Published online Dec 18, 2023. doi: 10.5500/wjt.v13.i6.357
Peer-review started: August 25, 2023
First decision: September 14, 2023
Revised: October 14, 2023
Accepted: November 28, 2023
Article in press: November 28, 2023
Published online: December 18, 2023
Processing time: 114 Days and 13.9 Hours
Early hospital readmissions (EHRs) after kidney transplantation range in incidence from 18%-47% and are important and substantial healthcare quality indicators. EHR can adversely impact clinical outcomes such as graft function and patient mortality as well as healthcare costs. EHRs have been extensively studied in American healthcare systems, but these associations have not been explored within a Canadian setting. Due to significant differences in the delivery of healthcare and patient outcomes, results from American studies cannot be readily applicable to Canadian populations. A better understanding of EHR can facilitate improved discharge planning and long-term outpatient management post kidney transplant.
To explore the burden of EHR on kidney transplant recipients (KTRs) and the Canadian healthcare system in a large transplant centre.
This single centre cohort study included 1564 KTRs recruited from January 1, 2009 to December 31, 2017, with a 1-year follow-up. We defined EHR as hospitalizations within 30 d or 90 d of transplant discharge, excluding elective procedures. Multivariable Cox and linear regression models were used to examine EHR, late hospital readmissions (defined as hospitalizations within 31-365 d for 30-d EHR and within 91-365 d for 90-d EHR), and outcomes including graft function and patient mortality.
In this study, 307 (22.4%) and 394 (29.6%) KTRs had 30-d and 90-d EHRs, respectively. Factors such as having previous cases of rejection, being transplanted in more recent years, having a longer duration of dialysis pretransplant, and having an expanded criteria donor were associated with EHR post-transplant. The cumulative probability of death censored graft failure, as well as total graft failure, was higher among the 90-d EHR group as compared to patients with no EHR. While multivariable models found no significant association between EHR and patient mortality, patients with EHR were at an increased risk of late hospital readmissions, poorer kidney function throughout the 1st year post-transplant, and higher hospital-based care costs within the 1st year of follow-up.
EHRs are associated with suboptimal outcomes after kidney transplant and increased financial burden on the healthcare system. The results warrant the need for effective strategies to reduce post-transplant EHR.
Core Tip: Early hospital readmissions post-transplant are associated with suboptimal patient outcomes and increased financial burden on the healthcare system. The 90-d window for defining early hospital readmissions, in addition to the frequently used 30-d period, provides a novel opportunity to evaluate the risks for kidney transplant recipients.
- Citation: Famure O, Kim ED, Li Y, Huang JW, Zyla R, Au M, Chen PX, Sultan H, Ashwin M, Minkovich M, Kim SJ. Outcomes of early hospital readmission after kidney transplantation: Perspectives from a Canadian transplant centre. World J Transplant 2023; 13(6): 357-367
- URL: https://www.wjgnet.com/2220-3230/full/v13/i6/357.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i6.357
Kidney transplantation is widely accepted as the best treatment option for the majority of patients with end-stage renal disease[1]; however, it carries a risk of complications and subsequent hospital readmissions in the post-transplant period[2]. Early hospital readmissions (EHRs), commonly defined as any new hospitalization occurring within 30 d after initial transplant discharge, is an indicator of healthcare quality and an important outcome measure after transplantation[2,3]. In the United States, approximately 30% of kidney transplant recipients (KTRs) have EHR, with rates ranging from 18% to 47% between transplant centres[4,5]. More recently, a single-centre Brazilian study reported an EHR incidence of 27% among 1175 KTRs from 2011 to 2012[3], while a population-based Canadian study reported a cumulative EHR incidence of 21% among 5437 KTRs from 2002 to 2014[2].
The relatively high incidence of post-transplant EHR is concerning since EHRs have been associated with a severe reduction in health status and substantial healthcare costs. Several kidney transplant studies observed an increased risk of graft failure, patient mortality, and suboptimal graft function with EHR[6-10]. EHRs were also associated with more late hospital readmissions (LHRs), defined as subsequent readmissions within the 1st year of transplantation after the EHR time frame. Furthermore, EHRs had a mean cost of approximately 10000 USD per KTR, which can create a significant burden on healthcare delivery systems[2].
Factors that interfere with post-transplant recovery and increase the risk of EHR include patient demographics (e.g., older age, African American race), pre-existing comorbidities (e.g., obesity, diabetes, heart disease, chronic obstructive pulmonary disease), transplant characteristics (e.g., expanded criteria donor transplants, lack of induction therapy, longer initial hospital stay, surgical complications), and frailty, a measure of physiologic reserve in aging populations[7,8,11-13]. Alternatively, EHR could potentially reflect deficits in discharge planning and outpatient management, calling for improvements in transplant care practices[8].
While EHRs have been studied extensively in American transplant settings, there is a paucity of EHR data collected in Canadian transplant populations. One Canadian study recently examined secular trends in post-transplant EHR incidence; however, it did not report on the impact of these findings on patients and healthcare delivery systems[2]. Due to significant differences in the delivery of healthcare services and patient outcomes between American and Canadian transplant centres[2], results from American studies cannot be readily extrapolated to Canadian populations[2].
The objectives of our study were to examine the impact of EHR on graft outcomes, patient mortality, LHR, and hospital costs in a Canadian transplant setting. We also considered how the impact on outcomes would change with an expanded EHR definition that included hospitalizations within 90-d of transplant discharge. With this information, we hoped to generate knowledge that may be useful in developing strategies to reduce post-transplant EHR.
We conducted a single-centre observational cohort study at the University Health Network (UHN) in Toronto, Ontario. Approval was obtained from the institutional Research Ethics Board.
