Published online Mar 18, 2024. doi: 10.5500/wjt.v14.i1.87752
Peer-review started: October 7, 2023
First decision: November 17, 2023
Revised: November 29, 2023
Accepted: December 19, 2023
Article in press: December 19, 2023
Published online: March 18, 2024
Processing time: 160 Days and 2.4 Hours
Liver transplantation (LT) is a life-saving procedure for patients with end-stage liver disease and has become the standard and most effective treatment method for these patients. There are many indications for LT that vary between countries and settings. The outcome of LT depends on the available facilities and surgical expertise, as well as the types of liver graft donors available.
To assess the clinical characteristics of patients from Bahrain who underwent LT overseas, and analyze factors affecting their survival.
In this retrospective cohort study, we reviewed the medical records and overseas committee registry information of all pediatric and adult patients who were sent overseas to undergo LT by the Pediatric and Medical Departments of Salmaniya Medical Complex and Bahrain Defence Force Hospital via the Overseas Treatment Office, Ministry of Health, Kingdom of Bahrain, between 1997 and 2023. Demo
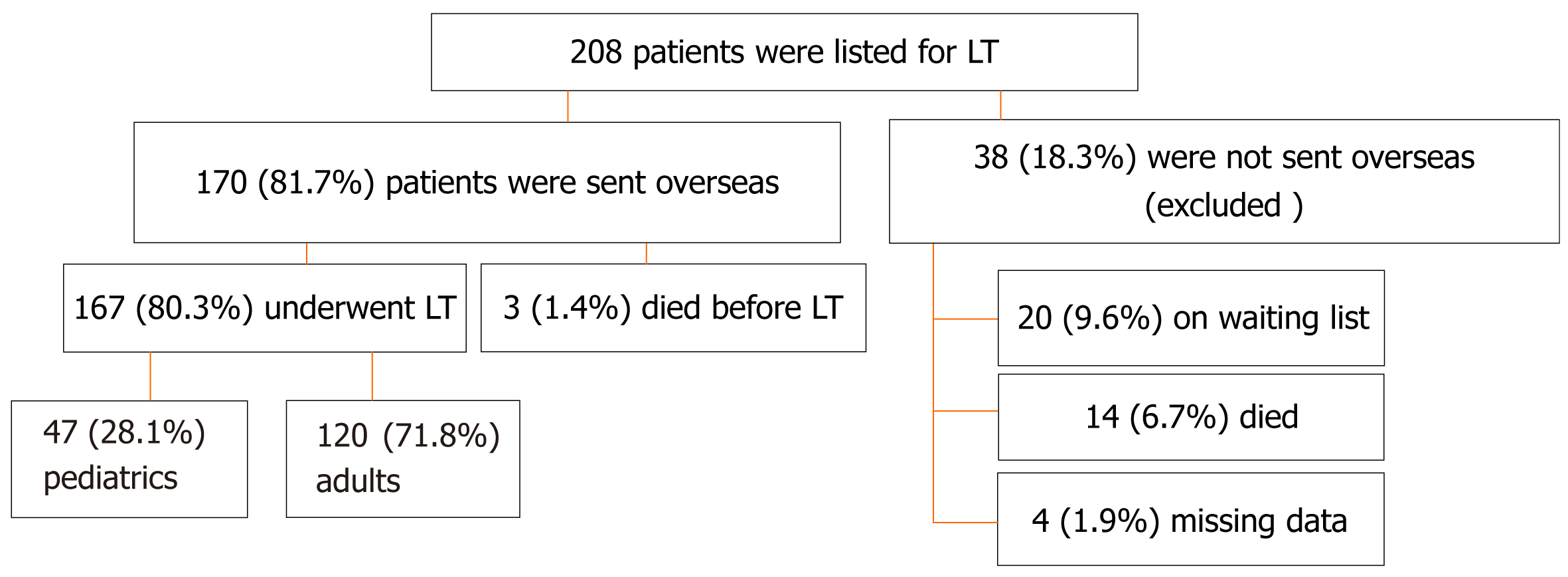
Of the 208 eligible patients, 170 (81.7%) were sent overseas to undergo LT while 38 (18.3%) remained on the waiting list. Of the 170 patients, 167 (80.3%) underwent LT and were included in the study. The majority of the patients were Bahraini (91.0%), and most were males (57.5%). One-hundred-and-twenty (71.8%) were adults and 47 (28.3%) were children. The median age at transplant was 50.0 [interquartile range (IQR): 14.9–58.4] years. The main indication for pediatric LT was biliary atresia (31.9%), while that of adult LT was hepatitis C-related cirrhosis (35.0%). Six (3.6%) patients required re-transplantation. Most patients received a living-related liver graft (82%). Pediatric patients received more living and related grafts than adults (P = 0.038 and P = 0.041, respectively), while adult patients received more cadaveric and unrelated grafts. Most patients required long-term immunosuppressive therapy after LT (94.7%), of which tacrolimus was the most prescribed (84.0%), followed by prednisolone (50.7%), which was prescribed more frequently for pediatric patients (P = 0.001). Most patients developed complications (62.4%) with infectious episodes being the most common (38.9%), followed by biliary stricture (19.5%). Tonsilitis and sepsis (n = 12, 8.1% for each) were the most frequent infections. Pediatric patients experienced higher rates of infection, rejection, and early poor graft function than adult patients (P < 0.001, P = 0.003, and P = 0.025, respectively). The median follow-up time was 6.5 (IQR: 2.6–10.6) years. The overall survival rate was 84.4%, the 5-year survival rate, 86.2%, and the mortality rate, 15.6%. Younger patients had significantly better odds of survival (P = 0.019) and patients who survived had significantly longer follow-up periods (P < 0.001).
Patients with end-stage liver disease in Bahrain shared characteristics with those from other countries. Since LT facilities are not available, an overseas LT has offered them great hope.
Core Tip: The clinical characteristics, management, and outcomes of patients from Bahrain with end-stage liver disease who underwent an overseas liver transplantation (LT) have not been studied previously. In this retrospective cohort study, we found that biliary atresia in children and hepatitis C infection in adults were the main indications. This was comparable to literature from neighboring countries and worldwide. Most patients received living-related grafts. The overall survival rate was 84.4% and was significantly better in younger patients. Therefore, in countries where LT facilities are not available, an overseas LT can offer great hope for this group of patients.
- Citation: Isa HM, Alkharsi FA, Khamis JK, Hasan SA, Naser ZA, Mohamed ZN, Mohamed AM, Altamimi SA. Pediatric and adult liver transplantation in Bahrain: The experiences in a country with no available liver transplant facilities. World J Transplant 2024; 14(1): 87752
- URL: https://www.wjgnet.com/2220-3230/full/v14/i1/87752.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i1.87752
The first successful human liver transplantation (LT) in the world took place in 1967 by Starzl et al[1]; after which LT became a standard treatment for patients with acute or chronic hepatic failure of various etiologies.
There are many indications for LT. In children, the most common indication for LT is biliary atresia, while in adults, the hepatitis C virus (HCV) infection is the most common[2]. Yet, LT indications can vary between countries and settings[3,4].
The outcome of LT depends on the available facilities, surgical expertise as well as the types of liver graft donor[3,4]. The improvement in surgical techniques as well as immunosuppression have improved patient survival and their overall quality of life[3,4].
LT is an essential surgical service that should be available in all countries that have the capabilities. In most of the developed countries, LT centers are available and providing LT services to their patients[3,4]. However, some small and developing countries are lacking LT facilities. Patients in these countries either die from the complications of acute and chronic liver failure, or if a suitable donor is found, they are sent overseas to undergo an expensive LT. The Kingdom of Bahrain is an example for the latter. Since the 90s, the Overseas Treatment Office of the Ministry of Health in Bahrain sends pediatric and adult patients with end-stage liver failure overseas to undergo LT when a suitable liver donor is found. Referral for LT may be emergent, urgent, or anticipatory, as the time of referral varies depending on the patient’s clinical circumstances and donor availability.
Many countries have built respectable reputations and experience in LT, including Turkey and India[5,6]. Moreover, some Gulf Cooperation Council countries and neighboring countries, including Saudi Arabia and Iran, have provided this surgical service for the public for many years[9-7]. Other countries have recently started developing their capabilities to provide LT services, such as Kuwait and Oman[10,11]. In Bahrain, an arrangement was made with multiple overseas LT centers from countries including Turkey, India, and Saudi Arabia, whereby they agreed to take care of patients from Bahrain.
Multiple reports about LT experiences have been published from several countries worldwide[3,4,7]. However, there are no reports studying the details of patients from Bahrain who went overseas for LT. The aim of this study was to review the clinical characteristics, indications, medical therapies, complications, and outcomes of pediatric and adult patients from Bahrain requiring LT, and assess the possible predictors of survival following overseas LT.
