Published online Dec 18, 2023. doi: 10.5500/wjt.v13.i6.344
Peer-review started: July 20, 2023
First decision: September 5, 2023
Revised: October 21, 2023
Accepted: November 13, 2023
Article in press: November 13, 2023
Published online: December 18, 2023
Processing time: 150 Days and 19.7 Hours
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is a global pandemic that is associated with a high risk of morbidity and mortality among recipients of solid organ transplantation. In the course of acute SARS-CoV-2 infection, various laboratory markers have been identified as predictors for high risk of mortality.
To risk stratify renal transplant recipients (RTxR) using general demographic parameters, comorbidities and routine laboratory markers for the severity of the disease and its outcomes. We believe that learning about these routinely moni
This present study includes RTxR who acquired SARS-CoV-2 infection from March 2020 to February 2021. We recorded the basic demographics, comorbidities and routine laboratory markers. We investigated the impact of SARS-CoV-2 infection on RTxRs and risk-stratified the progression of disease severity and outcomes in terms of recovery or mortality.
From 505 RTxRs in our renal transplant follow-up program, 29 (7.75%) RTxRs had PCR-positive SARS-CoV-2 infection. We recorded 8 deaths from SARS-CoV-2 infection giving an overall mortality rate of 1.6% but a significant 27.6% mortality in SARS-CoV-2 positive recipients. Age more than 68 years, non-Caucasian ethnicity and male gender were associated with a significant drop in survival probability; P ≤ 0.001. < 0.001 and < 0.0001 respectively. 87.5% of the deceased were diabetic; P ≤ 0.0.0001. Estimated glomerular filtration rate of less than 26 mL/min/1.73 m2, serum albumin less than 20 g/L, Hemoglobin less than 9.6 g/L and serum calcium less than 1.70 mmol/L were all associated with significantly increased risk of mortality; P = 0.0128, < 0.001, < 0.0001 and 0.0061 respectively.
This study has identified some routinely used modifiable parameters in predicting a higher risk of mortality and morbidity. This knowledge can be used in RTxR follow-up programs by addressing these parameters early to help reduce the morbidity and mortality in RTxRs.