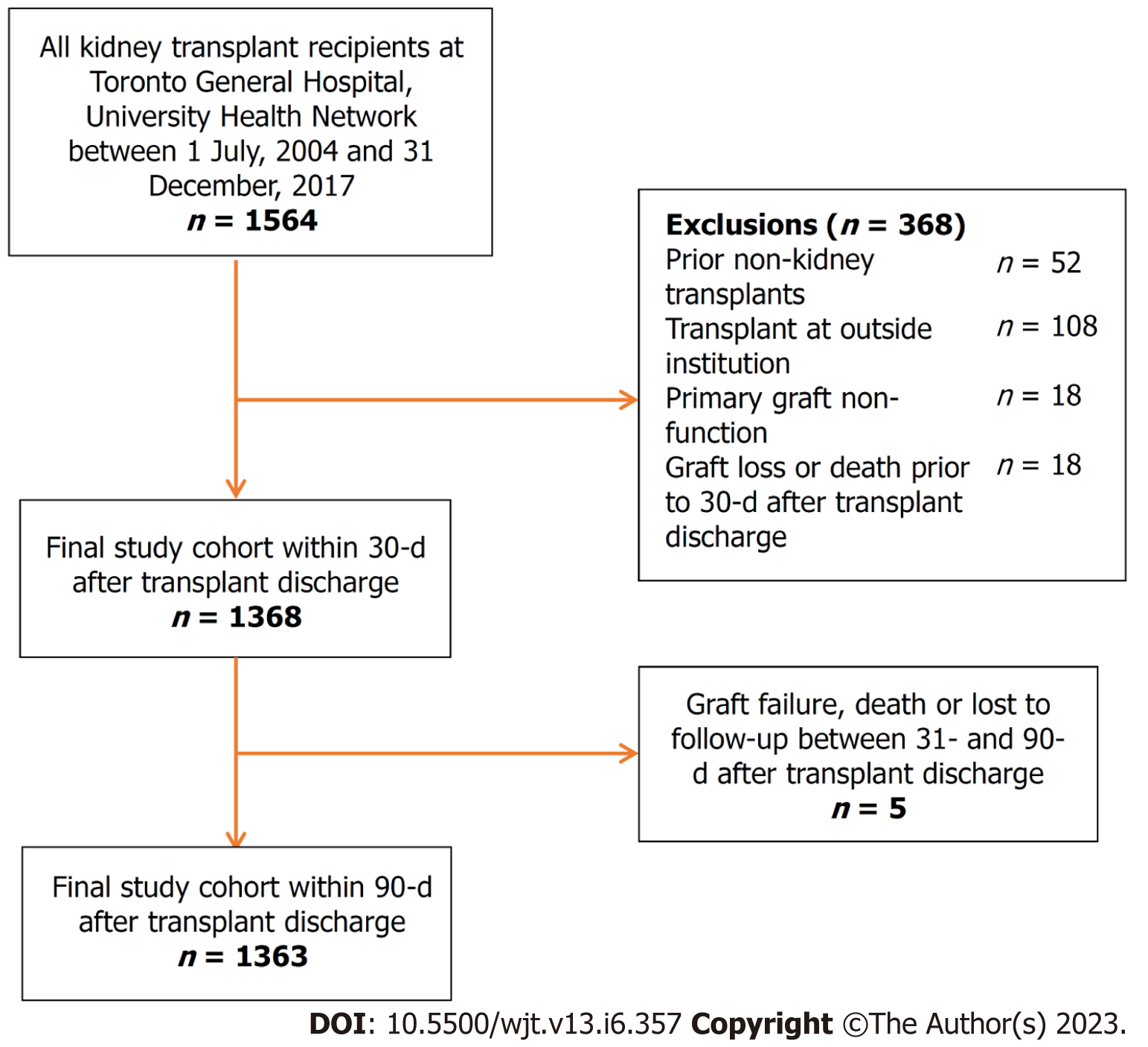
We included all adult (age ≥ 18 years) KTRs who received a kidney transplant from January 1, 2009 to December 31, 2017 (with follow-up until December 31, 2018) at the Toronto General Hospital, UHN. KTRs were excluded if they: (1) Were multiorgan transplant recipients; (2) Were transplanted at another transplant facility; (3) Experienced primary graft non-function; or (4) Experienced graft loss, death, or had their last follow-up before the study origin (i.e. 30 d after discharge from their transplant hospitalization).
Patient data was obtained from electronic hospital health records in the Organ Transplant Tracking Record and subsequently stored in the in-centre research database, the Comprehensive Renal Transplant Information System (CoReTRIS)[14]. CoReTRIS consists of recipient, donor, transplant, treatment, and follow-up data for all KTRs at UHN since January 2000 and has been audited for completeness and accuracy. All participants provided informed written consent for their health record information to be stored, collected, and used in CoReTRIS.
The main exposure of interest was EHR, defined as any hospitalization occurring within 30 d after discharge from the transplant hospitalization. We also examined an extended window of 90 d after discharge. The hospitalization must have been documented, either as an electronic summary in the Organ Transplant Tracking Record or as a paper discharge summary faxed from a non-UHN hospital. Hospitalization data was captured by a team of research assistants using a systematic review of medical records. Any discrepancies during data collection were later validated and resolved by a trained clinician.