A retrospective review was conducted of medical records of all pediatric and adult patients who were listed for an overseas LT by the Department of Pediatrics and the Department of Medicine at Salmaniya Medical Complex and Bahrain Defense Force Hospital via the Overseas Treatment Office, Supreme Committee for Treatment Abroad, Ministry of Health, Manama, Kingdom of Bahrain, between January 1, 1997 and August 1, 2023. All patients who underwent LT were included in the study while those who died before LT, those who remained on the waiting list, and those with missing relevant data, were excluded. Prior to LT, patients with end-stage liver failure were evaluated by their pediatric or adult gastroenterology consultant and the parents/guardians or the patient were asked to provide one or more LT donors.
According to our protocol, a dedicated LT nurse meets the donors, checks their body mass index, orders the basic laboratory tests and radiological imaging (vascular imaging to assess the hepatic arterial anatomy), and fills the donor check list. Following a satisfactory medical and psychological examination by the caring physicians, the donor’s results and the check list are reviewed and approved for donation fitness. The acceptance of a potential donor requires the following: Donors should be 18-55 years of age, have a compatible blood type with the recipient, normal or only slightly altered liver function tests, and hemodynamic stability. Once the donor is ready, a request letter along with a detailed patient medical report are sent to the Head of the Overseas Treatment Office who communicates with multiple overseas LT centers to get their approval. After approval, the patient, the donor, and two direct family members are sent to the overseas LT center by airplane. A senior doctor and a nurse escort sick patients. If more than one center accepts the patient, the choice of center will be based on the patient/guardian’s preference and the quoted cost of the LT.
Patients’ data were collected by reviewing paper-based and electronic medical records along with the overseas committee registry. Important missing data were retrieved by direct contact with the adult patient or the patient's parents/guardians in case of a child or via telephone calls. Demographic data including sex, nationality, area of residence, age at LT, weight and height at LT, presence of associated diseases, any previous surgeries, and family history of liver diseases were collected.
The underlying liver disease that led to liver failure requiring LT were reviewed. The LT indications included but were not limited to the following causes: (1) Extrahepatic cholestasis: Biliary atresia and choledochal cyst; (2) Intra-hepatic cholestasis: Primary sclerosing cholangitis, Alagille’s syndrome, and progressive familial intrahepatic cholestasis; (3) Infections: Intrauterine viral hepatitis, and viral hepatitis B and C; (4) Metabolic diseases: Wilson’s disease, Crigler-Najjar syndrome, inborn error of bile acid metabolism, tyrosinemia, galactosemia, disorders of the urea cycle, organic acidemia, and disorders of carbohydrate metabolism; (5) Acute liver failure; and (6) Other: Autoimmune hepatitis, primary liver tumor, hepatocellular carcinoma (HCC), cystic fibrosis, nonalcoholic steatohepatitis, and alcoholic liver disease.
The donor-recipient relationship, the type of graft (living or cadaveric), the LT center, and the surgical approach were also gathered. Based on the availability of a deceased donor, the LT team might select a cadaveric graft in the absence of a suitable living-related donor or if an early poor graft function developed after the first LT. In the latter case, the patient’s name is moved to the top of the LT waiting list.
Post-LT medical therapy was also reviewed. The use of immunosuppressive medications such as tacrolimus, pre
Development of LT-related complications like bleeding, hypovolemia, post-LT dialysis, early poor graft function, need for re-transplantation, hepatic surgical complications, infections, rejection, surgical wound complications, hepatic artery, or portal vein thrombosis, etc. were collected.
Follow-up duration was measured from the date of LT until death or the study end date. The patient outcomes were assessed based on the overall survival rate, 5-year survival rate, and mortality rate. The LT cost was presented in United States dollars (USD).
Patients’ data were analyzed using the Statistical Package for Social Sciences program (SPSS) version 21 (IBM Corp., Armonk, NY, United States). The patients were divided into pediatric and adult groups and compared in terms of clinical characteristics, LT indications, donor-recipient relationship, medications used, complications, and outcome. The frequencies and percentages were calculated for cate
Until August 2023, a total of 208 pediatric and adult patients were listed for possible LT, and 170 (81.7%) were sent overseas to undergo LT surgery. Of the latter, 167 (80.3%) patients underwent LT, and were included in the study, while 38 (18.3%) were excluded (Figure 1). Most patients were adults (adult: n = 120, 71.8%; pediatric: n = 47, 28.3%). Clinical characteristics of the included patients are shown in Table 1. Ninety-six (57.5%) patients were males. The majority were Bahraini (n = 152, 91.0%) while of the remaining 15 (9.0%), four were from Yemen, two from each of Sudan, Syria, Iran, and India, and one patient from each of Qatar, Egypt, and Pakistan. The median age at transplant was 50.0 (IQR: 14.9–58.4) years. There was no significant difference between males and females in terms of the median age at LT (P = 0.793) or age groups (P = 0.515).
| Patient demography | Total, n = 167 (100) | Pediatric, n = 47 (28.1) | Adult, n = 120 (71.8) | P value |
| Sex | 0.492a | |||
| Male | 96 (57.5) | 25 (53.2) | 71 (59.7) | |
| Female | 71 (42.5) | 22 (46.8) | 49 (40.8) | |
| Nationality | < 0.001a | |||
| Bahraini | 152 (91.0) | 36 (76.6) | 116 (96.7) | |
| Non-Bahraini | 15 (9.0) | 11 (23.4) | 4 (3.3) | |
| Governorate | 0.369b | |||
| Northern | 73 (43.7) | 21 (44.7) | 52 (43.3) | |
| Capital | 35 (20.9) | 6 (12.8) | 29 (24.2) | |
| Southern | 34 (20.4) | 12 (25.5) | 22 (18.3) | |
| Muharraq | 25 (15.0) | 8 (17.0) | 17 (14.2) | |
| Age at transplant (yr) | 50.0 (14.9-58.4) | 3.7 (1.0-9.0) | 55.2 (48.4-60.5) | < 0.001c |
| Weight at transplant (kg), (n = 83) | 52 (15.0-70.0) | 11.0 (7-23) | 69 (52-80) | < 0.001c |
| Height at transplant (cm), (n = 75) | 163 (138-169) | 82.0 (69-120) | 167 (159-172) | < 0.001c |
| Presence of associated diseases1 | 130/162 (80.3) | 25 (53.2) | 105/115 (91.3) | < 0.001a |
| Previous liver biopsy | 67/145 (46.2) | 20/46 (43.5) | 47/99 (47.5) | 0.722a |
| Previous surgeries | 52/145 (35.9) | 17/46 (37.0) | 35/99 (35.4) | 0.855a |
| Kasai procedure | 11/145 (7.6) | 10/46 (21.7) | 1/99 (1.0) | < 0.001a |
| Other surgeries | 45/145 (31.0) | 10/46 (21.7) | 35/99 (35.4) | 0.124a |
| Family history of liver disease | 38/145 (26.2) | 17/46 (37) | 21/99 (21.2) | 0.067a |
| Follow up duration (yr) | 6.5 (2.6-10.6) | 8.1 (1.3-10.6) | 6.1 (3.3-10.3) | 0.976c |
| Number of overseas visits | 3 (2.0-8.0) | 4 (2.0-10.0) | 3 (2.0-6.0) | 0.299c |
Most of the patients presented with chronic liver disease (n = 164, 98.2%), while three (1.8%) patients had acute liver failure. In patients with chronic liver diseases, 117 (71.3%) were adults while 47 (28.7%) were children, and all patients with acute liver failure were adults (n = 3, 2.5%). There was no significant difference between pediatric and adult patients in terms of disease onset (acute or chronic) (P = 0.560). Forty-one (24.6%) patients had documented liver cirrhosis prior to the LT with no difference between adult patients (n = 33, 19.8%) and children (n = 8, 17.0%) (P = 0.230).