Core Tip: In this present study, we aim to risk stratify renal transplant recipients (RTxR) using general demographic parameters, comorbidities and routine laboratory markers for the severity of the disease and its outcomes. We believe that learning about these routinely monitored parameters can help us to plan better strategies for RTxR follow-up program.
- Citation: Ghazanfar A, Abbas M, Hussain MW, Kayal M. Risk stratification of renal transplant recipients using routine parameters: Implication of learning from SARS-CoV-2 into transplant follow-up program. World J Transplant 2023; 13(6): 344-356
- URL: https://www.wjgnet.com/2220-3230/full/v13/i6/344.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i6.344
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was first identified in December 2019[1] and subse
The recipients of solid organ transplantation (SOT) are known to be more vulnerable to opportunistic infections[7] including several common respiratory virus infections[8], due to a weakened T-cell mediated immune response[9]. Globally, the recipients of SOT were included among patients at increased risk for severe illness from SARS-CoV-2[10,11]. The reported mortality among Renal Transplant Recipients (RTxRs) from SARS-CoV-2 varied between 10% to 33%, in different studies across the world[12-17]. This increased risk of mortality is not only because of immunosuppression but also secondary to associated comorbidities[5,6]. In the course of acute SARS-CoV-2 infection, various laboratory markers have been identified as predictors for high risk of mortality including lymphopenia, high C-reactive protein levels, D-dimer, lactate dehydrogenase and ferritin[18-20]. However, some of the other routinely monitored parameters have not been studied in detail in RTxRs when compared with the general population. These include blood pressure control[21], Haemoglobin (Hb)[22], serum albumin[23], serum calcium levels[24] and function of the renal allograft, being measured as estimated glomerular filtration rate (eGFR)[25]. In this present study, we investigate these routine parameters with an aim to risk stratify RTxRs in high or low-risk groups using general demographic parameters, comorbidities and routine laboratory markers. The aim is to identify relevant routinely done parameters to identify high-risk RTxRs at an early stage. We believe that identification and correction of these parameters can significantly reduce long-term morbidity and mortality in RTxRs from SARS-CoV-2 as well as non-SARS-CoV-2 related infections.
