Ushijima K, Sugimoto K, Ohmori M, Ohtomo S, Sakamoto K, Nakata M, Yano T, Yamamoto H, Kobayashi E, Yasuda Y, Kawarasaki H. Living-donor liver transplantation in 126 patients with biliary atresia: single-center experience. Transplant Proc. 2010;42:4127-4131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Chen CL, Concejero A, Wang CC, Wang SH, Lin CC, Liu YW, Yong CC, Yang CH, Lin TS, Chiang YC, Jawan B, Huang TL, Cheng YF, Eng HL. Living donor liver transplantation for biliary atresia: a single-center experience with first 100 cases. Am J Transplant. 2006;6:2672-2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Andraus W, Canedo BF, D'Alburquerque LA. Living donor liver transplantation in Brazil-current state. Hepatobiliary Surg Nutr. 2016;5:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Associação Brasileira de Transplante de Órgãos. Data from Brazilian Organ Transplantation Association. Available from: http://www.abto.org.br. |
| 24. | Fuchs J, Mrad C, Gonzales E, Ndiaye D, Fouquet V, Héry G, Baujard C, Guérin F, Branchereau S. Biliary drainage surgery before or after 3 mo of life vs primary liver transplantation in children with biliary atresia: comparative cohort study. BJS Open. 2023;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Lemoine CP, LeShock JP, Brandt KA, Superina R. Primary Liver Transplantation vs. Transplant after Kasai Portoenterostomy for Infants with Biliary Atresia. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |