Published online Dec 18, 2023. doi: 10.5500/wjt.v13.i6.379
Peer-review started: September 22, 2023
First decision: October 24, 2023
Revised: November 1, 2023
Accepted: December 1, 2023
Article in press: December 1, 2023
Published online: December 18, 2023
Processing time: 87 Days and 4.8 Hours
Numerous reports have demonstrated that the pathophysiology of graft-versus-host disease (GVHD) during hematopoietic stem cell transplantation (HSCT) is closely related to vascular endothelial disorders and coagulation abnormalities. We previously presented the discovery of a principle and the development of a novel instrument for measuring whole blood coagulation. This was achieved by assessing the variations in the dielectric properties of whole blood.
To investigate how GVHD affects the changes of dielectric properties of whole blood in patients with HSCT.
We examined the changes of dielectric properties of whole blood and erythrocyte proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis sequentially in patients with HSCT and compared it with clinical symptoms and inflammatory parameters of GVHD.
During severe GVHD, the dielectric relaxation strength markedly increased and expression of band3 decreased. The dielectric relaxation strength normalized with the improvement of GVHD. In vitro analysis confirmed that the increase of relaxation strength was associated with severe erythrocyte aggregates, but not with decreased expression of band3.
Severe erythrocyte aggregates observed in GVHD may cause coagulation abnor
Core Tip: The pathophysiology of graft-versus-host disease (GVHD) is complex. Examination of changes in the dielectric properties of whole blood revealed that erythrocytes formed risky levels of rouleaux and aggregates in severe GVHD. In severe GVHD, oxidative stress causes degradation of erythrocyte band3 and truncation of the C-terminus of peroxiredoxin 2, resulting in decreased plasticity, increased fragility, and reduced oxygen-carrying capacity. These phenomena may underlie persistent refractory coagulopathy and circulation disorder, leading to organ damage in severe GVHD.
- Citation: Nagasawa M. Pathophysiology of acute graft-versus-host disease from the perspective of hemodynamics determined by dielectric analysis. World J Transplant 2023; 13(6): 379-390
- URL: https://www.wjgnet.com/2220-3230/full/v13/i6/379.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i6.379
The graft-versus-host (GVH) reaction is the most serious complication of hematopoietic stem cell transplantation (HSCT) and the most important reaction that eradicates malignant cells[1,2]. When the GVH reaction is excessively induced leading to organ dysfunction, it is designated as graft-versus-host disease (GVHD) and is a target for treatment. Moreover, GVHD progresses in three phases[3]. The first is cell damage due to pretreatment with anticancer drugs and radiation, which induces inflammatory reactions and the production of inflammatory cytokines. The second mechanism involves the recognition and activation of host antigens by transplanted immune cells. Inflammatory cytokines produced in the first phase promote the response in phase 2 by inducing an increased expression of alloantigens. In phase 3, immunocompetent cells activated in phase 2 attack the host cells, induce new inflammation and initiate a vicious cycle of excessive inflammatory responses. The treatment for GVHD involves suppressing and controlling activated immunocompetent cells induced in phases 2 and 3[3]. Uncontrolled and persistent GVHD leads to the insidious progression of severe coagulation disorders and microcirculation disturbances, resulting in irreversible organ failure and tissue damage directly induced by the attack of activated immunocompetent cells[4]. Early and persistent coagulopathy has been reported to be associated with HSCT prognosis[4].
Coagulation abnormalities and circulatory failure in the peripheral circulation are not always caused by GVHD after HSCT. In some cases, the main causes of coagulation disturbances stem from treatment with anticancer drugs, radiation, or vascular endothelial damage caused by calcineurin inhibitors, which are used as immunosuppressants to prevent GVHD[1,5,6]. Furthermore, cytomegalovirus infection/reactivation induced in an immunodeficient state may also be involved in the aforementioned disturbances[7,8]. Additionally, clinically determining whether coagulation abnormalities and vascular endothelial disorders that develop after HSCT are caused by GVHD is often difficult[9,10]. The assessment of peripheral blood circulation abnormalities and coagulation disorders following HSCT, specifically concerning pathological alterations in erythrocytes, has received limited attention in the context of GVHD.
The measurement of the complex dielectric properties of biomaterials at radio frequency has gained increasing importance not only in material science, microwave circuit design, and absorber development but also in biological research[11-13]. Dielectric measurement is important for providing the electrical or magnetic characteristics of materials in a noninvasive manner and has proven useful in many research and development fields. We performed dielectric measurements of the coagulation process in whole human blood, clarified the principle, and reported its usefulness as a new whole-blood coagulation measurement method[14,15]. Moreover, we examined the changes in the dielectric properties of whole blood after HSCT in 16 patients and discussed the relationship between the changes in the dielectric properties of whole blood and GVHD.
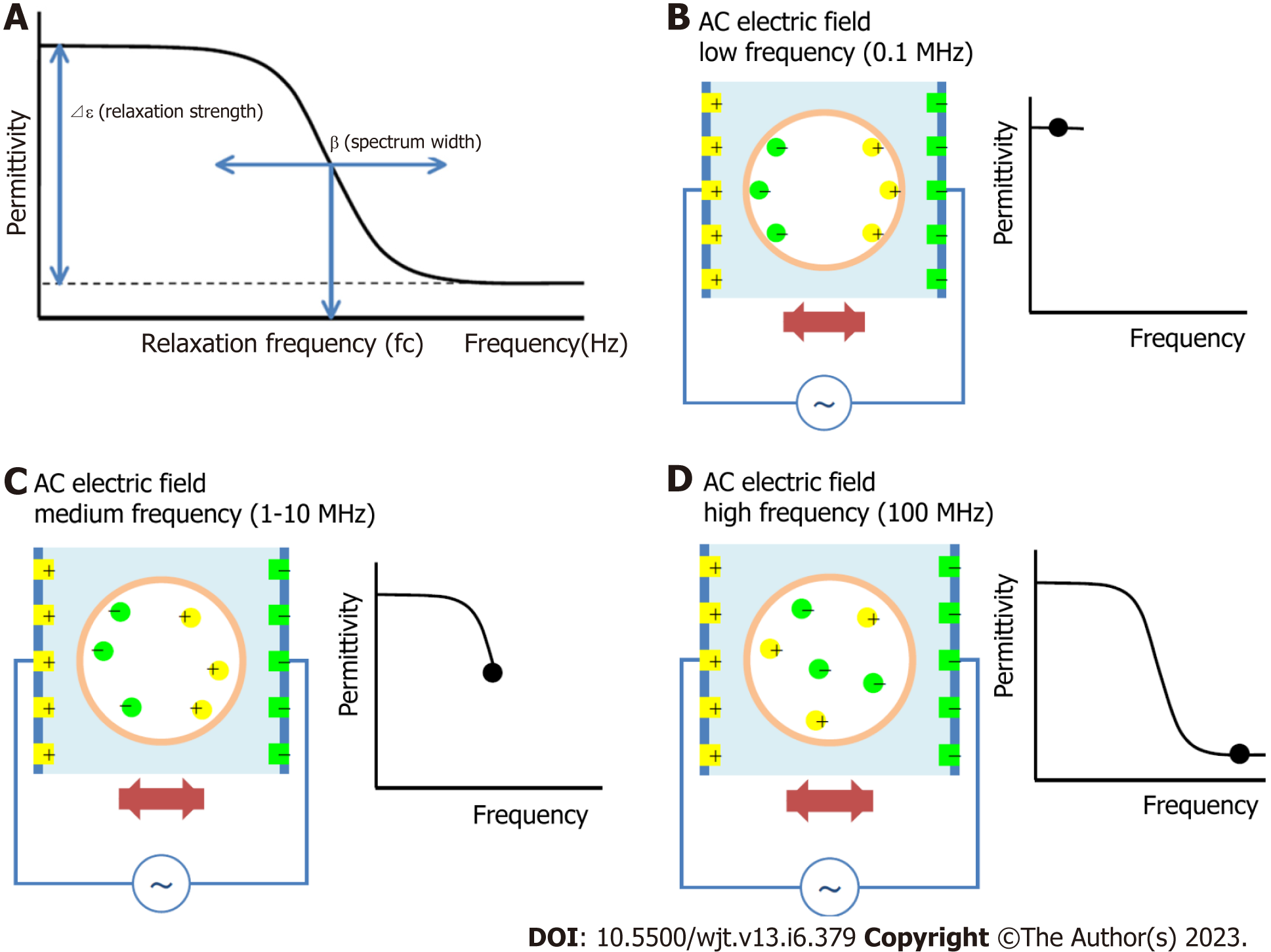
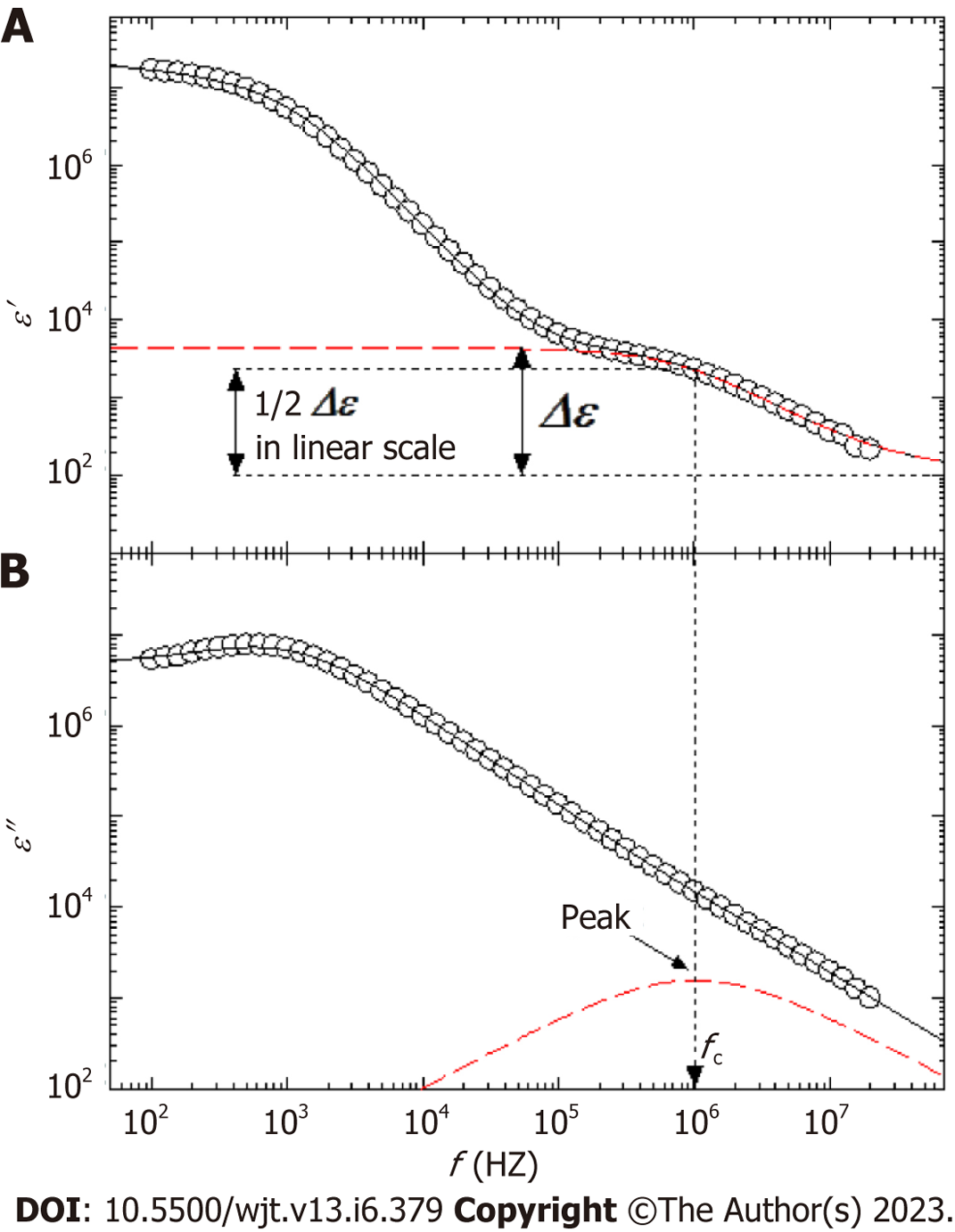
The principle of the dielectric measurement of whole blood is displayed in Figure 1A-D. Whole blood comprises plasma and blood cells (mainly erythrocytes), each of which is electrically charged. When an alternating electric field is applied to whole blood, sufficient ionization is achieved at low frequencies. However, as the frequency increases, ionization fails to keep pace sufficiently. Beyond a certain frequency, ionization is no longer possible and the dielectric constant does not change (Figure 1B-D). The dielectric properties of each material can be represented by the relaxation strength and frequency, as displayed in Figure 1A. The dielectric constant of whole blood was determined as the sum of the plasma (solvent) and blood cell components. As the electrode polarization of the solution component had a strong effect in the low-frequency region, the interfacial polarization of the blood cell component was calculated by correcting for this effect. Theoretically, the dielectric properties of blood cell components are considered to be significantly affected in the high-frequency range (Figure 2).
Venous blood was drawn in a blood collection tube containing Ethylenediamine-N, N, N', N'-tetra acetic acid dipotassium salt dihydrate (EDTA-2K·2H2O) and diluted to 10% hematocrit (Hct) with phosphate buffer saline (PBS). After 30 min of incubation at room temperature with gentle stirring, the dielectric properties were measured by our developed equipment. Diluted blood samples (200 L) were adjusted to 10% Hct using PBS and measured at 57 frequencies from 100 Hz to 40 MHz[14]. The measurement time for each frequency was < 0.2 s and the total frequency scan time for one dielectric relaxation measurement was 10 s. Measurements were performed within 1 h of blood sample collection.
Clinical samples were obtained from 16 patients who underwent allogeneic HSCT. The clinical information for each patient is summarized in Table 1. A portion (1 mL) of blood collected during routine clinical examinations performed 2–3 times per week after HSCT was used for the research. Information such as clinical symptoms and blood data were collected as anonymized information from medical records.
| Patient | Disease | Acute GVHD | Stage | Conditioning | Stem cell source | Decreased band3 | Truncated PRDX2 | ||
| No | Skin | Gut | Liver | ||||||
| 1 | AML | Ⅰ | 1 | 0 | 0 | MAC | URCB | No | No |
| 2 | HIM | 0 | 0 | 0 | 0 | RIC | URBMT | No | No |
| 3 | SCID | Ⅲ | 2 | 3 | 1 | RIC | URCB | Yes | Yes |
| 4 | SCID | Ⅲ | 1 | 3 | 1 | RIC | URCB | Yes | Yes |
| 5 | AML | Ⅰ | 1 | 0 | 0 | MAC | MSBMT | No | No |
| 6 | AML | Ⅱ | 3 | 0 | 0 | MAC | URBMT | No | No |
| 7 | DKC | Ⅲ | 2 | 3 | 1 | RIC | URBMT | Yes | Yes |
| 8 | ALL | 0 | 0 | 0 | 0 | MAC | URBMT | No | No |
| 9 | ALL | Ⅱ | 3 | 0 | 0 | MAC | URCB | No | No |
| 10 | ALL | Ⅱ | 3 | 0 | 0 | MAC | MSBMT | No | No |
| 11 | AML | Ⅰ | 1 | 0 | 0 | MAC | MSBMT | No | No |
| 12 | AML | Ⅲ | 1 | 3 | 1 | MAC | mis-RBMT | Yes | Yes |
| 13 | ALL | Ⅲ | 1 | 2 | 1 | MAC | URBMT | Yes | Yes |
| 14 | ALL | Ⅲ | 1 | 2 | 1 | MAC | URBMT | Yes | No |
| 15 | ALL | Ⅱ | 3 | 0 | 0 | MAC | mis-RBMT | No | No |
| 16 | SCN | Ⅰ | 1 | 0 | 0 | RIC | MSBMT | No | No |
Cells were washed with PBS and then lysed at 4 ℃ in a buffer containing 10 mmol/L Tris-hydrochloric acid, 50 mmol/L sodium chloride, 0.5% sodium deoxycholate, 0.2% SDS, 1% Nonidet P-40, 1 mmol/L phenylmethylsulfonyl fluoride, 50 mg/mL aprotinin, 50 mmol/L leupeptin, and 0.1 mmol/L sodium orthovanadate. After removing cellular debris by centrifugation, lysates were prepared for electrophoresis, and PAGE was performed as described previously[16]. Further, proteins separated on the gel were stained using silver staining.
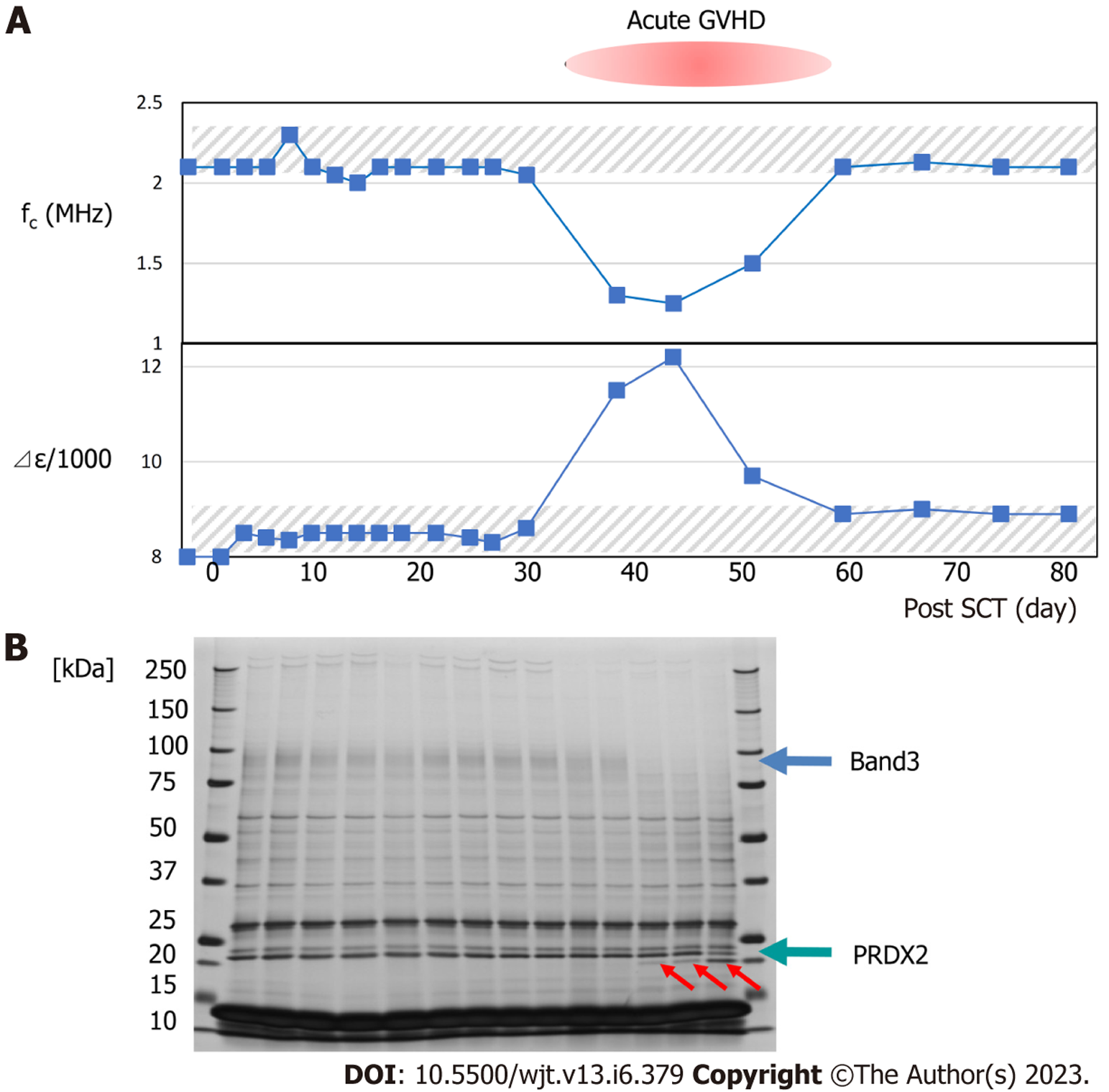
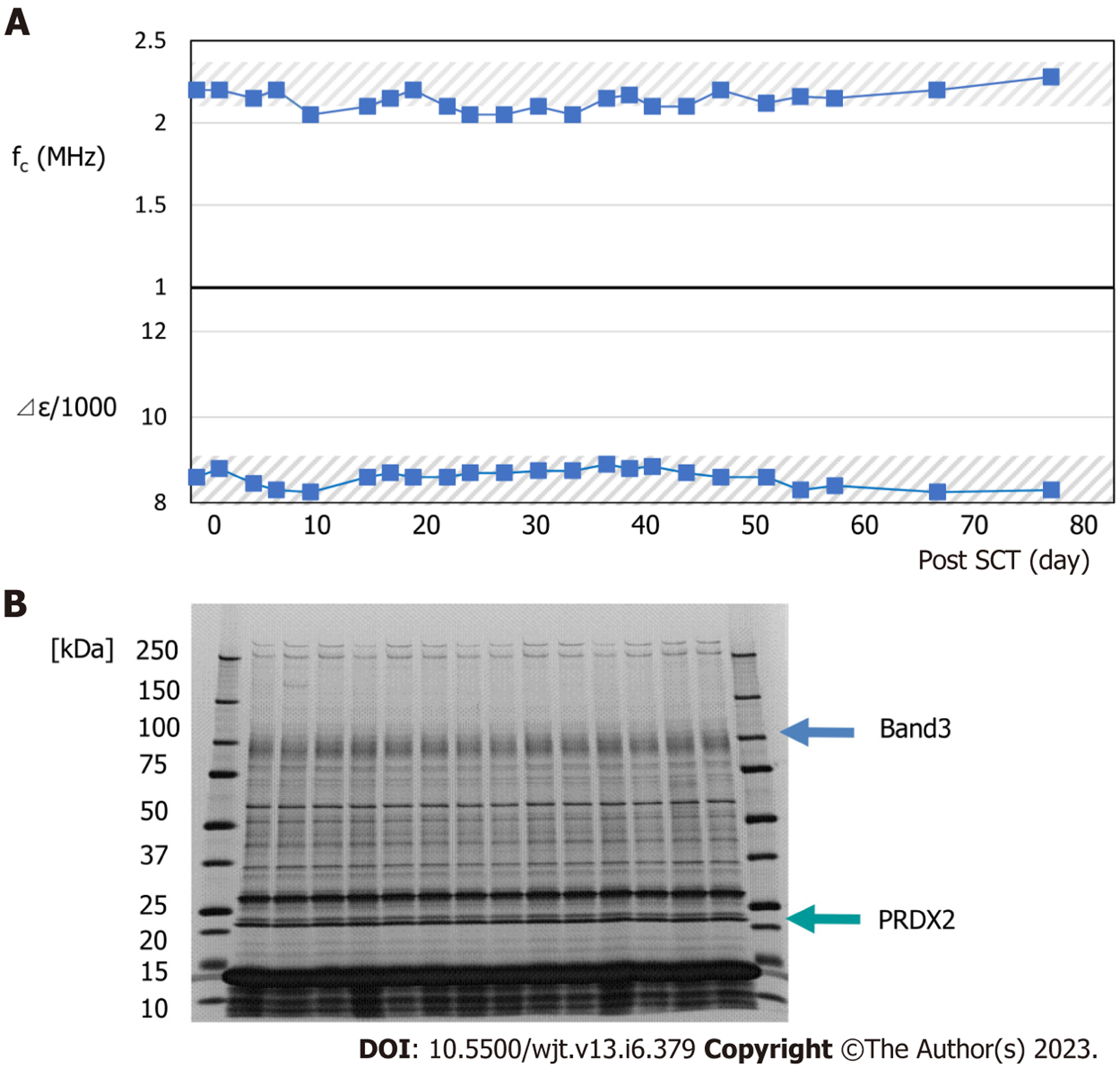
Typical changes in dielectric relaxation strength and frequency are depicted in patients who developed acute GVHD of grade 3 or more (patient 4) and those who did not (patient 2). During GVHD, a sharp increase in the dielectric relaxation strength and a decrease in the relaxation frequency intensity were observed (Figures 3 and 4). Overall, the dielectric relaxation strength and frequency changed in a complementary manner.
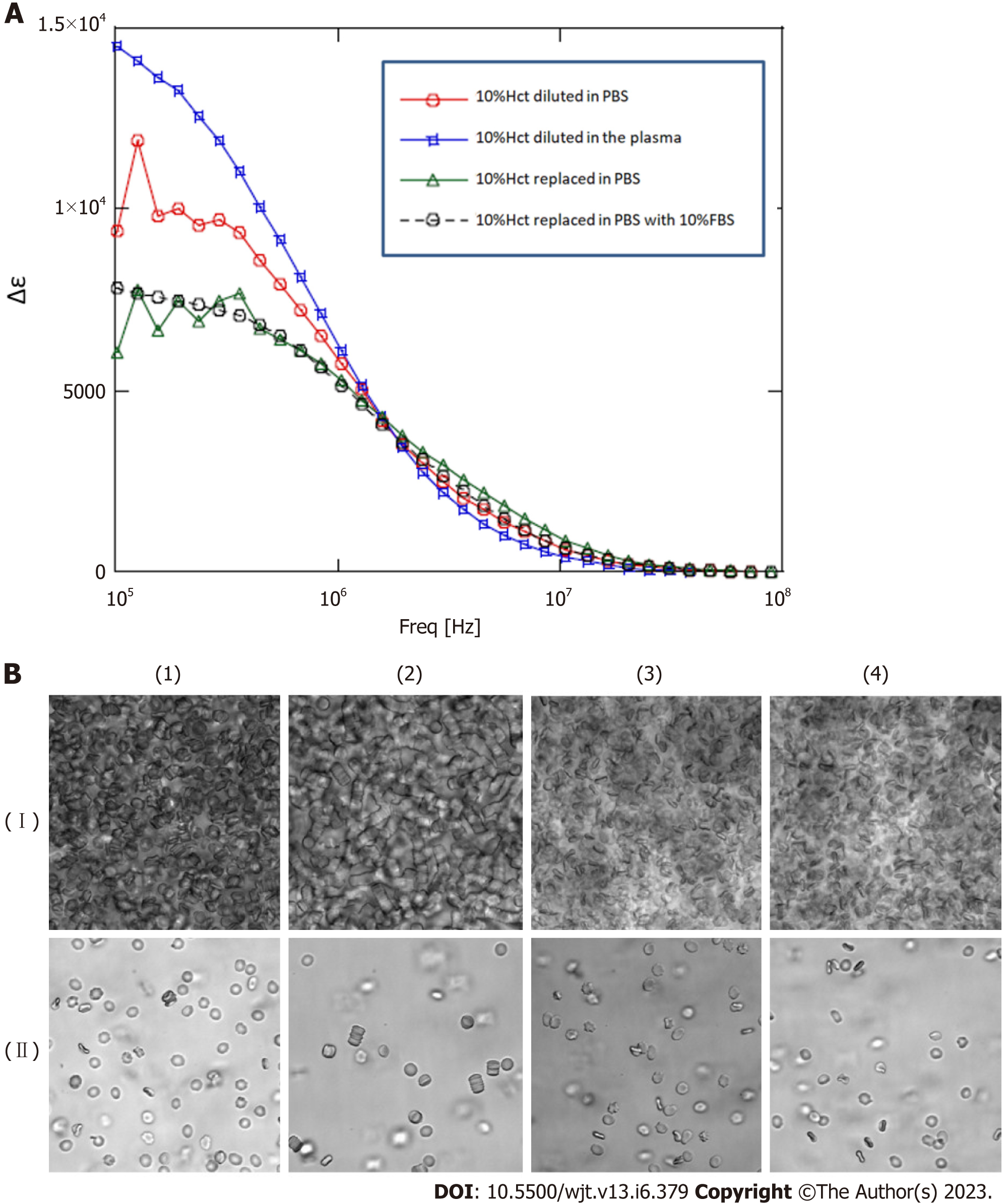
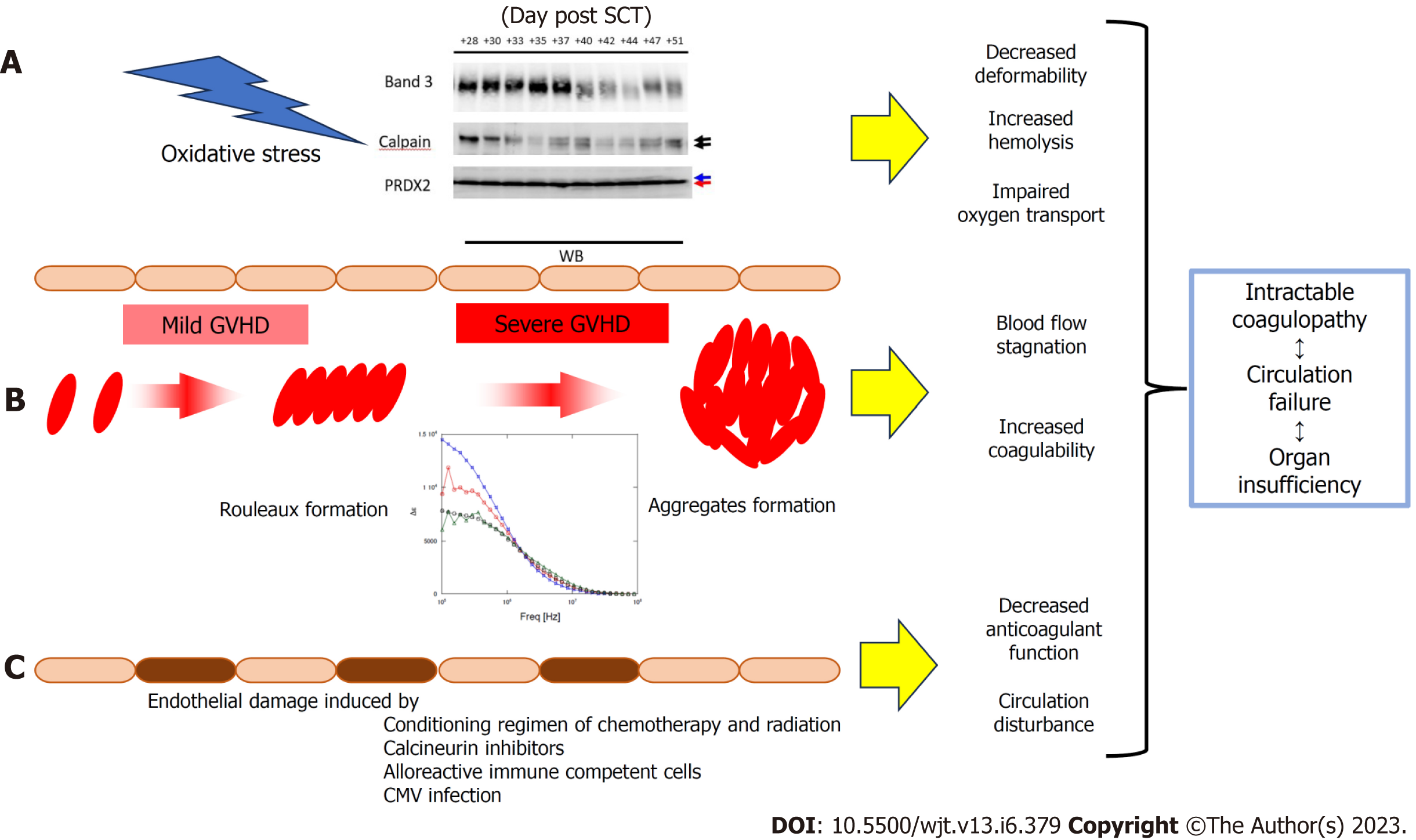
To investigate whether the change in dielectric properties was due to blood cells or plasma components, the expression of erythrocyte membrane proteins was analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). As demonstrated in Figure 3, a decrease in band 3 expression was observed in line with GVHD onset (Figure 3). Decreased expression of band 3 is one of the mechanisms of hereditary spherocytosis, in which erythrocytes are not normally concave-discoid-shaped, however, they morphologically change into spherocytes, exhibiting osmotic fragility. However, our previous study on dielectric relaxation frequency intensity changes in various erythrocyte morphologies displayed that the relaxation frequency increased during spherocytosis[12,17]. No significant changes in the dielectric properties of the plasma components were observed with or without GVHD (data not displayed). Therefore, we conducted a replacement experiment for the blood cells and plasma components. As demonstrated in Figure 5, the relaxation strength depends on the plasma components (Figure 5A). Observation of erythrocyte states under each condition using a phase-contrast microscope revealed the formation of rouleaux or aggregates in the presence of plasma components during the progression of GVHD (Figure 5B). Although the data are not displayed, rouleaux formation of erythrocytes was observed at a high frequency in the blood, in which the dielectric relaxation strength increased after HSCT.
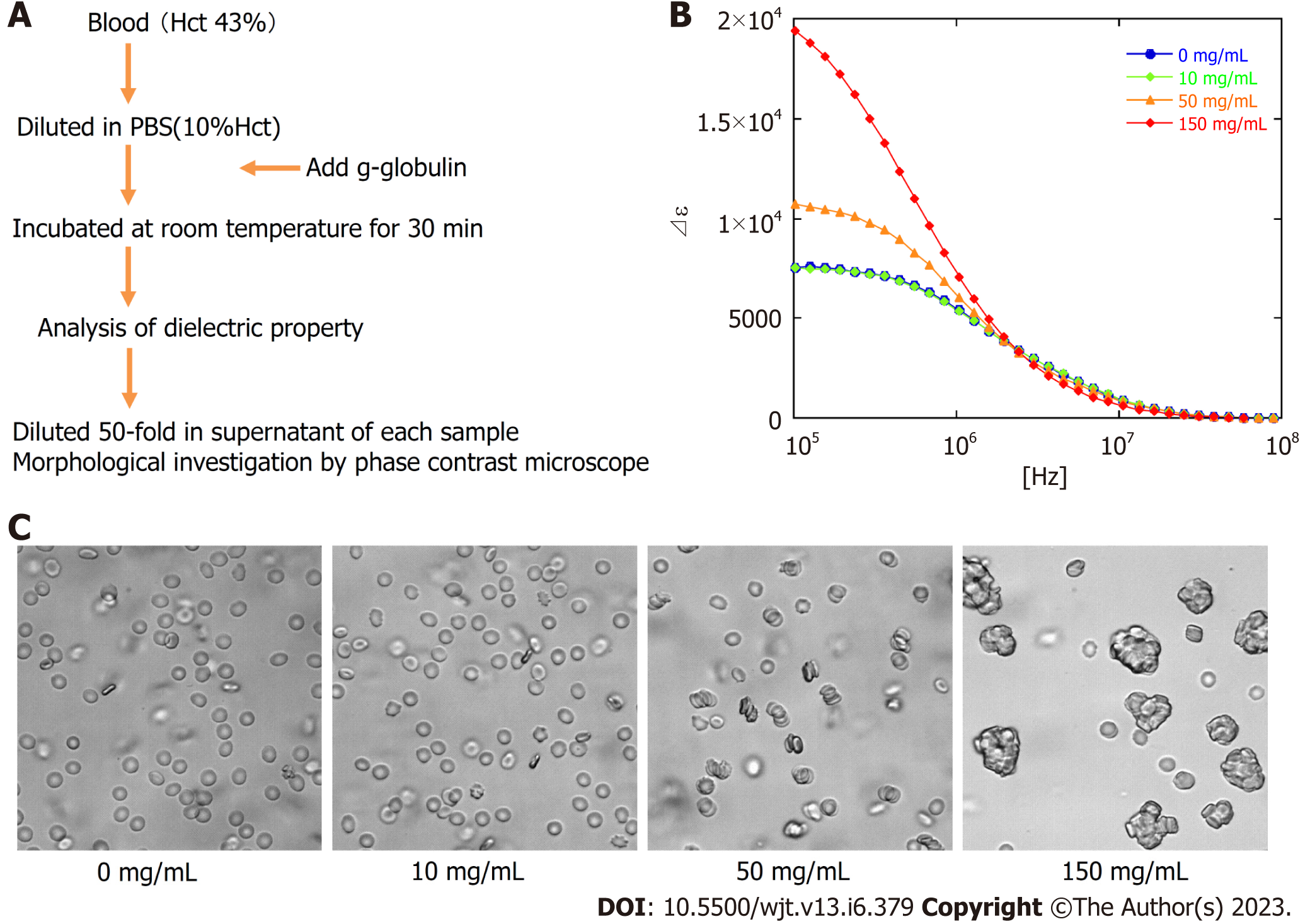
Next, to estimate the quantitative relationship between the dielectric relaxation strength change and the rouleaux formation of erythrocytes, experiments with the addition of gamma (γ)-globulin were carried out. Blood obtained from healthy individuals was washed with PBS, a 10% Hct erythrocyte suspension solution was created, and then γ-globulin was added to measure the dielectric properties and confirm the morphology at several concentration settings. The serum concentration of γ-globulin in healthy individuals is considered to be approximately 10 mg/mL, but little change was observed in dielectric relaxation strength and morphology under conditions between 0 mg/mL and 10 mg/mL of g-globulin. A rouleaux formation of erythrocytes was observed at a g-globulin concentration of 50 mg/mL, and large erythrocyte aggregates were observed at a concentration of 150 mg/mL. For dielectric relaxation measurement, the patient’s whole blood was diluted to approximately 1/3 in PBS to match 10% Hct. The observed rate of change in the dielectric constant in our study suggests that the acute phase of grade 3 or more GVHD was comparable to a state where the γ-globulin concentration exceeded 100 mg/mL, which is ten-fold higher than the normal value (Figure 6) (comparing with the data displayed in Figure 5A).
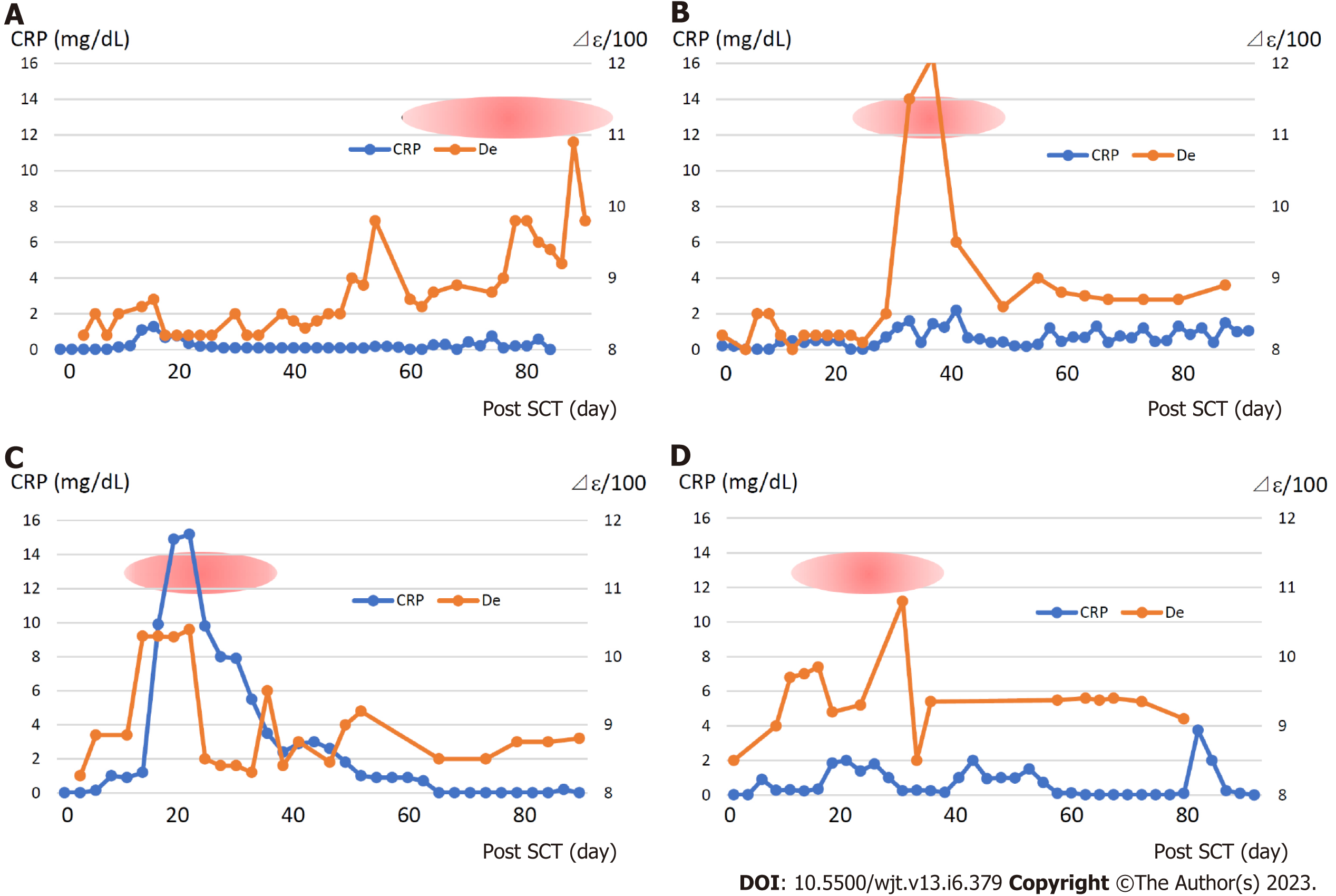
Figure 7 displays the changes in the dielectric relaxation strength and C-reactive protein (CRP), a biomarker of inflammation, in patients who developed grade 3 or more of GVHD. The dielectric relaxation strength and CRP levels demonstrated a correlation in some cases, but they did not necessarily match. In addition, the dielectric relaxation strength may fluctuate significantly, even with a slight change in the CRP. Interestingly, the dielectric relaxation strength increased gradually with minimal CRP changes in patient 3, who developed severe gastrointestinal GVHD. This indicates that the dielectric relaxation strength is affected by persistent or chronic inflammatory reactions even if the CRP level is elevated.
Erythrocytes account for two-thirds of all human cells. They are constantly produced in the bone marrow of hematopoietic stem cells and supplied to the blood[18]. The main role of erythrocytes is to transport oxygen to every corner of the body; therefore, the cell has an optimal structure for efficiently transporting oxygen. Erythrocytes are often subjected to oxidative stress due to their role in transporting high concentrations of oxygen and therefore have a strong scavenger function against reactive oxygen species[19-21]. A typical example is peroxiredoxin 2 (PRDX2), which is present in high concentrations in red blood cells and is maintained by the pentose phosphate pathway and glutathione system[22]. A concave discoid-like shape without a nucleus is the optimal structure that can efficiently take in oxygen and move inside the capillaries to every corner of the tissue with strong deformability[23]. Furthermore, erythrocytes have a strong buffering capacity to maintain the acid-base balance in body fluids[24] and abundant scavenger functions against oxidative stress[19-21].
On the other hand, the coagulation system is known to be phylogenetically closely related to inflammatory and immune responses[25,26]. The system is considered to have evolved as a biological defense mechanism that not only stops bleeding but also traps pathogens and prevents them from spreading to the surrounding area. In vivo, the coagulation system is initially considered to be activated on the cell surface[27], and evidence indicates that stagnation of blood flow can be a trigger[28].
Based on the principle of our newly developed in vitro whole blood coagulation measurement system utilizing whole blood permittivity measurements, this process can be divided into three stages[14]. First, an increase was observed in relaxation strength due to rouleaux formation, followed by a further increase in relaxation strength due to aggregation. Additionally, a rapid decrease in relaxation strength due to changes in erythrocyte morphology and tertiary structure caused by platelet secondary aggregation and clot retraction due to fibrin formation and polymerization was also observed. This result suggests the importance of erythrocyte rouleaux and aggregate formation as an initial reaction or precursor state for the activation of the coagulation system.
As is well known, hypergammaglobulinemia induces rouleaux formation by neutralizing the negative charge on the surface of erythrocytes. Venous thrombosis frequently occurs in multiple myeloma presenting with pathological hypergammaglobulinemia[29,30]. Many reports of thrombosis, likely due to high-dose g-globulin therapy[31-33]. The high-viscosity state caused by rouleaux formation causes stagnation of blood flow and interacts with tissue factors produced by GVHD-induced inflammatory reactions to lower the threshold of initial coagulation activation. According to this study, the change in whole blood dielectric constant in acute GVHD was considered equivalent to the change in hypergammaglobulinemia from 10 to 15 mg/mL, which is a high-risk level that progresses to thrombus formation and renal failure when replaced with multiple myeloma patients.
On the other hand, oxidative stress has been reported to be enhanced in acute GVHD[34,35], in addition to the oxidative stress induced by conditioning chemotherapy and radiation therapy[36]. We recently reported that calpain activated by oxidative stress may cause the degradation of band 3 and PRDX2[37]. Degradation of band 3 causes a decrease in erythrocyte plasticity, making the cell more susceptible to hemolysis[38,39]. The degradation of PRDX2 irreversibly disrupts the scavenging function of erythrocytes and reduces the resistance of the cell against oxidative stress[40-42]. Phosphatidylserine, which appears on the cell surface due to abnormal erythrocyte membranes, acts as a procoagulant[43]. Free hemoglobin released by hemolysis scavenges nitrogen oxide (NO)[26], which has a vasodilatory effect, and the released erythrocyte arginase reduces NO production[44]. These pathological conditions overlap to form a vicious circle, and it is thought that the progression of microcirculatory failure and the accompanying organ failure result in an irreversible state leading to fatality.
The differences between rouleaux formation, which is indicated by changes in dielectric relaxation strength, and the erythrocyte sedimentation rate (ESR), which has been classically used as a biomarker of inflammation, could not be examined in detail in this study. Moreover, ESR requires 30–60 min or more for measurement, whereas dielectric properties can be measured within 10 s, and it is believed that the condition of erythrocytes can be accurately depicted with minimal external influences during the assay. Furthermore, ESR is considered to be affected by many factors other than changes in the dielectric relaxation strength and does not necessarily reflect the same factors. Additionally, ESR is also affected by anemia and polycythemia. In this regard, our previous study on Hct and dielectric relaxation strength identified an increase in dielectric relaxation strength with increasing Hct[45]. An increase in Hct results in a decrease in ESR, which indicates that the phenomena of an increase in the dielectric relaxation strength and enhancement of ESR are not necessarily the same.
As demonstrated in Figure 3, when the dielectric relaxation strength was higher than a certain level, a decrease in band 3 of red blood cells and fragmentation of the C-terminus of PRDX2 were observed, possibly due to oxidative stress-induced calpain activation[37]. In a study of 16 transplant patients, these changes were observed only in cases of severe GVHD with grade 3 or higher, and not in grade 1–2 GVHD[37]. This suggests that changes in the intensity of the total blood dielectric relaxation after HSCT reflect the severity of GVHD and oxidative stress. By comprehensively considering the results of this study and the principle of dielectric coagulation measurement, a conclusion can be reached that the dielectric relaxation strength sensitively reflects the distribution pattern of blood cells in the solvent and their shape change[14]. Based on clinical data and in vitro experiments, the stage from rouleux to aggregate formation may be an indicator of serious illness. From this perspective, the experimental results displayed in Figure 6 provide important information for the quantitative interpretation of the dielectric relaxation strength. Figure 8 displays a schematic illustration of the mechanism of circulatory and coagulopathy development in GVHD after HSCT, as proposed in this study, of changes in the dielectric relaxation strength and membrane protein changes in erythrocytes.
In this study, we could not compare various previously reported GVHD biomarkers with changes in the dielectric properties of whole blood. This report does not indicate that changes in the dielectric properties of blood are superior to those in previously reported GVHD biomarkers[46]. The changes in the dielectric properties of blood are not specific to GVHD. A modification in the dielectric properties of blood is believed to arise due to conditions that induce alterations in plasma composition, fostering the development of erythrocyte aggregations. One of these includes classically recognized acute inflammatory alterations, and as previously mentioned, the parameter known as ESR is believed to indirectly mirror similar phenomena. The pathway by which severe GVHD leads to irreversible organ failure is not necessarily limited to direct organ damage caused by the GVH reaction through the alloimmune response; secondary and latent circulatory disorders are also involved. From this perspective, we believe that the changes in the dielectric properties of blood examined in this study provide a new perspective for understanding and managing GVHD.
The pathological significance of the dynamic changes in blood dielectric relaxation strength in acute GVHD identified in this study requires further investigation. In the future, we believe that more detailed quantitative analysis of dielectric relaxation strength and consideration of its relationship with other clinical parameters will be necessary. Furthermore, the clinical usefulness of dielectric relaxation strength as an interesting and unique biomarker as well as a target for therapeutic intervention should be duly considered.
We previously presented the discovery of a principle and the development of a novel instrument for measuring whole blood coagulation. This was achieved by assessing the variations in the dielectric properties of whole blood.
This assay of dielectric properties of whole blood may be useful for evaluation of coagulation abnormalities observed in graft-versus-host disease (GVHD).
To investigate how GVHD affects the changes of dielectric properties of whole blood in patients with hematopoietic stem cell transplantation (HSCT) and pathological significance of dielectric properties of whole blood in GVHD.
We examined the changes of dielectric properties of whole blood and erythrocyte proteins by sodium dodecyl sulfate-polyacrylamide electrophoresis sequentially in patients with HSCT and compared it with clinical symptoms and inflammatory parameters of GVHD.
During severe GVHD, the dielectric relaxation strength markedly increased and expression of band3 decreased. The dielectric relaxation strength normalized with the improvement of GVHD. In vitro analysis confirmed that the increase of relaxation strength was associated with severe erythrocyte aggregates, but not with decreased expression of band3.
Severe erythrocyte aggregates observed in GVHD may cause coagulation abnormalities and circulatory failure, which, together with the irreversible erythrocyte dysfunction we recently reported, could lead to organ failure.
The pathological significance of the dynamic changes in blood dielectric relaxation strength in acute GVHD identified in this study requires further investigation. Furthermore, the clinical usefulness of dielectric relaxation strength as an interesting and unique biomarker as well as a target for therapeutic intervention should be duly considered.