In this retrospective observational study, we analysed the data of our renal transplant follow-up program. At the time of the present study, we had 505 RTxRs registered under the follow-up program at St Georges University Hospitals NHS Foundation Trust, London, United Kingdom. Since the start of the pandemic in March 2020, on the advice of National Health Services England, National Health Services Blood and Transplant and the British Transplantation Society, we recommended shielding for all our RTxRs in our follow-up program. In this present study, we included all RTxR who acquired SARS-CoV-2 infection from March 2020 to February 2021. It included both symptomatic and asymptomatic patients. There were 29 patients in our RTxRs follow-up program who acquired SARS-CoV-2 infection during this period which were included in study group cohort A; leaving 476 patients in the control group, cohort B. We recorded the basic demographics, comorbidities and routine laboratory markers. We compared these two groups to identify any significant factors responsible for predisposing RTxRs to the severity of SARS-CoV-2 infection. We then further investigated 29 RTxRs with SARS-CoV-2 infection to stratify the progression of disease severity and outcomes in terms of recovery or mortality. We subdivided patients in cohort A into A1 (n = 21) where they recovered from SARS-CoV-2 infection and A2 (n = 8) which resulted in mortality. We used Prism 9 and MedCalc statistical software programs for the data analyses. Baseline characteristics were compared using a t-test, Fisher exact test, Chi-square test or Mann-Whitney U-test where appropriate. Box-Whisker plots were used to describe means, standard deviations and standard error of means. Survival probabilities were recorded for individual risk factors. Univariate and multivariate analyses were performed to record the impact of various factors on each other. Survival analysis was carried out using Kaplan–Meier estimates and for differences in survival, a log-rank test was used.
From 505 RTxRs in our renal transplant follow-up program 29 (7.75%) RTxRs had PCR-positive SARS-CoV-2 infection (cohort A), leaving 476 patients in control cohort B. We recorded 8 deaths in cohort A giving a mortality rate of 1.6% for the overall follow-up population but a significant 27.6% mortality in SARS-CoV-2 positive patients. There was no death recorded in cohort B during the same period.