The main indication for pediatric LT was biliary atresia (n = 15, 31.9%), followed by progressive familial intrahepatic cholestasis (n = 9, 19.1%), while the main indication for adult LT was HCV-related cirrhosis (n = 42, 35.0%), followed by nonalcoholic steatohepatitis (n = 19, 15.8%) (Table 2).
| Indications of liver transplantationa | Total, n (%) |
| Pediatric indications | 47 (28.1) |
| Biliary atresia | 15 (31.9) |
| Progressive familial intrahepatic cholestasis | 9 (19.1) |
| Metabolic diseasesb | 7 (14.9) |
| Alagille’s syndrome | 3 (6.4) |
| Autoimmune hepatitis | 3 (6.4) |
| Primary sclerosing cholangitis | 3 (6.4) |
| Cystic fibrosis liver disease | 2 (4.3) |
| Hepatocellular carcinoma | 2 (4.3) |
| Cytomegalovirus hepatitis | 2 (4.3) |
| Othersc | 8 (17.0) |
| Adult indications | 120 (71.9) |
| Hepatitis C-related cirrhosis | 42 (35.0) |
| Nonalcoholic steatohepatitis | 19 (15.8) |
| Hepatocellular carcinoma | 18 (15.0) |
| Primary sclerosing cholangitis | 17 (14.2) |
| Hepatitis B virus | 15 (12.5) |
| Cryptogenic cirrhosis | 13 (10.8) |
| Autoimmune hepatitis | 9 (7.5) |
| Alcoholic liver cirrhosis | 4 (3.3) |
| Othersd | 5 (4.2) |
Six (3.6%) patients required re-transplantation, of whom four (3.3%) were adults and two were children (4.3%) (P = 0.674). Two of the four adults were re-transplanted after three years from the first LT, while one underwent re-transplantation after four years, and another after nine years. One pediatric patient was re-transplanted after one week and the other after one month. The indications for re-transplantation in adults were early cirrhosis due to reinfection with HCV (n = 2), recurrence of primary sclerosing cholangitis (n = 1), and liver failure due to ductopenic chronic rejection (n = 1), while in the two pediatric patients the indication was early allograft dysfunction.
Of 173 LT surgeries, donor type data was available in 150 (86.7%) [144 (96%) single LT surgeries and the six (4%) re-transplantations]. The donor-recipient relationships are shown in Table 3. Most patients received a living-related liver graft (n = 123/150, 82%). Pediatric patients received more living grafts than adults [47/48 (97.9%) vs 88/102 (86.3%), respectively] while adult patients received more cadaveric [14/102 (13.7%) vs 1/48 (2.1%), respectively], (P = 0.038). Pediatric patients received more related grafts than adults [44/48 (91.7%) vs 79/102 (76.5%), respectively] while adult patients received more unrelated grafts [23/102 (23.5%) vs 4/48 (8.3%), respectively], (P = 0.041). The median hospitalization duration was 30 (IQR: 14–60) days.
| Donor | Total LT, n = 150/173 (86.7) | Pediatrics, n = 48/49 (98.0) | Adults, n = 102/124 (82.3) | P value |
| Related living donors | 123 (82) | 44 (91.7) | 79 (76.5) | 0.041a |
| 1st degree | 65 (52.8) | 29 (65.9) | 36 (45.6) | 0.020b |
| 2nd degree | 19 (15.5) | 3 (6.8) | 16 (20.3) | |
| 3rd degree | 21 (17.1) | 10 (22.7) | 11 (13.9) | |
| 4th degree | 17 (13.8) | 2 (4.5) | 15 (18.9) | |
| Unspecified relation | 1 (0.8) | 0 (0.0) | 1 (1.3) | |
| Unrelated donors | 27 (18) | 4 (8.3) | 23 (22.5) | 0.041a |
| Living | 12 (8.0) | 3 (6.3) | 9 (8.8) | 0.753a |
| Cadaveric | 15 (10.0) | 1 (2.1) | 14 (13.7) | 0.038a |
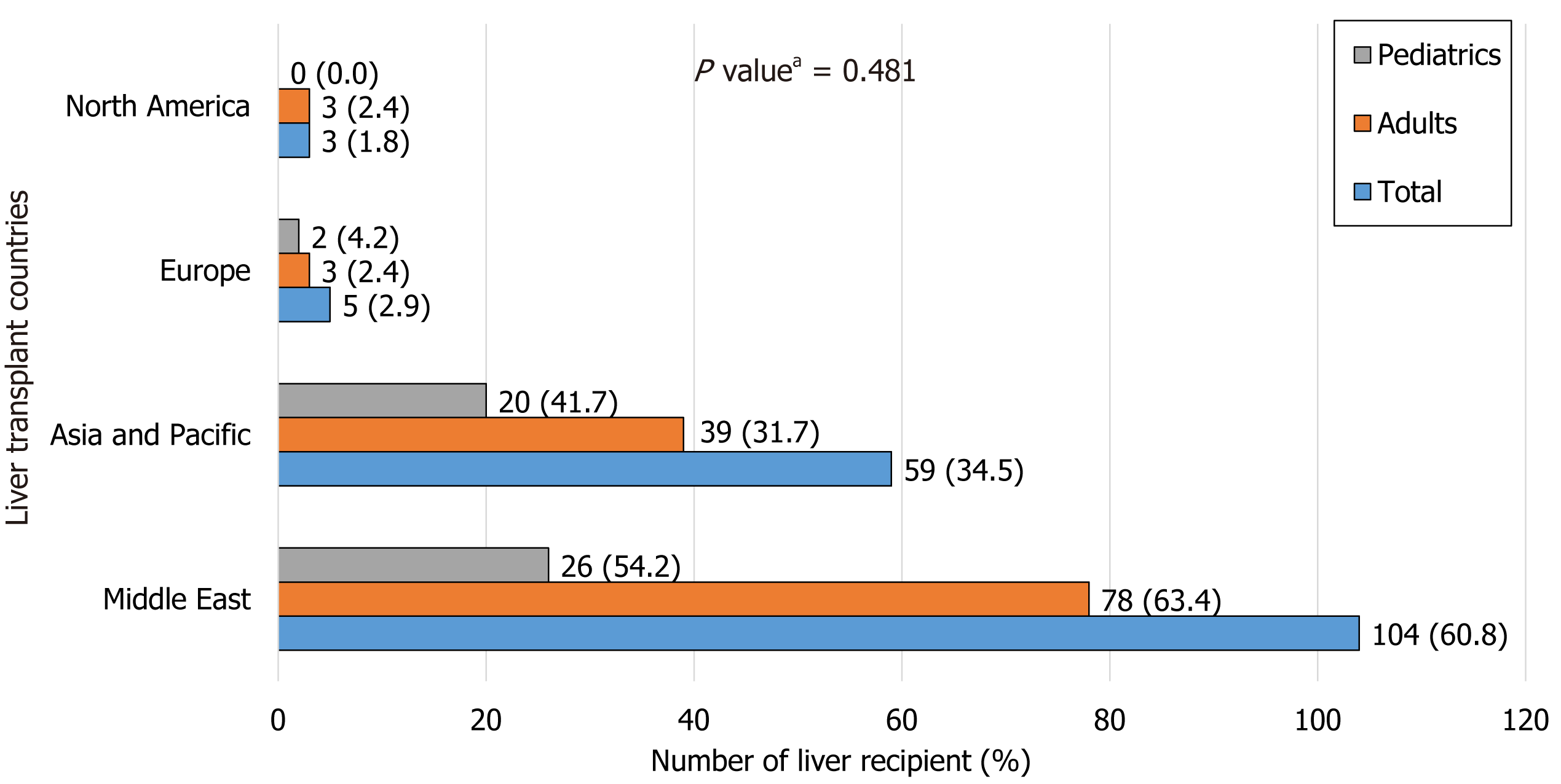
The main countries receiving patients from Bahrain for LT are shown in Figure 2. Most of the patients underwent LT in Turkey (n = 70/171, 40.9%), followed by India (n = 52/171, 30.4%), then Saudi Arabia (n = 22/171, 12.9%). There was no significant difference between the pediatric and adult patients in terms of LT center location (P = 0.481).
Of 173 LT surgeries, data about the surgical approach was available for 145 surgeries. The Mercedes incisions was the most common approach (n = 100, 69.0%) followed by the L-shaped (n = 24, 16.6%) and transverse incisions (n = 21, 14.5%). In pediatric patients, most incisions were either transverse or Mercedes (n = 20/44, 45.5% each), followed by L-shaped (n = 4/44, 9.0%) while in adult patients, most were Mercedes incisions (n = 80/101, 79.2%), followed by L-shaped (n = 20/101, 19.8%) and transverse (n = 1/101, 0.9%). This difference was statistically significant (P < 0.001). Of six patients who underwent re-transplantation, five (83.3%) had the same Mercedes incision while one (16.7%) patient had a transverse followed by an L-shaped incisional approach.