I thank Tomohiro Hayakawa, Yoichi Katsumoto, Yoshihide Hayashi, and Shinji Omori (LOC Development Department, R&D Division, Medical Business Unit, Sony Corporation, Japan) for their help in the dielectric analysis of whole blood and the analysis of erythrocyte proteins, and pediatric SCT team members of Tokyo Medical and Dental University for providing blood samples.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Taheri S, Iran; Gonzalez FM, Chile S-Editor: Liu JH L-Editor: A P-Editor: Zhang YL
| 1. | Woywodt A, Haubitz M, Buchholz S, Hertenstein B. Counting the cost: markers of endothelial damage in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;34:1015-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Gale RP, Butturini A. Bone marrow transplantation in acute lymphoblastic leukemia. Cancer Treat Res. 1990;50:223-233. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1987] [Cited by in RCA: 1841] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 4. | Nagasawa M, Ohkawa T, Endo A, Mitsuiki N, Ono T, Aoki Y, Isoda T, Tomizawa D, Takagi M, Kajiwara M, Morio T, Mizutani S. Early coagulation disorder after allogeneic stem cell transplantation is a strong prognostic factor for transplantation-related mortality, and intervention with recombinant human thrombomodulin improves the outcome: a single-center experience. Int J Hematol. 2013;98:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Nickel T, Schlichting CL, Weis M. Drugs modulating endothelial function after transplantation. Transplantation. 2006;82:S41-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Gavriilaki E, Sakellari I, Anagnostopoulos A, Brodsky RA. Transplant-associated thrombotic microangiopathy: opening Pandora's box. Bone Marrow Transplant. 2017;52:1355-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | van Burik JA, Lawatsch EJ, DeFor TE, Weisdorf DJ. Cytomegalovirus enteritis among hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2001;7:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Matsuda K, Ono S, Ishikawa M, Miyamoto S, Abiko S, Tsuda M, Yamamoto K, Kudo T, Shimizu Y, Hayase E, Hashimoto D, Teshima T, Matsuno Y, Sakamoto N. Cecum ulcer is a reliable endoscopic finding in cytomegalovirus colitis concomitant with graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2018;97:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Inamoto Y, Ito M, Suzuki R, Nishida T, Iida H, Kohno A, Sawa M, Murata M, Nishiwaki S, Oba T, Yanada M, Naoe T, Ichihashi R, Fujino M, Yamaguchi T, Morishita Y, Hirabayashi N, Kodera Y, Miyamura K; Nagoya Blood and Marrow Transplantation Group. Clinicopathological manifestations and treatment of intestinal transplant-associated microangiopathy. Bone Marrow Transplant. 2009;44:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Wong NA. Gastrointestinal pathology in transplant patients. Histopathology. 2015;66:467-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Hosokawa M, Hayata T, Fukuda Y, Arakaki A, Yoshino T, Tanaka T, Matsunaga T. Size-selective microcavity array for rapid and efficient detection of circulating tumor cells. Anal Chem. 2010;82:6629-6635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 12. | Hayashi Y, Oshige I, Katsumoto Y, Omori S, Yasuda A, Asami K. Dielectric inspection of erythrocyte morphology. Phys Med Biol. 2008;53:2553-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Katsumoto Y, Omori S, Sato K, Umetsu T, Brun MA, Soma H, Hayakawa T, Lee SM, Sakai K, Hayashi Y, Tasuda A, Nagasawa M, Morio T, Mizutani S. Single cell dielectric spectroscopy in a micro-channel 15th International Conference on Miniaturized Systems for Chemistry and Life Scieneces, 2011; 613-616. [DOI] [Full Text] |
| 14. | Hayashi Y, Brun MA, Machida K, Nagasawa M. Principles of dielectric blood coagulometry as a comprehensive coagulation test. Anal Chem. 2015;87:10072-10079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Hayashi Y, Katsumoto Y, Omori S, Yasuda A, Asami K, Kaibara M, Uchimura I. Dielectric coagulometry: a new approach to estimate venous thrombosis risk. Anal Chem. 2010;82:9769-9774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Terada N, Lucas JJ, Gelfand EW. Differential regulation of the tumor suppressor molecules, retinoblastoma susceptibility gene product (Rb) and p53, during cell cycle progression of normal human T cells. J Immunol. 1991;147:698-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 17. | Hayashi Y, Katsumoto Y, Oshige I, Omori S, Yasuda A, Asami K. The effects of erythrocyte deformability upon hematocrit assessed by the conductance method. Phys Med Biol. 2009;54:2395-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Corrons JLV, Casafont LB, Frasnedo EF. Concise review: how do red blood cells born, live, and die? Ann Hematol. 2021;100:2425-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 19. | Gell DA. Structure and function of haemoglobins. Blood Cells Mol Dis. 2018;70:13-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 20. | Sundd P, Gladwin MT, Novelli EM. Pathophysiology of Sickle Cell Disease. Annu Rev Pathol. 2019;14:263-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 419] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 21. | Bettiol A, Galora S, Argento FR, Fini E, Emmi G, Mattioli I, Bagni G, Fiorillo C, Becatti M. Erythrocyte oxidative stress and thrombosis. Expert Rev Mol Med. 2022;24:e31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 22. | Low FM, Hampton MB, Peskin AV, Winterbourn CC. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood. 2007;109:2611-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 233] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Risinger M, Kalfa TA. Red cell membrane disorders: structure meets function. Blood. 2020;136:1250-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, Johnson DE, Casey JR. The bicarbonate transport metabolon. J Enzyme Inhib Med Chem. 2004;19:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Iba T, Hashiguchi N, Nagaoka I, Tabe Y, Murai M. Neutrophil cell death in response to infection and its relation to coagulation. J Intensive Care. 2013;1:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Luo S, Hu D, Wang M, Zipfel PF, Hu Y. Complement in Hemolysis- and Thrombosis- Related Diseases. Front Immunol. 2020;11:1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Hoffman M. A cell-based model of coagulation and the role of factor VIIa. Blood Rev. 2003;17 Suppl 1:S1-S5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 189] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Pifarré R. Thrombosis and cardiovascular disease. Med Clin North Am. 1998;82:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | van de Donk NWCJ, Pawlyn C, Yong KL. Multiple myeloma. Lancet. 2021;397:410-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 494] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 30. | Covut F, Ahmed R, Chawla S, Ricaurte F, Samaras CJ, Anwer F, Garcia AVM, Angelini DE, Mazzoni S, Faiman B, Valent J, Khouri J. Validation of the IMPEDE VTE score for prediction of venous thromboembolism in multiple myeloma: a retrospective cohort study. Br J Haematol. 2021;193:1213-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Zaidan R, Al Moallem M, Wani BA, Shameena AR, Al Tahan AR, Daif AK, Al Rajeh S. Thrombosis complicating high dose intravenous immunoglobulin: report of three cases and review of the literature. Eur J Neurol. 2003;10:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Marie I, Maurey G, Hervé F, Hellot MF, Levesque H. Intravenous immunoglobulin-associated arterial and venous thrombosis; report of a series and review of the literature. Br J Dermatol. 2006;155:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Lorenzana A, Armin S, Sharma A, Allarakhia I, Witkowski A. Cerebral infarctions after intravenous immunoglobulin therapy for ITP in a child. Pediatr Neurol. 2014;50:188-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Sari I, Cetin A, Kaynar L, Saraymen R, Hacioglu SK, Ozturk A, Kocyigit I, Altuntas F, Eser B. Disturbance of pro-oxidative/antioxidative balance in allogeneic peripheral blood stem cell transplantation. Ann Clin Lab Sci. 2008;38:120-125. [PubMed] |
| 35. | Amer J, Weiss L, Reich S, Shapira MY, Slavin S, Fibach E. The oxidative status of blood cells in a murine model of graft-versus-host disease. Ann Hematol. 2007;86:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Sabuncuoğlu S, Kuşkonmaz B, Uckun Çetinkaya D, Ozgüneş H. Evaluation of oxidative and antioxidative parameters in pediatric hematopoietic SCT patients. Bone Marrow Transplant. 2012;47:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Nagasawa M. Degradation of band3 and PRDX2 in erythrocytes during severe acute GVHD. EJHaem. 2023;4:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 38. | Lang F, Abed M, Lang E, Föller M. Oxidative stress and suicidal erythrocyte death. Antioxid Redox Signal. 2014;21:138-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127:750-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 484] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 40. | Jara M, Vivancos AP, Hidalgo E. C-terminal truncation of the peroxiredoxin Tpx1 decreases its sensitivity for hydrogen peroxide without compromising its role in signal transduction. Genes Cells. 2008;13:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Silva-Herdade AS, Andolina G, Faggio C, Calado Â, Saldanha C. Erythrocyte deformability - A partner of the inflammatory response. Microvasc Res. 2016;107:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Koo KH, Lee S, Jeong SY, Kim ET, Kim HJ, Kim K, Song K, Chae HZ. Regulation of thioredoxin peroxidase activity by C-terminal truncation. Arch Biochem Biophys. 2002;397:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Wan J, Konings J, de Laat B, Hackeng TM, Roest M. Added Value of Blood Cells in Thrombin Generation Testing. Thromb Haemost. 2021;121:1574-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Caldwell RW, Rodriguez PC, Toque HA, Narayanan SP, Caldwell RB. Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiol Rev. 2018;98:641-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 318] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 45. | Hayashi Y, Brun MA, Machida K, Lee S, Murata A, Omori S, Uchiyama H, Inoue Y, Kudo T, Toyofuku T, Nagasawa M, Uchimura I, Nakamura T, Muneta T. Simultaneous assessment of blood coagulation and hematocrit levels in dielectric blood coagulometry. Biorheology. 2017;54:25-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Nagasawa M. Biomarkers of graft-vs-host disease: Understanding and applications for the future. World J Transplant. 2021;11:335-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (2)] |