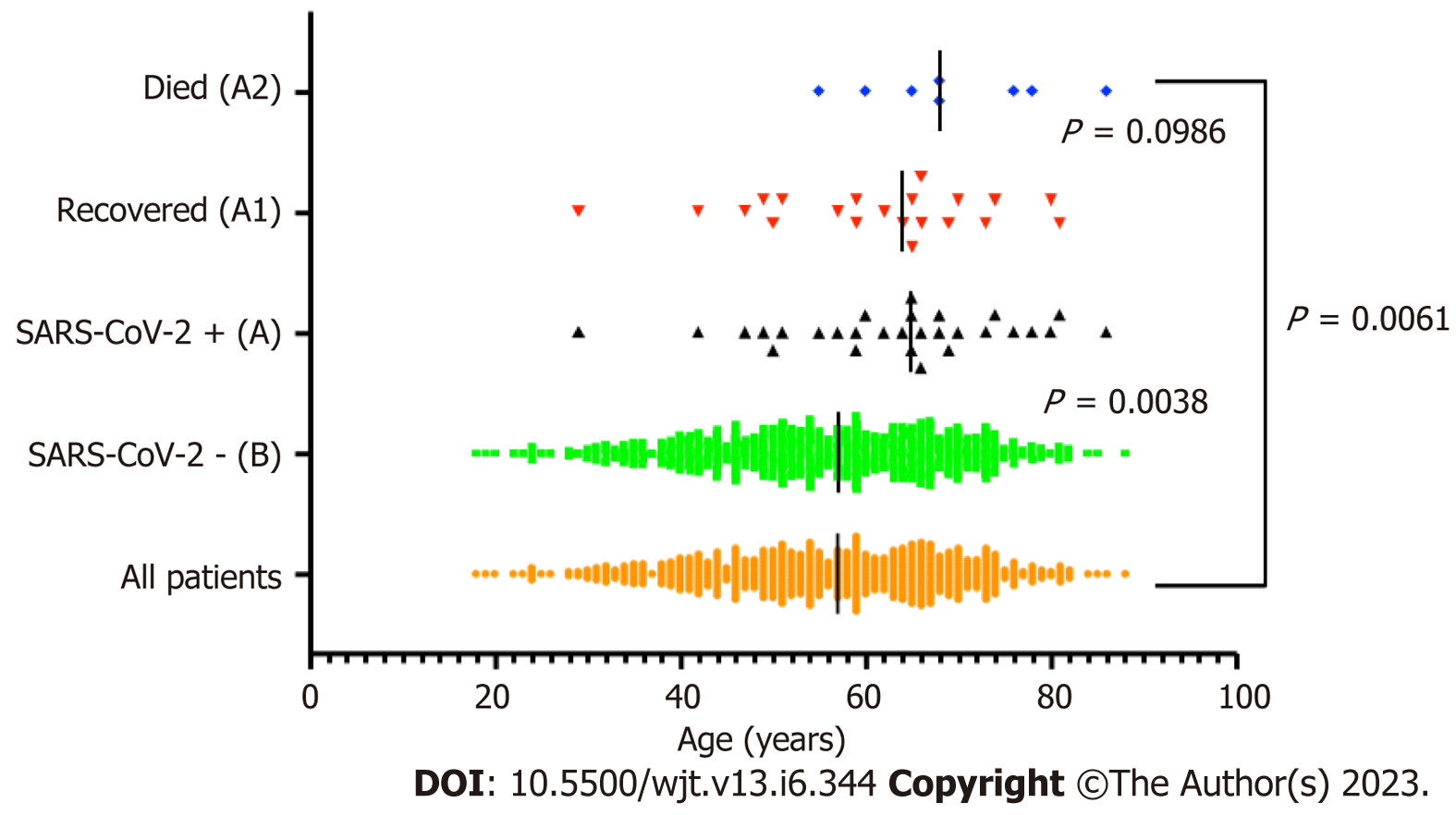
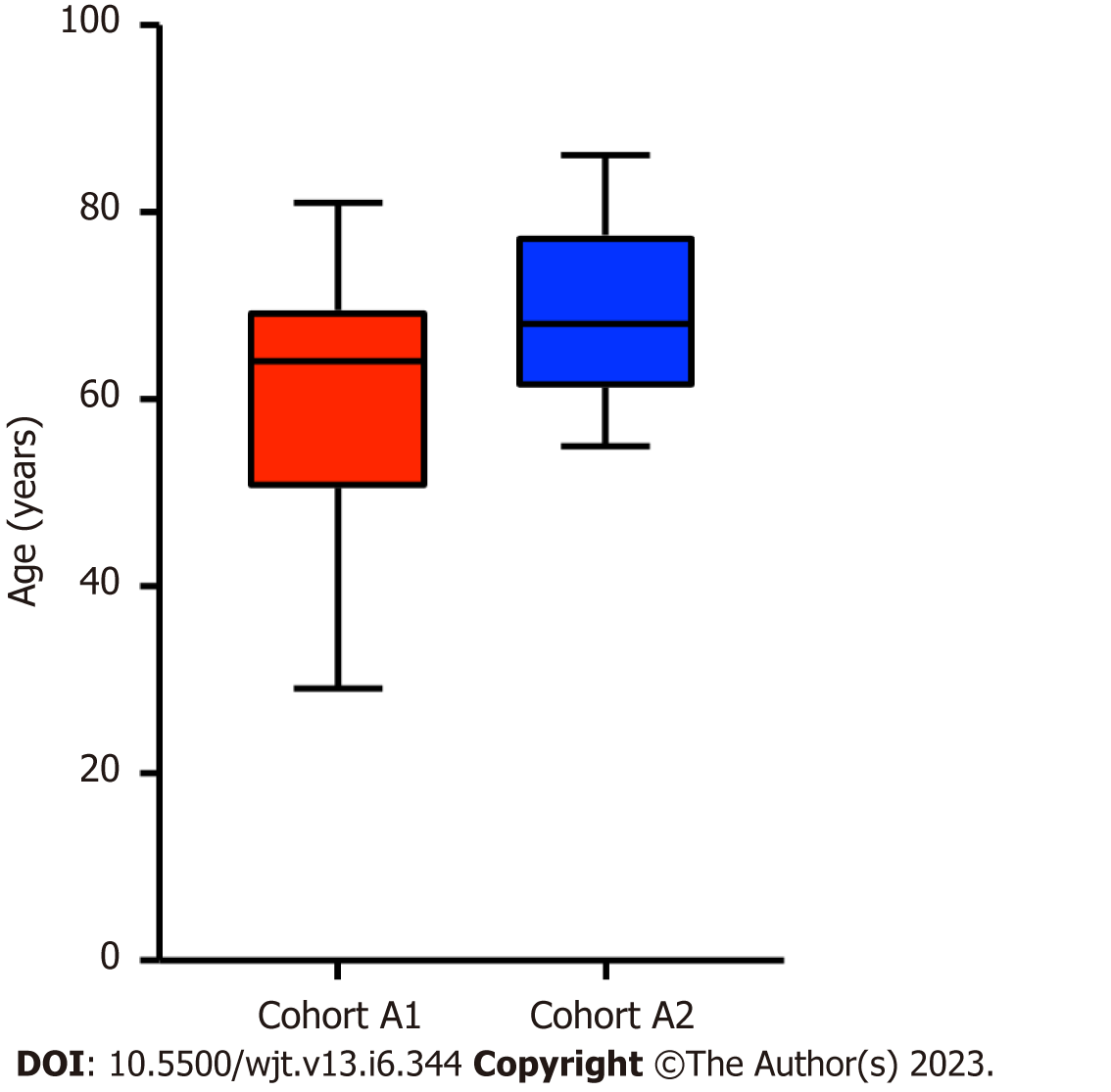
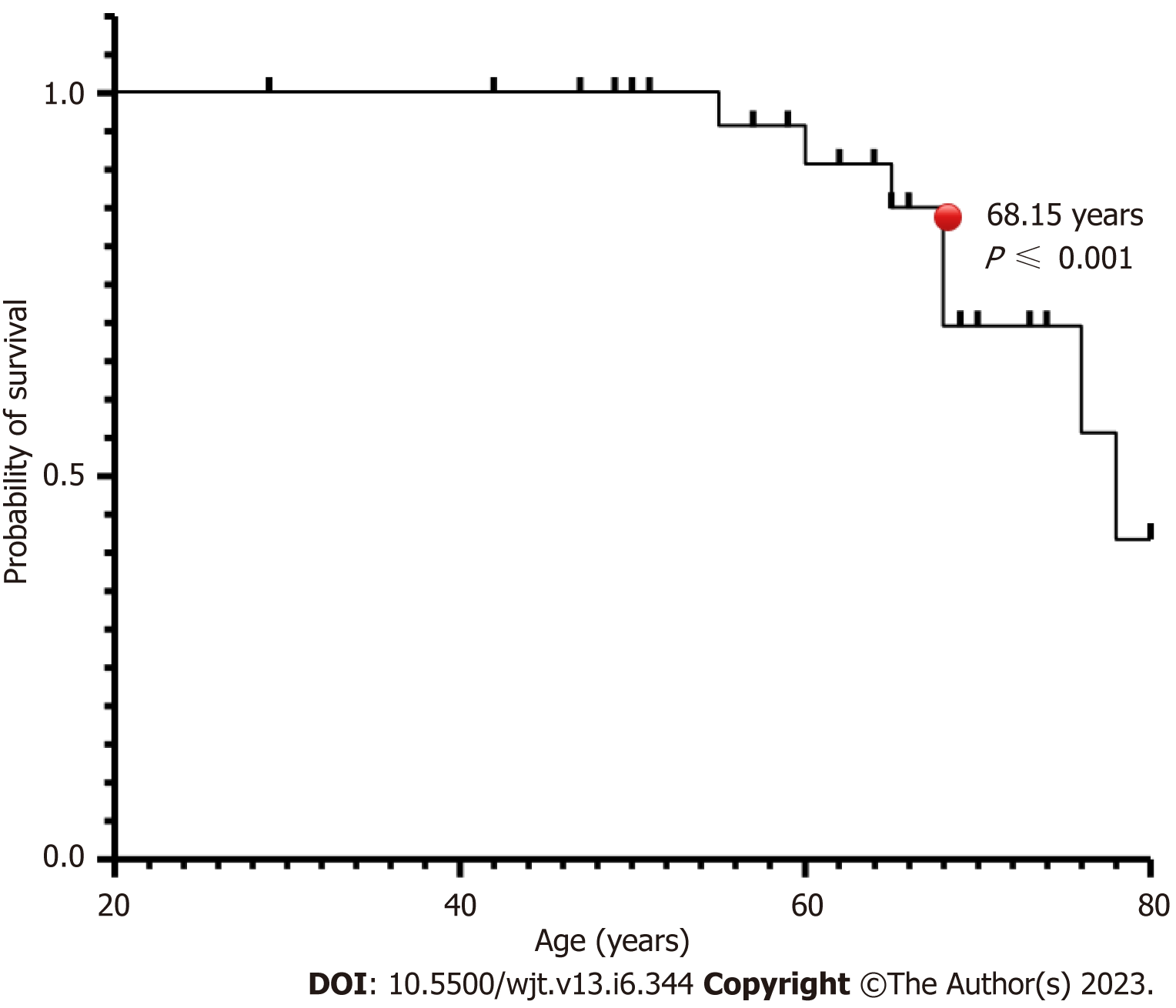
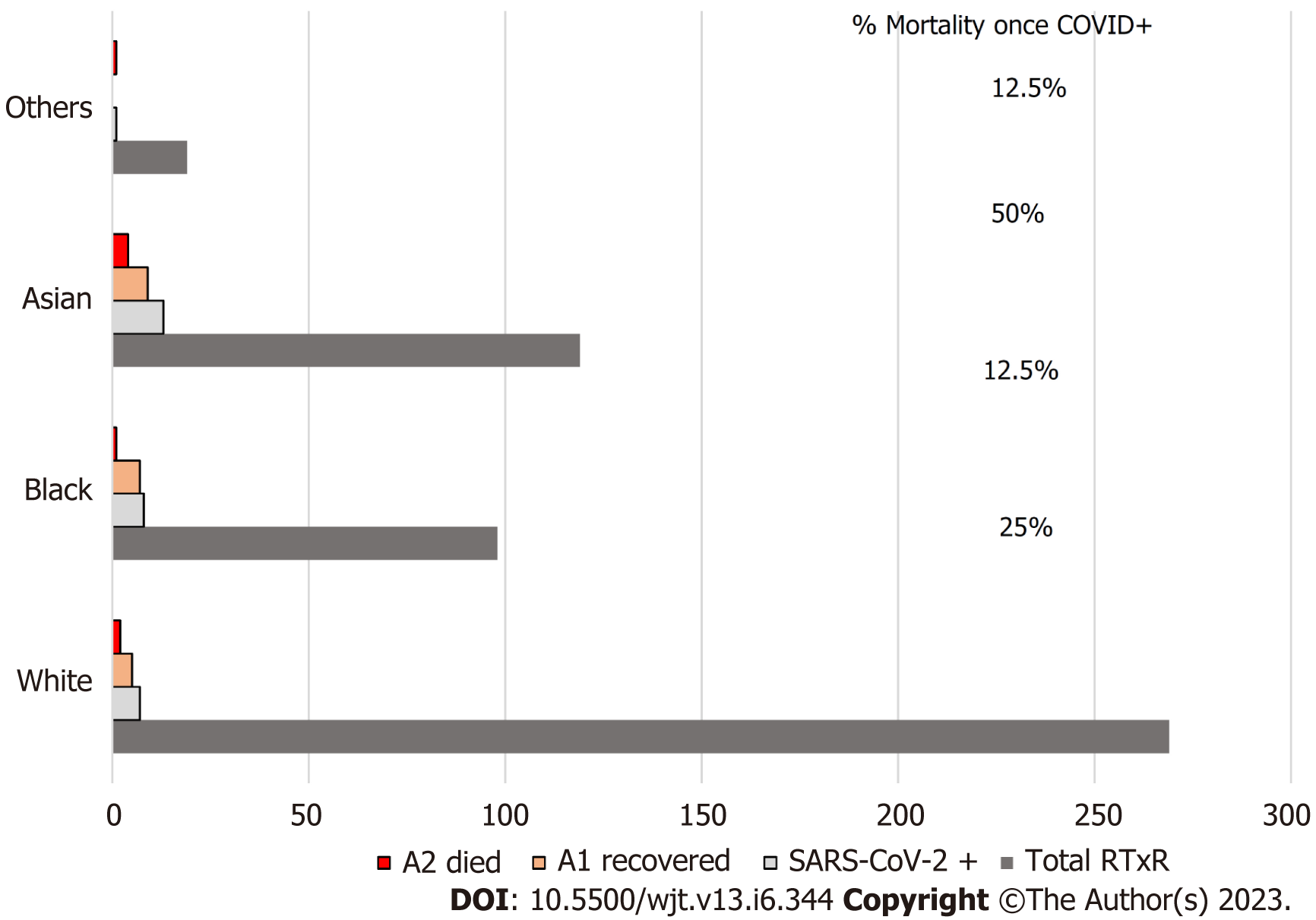
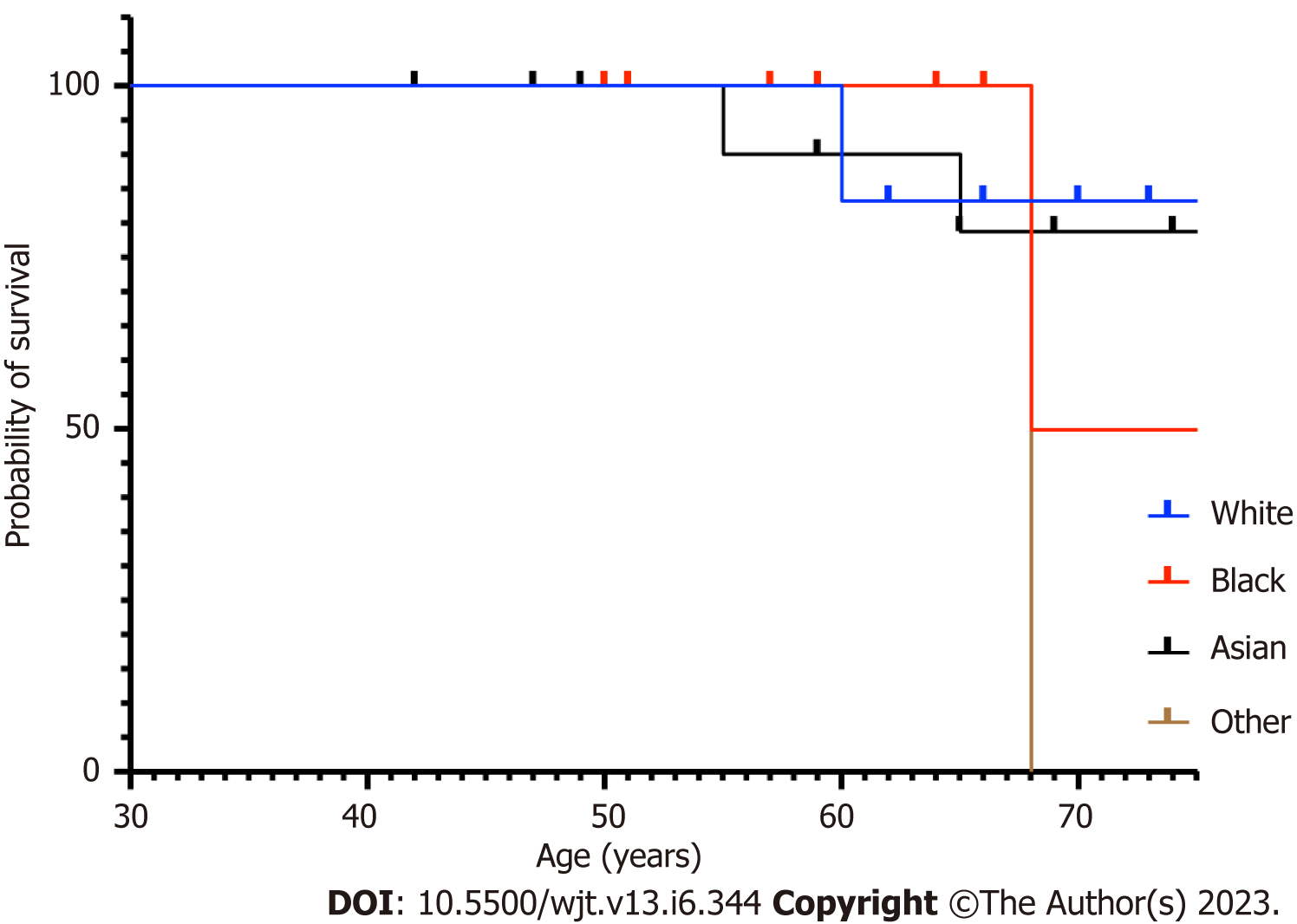
The patients who acquired SARS-CoV-2 infection were from a significantly older age group with a mean (SD) and median interquartile range (IQR) of 63.24 (12.57) and 65 (56-71.5) compared to rest of the group; P ≤ 0.001 (Table 1, Figure 1). In intra-cohort A analysis, where all patients were exposed to SARS-CoV-2 infection, the mean (SD) and median (IQR) age in years in cohort A1 and A 2 were 60.85 (12.5) and 64 (64-69.5) compared with 69.5 (9.5) and 68 (68-77); P = 0.0986 (Figure 2). However, on further analysis of survival probability, a direct correlation was noted between older age and mortality (Figure 3). There was a significant drop in survival probability recorded once patients crossed 68 years of age; P ≤ 0.001. When comparing gender distribution as a risk of SARS-CoV-2 infection there was no significance recorded between cohort A and B; P = 0.3056. However, when the risk of mortality was compared in the SARS-CoV-2 infection-positive group, there was a higher risk of mortality among male patients, with 75% of the deceased patients being male; P ≤ 0.0001 (Table 1). Non-Caucasian ethnicity was associated with high mortality risk once infected with SARS-CoV-2; P ≤ 0.001 (Figure 4). The survival probability was worst in older patients from Middle Eastern ethnicity followed by Black, Asian and White ethnicities (Figure 5).
| Demographic | Cohort A (n = 29), SARS-CoV-2 infection | Cohort B (n = 476), no infection | Significance |
| Age | |||
| Mean (SD) | 63.24 (12.57) | 55.70 (13.63) | < 0.001 |
| Median (IQR) | 65 (56-71.5) | 57 (46-66) | |
| Gender | |||
| Male | 14 (48.27) | 276 (57.8) | 0.3056 |
| Female | 15 (51.73) | 200 (42.2) | |
| Ethnicity | |||
| White | 7 (24.13) | 252 (52.94) | |
| Black | 8 (27.58) | 90 (18.9) | |
| Asian | 13 (44.82) | 126 (26.47) | < 0.001a |
| Others | 1 (3.44) | 8 (1.6) | |
| Cohort A1 | Cohort A2 | ||
| (Recovered; n = 21) | (Died; n = 8) | ||
| Age | |||
| Mean (SD) | 60.85 (12.5) | 69.5 (9.5) | 0.0986 |
| Median (IQR) | 64 (64-69.5) | 68 (68-77) | |
| Gender | |||
| Male | 8 (38) | 6 (75) | < 0.001 |
| Female | 13 (62) | 2 (25) | |
| Ethnicity | |||
| White | 5 (23.8) | 2 (25) | |
| Black | 7 (33.33) | 1 (12.5) | |