Medications used after LT surgery are shown in Table 4. Most patients required long-term immunosuppressive therapy (n = 142, 94.7%). Tacrolimus was the most prescribed (n = 126, 84.0%) followed by prednisolone (n = 76, 50.7%) which was significantly prescribed more for pediatric patients (P = 0.001). None of the patients received NAC prior to the LT. Ninety-three (62.4%) patients developed complications during or after LT. Infections were the most common complications (n = 58, 38.9%), followed by biliary stricture (n = 29, 19.5%) (Table 5). In general, pediatric patients had a higher rate of complications (n = 33/46, 71.7%) than adult patients (n = 60/103, 58.3%) but this difference was not statistically significant (P = 0.144). However, pediatric patients showed a significantly higher rate of infectious episodes, rejection, and early poor graft function than adult patients (P < 0.001, P = 0.003, and P = 0.025, respectively). Pediatric patients had significantly more tonsilitis and acute gastroenteritis than adults (P < 0.001 and P = 0.035, respectively) who had more septic episodes but with no significant difference (P = 0.755). None of the patients developed hypovolemia or bowel perforation.
| Medicationsa | Total, n = 150/167 (89.8) | Pediatrics, n = 44/47 (93.6) | Adults, n = 106/120 (88.3) | P valueb |
| Immunosuppressive medications | 142 (94.7) | 40 (90.9) | 102 (96.2) | 0.234 |
| Tacrolimus | 126 (84.0) | 38 (86.4) | 88 (83.0) | 0.807 |
| Prednisolone | 76 (50.7) | 32 (72.7) | 44 (41.5) | 0.001 |
| Mycophenolic acid | 73 (48.7) | 14 (31.8) | 59 (55.7) | 0.012 |
| Cyclosporine A | 13 (8.7) | 4 (9.1) | 9 (8.5) | 1.000 |
| Azathioprine | 8 (5.3) | 5 (11.4) | 3 (2.8) | 0.048 |
| Everolimus | 7 (4.7) | 0 (0.0) | 7 (6.6) | 0.106 |
| Baziliximab | 1 (0.7) | 1 (2.3) | 0 (0.0) | 0.293 |
| Dietary supplementations | 109 (72.7) | 37 (84.1) | 72 (67.9) | 0.046 |
| Calcium | 77 (51.3) | 18 (40.9) | 59 (55.7) | 0.110 |
| Vitamin D | 63 (42.0) | 22 (50.0) | 41 (38.7) | 0.210 |
| Magnesium | 35 (23.3) | 20 (45.5) | 15 (14.2) | < 0.001 |
| Multivitamin | 32 (21.3) | 27 (61.4) | 5 (4.7) | < 0.001 |
| Folic acid | 30 (20.0) | 14 (31.8) | 16 (15.1) | 0.026 |
| Iron | 22 (14.7) | 11 (25.0) | 11 (10.4) | 0.040 |
| Biotin | 4 (2.7) | 4 (9.1) | 0 (0.0) | 0.007 |
| Carnitine | 2 (1.3) | 2 (4.5) | 0 (0.0) | 0.085 |
| Antiviral (valganciclovir) | 23 (15.3) | 20 (45.5) | 3 (2.8) | < 0.001 |
| Antibiotics | 20 (13.3) | 18 (40.9) | 2 (1.9) | < 0.001 |
| Aminoglycosides | 15 (10.0) | 15 (34.1) | 0 (0.0) | < 0.001 |
| Co-trimoxazole | 12 (8.0) | 10 (22.7) | 2 (1.9) | < 0.001 |
| Antifungal medications | 9 (6.0) | 8 (18.2) | 1 (0.9) | < 0.001 |
| Fluconazole | 7 (4.7) | 6 (13.6) | 1 (0.9) | 0.003 |
| Amphotericin B | 2 (1.3) | 2 (4.5) | 0 (0.0) | 0.085 |
| Other medications | 135 (90.0) | 38 (86.4) | 97 (91.5) | 0.375 |
| Proton pump inhibitors | 96 (64.0) | 21 (47.7) | 75 (70.8) | 0.009 |
| Urosodeoxycholic acid | 91 (60.7) | 27 (61.4) | 64 (60.4) | 1.000 |
| N-acetylcysteine | 66/118 (55.9) | 10/29 (34.5) | 56/89 (62.9) | 0.010 |
| Aspirin | 57 (38.0) | 22 (50.0) | 35 (33.0) | 0.065 |
| Complicationsa | Total, n = 93/149 (62.4) | Pediatrics, n = 33/46 (71.7) | Adults, n = 60/103 (58.3) | P valueb |
| Infection episodes | 58 (38.9) | 29 (63.0) | 29 (28.2) | < 0.001 |
| Tonsillitis | 12 (8.1) | 10 (21.7) | 2 (1.9) | < 0.001 |
| Sepsis | 12 (8.1) | 3 (6.5) | 9 (8.7) | 0.755 |
| Acute gastroenteritis | 11 (7.4) | 7 (15.2) | 4 (3.9) | 0.035 |
| Cytomegalovirus | 7 (4.7) | 7 (15.2) | 0 (0.0) | < 0.001 |
| Fever of unclear cause | 7 (4.7) | 1 (2.2) | 6 (5.8) | 0.437 |
| Pneumonia | 7 (4.7) | 5 (10.9) | 2 (1.9) | 0.029 |
| Other infections1 | 31 (20.8) | 16 (34.8) | 15 (14.6) | 0.016 |
| Biliary stricture | 29 (19.5) | 5 (10.9) | 24 (23.3) | 0.115 |
| Rejection | 15 (10.1) | 10 (21.7) | 5 (4.9) | 0.003 |
| Early poor graft function | 9 (6.0) | 6 (13.0) | 3 (2.9) | 0.025 |
| Incisional hernia | 9 (6.0) | 3 (6.5) | 6 (5.8) | 1.000 |
| Surgical wound complications | 8 (5.4) | 2 (4.3) | 6 (5.8) | 1.000 |
| Bleeding | 4 (2.7) | 2 (4.3) | 2 (1.9) | 0.587 |
| Hepatic artery complications | 2 (1.3) | 1 (2.2) | 1 (0.9) | 0.524 |
| Portal vein thrombosis | 2 (1.3) | 1 (2.2) | 1 (0.9) | 0.524 |
| Gastric perforation | 2 (1.3) | 2 (4.3) | 0 (0.0) | 0.094 |
| Chylous ascites | 2 (1.3) | 1 (2.2) | 1 (0.9) | 0.524 |
| Other complications2 | 9 (6.0) | 3 (6.5) | 6 (5.6) | 1.000 |
Patients were seen at the liver clinic in Bahrain within two weeks of their overseas LT, with close follow-up in the first three months. Afterward, regular follow-up visits continued at every three months in the first year and every six months in the second year. The median follow-up time was 6.5 (IQR: 2.6–10.6) years and the median number of overseas follow-up visits was three (IQR: 2–8). Most patients were sent back to the overseas LT center for follow-up every six months during the first-year post LT.
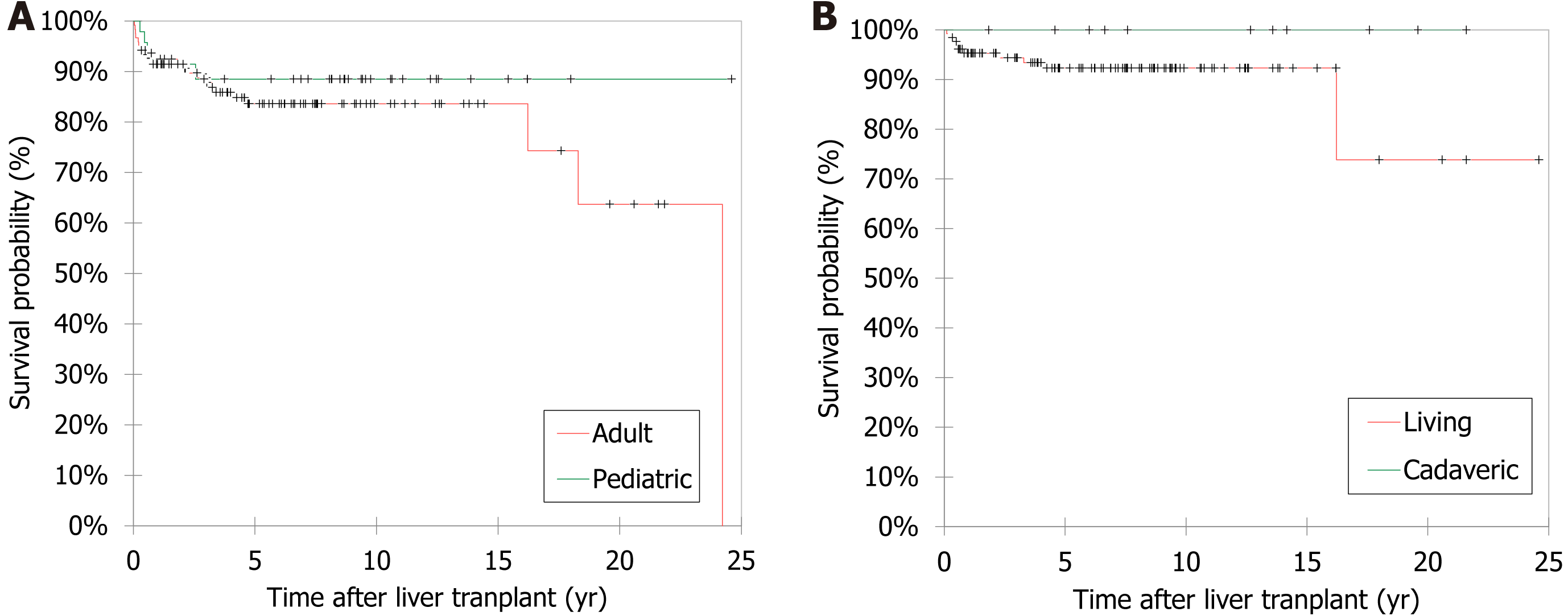
The results of post-LT survival analysis using the Kaplan-Meier method are shown in Figure 3. The overall survival rate was 84.4% (n = 141/167), 5-year survival rate was 86.2%, and the mortality rate was 15.6% (26 patients died; 21 adults and five children). Pediatric patients had better survival outcomes (n = 42, 89.4%) compared to adult patients (n = 99, 82.5%). However, this difference was not statistically significant (P = 0.346). The median survival age was 57.1 (IQR: 26–65.2) years; 11.1 (IQR: 7.4–17.5) years for pediatric patients and 61.6 (IQR: 54.9–67.6) years for adults. Younger patients had better survival outcome (P = 0.019) (Table 6). Patients who survived had a significantly longer period of follow up compared to those who died (P < 0.001). None of the other variables such as sex, nationality, area of residency, weight and height at LT, presence of associated diseases (Supplementary Table 1), type of graft, donor-recipient relationship, indication for LT, intra- and post-LT complications, and the location of the LT center had a statistically significant impact on survival. On comparing the main three centers regarding the patient outcomes, the overall survival was 100% in Saudi Arabia, 88.2% in India, and 76.1% in Turkey and this difference was statistically significant (P = 0.021). On comparing the survival between pediatric and adult patients according to the LT center, after excluding Iran (81.8% survival) as they transplanted adult patients only, the ranking was in favor of Saudi Arabia, followed by India, then Turkey with no difference between pediatric and adult patients (Supplementary Table 2). In univariate and multivariate analyses, none of the selected variables were found to be significant predictors of LT outcome (Table 7).