The primary clinical outcome of interest was the composite of graft failure or death with graft function. Graft failure and death with graft function were also examined separately as our secondary outcomes. The time of origin for the analyses was defined as either 30 d or 90 d after transplant discharge. Therefore, we excluded KTRs who experienced death or graft failure or were lost to follow-up prior to this time. Other clinical outcomes included: (1) Graft function, which was measured using estimated glomerular filtration rate, calculated using the Chronic Kidney Disease Epidemiology Collaboration equation, at 6-mo and 1-year post-transplant; and (2) LHR, defined as any hospitalization occurring between 31 and 365 d for 30-d EHR or 91 to 365 d for 90-d EHR. The financial outcome of interest was the average cost of hospital-based care (inpatient and outpatient) per KTR over the 1st year of follow-up. This included all billed patient expenditures at each department of all hospitals that are part of UHN. Inpatient and outpatient cost data were provided by the UHN Accounting Centre and evaluated using a single-centre perspective.
To assess the independent association between the exposure and outcomes, covariates were chosen based on the literature and clinical experience. Recipient factors (i.e. age, sex, race, body mass index at time of transplant discharge, smoking history, diabetes mellitus, chronic lung disease, cardiovascular disease, baseline estimated glomerular filtration rate, and time on dialysis), donor factors (i.e. donor age, body mass index at time of donation, donation type, and expanded-criteria status), and transplant factors (i.e. peak panel reactive antibody, delayed graft function, acute rejection within 30 d of discharge, and transplant era) were considered in multivariable analyses.
Categorical variables were described using frequencies and percentages. Continuous variables were described using mean ± standard deviation if normally distributed and median [interquartile range (IQR)] if skewed. Baseline characteristics were compared between patients who experienced EHR and patients who did not experience EHR, using the χ2 test for categorical variables, the Student t-test for normally distributed continuous variables, and Wilcoxon rank-sum test for skewed continuous variables. The Kaplan-Meier product limit method was used to assess time from 30 d post-discharge to graft failure, death, or the composite by EHR status. Multivariable Cox proportional hazard models were used to estimate the independent association of EHR with graft failure, mortality, and LHR. Linear regression models were used to estimate the association between EHR and graft function during 1-year of post-transplant follow-up. Multiple imputation by chained equations method was used to address the degree of absence of all outcome variables[15]. Two-tailed P values < 0.05 were considered statistically significant. Data management and analyses were performed using Stata/MP 12.0 (StataCorp, College Station, TX, United States). Statistical review of the study was performed by a biomedical statistician (Li Y from Toronto General Hospital).
A total of 1564 KTRs were eligible for inclusion in the study cohort. Application of the prespecified exclusion criteria resulted in a final study cohort of 1368 KTRs for 30-d EHR analyses (Figure 1). A final study cohort of 1333 KTRs was used for the 90-d EHR analyses, as 5 KTRs experienced death or graft failure or were lost to follow-up between 31-d and 90-d post-transplant. For the 30-d EHR analysis, the median follow-up time was 5.11 years (IQR: 3.16, 7.59), with 329 cases of graft failure, 145 cases of death, and 439 cases of LHR in the 1st year starting from 30 d after transplant discharge. For the 90-d EHR analysis, the median follow-up time was 5.05 years (IQR: 3.12, 7.52), with 324 cases of graft failure, 140 cases of death, and 368 cases of LHR in the 1st year starting from 90 d after transplant discharge.