| Asian | 9 (42.85) | 4 (50) | < 0.001b |
| Others | 0 | 1 (12.5) | |
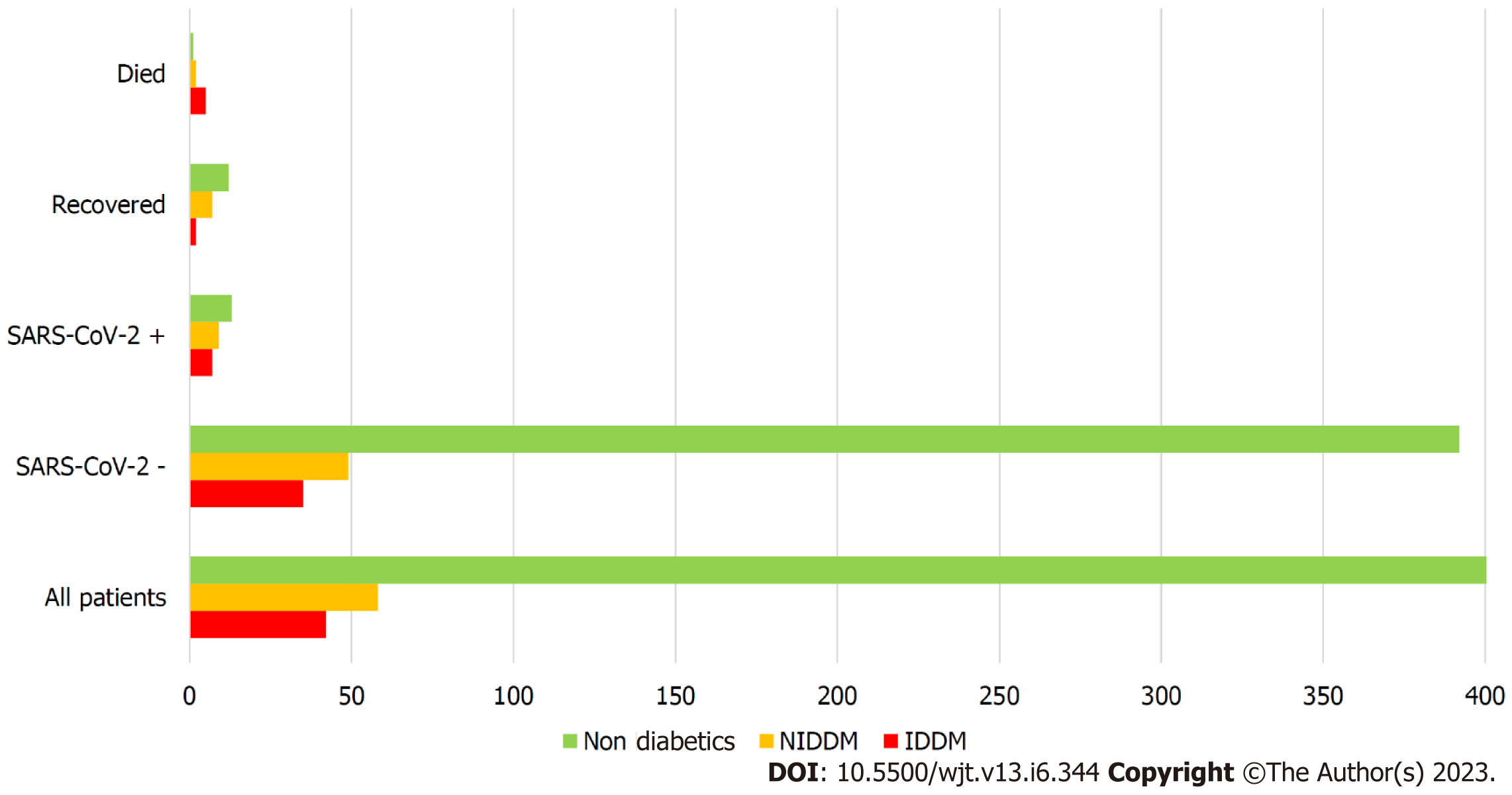
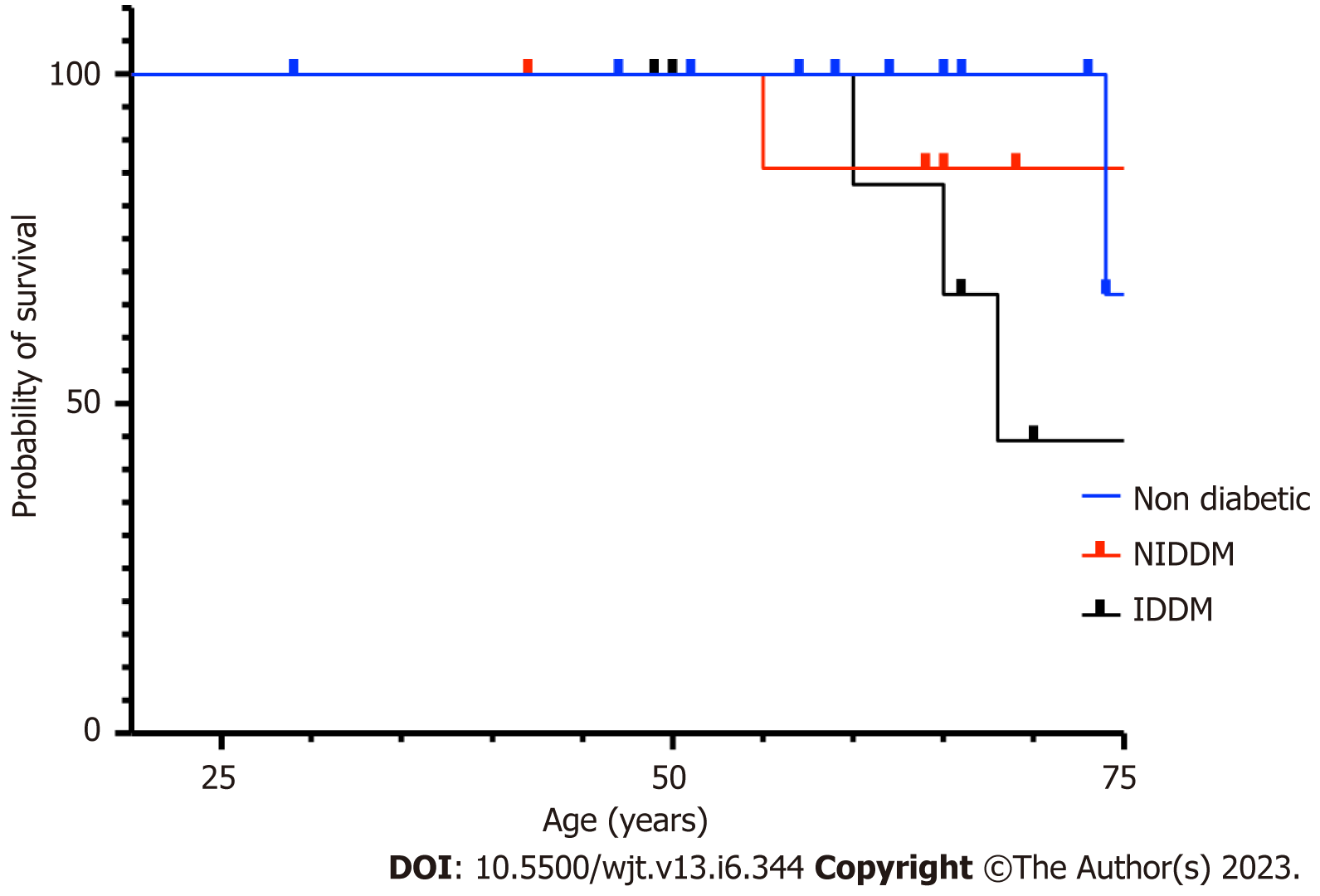
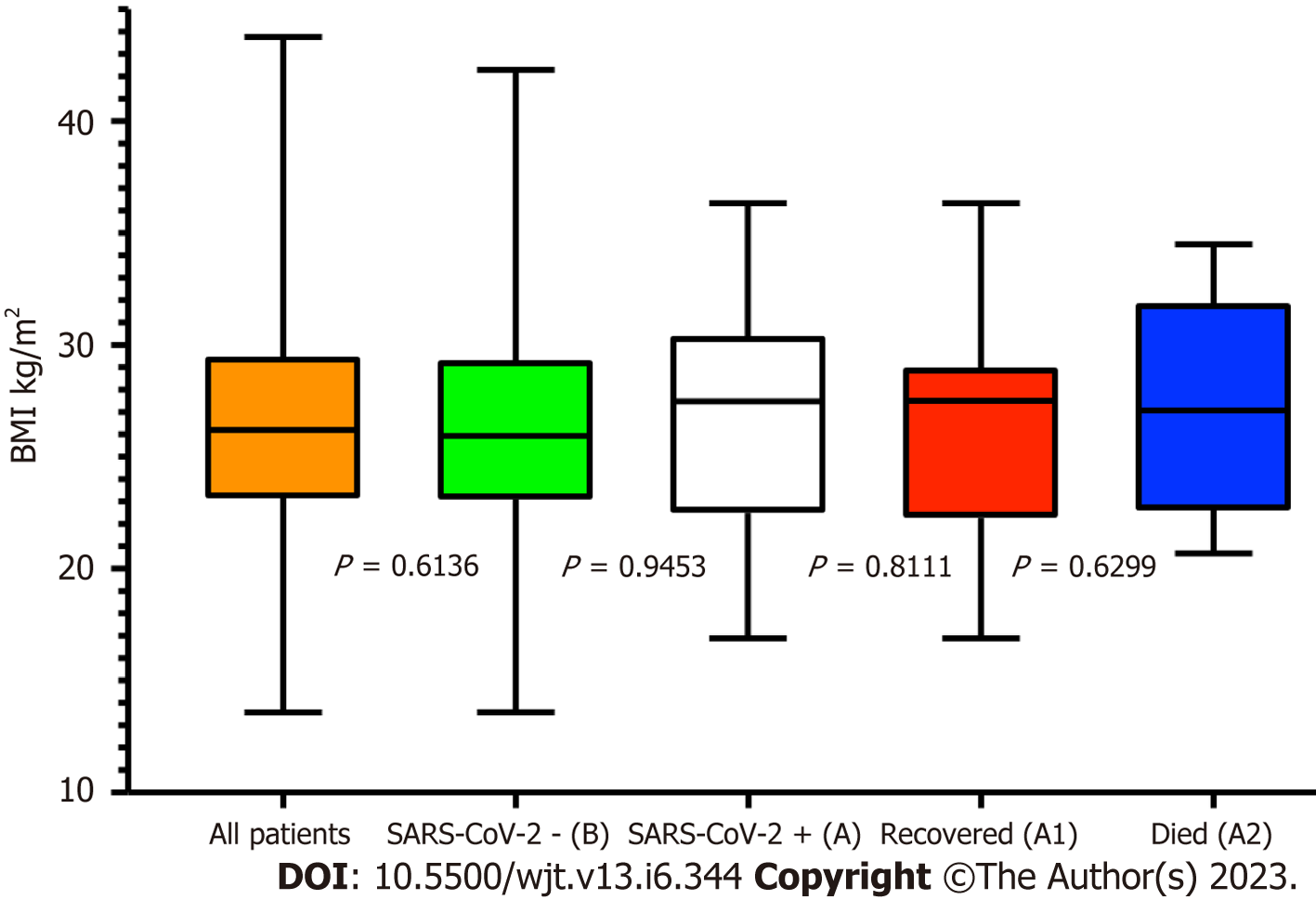
The overall prevalence of diabetes in our RTxR follow-up patients was 20%. Of the patients who acquired SARS-CoV-2 infection, 55% were diabetic (Cohort A) with 87.5% among deceased (Cohort A2); P ≤ 0.0.0001 (Figure 6). This suggests diabetes is a major risk factor for SARS-CoV-2-related mortality in renal transplant patients. We further analysed survival probability depending on the recipient's age and diabetic status and found a direct correlation between old age, diabetes and mortality (Figure 7). The mean (SD) body mass index (BMI) kg/m2 of the overall RTxR population was 26.60 (4.81). There were no significant differences recorded in BMI across the RTxR population (Figure 8). There was no impact of BMI on mortality. In our RTxR follow-up cohort hypertension and history of ischemic heart disease were also not indep
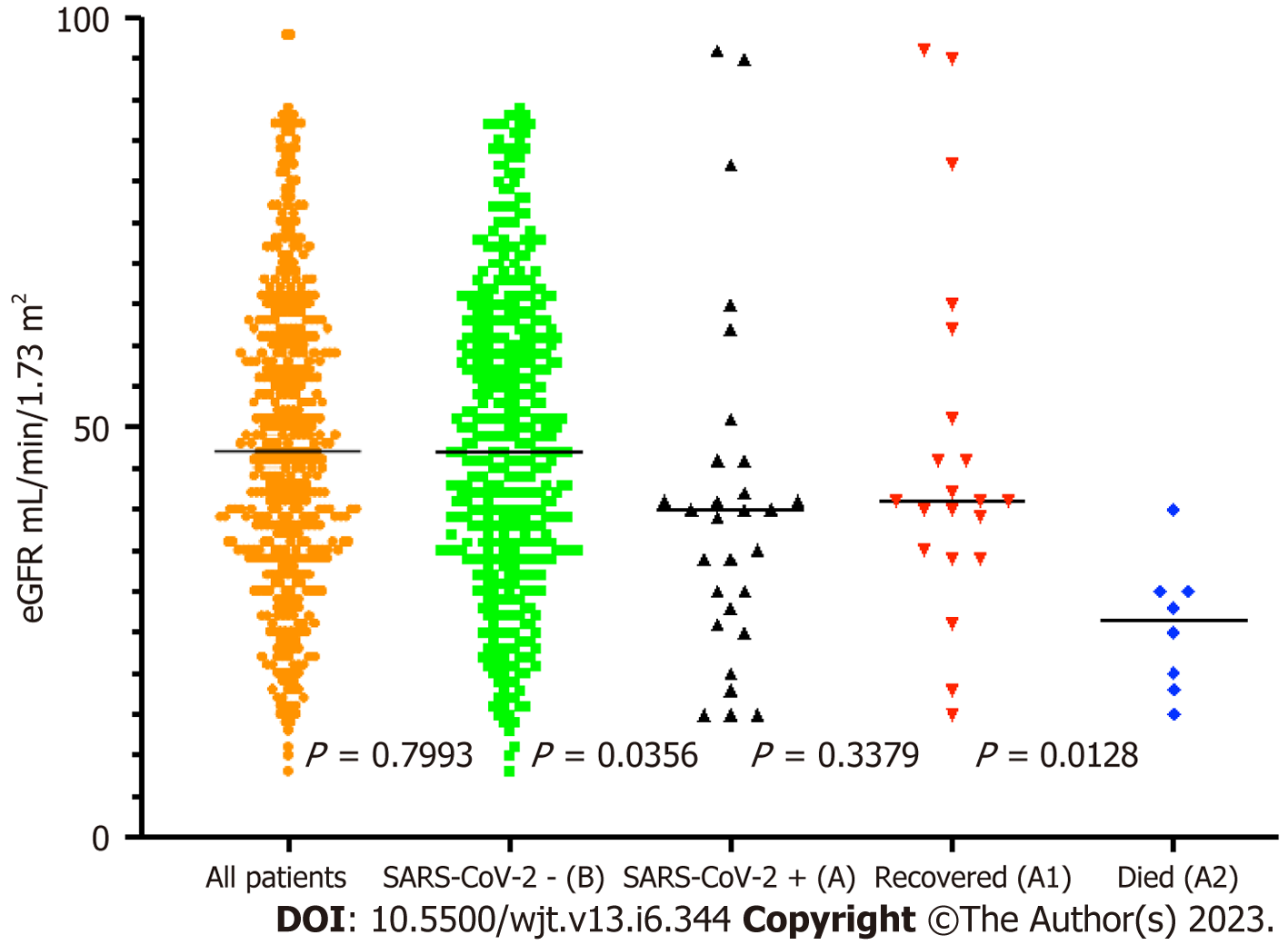
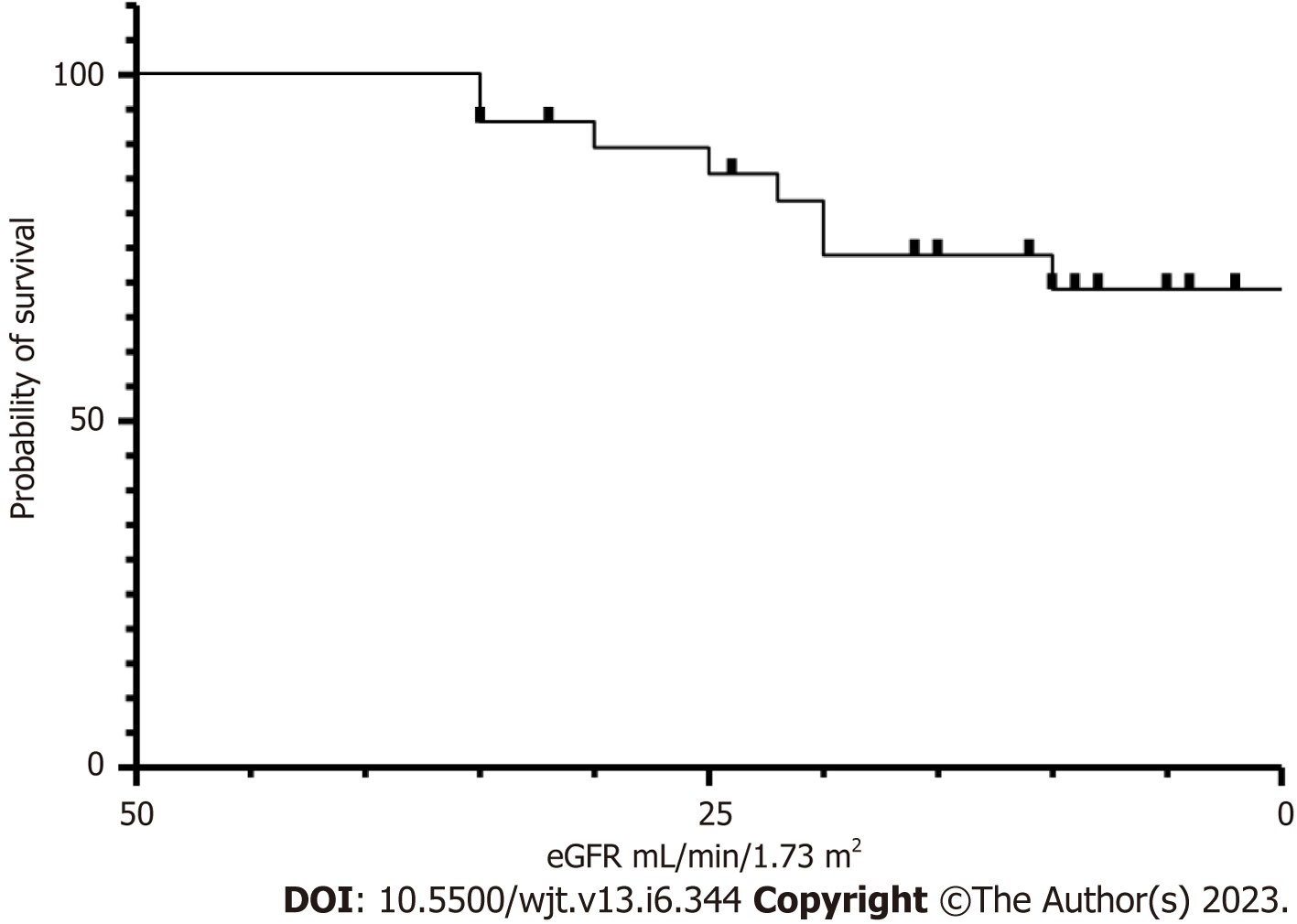
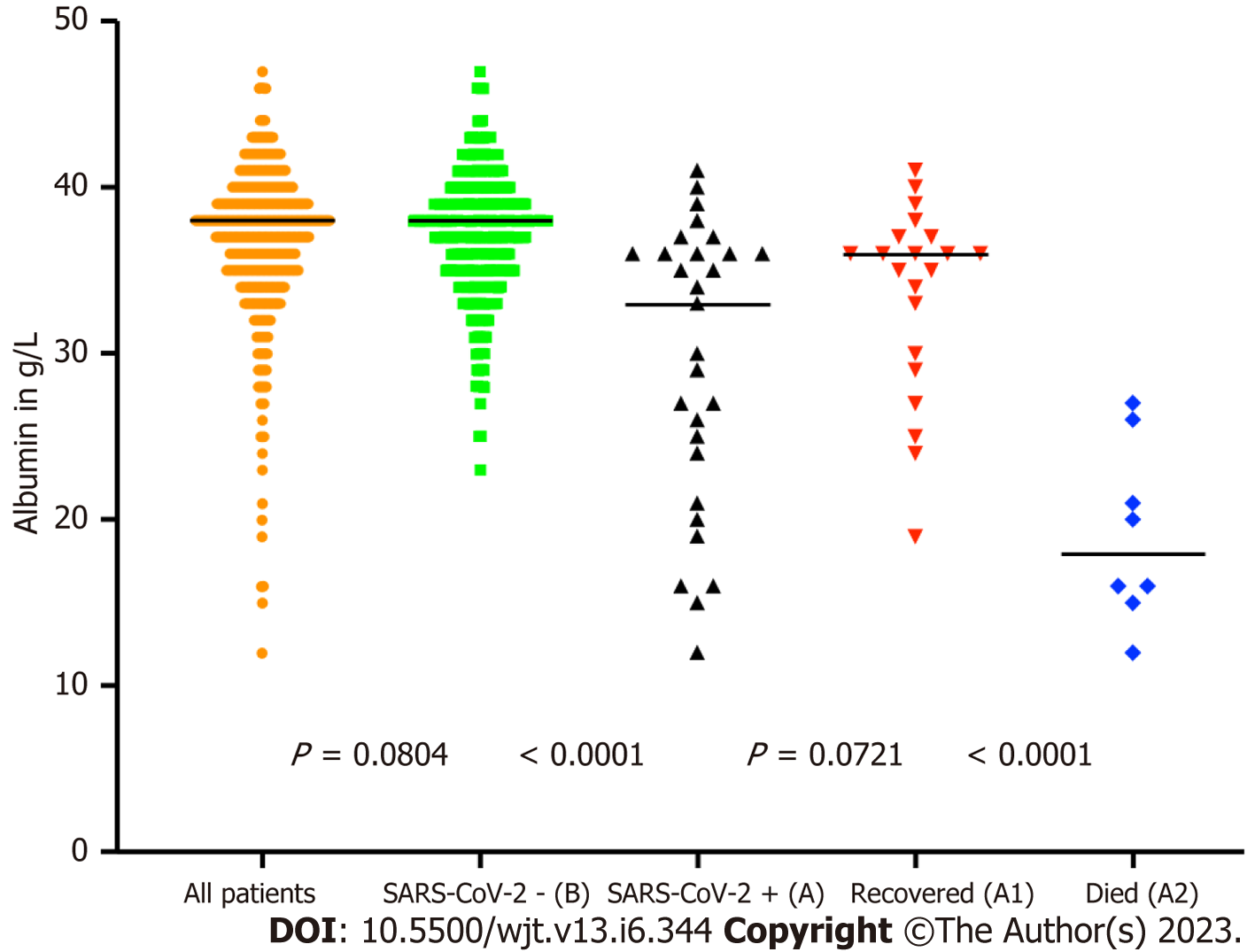
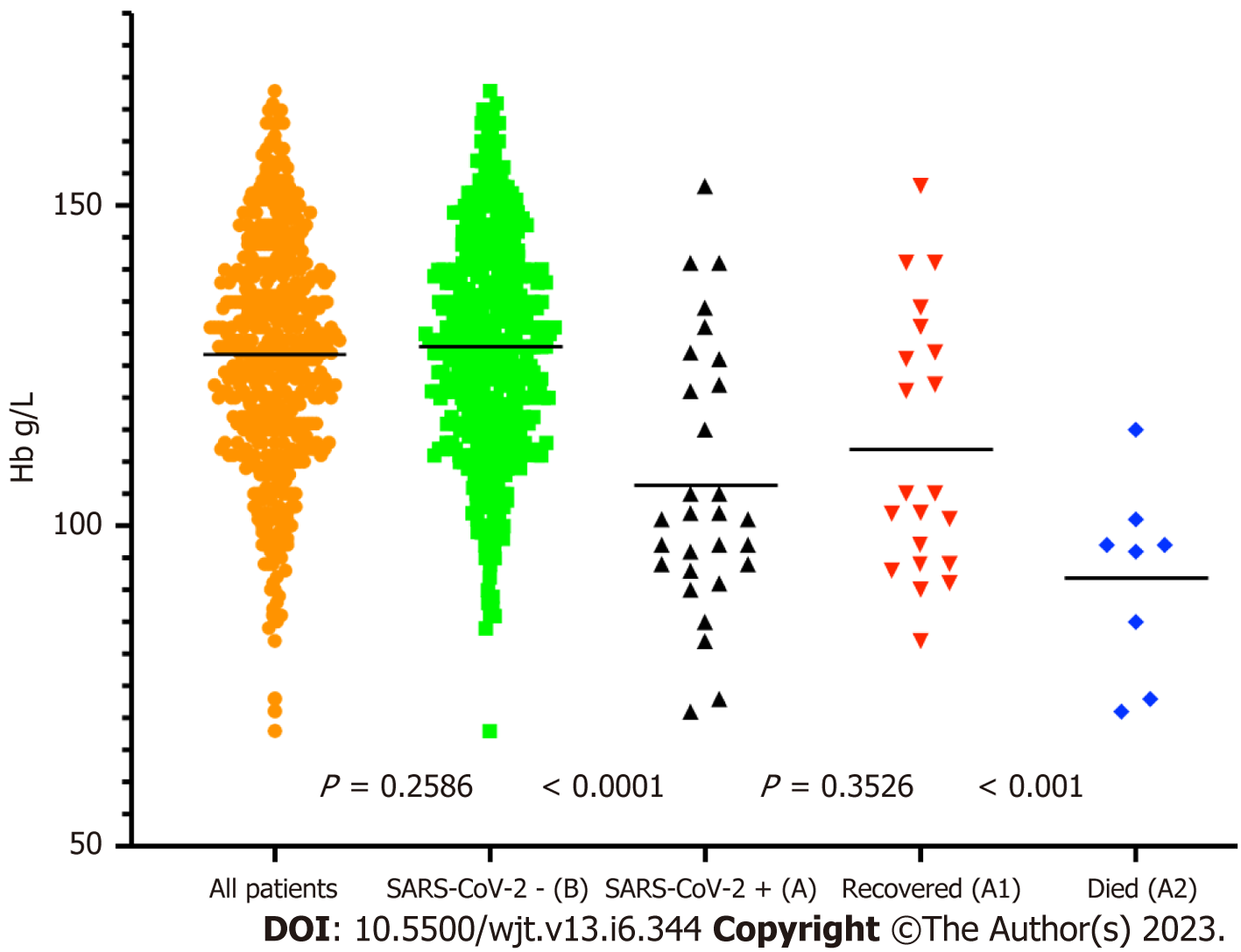
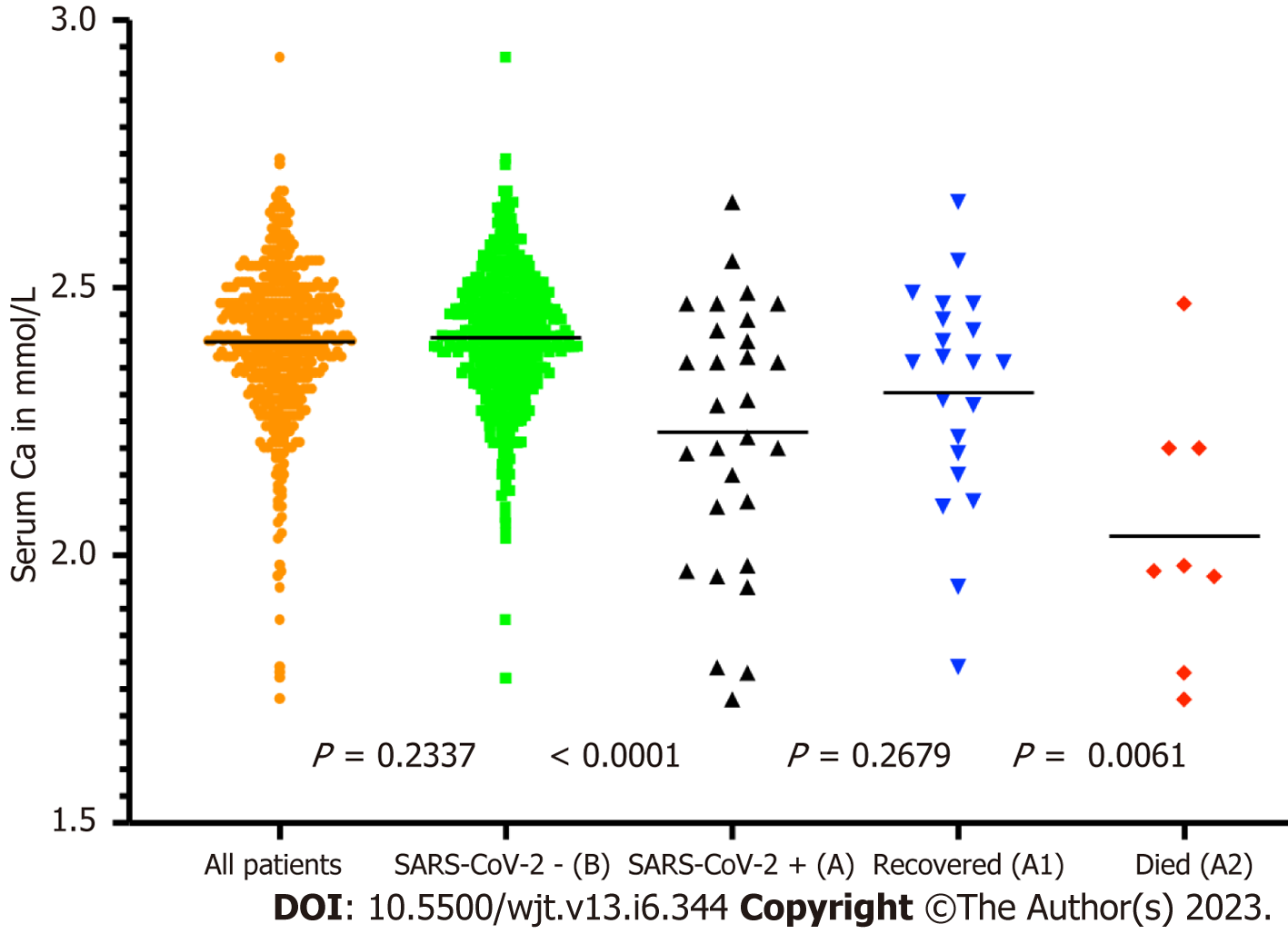
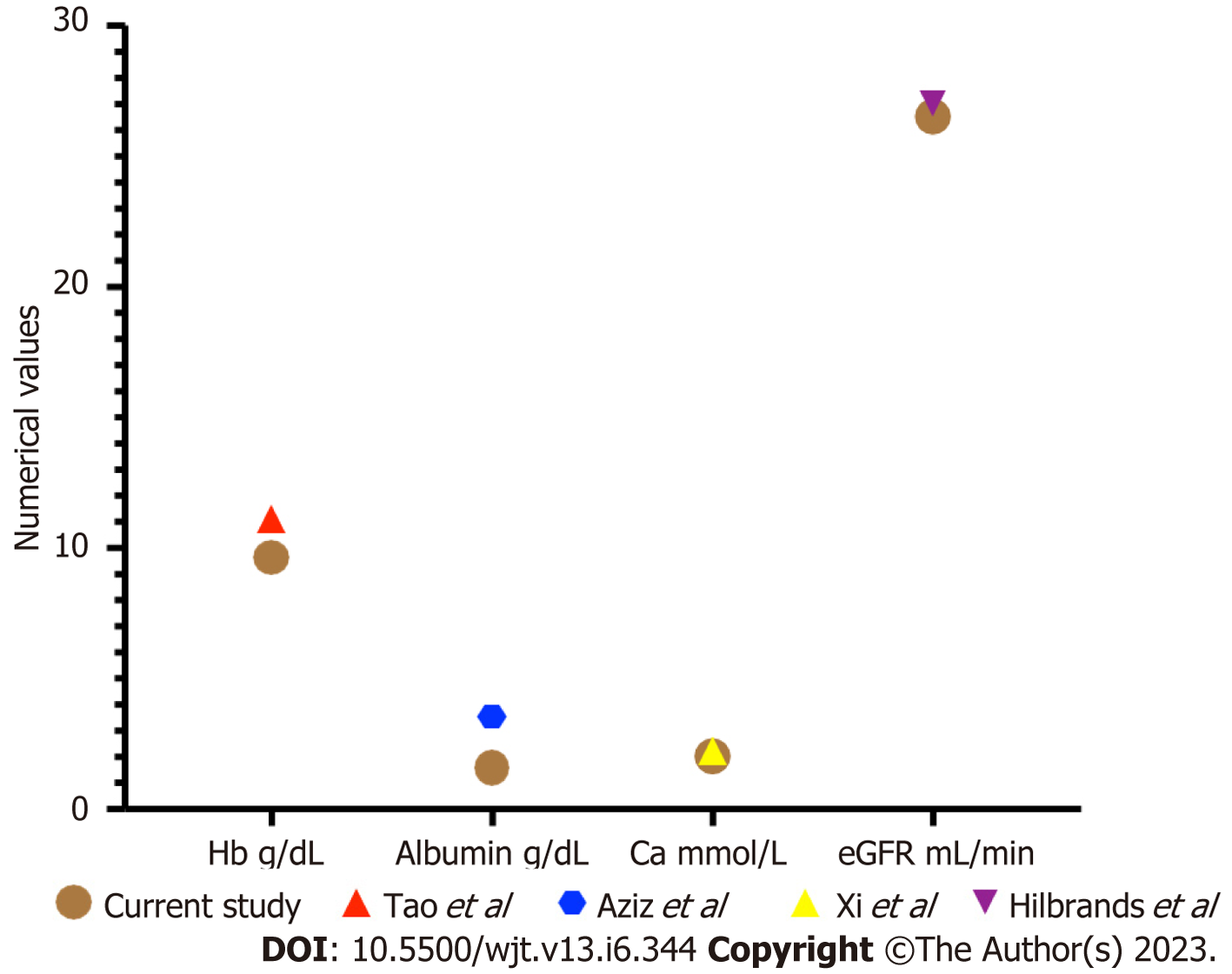
We analysed some of the routinely monitored laboratory investigations to identify the severity of SARS-CoV-2 infection. We analysed the latest laboratory markers just prior to SARS-CoV-2 infection to avoid any impact of acute infection on these markers. We investigated the patient's renal allograft function recorded as an eGFR in mL/min/1.73 m2). The mean (SD) and median (IQR) in cohort A1 and A2 were 47 (21) and 41 (41-56.5); and 25.75 (7.5) and 26.5 (26.5-30) respectively, P = 0.0128 (Figure 9). The poor functional quality of the renal allograft was directly related to a higher risk of mortality (Figure 10). The second marker we investigated was serum albumin level. The mean (SD) and median (IQR) of serum albumin in g/L of all RTxR patients was 36.81 (4.36) and 38 (35-39) which was within the normal range (Figure 11). On review, there was a significant difference between serum albumin levels of patients who recovered from SARS-CoV-2 infection as compared to those who died; P ≤ 0.0001. There was also a significant relation noted between low albumin levels and a high risk of mortality, particularly when serum albumin was less than 20g/L P ≤ 0.001. The mean (SD) and median (IQR) (Hb g/L) of overall RTxR were 126.9 (18.07) and 127 (115-140) compared to 106.4 (20.8) in group A and 101 (93-124) in group A2; P ≤ 0.001 (Figure 12). On further comparison between patients who recovered (A1) with patients who died (A2), a significant difference was recorded; P ≤ 0.0001. Looking at survival probability Hb less than 7 was associated with higher mortality. Finally, we looked at serum calcium levels across our RTxR. The mean (SD) and median (IQR) values of serum calcium in mmol/L of the overall RTxR cohort were 2.39 (0.14) and 2.41 (2.3-2.4). There was a significant difference in serum calcium recorded between all RTxR and SARS-CoV-2 positive; P ≤ 0.001 and also between patients who recovered and those who died; P = 0.0061 (Figure 13). We found a significant correlation between low serum calcium levels and mortality once the level falls below 1.70 mmol/L < 0.001.
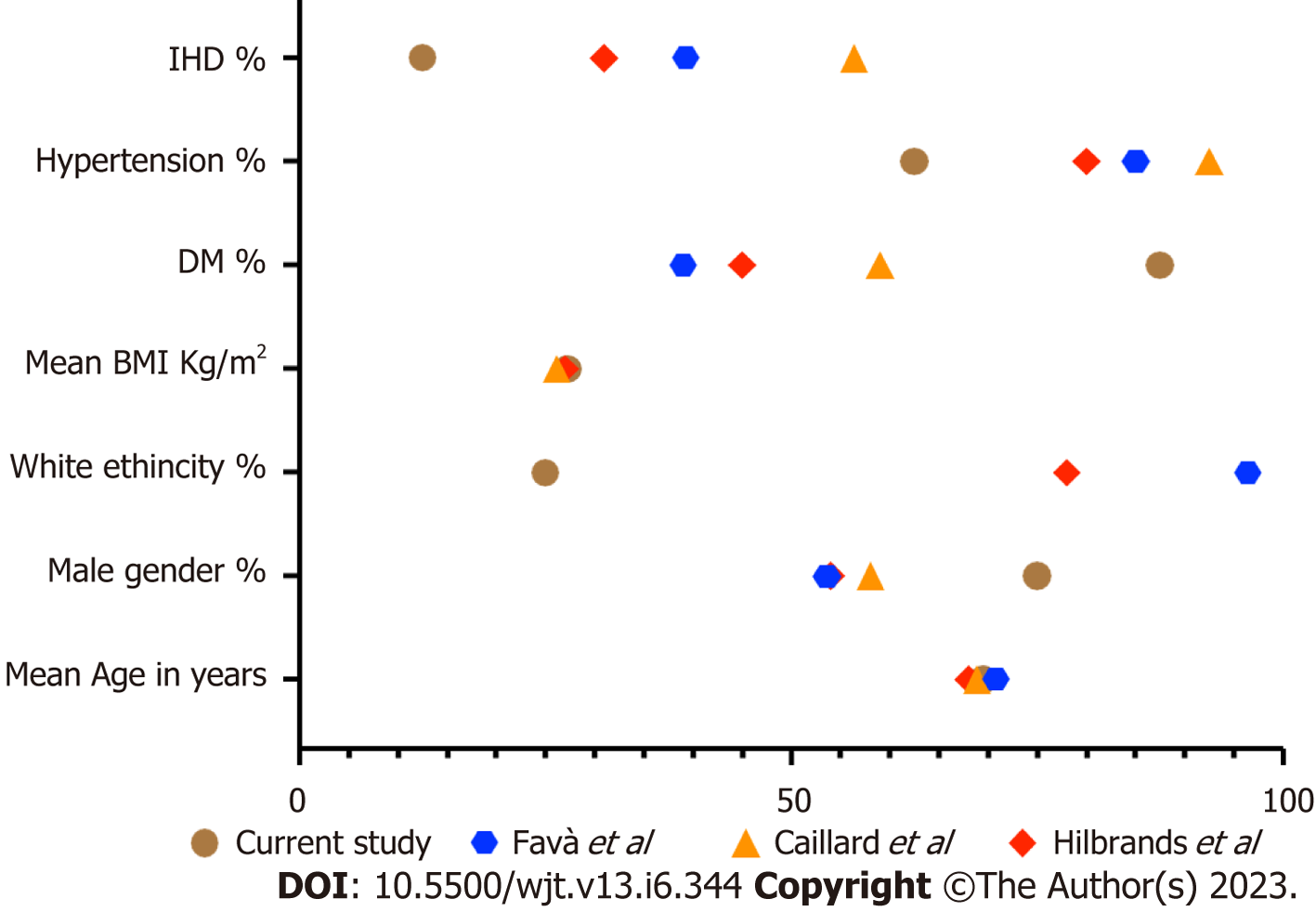
The recipients of solid organ transplantation are more vulnerable to opportunistic infections due to immunosuppressant medication. They demonstrate reduced resistance to infection, rapid progression of pathology, atypical clinical presentations and high risk of morbidity and mortality[26]. In addition to these factors, recovery from infection is dependent on various factors including the general well-being of the recipient and associated comorbidities. In renal transplant recipients, the functional status of the renal allograft also plays a vital part in the recovery phase, particularly when medication dosage is dependent on renal function. In addition to this, treatment regimens may be complicated by drug interactions and the need to maintain immunosuppression to prevent rejection. This complex interconnection between high-risk of infection, allograft function, limited treatment choices and associated comorbidities makes post-transplant infections the leading cause of morbidity and mortality[27]. During SARS-CoV-2 pandemic, the reported mortality among renal transplant recipients was as high as 33%[17]. In various general population studies, greater than 75 years of age, male gender and BMI greater than 40 are associated with significant mortality[28]. In our study, the mean (SD) age of recipient mortality was 69.5 (± 9.5) with a significantly increased risk of mortality after age 68 years and above. This finding is comparable to other studies in the renal transplant population[11,17,28] (Figure 14). These findings confirm that renal transplant recipients are at high risk of mortality at an earlier age when compared to the general population. We recorded a higher rate of mortality among male patients. This is also comparable with other published data[11,17,27,29]. Comparing BMI, there was no significant difference recorded in various groups in our study. The mean (SD) BMI in our mortality group was 27.20 (4.97) which is comparable to other published studies in the transplant population[11,17,29]. In general population studies, there was a high risk of mortality recorded in patients with a BMI greater than 40, but this BMI range is uncommon in renal transplant recipients. Surprisingly, we did not find any significant impact of hyper
It would be very easy and cost-effective to incorporate the findings of this study into any post-operative follow-up pathway and protocol for RTxRs. These simple parameters can help to risk stratify RTxRs into high and low-risk categories. In addition to this, despite having a failing renal transplant, early intervention to improve a patient’s anaemia, hypercalcemia and hypoalbuminemia could reduce their risk of morbidity and mortality. Early identification of at-risk sub-groups within those already identified as being high-risk, can further reduce the risk of infection-associated mortality, with timely interventions.
Various studies have been done to separately study routine laboratory markers to stratify patients with high risk of morbidity and mortality but very little is known in renal transplant patients.
This study provides a new way of looking at the significance of routine laboratory tests with an aim to risk stratify renal transplant recipients into high-risk sub-groups.
This study will help in shaping new policies and guidelines by providing individualized shielding advice, self-isolation guidance and booster coronavirus disease 2019 vaccination. Moreover, this will also help to plan better follow-up strategies for transplant patient. Addressing and correcting these parameters during a follow-up program can reduce the risk of morbidity and mortality in renal transplant recipients (RTxR).
Retrospective observational study to analyze the data of our renal transplant follow-up program for various routine parameters and their impact of patient outcomes.