| Variable | Survived, n = 141 (84.4) | Died, n = 26 (15.6) | P value |
| Sex | 0.130a | ||
| Male | 85 (60.3) | 11 (42.3) | |
| Female | 56 (39.7) | 15 (57.7) | |
| Nationality | 0.471a | ||
| Bahraini | 127 (90.1) | 25 (96.2) | |
| Non-Bahraini | 14 (9.9) | 1 (3.8) | |
| Area of residency | 0.118b | ||
| Northern | 63 (44.7) | 10 (38.5) | |
| Capital | 29 (20.6) | 6 (23.1) | |
| Southern | 25 (17.7) | 9 (34.6) | |
| Muharraq | 24 (17.0) | 1 (3.8) | |
| Age at liver transplant (yr) | 48.8 (13.2-58.0) | 57.5 (47.9-65.2) | 0.019c |
| Age group | 0.346a | ||
| Pediatric | 42 (29.8) | 5 (19.2) | |
| Adult | 99 (70.2) | 21 (80.8) | |
| Weight at transplant (kg), (n = 83) | 52.0 (20.0-70.0) | 8.0 (5.0-46.0) | 0.144c |
| Height at transplant (cm), (n = 76) | 163.0 (149.0-169.0) | 138.0 (74.0-149.0) | 0.101c |
| Presence of associated diseases | 139 (98.6) | 23 (88.5) | 0.255a |
| Yes | 109 (78.4) | 21 (91.3) | |
| No | 30 (21.6) | 2 (8.7) | |
| Type of graft (n = 150) | 137 (97.2) | 13 (50.0) | 0.364a |
| Living | 122 (89.1) | 13 (100) | |
| Cadaveric | 15 (10.9) | 0 (0.0) | |
| Donor-recipient relationship (n = 150) | 137 (97.2) | 13 (50.0) | 0.704a |
| Related donors | 113 (82.5) | 10 (76.9) | |
| Unrelated donors | 24 (17.5) | 3 (23.1) | |
| Indications of liver transplantation | |||
| Hepatitis C virus | 37 (26.2) | 5 (19.2) | 0.623a |
| Primary sclerosing cholangitis | 18 (12.8) | 2 (7.7) | 0.743a |
| Nonalcoholic steatohepatitis | 15 (10.6) | 4 (15.4) | 0.503a |
| Hepatic cellular carcinoma | 14 (9.9) | 6 (23.1) | 0.092a |
| Biliary atresia | 13 (9.2) | 3 (11.5) | 0.718a |
| Hepatitis B virus | 13 (9.2) | 2 (7.7) | 1.000a |
| Autoimmune hepatitis | 11 (7.8) | 1 (3.8) | 0.694a |
| Metabolic diseases | 7 (5.0) | 0 (0.0) | 0.597a |
| Intra- or post-LT complications (n = 150) | 138 (97.9) | 11 (42.3) | 0.537a |
| Yes | 85 (61.6) | 8 (72.7) | |
| No | 53 (38.4) | 3 (27.7) | |
| Post-LT N-acetylcysteine use | 50/66 (75.8) | 46/52 (88.5) | 0.098 |
| Liver transplant countries | 0.582b | ||
| Middle East | 86 (61.0) | 19 (73.1) | |
| Asia & Pacific | 51 (36.2) | 8 (30.7) | |
| Europe | 4 (2.8) | 0 (0.0) | |
| North America | 3 (2.1) | 0 (0.0) | |
| Follow-up duration (yr) | 7.5 (3.9-10.6) | 1.5 (0.3-3.2) | < 0.001c |
| Variable | Univariate analysis | Multivariate analysis | ||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | |
| Male sex | 0.483 (0.207-1.128) | 0.093 | 0.617 (0.127-2.999) | 0.549 |
| Bahraini nationality | 0.363 (0.046-2.886) | 0.338 | 2.001 (0.124-32.414) | 0.625 |
| Governorate (Northern vs others) | 0.774 (0.328-1.823) | 0.558 | 0.912 (0.179-4.652) | 0.912 |
| Age at LT (yr) | 0.980 (0.960-1.001) | 0.059 | 1.047 (0.955-1.148) | 0.329 |
| Age group (pediatrics vs adults) | 0.561 (0.198-1.588) | 0.276 | 0.073 (0.001-8.750) | 0.284 |
| Weight at LT (kg) | 1.009 (0.990-1.029) | 0.362 | 1.047 (0.965-1.136) | 0.268 |
| Height at LT (cm) | 1.007 (0.994-1.022) | 0.293 | 1.000 (0.969-1.032) | 0.989 |
| Presence of associated diseases | 0.346 (0.077-1.560) | 0.167 | 1.131 (0.136-9.415) | 0.909 |
| Related versus unrelated donor | 1.865 (0.225-15.447) | 0.563 | 1.979 (0.149-26.338) | 0.605 |
| Hepatitis C virus | 1.494 (0.526-4.249) | 0.451 | 0.275 (0.026-2.914) | 0.284 |
| Biliary atresia | 0.779 (0.206-2.949) | 0.713 | 1.474 (0.124-17.462) | 0.759 |
| Presence of complications | 0.601 (0.153-2.368) | 0.467 | 0.222 (0.020-2.458) | 0.220 |
| LT countries (Middle East versus others) | 2.096 (0.784-5.602) | 0.140 | 2.699 (0.383-19.017) | 0.319 |
The LT cost varied between centers. The average cost of LT surgery was 60000 USD per patient, ranging from 42500 USD to 84000 USD. For the donor preparation, the cost ranged from 10000 USD to 20000 USD.
This study found that most patients who required LT were adults (71.8%). Similarly, several studies reported that a higher number of adult patients underwent LT than pediatric patients[2,7,12,13]. The reason behind this finding might be related to the fact that most centers started LT services in adults first, followed by the pediatric population. Subsequently, the adult LT programs are predominant compared to those for pediatric patients[4]. Moreover, the rapid rise in the prevalence of nonalcoholic steatohepatitis in adults makes them more likely to require LT[4].
In the present study, most of the LT patients were males (57.5%). This is similar to several other studies where a male predominance ranged from 52.8% to 85.0%[2,8-10,12,14-17]. This male predominance might be attributed to the risky behaviors of males, such as alcohol consumption, tobacco smoking, and addiction to intravenous drug use which may increase their risk of becoming infected with HCV[18]. Moreover, HCC is approximately three times more prevalent in males than females, attributed to their hormonal pattern[19]. Furthermore, males have a higher prevalence of obesity and metabolic dysfunction-associated fatty liver disease[4]. In contrary to our study, three studies from Korea and one study from the United States reported that most of the patients were females[20-23].