Baseline recipient, donor, and transplant characteristics of both study cohorts are summarized in Table 1. The 30-d EHR study population was 60.0% male and 46.7% White. The 90-d EHR study population was 60.2% male and 47.4% White. A total of 307 (22.4%) and 394 (29.6%) KTRs experienced 30-d and 90-d EHRs, respectively. KTRs who experienced an EHR were more likely to have a longer duration of dialysis, an expanded donor criteria donor, and a previous case of biopsy-proven acute rejection and received transplantation between 2015-2017. Particularly, KTRs with 90-d EHR were older, more likely to have a history of diabetes, and more likely to have a previous case of rejection, spent a longer time on dialysis before transplant, had older donors, and received transplantation between 2015-2017. Other characteristics were similar between the EHR and non-EHR groups.
| Variables | EHR within 30 d after transplant discharge | EHR within 90 d after transplant discharge | ||||||
| Number of patients, n = 1368 | Yes, n = 307 | No, n = 1061 | P value | Number of patients, n = 1333 | Yes, n = 394 | No, n = 939 | P value | |
| Recipient characteristics | ||||||||
| Recipient age at transplant in yr, mean (± SD) | 1368 | 52.4 (13.4) | 51.4 (13.6) | 0.26 | 1333 | 52.7 (13.4) | 51.1 (13.6) | 0.05 |
| Recipient sex | ||||||||
| Male | 821 | 185 (60.3%) | 636 (59.9%) | 0.92 | 802 | 235 (59.6%) | 567 (60.4%) | 0.80 |
| Female | 547 | 122 (39.7%) | 425 (40.1%) | 531 | 159 (40.4%) | 372 (39.6%) | ||
| Recipient race | ||||||||
| White | 639 | 134 (56.3%) | 505 (59.1%) | 0.44 | 632 | 176 (57.1%) | 456 (59.1%) | 0.56 |
| Non-white | 454 | 104 (43.7%) | 350 (40.9%) | 448 | 132 (42.9%) | 316 (40.9%) | ||
| History of smoking | ||||||||
| Smoker | 584 | 137 (44.8%) | 447 (42.3%) | 0.42 | 569 | 174 (44.5%) | 395 (42.1%) | 0.42 |
| Non-smoker | 780 | 169 (55.2%) | 611 (57.8%) | 760 | 217 (55.5%) | 543 (57.9%) | ||
| History of diabetes mellitus | 467 | 111 (36.2%) | 356 (33.6%) | 0.40 | 455 | 151 (38.3%) | 304 (32.4%) | 0.04 |
| History of chronic lung disease | 57 | 14 (4.6%) | 43 (4.1%) | 0.70 | 56 | 21 (5.3%) | 35 (3.7%) | 0.19 |
| History of cardiovascular disease | 377 | 82 (26.7%) | 295 (27.8%) | 0.70 | 368 | 118 (30.0%) | 250 (26.7%) | 0.22 |
| Recipient body mass index at transplant discharge in kg/m2, mean (± SD) | 1074 | 27.2 (6.0) | 26.9 (5.9) | 0.49 | 1333 | 52.7 (13.4) | 51.1 (13.6) | 0.05 |
| Recipient eGFR at baseline in mL/min, mean (± SD) | 1085 | 62.7 (26.5) | 63.0 (28.0) | 0.88 | 1072 | 60.7 (27.0) | 64.0 (27.7) | 0.08 |
| Time on dialysis in yr, mean (± IQR) | 1368 | 3.4 (1.2, 5.6) | 3.2 (1.2, 5.3) | 0.46 | 1333 | 3.5 (1.6, 5.8) | 3.1 (1.1, 5.3) | 0.02 |
| Donor characteristics | ||||||||
| Donor age at donation in yr, mean (± SD) | 1359 | 48.9 (14.2) | 47.3 (15.0) | 0.10 | 1325 | 49.3 (14.4) | 47.0 (14.9) | 0.01 |
| Donor body mass index in kg/m2, (mean ± SD) | 1339 | 26.9 (5.6) | 26.7 (5.4) | 0.56 | 1305 | 26.7 (5.4) | 26.8 (5.4) | 0.70 |
| Type of donation | ||||||||
| Deceased | 766 | 178 (58.0%) | 588 (55.4%) | 0.43 | 742 | 234 (59.4%) | 508 (54.1%) | 0.08 |
| Living | 602 | 129 (42.0%) | 473 (44.6%) | 591 | 160 (40.6%) | 431 (45.9%) | ||
| Expanded criteria donor | 256 | 70 (22.8%) | 186 (17.5%) | 0.04 | 249 | 94 (23.9%) | 155 (16.5%) | 0.002 |
| Transplant characteristics | ||||||||
| Peak PRA | ||||||||
| 0% | 709 | 161 (52.6%) | 548 (51.7%) | 0.78 | 686 | 211 (53.7%) | 475 (50.6%) | 0.31 |
| > 0% | 657 | 145 (47.4%) | 512 (48.3%) | 645 | 182 (46.3%) | 463 (49.4%) | ||
| Delayed graft function | 276 | 57 (18.6%) | 219 (20.6%) | 0.43 | 265 | 79 (20.1%) | 186 (19.8%) | 0.92 |
| Biopsy-proven acute rejection | 85 | 46 (15.0%) | 39 (3.7%) | < 0.001 | 98 | 59 (15.0%) | 39 (4.2%) | < 0.001 |
| Transplant era | ||||||||
| 2009-2011 | 429 | 94 (30.6%) | 335 (31.6%) | 0.002 | 429 | 131 (33.3%) | 298 (31.7%) | < 0.001 |
| 2012-2014 | 431 | 75 (24.4%) | 356 (33.6%) | 430 | 96 (24.4%) | 334 (35.6%) | ||
| 2015-2017 | 508 | 138 (45.0%) | 370 (34.9%) | 474 | 167 (42.4%) | 307 (32.7%) | ||
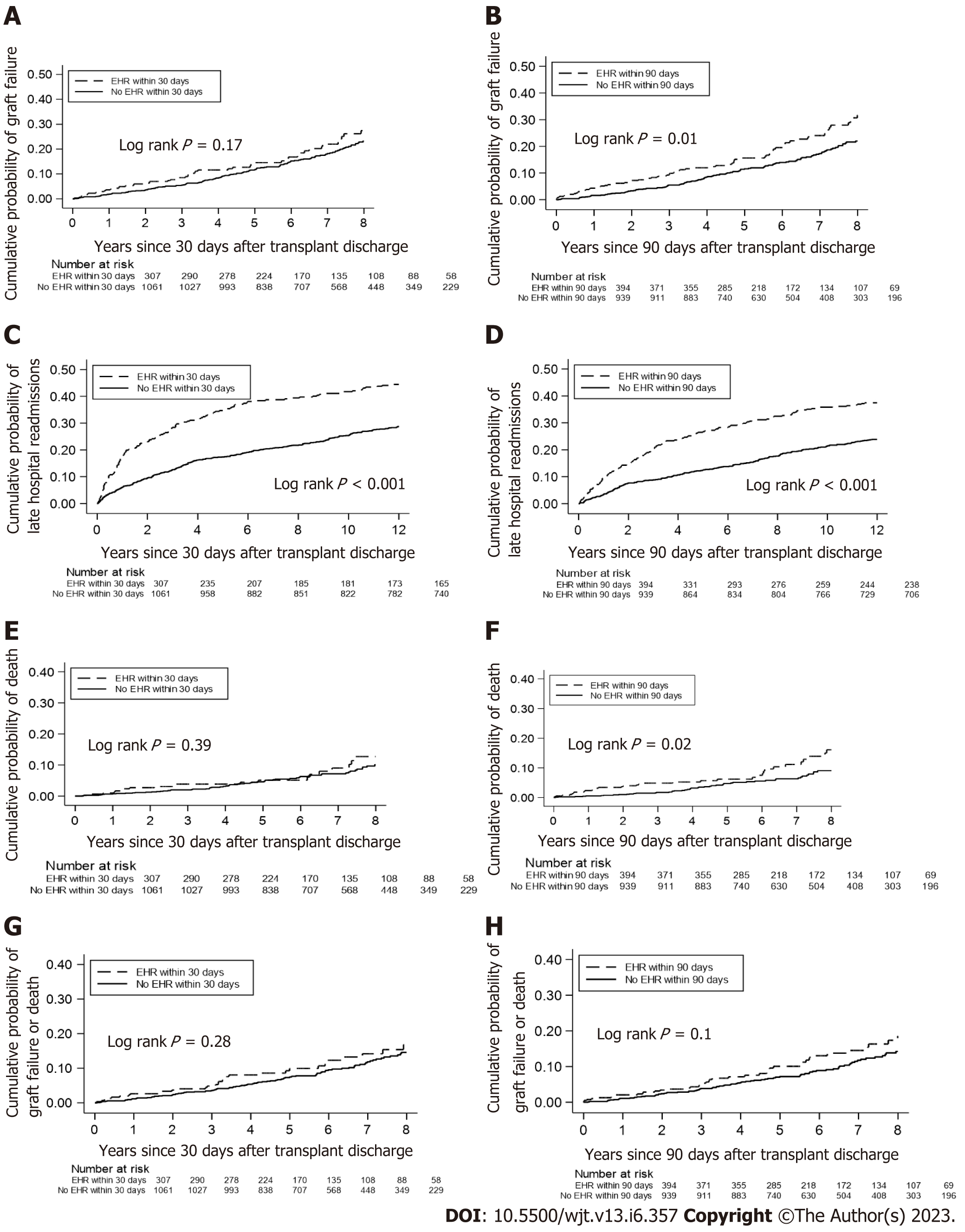
The 30-d EHR group did not have a higher cumulative probability of death-censored graft failure as compared to non-EHR KTR (Figure 2A); in contrast, the 90-d EHR group did show a greater probability of death-censored graft failure (log rank P = 0.01, Figure 2B). The 30-d and 90-d EHR groups had greater cumulative probabilities of LHR within 1 year (Log rank P < 0.001, Figure 2C and D) compared to the non-EHR group. The 30-d EHR group did not have a higher probability of death vs the non-EHR group (Figure 2E), whereas the 90-d EHR group displayed a higher probability of death (log rank P = 0.02, Figure 2F). Neither EHR group had a higher probability of the composite outcome of graft failure or death vs the non-EHR group (Figure 2G and H).
The 30-d and 90-d EHRs were independent predictors of LHR hazard ratio (HR): 1.73; 95% confidence interval (CI): 1.40, 2.13 for 30-d EHR and HR: 1.58; 95%CI: 1.27 to 1.97 for 90-d EHR (Table 2). Neither 30-d nor 90-d EHRs were associated with the other outcomes of interest in the multivariable Cox models. In multivariable linear regression models (Table 3), 30-d and 90-d EHRs were associated with lower graft function at 3 mo (HR: -2.60; 95%CI: -4.90 to -0.30) and 12 mo (HR: -3.11; 95%CI: -5.62 to -0.60) for 30-d EHR and lower function at 3 mo (HR: -3.08; 95%CI: -5.17 to -0.99), 9 mo (HR: -2.81; 95%CI: -5.24 to -0.39), and 12 mo (HR: -3.77; 95%CI: -6.15 to -1.38) for 90-d EHR.
| Outcome | 30-d EHR | 90-d EHR | ||||
| Number of outcomes | Full model1 | Number of outcomes | Full model1 | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |||
| Graft failure or death | 237 | 1.06 (0.77-1.45) | 0.73 | 232 | 1.21 (0.91-1.61) | 0.19 |
| Graft failure | 92 | 1.05 (0.62-1.77) | 0.86 | 92 | 1.45 (0.92-2.29) | 0.11 |
| Death | 145 | 1.08 (0.73-1.60) | 0.71 | 140 | 1.09 (0.75-1.57) | 0.66 |
| LHR | 439 | 1.73 (1.40-2.13) | < 0.001 | 368 | 1.58 (1.27-1.97) | < 0.001 |
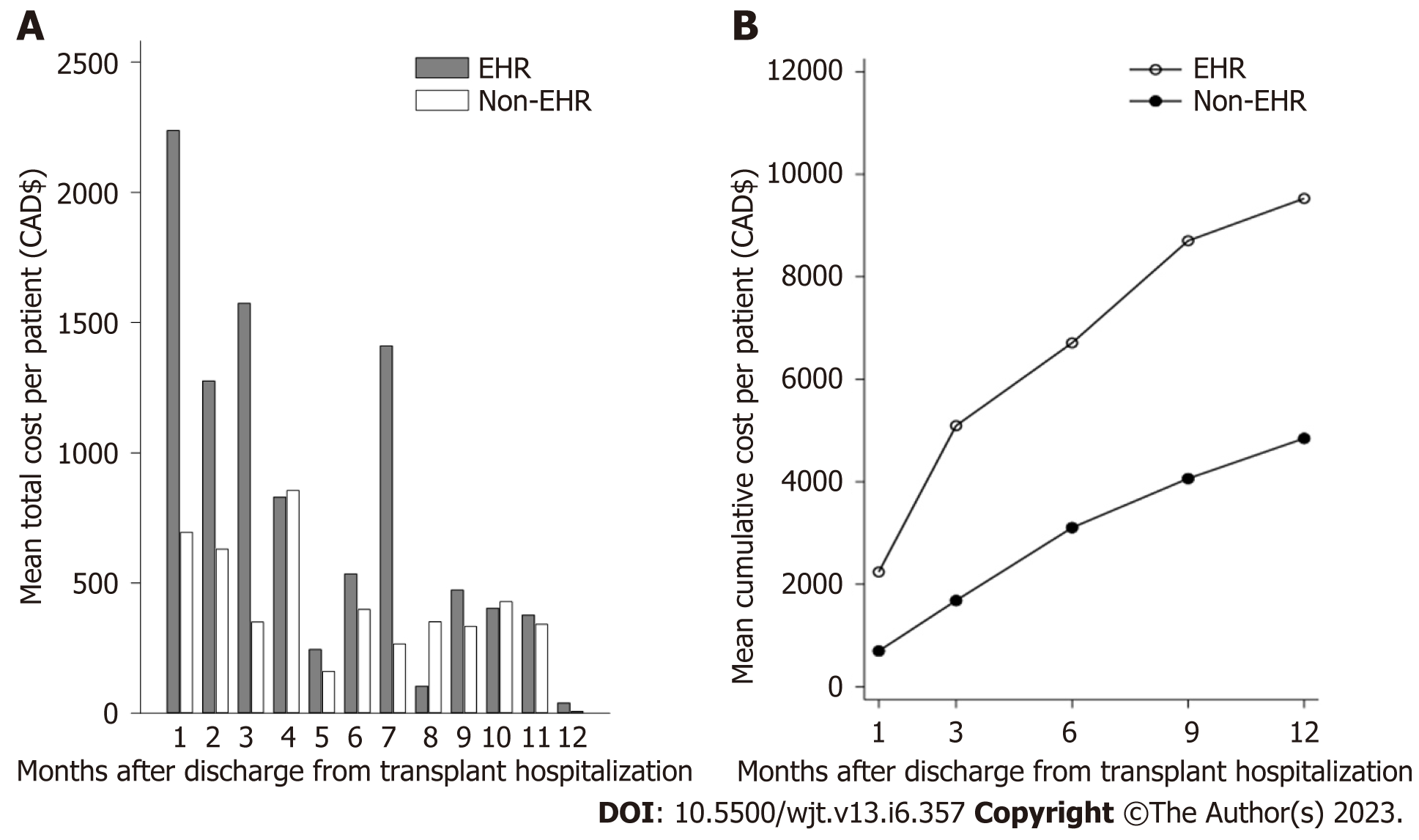
The mean cost of hospital-based care per KTR in the 1st year post-transplant is shown in Figure 3. In the first 3 mo, the mean cost of care for KTR with an EHR was nearly three times higher than for those without EHR (Figure 3A). After 3 mo, the mean cost of care for the EHR group declined to levels comparable to the non-EHR group, with an exception at month 7. Similarly, the mean number of readmissions for the EHR group decreased after the first 3 mo post-transplant, though the EHR group had more readmissions than the non-EHR group overall (Supplementary Figure 1). When the cost of hospital-based care was examined cumulatively, the mean post-transplant cost was consistently higher for the EHR group than the non-EHR group (Figure 3B).
In our patient cohort, the incidence of 30-d and 90-d EHRs was 22.4% and 29.5%, respectively. The 30-d EHR incidence was lower than those reported in the American studies by Luan et al[8] (36%) and McAdams-DeMarco et al[9] (31%). This may be related to differences in the study population as well as specific threshold and institutional criteria for admitting KTRs rather than outpatient care. Our results were comparable with the 30-d EHR incidence of 21% that was reported in a recent population-based Canadian study[2]. However, Naylor et al[2] found that 30-d EHR rates can vary even across different transplant centres within the province of Ontario, ranging from 16% to 27%.
After accounting for potential confounders, 30-d and 90-d EHRs were shown to be an independent predictor of LHR at 1-year post-transplant and poorer graft function. Our results corroborated recent observational studies that associated EHR with negative clinical outcomes among KTRs and other high-risk patient populations[7,16-19,20]. More specifically, Luan et al[8] and McAdams-DeMarco et al[9] also demonstrated that EHRs are associated with a higher risk of LHR and graft failure in KTRs. However, contrary to these studies, we did not find a statistically significant association between EHR and patient mortality. Additionally, while most studies focused on the impact of 30-d EHR[9,21], we expanded our EHR definition and were able to demonstrate that hospitalizations occurring within the first 3 mo after transplantation discharge are also associated with rehospitalizations up to 1 year post-transplant.
The relationship between EHR and inferior patient outcomes can be explained in several ways. Post-transplant conditions or complications that necessitate EHR could directly result in clinical events like graft failure or frequent hospital readmissions[8]. Alternately, KTRs with EHR may already possess pre-existing medical comorbidities (e.g., diabetes mellitus, cardiovascular disease) that increase the likelihood of adverse clinical events after transplantation. In our study, the patients in the EHR group were more likely to have an expanded criteria donor and a history of acute rejection, which have been previously linked with mortality, graft failure, and hospitalizations after kidney transplant[5,22]. EHRs are also associated with frailty, which is a marker of suboptimal transplant outcomes[12,23-26]. This factor may become increasingly important over time with an aging and subsequently a KTR population with more comorbidities[7]. Moreover, patients transplanted in the more recent era (2015-2017) were more likely to have EHRs as compared to earlier transplant years. The transplant program at our centre has been expanding its pool of patients among both recipients and donors in more recent years to include more medically complicated patients such as expanded criteria donors.
EHR not only affects patient outcomes but is detrimental from a financial perspective. We observed that, on average, twice as much money was spent on EHR patients as compared to non-EHR patients. Hospital readmissions increase the financial burden on the healthcare system, costing 1.8 billion CAD annually (11% of annual inpatient costs)[27]. Moreover, the average cost of a second hospitalization is often greater than the first[27], which is particularly relevant to our finding that EHR increase the risk of LHR. Our analysis only focused on costs at a single transplant centre, thus the financial consequence might have been more significant if expenditure at other tertiary care centres and community-based hospitals were also taken into consideration.