This study has identified some routinely used modifiable parameters in predicting a higher risk of mortality and morbidity.
This knowledge can be used in RTxR follow-up programs by addressing these parameters early to help reduce the morbidity and mortality in RTxRs.
This knowledge can be used in RTxR follow-up programs by addressing these parameters early to help reduce the morbidity and mortality in RTxRs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Capone D, Italy S-Editor: Liu JH L-Editor: A P-Editor: Zhang YL
| 1. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30049] [Article Influence: 6009.8] [Reference Citation Analysis (3)] |
| 2. | Listings of WHO's response to COVID-19. Accessed April 10, 2021. Available from: https://www.who.int/news/item/29-06-2020-covidtimeline. |
| 3. | WHO Coronavirus (COVID-19) Dashboard. Accessed June 22, 2021. Available from: https://covid19.who.int. |
| 4. | Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, Bonanomi E, Cabrini L, Carlesso E, Castelli G, Cattaneo S, Cereda D, Colombo S, Coluccello A, Crescini G, Forastieri Molinari A, Foti G, Fumagalli R, Iotti GA, Langer T, Latronico N, Lorini FL, Mojoli F, Natalini G, Pessina CM, Ranieri VM, Rech R, Scudeller L, Rosano A, Storti E, Thompson BT, Tirani M, Villani PG, Pesenti A, Cecconi M; COVID-19 Lombardy ICU Network. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 1052] [Article Influence: 210.4] [Reference Citation Analysis (0)] |
| 5. | Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, Gan H, Sun YL, Fu W, Li W, Liang HL, Cao YY, Yan Q, Cao C, Gao HY, Brüggen MC, van de Veen W, Sokolowska M, Akdis M, Akdis CA. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021;76:428-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 878] [Article Influence: 219.5] [Reference Citation Analysis (0)] |
| 6. | Phanish M, Ster IC, Ghazanfar A, Cole N, Quan V, Hull R, Banerjee D. Systematic Review and Meta-analysis of COVID-19 and Kidney Transplant Recipients, the South West London Kidney Transplant Network Experience. Kidney Int Rep. 2021;6:574-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Kumar R, Ison MG. Opportunistic Infections in Transplant Patients. Infect Dis Clin North Am. 2019;33:1143-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Kumar D, Ferreira VH, Blumberg E, Silveira F, Cordero E, Perez-Romero P, Aydillo T, Danziger-Isakov L, Limaye AP, Carratala J, Munoz P, Montejo M, Lopez-Medrano F, Farinas MC, Gavalda J, Moreno A, Levi M, Fortun J, Torre-Cisneros J, Englund JA, Natori Y, Husain S, Reid G, Sharma TS, Humar A. A 5-Year Prospective Multicenter Evaluation of Influenza Infection in Transplant Recipients. Clin Infect Dis. 2018;67:1322-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 9. | L'Huillier AG, Ferreira VH, Hirzel C, Nellimarla S, Ku T, Natori Y, Humar A, Kumar D. T-cell responses following Natural Influenza Infection or Vaccination in Solid Organ Transplant Recipients. Sci Rep. 2020;10:10104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Kompaniyets L, Pennington AF, Goodman AB, Rosenblum HG, Belay B, Ko JY, Chevinsky JR, Schieber LZ, Summers AD, Lavery AM, Preston LE, Danielson ML, Cui Z, Namulanda G, Yusuf H, Mac Kenzie WR, Wong KK, Baggs J, Boehmer TK, Gundlapalli AV. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020-March 2021. Prev Chronic Dis. 2021;18:E66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 207] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 11. | Mamode N, Ahmed Z, Jones G, Banga N, Motallebzadeh R, Tolley H, Marks S, Stojanovic J, Khurram MA, Thuraisingham R, Popoola J, Ghazanfar A, Game D, Sran K, Dor FJMF, Lucisano G, Sinha M, Olsburgh J, Willicombe M. Mortality Rates in Transplant Recipients and Transplantation Candidates in a High-prevalence COVID-19 Environment. Transplantation. 2021;105:212-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Zhu L, Gong N, Liu B, Lu X, Chen D, Chen S, Shu H, Ma K, Xu X, Guo Z, Lu E, Ge Q, Cai J, Jiang J, Wei L, Zhang W, Chen G, Chen Z. Coronavirus Disease 2019 Pneumonia in Immunosuppressed Renal Transplant Recipients: A Summary of 10 Confirmed Cases in Wuhan, China. Eur Urol. 2020;77:748-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 13. | Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, Ross M, Forest S, Goldstein YD, Ajaimy M, Liriano-Ward L, Pynadath C, Loarte-Campos P, Nandigam PB, Graham J, Le M, Rocca J, Kinkhabwala M. Covid-19 and Kidney Transplantation. N Engl J Med. 2020;382:2475-2477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 639] [Article Influence: 127.8] [Reference Citation Analysis (0)] |
| 14. | Caillard S, Anglicheau D, Matignon M, Durrbach A, Greze C, Frimat L, Thaunat O, Legris T, Moal V, Westeel PF, Kamar N, Gatault P, Snanoudj R, Sicard A, Bertrand D, Colosio C, Couzi L, Chemouny JM, Masset C, Blancho G, Bamoulid J, Duveau A, Bouvier N, Chavarot N, Grimbert P, Moulin B, Le Meur Y, Hazzan M; French SOT COVID Registry. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 15. | Bossini N, Alberici F, Delbarba E, Valerio F, Manenti C, Possenti S, Econimo L, Maffei C, Pola A, Terlizzi V, Salviani C, Moscato M, Pasquali S, Zambetti N, Tonoli M, Affatato S, Pecchini P, Viola FB, Malberti F, Depetri G, Gaggiotti M, Scolari F; Brescia Renal COVID task force. Kidney transplant patients with SARS-CoV-2 infection: The Brescia Renal COVID task force experience. Am J Transplant. 2020;20:3019-3029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Sánchez-Álvarez JE, Pérez Fontán M, Jiménez Martín C, Blasco Pelícano M, Cabezas Reina CJ, Sevillano Prieto ÁM, Melilli E, Crespo Barrios M, Macía Heras M, Del Pino Y Pino MD. SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN). Nefrologia (Engl Ed). 2020;40:272-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 17. | Favà A, Cucchiari D, Montero N, Toapanta N, Centellas FJ, Vila-Santandreu A, Coloma A, Meneghini M, Manonelles A, Sellarés J, Torres I, Gelpi R, Lorenzo I, Ventura-Aguiar P, Cofan F, Torregrosa JV, Perelló M, Facundo C, Seron D, Oppenheimer F, Bestard O, Cruzado JM, Moreso F, Melilli E. Clinical characteristics and risk factors for severe COVID-19 in hospitalized kidney transplant recipients: A multicentric cohort study. Am J Transplant. 2020;20:3030-3041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 18. | Benotmane I, Perrin P, Vargas GG, Bassand X, Keller N, Lavaux T, Ohana M, Bedo D, Baldacini C, Sagnard M, Bozman DF, Chiesa MD, Cognard N, Olagne J, Delagreverie H, Marx D, Heibel F, Braun L, Moulin B, Fafi-Kremer S, Caillard S. Biomarkers of Cytokine Release Syndrome Predict Disease Severity and Mortality From COVID-19 in Kidney Transplant Recipients. Transplantation. 2021;105:158-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Gautier-Vargas G, Baldacini C, Benotmane I, Keller N, Perrin P, Moulin B, Caillard S. Rapid resolution of cytokine release syndrome and favorable clinical course of severe COVID-19 in a kidney transplant recipient treated with tocilizumab. Kidney Int. 2020;98:508-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Roberts MB, Izzy S, Tahir Z, Al Jarrah A, Fishman JA, El Khoury J. COVID-19 in solid organ transplant recipients: Dynamics of disease progression and inflammatory markers in ICU and non-ICU admitted patients. Transpl Infect Dis. 2020;22:e13407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Ran J, Song Y, Zhuang Z, Han L, Zhao S, Cao P, Geng Y, Xu L, Qin J, He D, Wu F, Yang L. Blood pressure control and adverse outcomes of COVID-19 infection in patients with concomitant hypertension in Wuhan, China. Hypertens Res. 2020;43:1267-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 22. | Tao Z, Xu J, Chen W, Yang Z, Xu X, Liu L, Chen R, Xie J, Liu M, Wu J, Wang H, Liu J. Anemia is associated with severe illness in COVID-19: A retrospective cohort study. J Med Virol. 2021;93:1478-1488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 23. | Aziz M, Fatima R, Lee-Smith W, Assaly R. The association of low serum albumin level with severe COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 24. | Zhou X, Chen D, Wang L, Zhao Y, Wei L, Chen Z, Yang B. Low serum calcium: a new, important indicator of COVID-19 patients from mild/moderate to severe/critical. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 25. | Hilbrands LB, Duivenvoorden R, Vart P, Franssen CFM, Hemmelder MH, Jager KJ, Kieneker LM, Noordzij M, Pena MJ, Vries H, Arroyo D, Covic A, Crespo M, Goffin E, Islam M, Massy ZA, Montero N, Oliveira JP, Roca Muñoz A, Sanchez JE, Sridharan S, Winzeler R, Gansevoort RT; ERACODA Collaborators. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973-1983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 26. | Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 509] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 27. | Ko KS, Cho DO, Ahn JH, Lee TW, Ihm CG, Chang SG, Chai SE, Park HC, Hong SH, Joo HZ. Infections after renal transplantation. Transplant Proc. 1994;26:2072-2074. [PubMed] |
| 28. | Booth A, Reed AB, Ponzo S, Yassaee A, Aral M, Plans D, Labrique A, Mohan D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS One. 2021;16:e0247461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 360] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 29. | Zhang XB, Hu L, Ming Q, Wei XJ, Zhang ZY, Chen LD, Wang MH, Yao WZ, Huang QF, Ye ZQ, Cai YQ, Zeng HQ. Risk factors for mortality of coronavirus disease-2019 (COVID-19) patients in two centers of Hubei province, China: A retrospective analysis. PLoS One. 2021;16:e0246030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Fontanet A, Autran B, Lina B, Kieny MP, Karim SSA, Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397:952-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 367] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 31. | Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2146] [Cited by in RCA: 1656] [Article Influence: 331.2] [Reference Citation Analysis (1)] |