In the current study, the median age at transplant was 50.0 (IQR: 14.9–58.4) years. However, multiple studies reported that most of the patients underwent LT at a younger age, ranging from 17.6 to 43 years[8,12,15,22]. Moreover, many studies reported LT among pediatric patients alone[5,9,20,23,24]. This can be related to the study population, design, and setting.
In this study, biliary atresia was the main indication for LT in pediatric patients (31.9%). Comparably, many published studies reported that biliary atresia was the most common indication for LT among the pediatric population, but with a higher percentage, ranging from 43% to 66.1%[2,3,13,20,25-27]. The reason behind this finding might be that most children with biliary atresia underwent the Kasai procedure that failed to re-establish effective biliary flow, which causes rapid evolution to secondary biliary cirrhosis[28]. In contrast, a Turkish study reported that Wilson disease was the main indication for LT in pediatric patients (16.3%) rather than biliary atresia (14.5%)[5].
HCV-related cirrhosis was the main LT indication in adult patients in this study (35%). This is comparable to other published studies from Argentina, the United States, and Saudi Arabia, where HCV was the most common LT indication in adult patients and represented 35%, 37.4%, and 38% of their patients, respectively[2,7,17]. However, the European Liver Transplant Registry reported a lower percentage of HCV-related cirrhosis (13%) among their population[3]. This variation might be related to the differences in the HCV infection prevalence between countries. The overall prevalence of HCV in Bahrain was 1.7% (1.0%–1.9%) in 2011 and reduced to 0.99% in 2014[29,30]. This prevalence is considered relatively low when compared to the total global HCV prevalence (2.5%)[31]. The reason behind the high incidence of HCV in adults is the history of blood transfusion (35%) which is a major risk factor in patients with thalassemia and sickle cell anemia, which are common in Bahrain[32]. Other reasons include intravenous drug use (16.9%), tattoos (4.9%), extramarital sexual contact (3.3%), hemodialysis for chronic renal failure (3.3%), previous surgery (1.6%), and bleeding disorders (1.6%)[32].
The difficulty in finding deceased donors is a serious universal problem especially in Asia for social, religious, and cultural reasons[10,16]. Religious beliefs may either reject or limit organ donation from deceased individuals[10]. Moreover, procurement of organs is considered as an act of body mutilation in some cultures[10]. In the current study, most patients received a living-related liver graft (82%). This figure was comparable to that reported from Korea (84.6%)[20]. However, two studies from Canada and Turkey reported a lower percentage (45% and 32%) of patients received liver allografts from living donors[5,24]. In contrast, most reported patients from Saudi Arabia received a cadaveric graft due to the difficulty in finding living donors who can fulfill all the required criteria for liver donation[12]. Nonetheless, a Korean study reported no significant difference between emergency LT with a deceased donor and elective LT with a living donor[22], which was also observed in our study. In the current study, pediatric patients received more related grafts (91.7%) than adults (76.5%) (P = 0.041). Similarly, a study from Japan stated that the parents were the main donors for pediatric cases (95%)[33]. Moreover, a study from Korea found that haplo-matched donors were predominant among pediatric patients, while unrelated donors were predominant among the adult group (P = 0.006)[22].
Most patients in the current study received long-term immunosuppressive therapy post-LT (n = 142, 94.7%). Tacrolimus-based immunosuppression was the most frequently prescribed (84.0%). Similarly, Kim et al[20] and Ng et al[26] reported that most LT recipients received tacrolimus as immunosuppressive therapy (94.4% and 68%, respectively). Tacrolimus is the most effective immunosuppressive medication used after LT, as it helps prevent organ rejection and, therefore, increases the survival rate[34,35]. Tacrolimus had become the standard immunosuppressive medication used after LT in adults and pediatric patients[2,26]. Adequate immunosuppression is needed to support graft function but must be balanced against the risks of side effects and potential over immunosuppression[35]. Tacrolimus and cyclo
In the current study, many patients developed LT-related complications (62.4%). Comparably, two studies reported a post-operative complication rate of 72.4% and 58.4%[9,21]. Infectious episodes were the most common complications in our study (38.9%). Similarly, Busuttil et al[2] found that infections were the most common complication after LT but with a lower percentage (13.7%). Early infectious complications tend to be related to surgical manipulations, technical complications of the surgery, catheters, and other foreign bodies[38]. Development of infection after LT may be related to the immunosuppressive drugs used to prevent rejection, which inhibit the activation of T lymphocytes, medullar cell proliferation, and macrophage functions[28]. This can create an optimal environment for development of infections[28]. Infectious complications had become the most common cause of morbidity and mortality after transplantation[28]. In the current study, tonsilitis and sepsis were the most frequent infectious complications (8.1% each) followed by acute gastroenteritis (7.4%). Bacteria are the main infectious agents in the first weeks after LT, with enterococci and gram-negative bacteria in the abdomen being the most frequent[39]. Signs of infection can vary from laboratory abnormalities without clinical manifestations to irreversible fulminant septic shock[39]. Septic shock was found in four (2.4%) patients in this study. Fever of unknown origin was found in seven (4.7%) patients, and the presence of fever may indicate the development of systemic inflammatory response syndrome or a hidden infection which requires blood or urine sampling for culture and further investigations to detect the focus of infection[39]. Moreover, immediate administration of either specific or broad-spectrum antibiotics is important[39]. Upon literature review, most of the studies focused on cytomegalovirus (CMV) and Epstein-Barr virus (EBV) infections post-LT. One review article reported that viral infections usually occur during the first month post-LT, with CMV being the most frequent infectious agent[39]. Another two studies reported that EBV infection was the most common type of infection after LT, followed by CMV[20,21]. EBV infection was documented in 0.7% (one of 149 patients) in the current study, while CMV infection was found in 4.7% of the patients. Campbell et al[35] stated that treatment of CMV with intravenous ganciclovir is recommended as initial therapy and can dramatically improve the outcomes. All our patients had received valganciclovir as a prophylactic measure initially as per the protocol but only 15.3% of them had documented valganciclovir therapy in their medical records either as treatment for active CMV infection or as a continuation of the prophylactic use.
Biliary stricture was the second most frequent complication (19.5%) in this study and the most common surgical problem. A study from Korea also reported that bile duct complications were the most frequent in the surgical aspect (17.1%)[21]. However, another study reported a lower rate of biliary stricture (11%) in their pediatric patients who underwent LT[5]. The majority of stenoses can be treated with dilation by percutaneous transhepatic cholangiography, which involves inserting a bile drain to shape the anastomosis for approximately six months[39].
Follow-up duration of patients post LT varies between studies based on the time of establishing the LT services, patients’ general condition, development of complications, and survival rate. The median follow-up time in this study was 6.5 (IQR, 2.6–10.6) years. Similarly, Busuttil et al[2] reported a median follow-up time of 6.7 (range, 0–20) years. However, other studies reported shorter mean/median follow-up period ranging 2–5.9 years[12,17,20,22]. In contrast, Thammana et al[23] reported longer median follow-up time (8.3 years).
The overall survival rate was 84.4% in the patients who underwent LT in the present study. Comparably, Al-Sebayel et al[12] reported a survival rate of 90% despite a shorter follow-up period of 736 days. In the current study, pediatric patients had better survival rate (89.4%) compared to adult patients (82.5%). Adam et al[3] also found that the 5-year survival rate in pediatric patients was significantly better than adult patients, 79% vs 70%, respectively (P < 0.0001). Many studies reported a higher survival rate among pediatric patients after LT[2,5,20,25]. One study reported that the overall survival rate within five years was 97% after pediatric orthotopic LT[25]. Another study reported that 1-year and 5-year survival rates of their pediatric patients were 87% and 84%, respectively[5]. On the other hand, a study from Korea[22] reported that there were no significant differences between pediatric and adult patients in terms of outcomes when the etiology was the same and the same surgical techniques were used at a single medical center. Nonetheless, LT outcomes are improving, and the number of candidates listed for transplantation has increased dramatically over the years[40].