Due to the risks and costs associated with EHR, there is considerable interest in clinical monitoring and prevention of EHR. However, despite the growing evidence in the literature, there are no specific clinical practice guidelines to manage and monitor KTRs with EHR. After transplant discharge, KTRs at the UHN Kidney Transplant Program are followed weekly for the 1st month, biweekly from months 2 to 3, monthly from months 4 to 6, bimonthly from months 7 to 12, every 3 to 4 mo from 13 to 24 mo, and then every 6 to 12 mo beyond 24 mo[28]. Like many other centres, a number of KTRs with stable kidney function from the UHN program are transferred from the hospital-based transplant unit to community-based general nephrology centres within the 1st year post-transplant. Thus, although there are standard practices in place for KTR management in general, there are no standardized strategies that are tailored specifically for those KTRs at risk of EHR.
KTRs who are at increased risk of EHR may benefit from multifaceted interventions that include: (1) Better educational strategies to improve medication knowledge and support capacity for self-care; (2) Collaborative care provided by transplant and general nephrologists; and (3) More frequent follow-up visits for an extended period of time[2,9,29]. Further investigation of these interventions would be required to determine the feasibility and efficiency of reducing EHR in KTRs. Previous studies have suggested that up to half of hospital readmissions for KTRs are preventable and can be reduced by early intervention[30]. Exploring the characteristics of KTRs with preventable EHR can inform the development and evaluation of prediction tools, which will aid clinicians in identifying high-risk patients[31].
With this study, we were able to extend the previous work on EHR and long-term outcomes of KTRs to a Canadian healthcare context. Our methodology involved a standardized and comprehensive collection of patient and hospitalization data for a relatively large study population of over 1000 KTRs[14]. Moreover, we benefited from exploring the use of 90-d EHR (in addition to the previously used 30-d EHR definition) for the assessment of outcomes. Nevertheless, some limitations to our study also warrant discussion. First, the generalizability of our findings may be limited by the single-centre study design. Second, this study was based on observational data. Therefore, we cannot confirm that changes in EHR would improve long-term outcomes. Third, non-UHN readmissions may have been missed since we relied on UHN clinical notes to determine readmissions within the 1st year. However, it is unlikely that many events were missed since patients are instructed to contact the transplant centre if they are admitted to any facilities outside of UHN, and hospitalization events are checked with each patient at every clinic visit. Finally, while patient and hospitalization data could be verified with patient charts and electronic records, our cost data was solely obtained from the UHN financial services and was difficult to verify independently. However, these cost data are used for hospital planning and budgeting and were sufficient for their intended purpose in our study.
In summary, EHR after kidney transplantation was associated with a greater risk of LHR at 1-year post-transplant, suboptimal kidney function, and higher hospital-based care costs. The 90-d window after discharge from transplant hospitalization, in addition to the frequently used 30-d post-transplant period, marks a novel opportunity to evaluate the risks for KTRs. Further studies are required to determine which EHRs are preventable and implement reliable tools that can reduce EHR after kidney transplantation.
Early hospital readmissions (EHRs) post-kidney transplantation adversely impact clinical outcomes such as graft function and patient mortality as well as healthcare costs. A better understanding of EHR can facilitate improved discharge planning and long-term outpatient management post kidney transplant.
Associations between EHR and suboptimal clinical outcomes post kidney transplant have not been extensively studied in a Canadian healthcare setting. We sought to explore the burden of EHR on kidney transplant recipients (KTRs) and the Canadian healthcare system in a large transplant centre.
The objectives of our study were to examine the impact of EHR on graft outcomes, patient mortality, late hospital readmissions (LHRs), and hospital costs in a Canadian transplant setting.
This was a single centre cohort study of 1564 KTRs transplanted between 2009-2017. Analyses were separated by patients with no EHRs, patients with EHRs within 30 d of transplant, and those with EHRs within 90 d of transplant. Multivariable Cox and linear regression models were used to examine EHR, LHR, and outcomes including graft function and patient mortality.
EHRs post kidney transplant were associated with subsequent LHRs, suboptimal kidney function, and a higher burden on the healthcare system.
EHRs post kidney transplant are associated with suboptimal patient outcomes and higher burdens on the healthcare system. Expanding the window of readmissions to 90 d post-transplant revealed an important target for reducing the risk of suboptimal outcomes.