In this study, a younger age at LT and longer follow-up duration appeared to have a positive effect on survival (P = 0.019 and P < 0.001, respectively). Similarly, Haseli et al[9] found patient age to be one of the effective factors on patient survival in the univariate analysis. However, children below one year old had the lowest survival rate compared to the other age groups[9]. In terms of the effect of LT center on patient survival, we found a significant variation between the main three centers (P = 0.021), which may lead us to recommend a LT center from Saudi Arabia for both pediatric and adult patients from Bahrain. This variation in the outcome might be attributed to the proximity of the center to our country which is the case for centers in Saudi Arabia, and the length of LT surgical experience, as for the centers in India and Turkey. In addition, Haseli et al[9] found that weight at LT, initial diagnosis, pediatric end-stage liver disease/model for end-stage liver disease score, type of graft, existence of post-LT complications, and year of LT were effective factors on patient survival. Busuttil et al[2] found that recipient survival was affected by operative parameters and the etiology of end-stage liver disease. Moreover, recipients of younger organs appeared to exhibit long-term survival advantage over recipients of older donors[2]. In comparison between living and cadaveric grafts recipients, two studies reported no significant difference between the two groups in graft and patient survival after long-term follow-up[12,22], which was similar to the findings of our study. Furthermore, the use of liver support medications such as NAC have shown better overall and post LT survival[36]. However, on analyzing the effect of NAC on the overall survival, we found no significant difference between patients who received it and those who did not (P = 0.098). Yet, this finding should be interpreted with caution especially as the data was available from only two centers, each with different NAC prescription protocols.
Like most of other retrospective studies, this study has limitations, such as missing patient data, including anthropometric data at the time of LT, previous surgical history, the donor-recipient relationship, medications used, and complications. Another limitation is that our study did not focus on those patients who died while on the waiting list for LT. Likewise, patients who could not afford to bring a suitable donor were not listed and were not accounted for in this study. This may underestimate the magnitude of the mortality related to end-stage liver disease in Bahrain. Moreover, the details of the cost of the overseas LT including donor preparation work-up, transportation, surgery, post-LT care, and follow-up visits were not analyzed in this study. Furthermore, compared to bacterial infections, viral infections were less documented in our study as viral serology was limited to CMV and EBV infections. In addition, upon an extensive literature search, we could not find published studies from countries lacking LT facilities to compare with our study. Despite these limitations, the findings of this study are important, being the first study focusing on patients from Bahrain undergoing LT. Our study included both pediatric and adult patients from the main two centers in Bahrain that send patients overseas for LT which makes our sample highly representative of the general population. This study is contributing to the body of literature, highlighting the effectiveness of pediatric and adult LT in improving the survival of patients with acute or chronic liver failure. The findings of this study might benefit centers in which LT facilities are not available. They can direct targeted ranking of patients at risk of liver failure and help implementing new interventional strategies in these high-risk groups.
LT remains a complex and costly procedure and initiating a LT program in any country can present several challenges including: (1) The availability of infrastructure and resources; (2) establishing effective organ procurement mechanisms; (3) recruiting and training healthcare professionals to formulate a multidisciplinary team; (4) navigating various regulatory and legal requirements; (5) careful financial planning; and (6) collaboration and networking with other transplant centers. Nonetheless, these challenges are not insurmountable, and many countries have successfully established LT programs. On January 19, 2020, the Health Minister for Bahrain announced that the preparations are underway to perform the first ever LT[41]. Recently, the Royal Medical Services (RMS) at King Hamad University Hospital initiated the Organ Transplantation Program in co-operation with the Supreme Committee for Treatment Abroad, Bahrain and King Fahad Specialist Hospital, Dammam, Saudi Arabia. On November 15, 2023, the RMS tran
Acute and chronic liver failure are conditions that carry a high mortality rate in both pediatric and adult populations. This study found that patients with end-stage liver disease in Bahrain shared comparable clinical characteristics to those published in reports from neighboring countries and worldwide. In a developing country like Bahrain, where LT facilities are not available, an overseas LT can offer great hope to patients with an end-stage liver disease, assuming the presence of a suitable donor. Greater attention must be made to identify patients at increased risk of developing liver failure and establishing strategies for early overseas LT is crucial. A multicenter prospective study is required to investigate the cost-effectiveness of the overseas LT in countries lacking this important facility.
Liver transplantation (LT) is a life-saving procedure for patients with end-stage liver disease and has become the standard and most effective way of treatment for these patients. There are many indications for LT that vary between countries and settings. The outcome of LT depends on the available facilities and surgical expertise, as well as the types of liver graft donors available.
Multiple reports about LT experiences have been published from several countries worldwide. However, there are no reports studying the details of patients from Bahrain who went overseas for LT. This gap of knowledge motivated us to study the experience of an overseas LT in our country.
To assess the clinical characteristics of patients from Bahrain who underwent LT overseas, and analyze factors affecting their survival.
We retrospectively reviewed the medical records and overseas committee registry information of all pediatric and adult patients who were sent overseas to undergo LT by the Pediatric and Medical Departments of Salmaniya Medical Complex and Bahrain Defence Force Hospital via the Overseas Treatment Office, Ministry of Health, Kingdom of Bahrain, between 1997 and 2023. Pediatric and adult patients were compared in terms of demographic data, LT indication, donor-recipient relationship, overseas LT center, graft type, post-LT medications, LT complications, and outcomes. Survival analysis was estimated, and predictors of survival were analyzed.
Up to August 2023, of the 208 listed patients, 170 (81.7%) were sent overseas to undergo LT. Of the latter, 167 (80.3%) underwent LT and were included. The majority were Bahraini (91.0%), and most were males (57.5%). One-hundred-and-twenty (71.8%) were adults and 47 (28.3%) were children. The median age at transplant was 50.0 [interquartile range (IQR): 14.9–58.4] years. The main indication for pediatric LT was biliary atresia (31.9%), while that of adult LT was hepatitis C-related cirrhosis (35.0%). Six (3.6%) patients required re-transplantation. Most patients received a living-related liver graft (82%). Pediatric patients received more living and related grafts than adults (P = 0.038 and P = 0.041, respectively), while adult patients received more cadaveric and unrelated grafts. Most patients required long-term immunosuppressive therapy after LT (94.7%), of which tacrolimus was the most prescribed (84.0%), followed by prednisolone (50.7%), which was prescribed more frequently for pediatric patients (P = 0.001). Most patients developed complications (62.4%) with infectious episodes being the most common (38.9%), followed by biliary stricture (19.5%). Tonsilitis and sepsis (n = 12, 8.1% for each) were the most frequent infections. Pediatric patients experienced higher rates of infection, rejection, and early poor graft function than adult patients (P < 0.001, P = 0.003, and P = 0.025, respectively). The median follow-up time was 6.5 (IQR: 2.6–10.6) years. The overall survival rate was 84.4%, the 5-year survival rate, 86.2%, and the mortality rate, 15.6%. Younger patients had significantly better odds of survival (P = 0.019) and patients who survived had significantly longer follow-up periods (P < 0.001).
Acute and chronic liver failure are conditions that carry a high mortality rate in both pediatric and adult populations. This study found that patients with end-stage liver disease in Bahrain shared comparable clinical characteristics to those published in reports from neighboring countries and worldwide. In a developing country like Bahrain, where LT facilities are not available, an overseas LT can offer great hope to patients with an end-stage liver disease, assuming the presence of a suitable donor.
Greater attention must be made to identify patients at increased risk of developing liver failure and establishing strategies for early overseas LT is crucial. A multicenter prospective study is required to investigate the cost-effectiveness of the overseas LT in countries lacking this important facility.
The authors gratefully acknowledge all medical staff in the Department of Pediatrics and the Department of Medicine, Salmaniya Medical Complex, Bahrain Defense Hospital, and the Overseas Treatment Office in the Ministry of Health, Manama, Kingdom of Bahrain for the care provided for liver transplant patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Al-Kawther Society for Social Care, 133; National Health Regulation Authority, 11002084.
Specialty type: Transplantation
Country/Territory of origin: Bahrain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Serafi I, United Arab Emirates; Ferrarese A, Italy; Shehta A, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Zhang YL
| 1. | Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, Moon JB, Blanchard H, Martin AJ Jr, Porter KA. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 441] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 2. | Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, Saab S, Han S, Durazo F, Weaver M, Cao C, Chen T, Lipshutz GS, Holt C, Gordon S, Gornbein J, Amersi F, Ghobrial RM. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg. 2005;241:905-16; discussion 916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 303] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 3. | Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, Pollard S, Lerut J, Paul A, Garcia-Valdecasas JC, Rodríguez FS, Burroughs A; All contributing centers (www. eltr.org); European Liver and Intestine Transplant Association (ELITA). Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 643] [Article Influence: 49.5] [Reference Citation Analysis (2)] |
| 4. | Kwong AJ, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Foutz J, Booker SE, Cafarella M, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2019 Annual Data Report: Liver. Am J Transplant. 2021;21 Suppl 2:208-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 259] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 5. | Basturk A, Yılmaz A, Sayar E, Dinçkan A, Aliosmanoğlu İ, Erbiş H, Aydınlı B, Artan R. Pediatric Liver Transplantation: Our Experiences. Eurasian J Med. 2016;48:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Choudhary NS, Bhangui P, Soin AS. Liver Transplant Outcomes in India. Clin Liver Dis (Hoboken). 2022;19:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Al MI, Abaalkhail FA, Bahili HA, Abdo AH, Elsiesy HA, Al MS, El Sheikh YM, Hegab BS, Kamel YM, AlGoufi TT, Hasssan HH, Burdelski MM, Al MA, Abdelfattah MR, Attallah KM, Mahmood TZ, Saleh YZ, Eldeen FZ, Broering DC. Liver transplantation at KFSHRC: achievement and challenges. Ann Saudi Med. 2014;34:103-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Khosravi B, Pourahmad S, Bahreini A, Nikeghbalian S, Mehrdad G. Five Years Survival of Patients After Liver Transplantation and Its Effective Factors by Neural Network and Cox Poroportional Hazard Regression Models. Hepat Mon. 2015;15:e25164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Haseli N, Hassanzadeh J, Dehghani SM, Bahador A, Malek Hosseini SA. Long-term survival and its related factors in pediatric liver transplant recipients of shiraz transplant center, shiraz, iran in 2012. Hepat Mon. 2013;13:e10257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Jamal M, AlMahmeed H, AlGhanem M, AlMatooq M, Sadek A, AlMousawi M, Al-Sabah S, Vilca Melendez H, Rela M, Heaton N, Jassem W. Organ Transplantation in Kuwait and the Recent Initiation of a Liver Program. Transplantation. 2021;105:2125-2127. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Al Adawi M, Al Harthi H, Al Hinai R, Al Haddabi S, Al Busaidi I, Al Siyabi O, Al Awaidy ST. Living-Donor Liver Transplant in Oman: A Quantitative Cross-Sectional Study of Donors' Experiences and Challenges. J Transplant. 2021;2021:4251814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Al-Sebayel M, Khalaf H, Al-Sofayan M, Al-Saghier M, Abdo A, Al-Bahili H, El-Sheikh Y, Helmy A, Medhat Y. Experience with 122 consecutive liver transplant procedures at King Faisal Specialist Hospital and Research Center. Ann Saudi Med. 2007;27:333-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Soyama A, Hara T, Matsushima H, Imamura H, Yamashita M, Adachi T, Miuma S, Miyaaki H, Nakao K, Eguchi S. Evolution of Liver Transplantation Over the Last 2 Decades Based on a Single-Center Experience of 300 Cases. Ann Transplant. 2023;28:e941796. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Eshraghian A, Imanieh MH, Dehghani SM, Nikeghbalian S, Shamsaeefar A, Barshans F, Kazemi K, Geramizadeh B, Malek-Hosseini SA. Post-transplant lymphoproliferative disorder after liver transplantation: Incidence, long-term survival and impact of serum tacrolimus level. World J Gastroenterol. 2017;23:1224-1232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Yuan D, Liu F, Wei YG, Li B, Yan LN, Wen TF, Zhao JC, Zeng Y, Chen KF. Adult-to-adult living donor liver transplantation for acute liver failure in China. World J Gastroenterol. 2012;18:7234-7241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Li QG, Wan P, Zhang JJ, Chen QM, Chen XS, Han LZ, Xia Q. Liver transplantation for biliary atresia: A single-center study from mainland China. World J Gastroenterol. 2015;21:9638-9647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Bruballa R, Sanchez Thomas D, de Santl'banes E, Ciardullo M, Mattera J, Pekolj J, de Santibanes M, Ardiles V. Liver Re-transplantation in Adults: Indications and Outcomes Analysis of a 23-year Experience in a Single Center in Argentina. Int J Organ Transplant Med. 2022;13:30-35. [PubMed] |
| 18. | Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res. 2008;32:1242-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Shaw JJ, Shah SA. Rising incidence and demographics of hepatocellular carcinoma in the USA: what does it mean? Expert Rev Gastroenterol Hepatol. 2011;5:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Kim JM, Kim KM, Yi NJ, Choe YH, Kim MS, Suh KS, Kim SI, Lee SK, Lee SG. Pediatric liver transplantation outcomes in Korea. J Korean Med Sci. 2013;28:42-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Byun J, Yi NJ, Lee JM, Suh SW, Yoo T, Choi Y, Ko JS, Seo JK, Kim H, Lee HW, Kim HY, Lee KW, Jung SE, Lee SC, Park KW, Suh KS. Long term outcomes of pediatric liver transplantation according to age. J Korean Med Sci. 2014;29:320-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Kim JS, Kim KM, Oh SH, Kim HJ, Cho JM, Yoo HW, Namgoong JM, Kim DY, Kim KH, Hwang S, Lee SG. Liver transplantation for metabolic liver disease: experience at a living donor dominant liver transplantation center. Pediatr Gastroenterol Hepatol Nutr. 2015;18:48-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Thammana RV, Knechtle SJ, Romero R, Heffron TG, Daniels CT, Patzer RE. Racial and socioeconomic disparities in pediatric and young adult liver transplant outcomes. Liver Transpl. 2014;20:100-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Alobaidi R, Anton N, Cave D, Moez EK, Joffe AR. Predicting early outcomes of liver transplantation in young children: The EARLY study. World J Hepatol. 2018;10:62-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 25. | Emre S, Umman V, Cimsit B, Rosencrantz R. Current concepts in pediatric liver transplantation. Mt Sinai J Med. 2012;79:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Ng VL, Alonso EM, Bucuvalas JC, Cohen G, Limbers CA, Varni JW, Mazariegos G, Magee J, McDiarmid SV, Anand R; Studies of Pediatric Liver Transplantation (SPLIT) Research Group. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: report of the studies of pediatric liver transplantation experience. J Pediatr. 2012;160:820-6.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 27. | Nishimura N, Kasahara M, Ishikura K, Nakagawa S. Current status of pediatric transplantation in Japan. J Intensive Care. 2017;5:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | McLin VA, Anand R, Daniels SR, Yin W, Alonso EM; SPLIT Research Group. Blood pressure elevation in long-term survivors of pediatric liver transplantation. Am J Transplant. 2012;12:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Daw MA, Dau AA. Hepatitis C virus in Arab world: a state of concern. ScientificWorldJournal. 2012;2012:719494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Ministry of Health of Kingdom of Bahrain. Basic Data on Infectious Diseases at Population Level. Bahrain; Ministry of Health. 2014. |
| 31. | Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824-7840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 514] [Cited by in RCA: 568] [Article Influence: 63.1] [Reference Citation Analysis (7)] |
| 32. | Abdulla MA, Al Qamish JR. Hepatitis C virus infection: a single center experience. Bahrain Medical Bulletin. 2008;30:3-8. |
| 33. | Umeshita K, Eguchi S, Egawa H, Haga H, Kasahara M, Kokudo N, Sakisaka S, Takada Y, Tanaka E, Eguchi H, Uemoto S, Ohdan H. Liver transplantation in Japan: Registry by the Japanese Liver Transplantation Society. Hepatol Res. 2019;49:964-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 34. | Kelly D. Safety and efficacy of tacrolimus in pediatric liver recipients. Pediatr Transplant. 2011;15:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Campbell KM, Yazigi N, Ryckman FC, Alonso M, Tiao G, Balistreri WF, Atherton H, Bucuvalas JC. High prevalence of renal dysfunction in long-term survivors after pediatric liver transplantation. J Pediatr. 2006;148:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Darweesh SK, Ibrahim MF, El-Tahawy MA. Effect of N-Acetylcysteine on Mortality and Liver Transplantation Rate in Non-Acetaminophen-Induced Acute Liver Failure: A Multicenter Study. Clin Drug Investig. 2017;37:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Jia D, Guo S, Jia Z, Gao Z, You K, Gong J, Li S. N-acetylcysteine in the Donor, Recipient, or Both Donor and Recipient in Liver Transplantation: A Systematic Review With Meta-analysis and Trial Sequential Analysis. Transplantation. 2023;107:1976-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Herlenius G, Fistouris J, Olausson M, Felldin M, Bäckman L, Friman S. Early renal function post-liver transplantation is predictive of progressive chronic kidney disease. Scand J Gastroenterol. 2008;43:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Tannuri U, Tannuri AC. Postoperative care in pediatric liver transplantation. Clinics (Sao Paulo). 2014;69 Suppl 1:42-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G; National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3156] [Cited by in RCA: 3212] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 41. | The daily tribune-news of Bahrain. SMC doctors may perform first liver transplant this year. The daily tribune-news of Bahrain. 19 Jan 2020. Cited 21 Nov 2023. Available from: https://www.zawya.com/en/Life/bahrain-prepares-for-first-liver-transplant-a9tujvfn. |
| 42. | Gulf daily news. Liver surgery success. GDN online. 16 Nov 2023. Cited 21 Nov 2023. Available from: https://www.gdnonline.com/Details/1295993. |