A better understanding of EHR can contribute to the development of prediction tools to identify those KTRs at risk of EHR and thus a standardized approach to manage and target these patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain S-Editor: Lin C L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Abecassis M, Bartlett ST, Collins AJ, Davis CL, Delmonico FL, Friedewald JJ, Hays R, Howard A, Jones E, Leichtman AB, Merion RM, Metzger RA, Pradel F, Schweitzer EJ, Velez RL, Gaston RS. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol. 2008;3:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 424] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 2. | Naylor KL, Knoll GA, Allen B, Li AH, Garg AX, Lam NN, McCallum MK, Kim SJ. Trends in Early Hospital Readmission After Kidney Transplantation, 2002 to 2014: A Population-Based Multicenter Cohort Study. Transplantation. 2018;102:e171-e179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Tavares MG, Tedesco-Silva Junior H, Pestana JOM. Early Hospital Readmission (EHR) in kidney transplantation: a review article. J Bras Nefrol. 2020;42:231-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Harhay M, Lin E, Pai A, Harhay MO, Huverserian A, Mussell A, Abt P, Levine M, Bloom R, Shea JA, Troxel AB, Reese PP. Early rehospitalization after kidney transplantation: assessing preventability and prognosis. Am J Transplant. 2013;13:3164-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL. Early hospital readmission after kidney transplantation: patient and center-level associations. Am J Transplant. 2012;12:3283-3288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Kang IC, Kim IK, Son S, Ju MK. Impact of Early Hospital Readmissions After Kidney Transplantation on Graft Function. Transplant Proc. 2018;50:2359-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Li AH, Lam NN, Naylor KL, Garg AX, Knoll GA, Kim SJ. Early Hospital Readmissions After Transplantation: Burden, Causes, and Consequences. Transplantation. 2016;100:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Luan FL, Barrantes F, Roth RS, Samaniego M. Early hospital readmissions post-kidney transplantation are associated with inferior clinical outcomes. Clin Transplant. 2014;28:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | McAdams-Demarco MA, Grams ME, King E, Desai NM, Segev DL. Sequelae of early hospital readmission after kidney transplantation. Am J Transplant. 2014;14:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Iqbal K, Hasanain M, Rathore SS, Iqbal A, Kazmi SK, Yasmin F, Koritala T, Thongprayoon C, Surani S. Incidence, predictors, and outcomes of early hospital readmissions after kidney transplantation: Systemic review and meta-analysis. Front Med (Lausanne). 2022;9:1038315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Altamimi AR, Alrzouq FK, Aljaafri ZA, Alahmadi F, Alsuwailem Y, Dendini F. Incidence and Causes of Early Hospital Readmissions After Living-Donor Renal Transplant at King Abdulaziz Medical City, Riyadh. Cureus. 2023;15:e40254. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Parajuli S, Astor BC, Lorden HM, O'Toole KA, Wallschlaeger RE, Breyer IC, Dodin B, Aziz F, Garonzik-Wang J, Mandelbrot DA. Analysis of individual components of frailty: Pre-transplant grip strength is the strongest predictor of post kidney transplant outcomes. Clin Transplant. 2022;36:e14827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Moein M, Vlassis IM, Kim L, Hanlon M, Saidi R. Early readmissions post kidney transplantation: lessons learned. Actas Urol Esp (Engl Ed). 2023;47:382-389. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Famure O, Phan NA, Kim SJ. Health information management for research and quality assurance: the Comprehensive Renal Transplant Research Information System. Healthc Manage Forum. 2014;27:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6773] [Cited by in RCA: 5884] [Article Influence: 420.3] [Reference Citation Analysis (0)] |
| 16. | Berman K, Tandra S, Forssell K, Vuppalanchi R, Burton JR Jr, Nguyen J, Mullis D, Kwo P, Chalasani N. Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Curtis JP, Schreiner G, Wang Y, Chen J, Spertus JA, Rumsfeld JS, Brindis RG, Krumholz HM. All-cause readmission and repeat revascularization after percutaneous coronary intervention in a cohort of medicare patients. J Am Coll Cardiol. 2009;54:903-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Greenblatt DY, Greenberg CC, Kind AJ, Havlena JA, Mell MW, Nelson MT, Smith MA, Kent KC. Causes and implications of readmission after abdominal aortic aneurysm repair. Ann Surg. 2012;256:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Lum HD, Studenski SA, Degenholtz HB, Hardy SE. Early hospital readmission is a predictor of one-year mortality in community-dwelling older Medicare beneficiaries. J Gen Intern Med. 2012;27:1467-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Yousufuddin M, Young N, Keenan L, Olson T, Shultz J, Doyle T, Ahmmad E, Arumaithurai K, Takahashi P, Murad MH. Effect of early hospital readmission and comorbid conditions on subsequent long-term mortality after transient ischemic attack. Brain Behav. 2017;7:e00865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Tavares MG, Cristelli MP, Ivani de Paula M, Viana L, Felipe CR, Proença H, Aguiar W, Wagner Santos D, Tedesco-Silva Junior H, Medina Pestana JO. Early hospital readmission after kidney transplantation under a public health care system. Clin Transplant. 2019;33:e13467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Lafranca JA, IJermans JN, Betjes MG, Dor FJ. Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Med. 2015;13:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 23. | Cheng XS, Lentine KL, Koraishy FM, Myers J, Tan JC. Implications of Frailty for Peritransplant Outcomes in Kidney Transplant Recipients. Curr Transplant Rep. 2019;6:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Hughes LD, Witham MD. Causes and correlates of 30 day and 180 day readmission following discharge from a Medicine for the Elderly Rehabilitation unit. BMC Geriatr. 2018;18:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Kahlon S, Pederson J, Majumdar SR, Belga S, Lau D, Fradette M, Boyko D, Bakal JA, Johnston C, Padwal RS, McAlister FA. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ. 2015;187:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 26. | Ross KH, Jaar BG, Lea JP, Masud T, Patzer RE, Plantinga LC. Long-term outcomes among Medicare patients readmitted in the first year of hemodialysis: a retrospective cohort study. BMC Nephrol. 2019;20:285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Canadian Institute for Health Information. All-cause readmission to acute care and return to the emergency department. 2012. [cited 14 October 2023]. Available from: https://publications.gc.ca/collections/collection_2013/icis-cihi/H118-93-2012-eng.pdf. |
| 28. | Wu WK, Famure O, Li Y, Kim SJ. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int. 2015;88:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 29. | Leppin AL, Gionfriddo MR, Kessler M, Brito JP, Mair FS, Gallacher K, Wang Z, Erwin PJ, Sylvester T, Boehmer K, Ting HH, Murad MH, Shippee ND, Montori VM. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174:1095-1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 585] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 30. | Hogan J, Arenson MD, Adhikary SM, Li K, Zhang X, Zhang R, Valdez JN, Lynch RJ, Sun J, Adams AB, Patzer RE. Assessing Predictors of Early and Late Hospital Readmission After Kidney Transplantation. Transplant Direct. 2019;5:e479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Taber DJ, Palanisamy AP, Srinivas TR, Gebregziabher M, Odeghe J, Chavin KD, Egede LE, Baliga PK. Inclusion of dynamic clinical data improves the predictive performance of a 30-day readmission risk model in kidney transplantation. Transplantation. 2015;99:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |