Published online Dec 18, 2023. doi: 10.5500/wjt.v13.i6.321
Peer-review started: September 19, 2023
First decision: October 9, 2023
Revised: October 10, 2023
Accepted: November 2, 2023
Article in press: November 2, 2023
Published online: December 18, 2023
Processing time: 89 Days and 20.7 Hours
Limbal stem cell deficiency (LSCD) causes severe vision impairment and can lead to blindness, representing one of the most challenging ocular surface disorders. Stem cell deficiency can be congenital or, more often, acquired. The categorization of ocular surface transplantation techniques is crucial to achieving treatment homogeneity and quality of care, according to the anatomic source of the tissue being transplanted, genetic source, autologous or allogenic transplantation (to reflect histocompatibility in the latter group), and cell culture and tissue engi
Core Tip: Limbal cell transplantation has been developed for the management of limbal stem cell (LSC) deficiency, to improve this condition and related complications, ameliorating visual acuity and quality of life of affected patients. Some of the limitations include the lack of specific markers and standardized methods to identify LSCs, as well as the need to standardize the choice of therapeutic options which have diversified over the years and have evolved in terms of technology, efficacy, and safety. This clinical update review is to enable clinicians with the best evidence and current recommendations for managing their patients within the most advanced limbal cell transplant techniques.
- Citation: Tonti E, Manco GA, Spadea L, Zeppieri M. Focus on limbal stem cell deficiency and limbal cell transplantation. World J Transplant 2023; 13(6): 321-330
- URL: https://www.wjgnet.com/2220-3230/full/v13/i6/321.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i6.321
The primary function of the cornea is to refract light, and its function directly depends on its transparency. One of the factors that is implied in the cornea’s transparency is epithelium integrity. The corneal epithelium is a non-keratinized multilayer cuboid epithelium that covers the cornea starting from the limbus, where the junction between the conjunctiva and cornea is. It is capable of self-renewing thanks to the presence of stem cells. Corneal epithelial limbal stem cells (LSCs) reside preferentially in the basal layer of the peripheral cornea in the limbal zone[1,2]. There are no current specific markers for these cells. The research methods used for the identification of these cells tend to be indirect. The presence of stem cell-associated markers such as p63 and the absence of differentiated cell markers such as chemokine (CK) 12 (for corneal epithelium) or CK19 (for conjunctiva) indicate the putative stem cells[3,4].
With regards to the predominant theory about corneal epithelium regeneration, LSCs asymmetrically divide into transient amplifying cells that migrate centripetally and anteriorly and differentiate into squamous cells[5]. Several current studies suggest that, in experimental models, some stem cells could reside outside the limbus[5-7].
The presence of LSCs is crucial to inhibit the proliferation of the conjunctival epithelium on the corneal surface, and the reduction of their number leads to conjunctivalisation of the corneal surface, persistent and recurrent epithelial defects, scarring, and ulceration of the cornea. This condition is called LSC deficiency (LSCD). LSCD can be primary or secondary. Primary causes can occur for genetic pathologies or idiopathically, while the acquired ones can occur for traumas or autoimmune pathologies (Table 1)[8]. LSCD presents nonspecific symptoms such as discomfort, pain, photophobia, and decreased vision in more severe cases. The signs of LSCD depend upon pathology severity, starting from focal areas with a stippled staining pattern, loss of clarity, epithelium hyperreflectivity on Anterior Segment Optical Coherence Tomography (AS-OCT), and flattening of Vogt palisades. In severe cases, it is possible to have conjunctivalisation of the cornea, whorl pattern in fluorescein staining, superficial corneal neovascularization, persistent epithelium defect, stromal scarring, or sterile melts[9].
| Genetic disease | Acquired immune-mediated | Acquired nonimmune-mediated | Others |
| Congenital aniridia | Steven-Johnson syndrome | Chemical/thermal injury | Ocular surface tumors |
| Keratitis ichthyosis deafness syndrome | Toxic necrolysis | Radiation injury | Drug-induced LSCD |
| Xeroderma pigmentosum | Mucous membrane pemphigoid | Contact lens wear | Idiopathic |
| Ectrodactyly-ectodermal dysplasia-clefting syndrome | Vernal/atopic keratoconjunctivitis | Multiple limbal surgeries | |
| Dyskeratosis congenita | Graft-vs-host disease | Bullous keratopathy | |
| Peter’s anomaly | Chronic lid diseases | ||
| Infectious ocular diseases |
These signs can affect just some portion of the cornea with a clear demarcation between normal and abnormal areas, accordingly with the extension of LSC damage. In the cases of traumatic etiologies, LSCD is commonly asymmetrical. In autoimmune and congenital etiologies, LSC damage is commonly symmetrical. LSCD diagnosis is clinical in frank cases, but it can be confirmed by diagnostic investigation in subtle situations. There are several reliable tests in the diagnosis of LSCD. Understanding the underlying cause of LSC damage and starting adequate therapy are fundamental to ensuring good outcomes of LSCD treatments.
The aim of this minireview is to briefly summarize the important issues regarding the clinical characteristics and management of patients with LSCD, in addition to summarizing the therapeutic outcomes to the development of novel therapeutic approaches, future challenges, and recent findings in the current literature.
We conducted a search of the literature published between January 1, 2002, to December 1, 2022, using MEDLINE (PubMed). The database was first searched using the following keywords: “Limbal Stem Cell Deficiency; Limbal Cell Transplantation; Limbal Stem Cell Deficiency and/or Limbal Cell Transplantation; LSCD, and/or Conjunctival limbal autograft (CLAU); LSCD and/or Conjunctival limbal allograft (CLAL); LSCD and/or Cultivated limbal epithelial transplantation (CLET); and, LSCD and/or Simple limbal epithelial transplantation (SLET)”. We considered only studies in English and those referring to humans and with an abstract, thus reducing the count to 301 papers. The reference lists of all retrieved articles were assessed to identify additional relevant studies. The research of articles was performed using PubMed (https://pubmed.ncbi.nlm.nih.gov) and Reference Citation Analysis (https://www.referencecitationanalysis.com) Only articles with an abstract were considered. A quality score was calculated for each article using a checklist. Each study was independently assessed by at least two reviewers (Tonti E and Zeppieri M), and rating decisions were based on the consensus of the reviewing authors. The most common surgical techniques highlighted in the most relevant studies are shown in Table 1.
A filter paper of nitrocellulose or acetate cellulose is applied over the cornea or the conjunctiva to obtain cells from the ocular surface. Repeating the sampling on the same area allows us to obtain cells from the deeper layers, and this makes the sampling more reliable. The specimens are processed with various stains searching for goblet cells[9-11]. The presence of these cells indicates the invasion of the conjunctival epithelium over the cornea, but their absence does not exclude LSCD. In fact, in some cases, such as Stevens-Johnson syndrome or chronic inflammatory diseases, the number of conjunctival goblet cells can be markedly reduced, and their identification can be difficult[11]. Differentiating corneal epithelial cells from conjunctival epithelial cells, instead, is possible only by immunohistochemistry. As mentioned before, CK2 and CK12 are specific for the mature corneal epithelium, CK3 for the conjunctiva and corneal epithelium, and CK7, CK13, and CK19 are specific for the conjunctival epithelium. Another used marker is mucin 5AC, but it has a low sensitivity. Impression cytology is also useful for analyzing the results of LSCD therapies[12].
With this exam, it is possible to acquire pictures of the corneal microstructures without collecting specimens. The presence of goblet cells in a corneal IVCM, as seen in impression cytology, confirms the diagnosis of LSCD, but their absence cannot exclude the diagnosis because this exam scans just a small area and the morphology of goblet cells can be difficult to recognize in IVCM[13]. In LSCD, the density of the basal cells of the corneal epithelium is decreased and the mean size of cells is increased, and these findings correlate with the severity of the pathology[14]. Other findings are intraepithelial cystic lesions surrounded by goblet cells and the decrease in the density of the sub-basal nervous plexus[15].
This is a non-invasive imaging tool with low operator dependence. LSCD has been associated with epithelial thinning at the cornea and limbus, but these signs are not specific to LSCD[16]. With volumetric scans, it is possible to study the status of Vogt palisades, and their thinning (or absence) is associated with areas with a thinned epithelium. The analysis of the reflectivity of the epithelium and stroma in LSCD shows that epithelial reflectivity varies more than stromal reflectivity, and the ratio between them could be a diagnostic tool for LSCD. Furthermore, this ratio tends to return to normal values after LSC transplantation, even if it does not return to normal values[11,17].
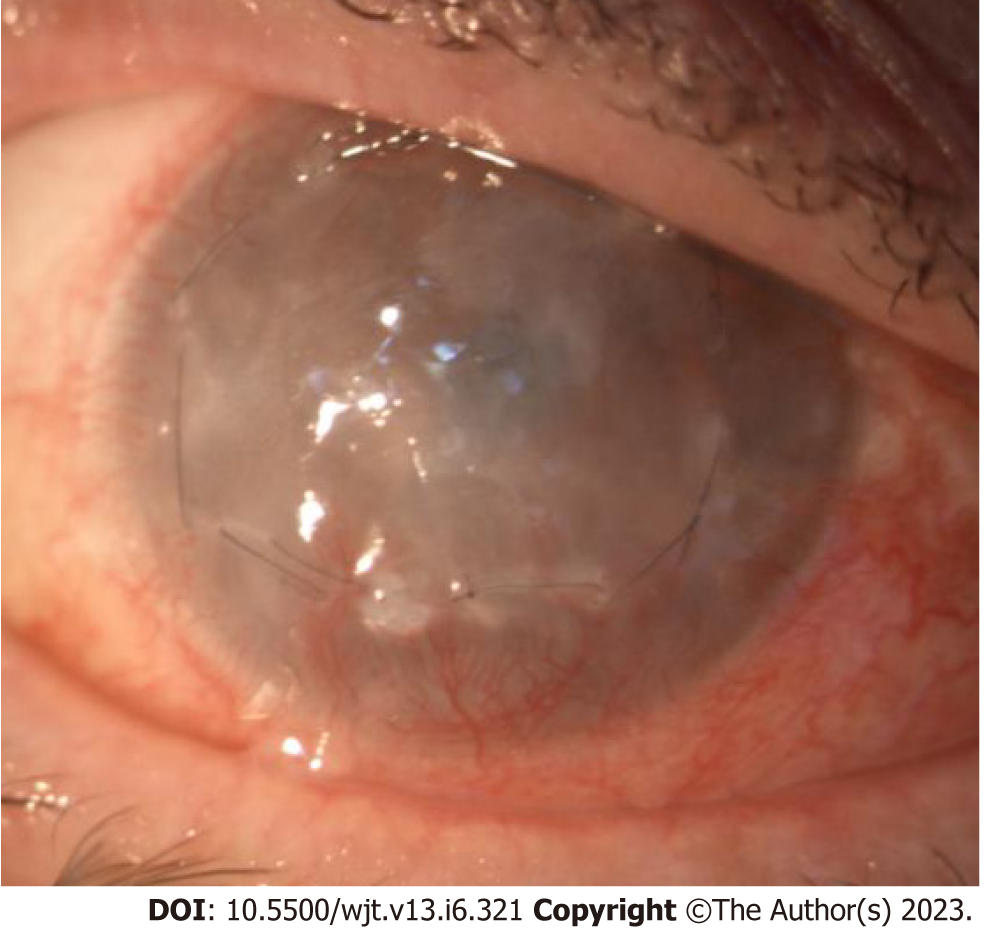
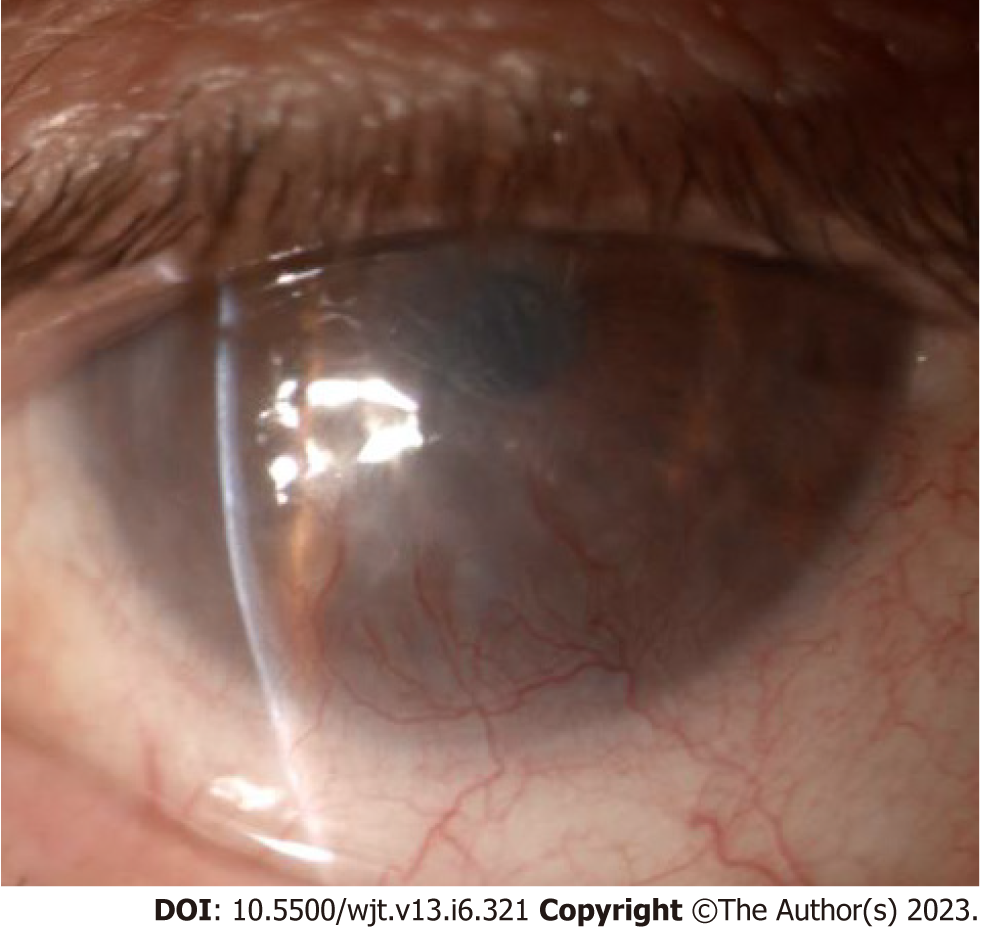
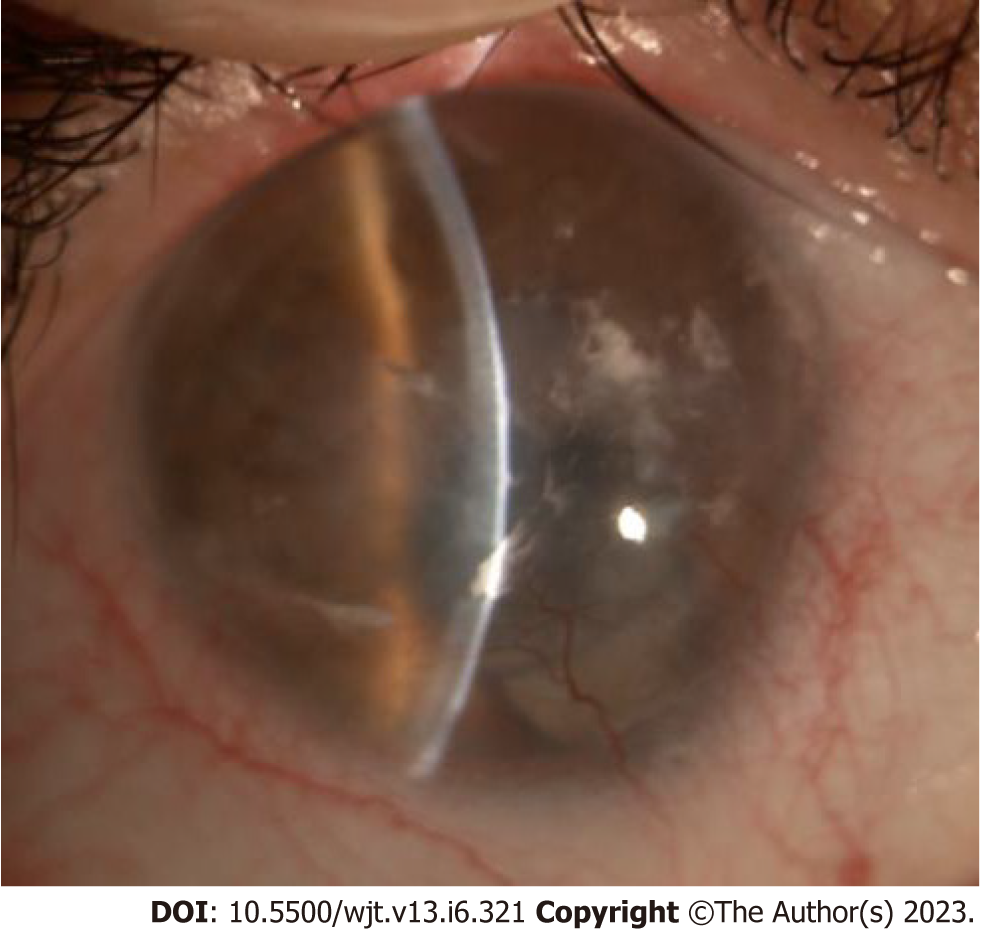
LSCD staging is based on the clinical presentation, and 5 mm central cornea involvement and limbal involvement are the two parameters evaluated (Table 2). The most important factor is corneal involvement; in the first stage central 5 mm is not involved, in the second it is partially affected, and in the final stage the entire corneal surface is involved. Every stage is divided into A when limbus involvement is less than 50%; B if it is more than 50% but non-complete; and C if the limbus is completely affected. Correct staging is useful for therapeutic decisions, but it is also important to evaluate palpebral and adnexa status and to control the underlying pathology if the LSCD is secondary to other pathologies[18]. Figures 1-3 show different stages of stem cell deficiency.
| Stage | A | B | C | |
| I | Central 5 mm of cornea not involved | Limbus involvement < 50% | Limbus involvement > 50% but < 100% | Limbus entirely involved |
| II | Central 5 mm of cornea involved | Limbus involvement < 50% | Limbus involvement > 50% but < 100% | |
| III | The entire corneal surface involved |
Over the past two decades, a variety of ocular surface rehabilitation treatments have been developed. The ocular surface is rehabilitated by improving the ocular surface environment, ensuring control of inflammation, good lubrication, and lid closure, and eliminating keratinization and symblepharon. A favorable environment is crucial for restoring the normal corneal phenotype and proper corneal clarity[18]. Corneal transplantation can be considered for corneal clarity restoration in patients with LSCD, but results and visual outcomes tend to be limiting over time because of the inability of the LSC to regenerate and maintain the transparency of the epithelium[19].
Several transplantation procedures have been used over the past years, and many of them have been labeled using different terminologies. These procedures include autologous and allograft conjunctival transplantation[20-22], keratoepithelioplasty[23], homotransplantation of limbal cells[24], limbal transplantation[25], homotransplantation of limbal cells[26], and autologous and allograft limbal transplantation (Table 3)[27-31].
| Procedure | Abbreviation | Tissue origin |
| Direct transplantation | ||
| Conjunctival limbal autograft | CLAU | Patient |
| Living-related conjunctival allograft | lr-CLAL | Relative donor |
| Keratolimbal allograft | KLAL | Cadaveric donor |
| Cincinnati procedure | Relative/cadaveric donor | |
| Modified Cincinnati procedure | Patient/cadaveric donor | |
| Stem cells transplantation | ||
| Simple limbal epithelium transplantation | SLET | Patient |
| Tissue engineering | ||
| Cultured limbal epithelial transplantation | CLET | Patient/living donor |
| Autologous conjunctival epithelial cells cultivated ex vivo | EVCAU | Patient |
| Cultivated oral mucosa epithelial transplantation | COMET | Patient |
CLAU, first described by Tseng and Kenyon[26] in 1989, is one of the most used techniques for LSC transplantation in unilateral LSCD. It consists of taking a portion of limbal conjunctiva (usually from 3 to 6 o’clock) from the fellow eye and implanting it in the affected eye, with or without amniotic membrane (AM) transplantation. CLAU has been successfully used to treat different pathologies and LSCD of different etiologies and different severities, even in cases of total corneal involvement[26]. The donor eye should be examined with particular attention to exclude any sign of LSCD to avoid an iatrogenic LSCD, even if this represents a remote possibility[32,33].
The inflammatory status of the graft can reduce the transplantation success rate, so topical steroids could be useful before the graft harvesting[34]. There is not a universally accepted consensus about the size of limbal grafts, generally harvested at 12 and 6 o’clock, where Vogt palisades are more developed. The first technique used a wide graft (8 o’clock), but successful results have been achieved with smaller grafts and new promising techniques use two grafts each at about 1 o’clock combined with AM transplantation (AMT)[35-37].
AM could be transplanted even in the donor's eye when large grafts are taken to reduce the risk of LSCD due to its capacity to facilitate the in-vivo expansion of LSCs[38,39]. Generally, the recipient bed is prepared by doing a peritomy 4-5 mm from the limbus and dissecting the corneal pannus, the dissection of corneal layers should be avoided, and stromal opacities are better treated afterward by keratoplasty[40]. The limbal epithelial graft is fixed around the cornea and the posterior margin is sutured to the conjunctiva. Between the graft and the ocular surface could be interposed a layer of AM that seems to increase the rates of success and the rapidity of the healing process, especially with little grafts, but a study with wide grafts showed no significant differences between the AMT group and the other without[41,42].
The great advantage of autologous transplantation is the immunosuppressive therapy sparing, so, generally, the only medication needed are topical antibiotics and topical corticosteroids. A scleral lens is applied to protect the graft from the mechanical stress of winking, and sometimes a temporary tarsorrhaphy can be considered[43]. A meta-analysis in 2020 found that the overall success rate of CLAU was 83.2%, with a 95%CI. In this study, “success” was defined as the reconstruction of an intact epithelium and a stable ocular surface. These data involved 16 articles, and 505 eyes, most of which had chemical or thermal injury[44].
In this technique, the surgical procedure is the same as CLAU, but the graft is harvested from a living related, and this makes it suitable even for severe bilateral LSCD. To decrease the chances of rejection, the donor must be the best human leukocyte antigen (HLA) matched available relative, generally a parent or a sibling. In 100% HLA compatibility cases, immunosuppressive therapy is not needed; in other cases, the administration of 6-12 mo of oral corticosteroids 10 mg/kg/d and oral cyclosporin A 10 mg/kg/d is required, subsequently tapered for maintenance dose during all the follow-up period. Some protocols added azathioprine to previous drugs and others used tacrolimus and mycophenolate mofetil[45,46]. Other authors administered oral cyclosporin for more than 6 mo and topical cyclosporin continued indefinitely unless toxic effects onset[47]. Patients under immunosuppressive therapy must be checked often for liver and kidney function.
KLAL is an allogenic transplant from a cadaveric donor. The graft is prepared by dissecting and removing the limbus and a peripheral portion of the cornea of the donor eye, then the stromal portion is dissected carefully to preserve the conjunctival and limbal epithelium. The graft is then sutured to the peripheral cornea and a patch of AM is generally transplanted to ensure better outcomes[48-50]. Tissue from the youngest possible donor with an upper limit of 50 years is recommended. Surgery should be performed within 72 h as the cells are expected to be more active and vital[51-53]. The recipient bed is prepared the same way as CLAU and lr-CLAL. HLA matching is recommended, and an immunosuppressive therapy, similar to lr-CLAL, is generally needed.
Holland[29] developed the Cincinnati procedure combining lr-CLAL and KLAL. In this technique, two portions of healthy limbus conjunctiva are harvested from an HLA-matched living donor, and the corneoscleral rim is taken from a cadaveric donor. The conjunctival tissue is placed at 12 and 6 o’clock in the same anatomical orientation, and the corneoscleral tissue is placed at 3 and 9 o’clock. With this procedure, ocular surface stability was achieved in 54.2% and an improvement was achieved in 33.3%, and 75% had an improvement in the visual acuity[54]. The same authors described even the modified Cincinnati procedure that combines CLAU with KLAL, achieving ocular surface stability in 82% of patients and ocular surface improvement in 18%[55]. Both techniques require an immunosuppressive therapy like that for KLAL[54,55].
In this group of techniques, a small portion of the corneal epithelium is taken from a donor, cultivated to expand its surface, and then transplanted. The advantage of these techniques is that with a limited amount of harvested tissue, it is possible to generate a considerable amount of epithelium to transplant. Thus, even in severe bilateral LSCD, it is possible to conduct autologous transplantations. However, the cultivation process needs an advanced laboratory and a relevant amount of resources, so just a few centers perform these kinds of surgeries. The harvested corneal tissue can belong to a living donor (the patient itself, a living relative, or a living nonrelative person) or from a cadaveric donor, but some techniques use other epithelia such as the oral one (ex vivo oral mucosa autograft, also called cultivated oral mucosa epithelial transplantation).
However, most of the techniques are still experimental procedures non-suitable for routine application except for Holoclar (ex vivo expanded autologous human corneal epithelial cells containing stem cells), first described in 1997 by Pellegrini et al[56] that achieved EMA authorization for commercial purposes in 2015.
Holoclar is a CLET procedure that starts with the enzymatical dissociation of the sample and the seeding of the cells in a layer of irradiated mouse feeder cells with growth factors and antibiotics. After this step, cells are cryopreserved and samples are tested; some of them are stored in case of failure of the first graft. Primary cultures are then seeded into antibiotic-free fibrin matrix discs and cultivated again. Epithelized discs are then shipped to the clinic, shaped by the surgeon, and implanted like in the CLAU technique. There is no standard procedure for CLET, and in fact, the cultivation procedure in the literature varies for the substrate used.
AM and cultivating milieu are mostly used, but the overall success rate of this technique is 71.8%. When cultivated cells are autologous, the ocular surface stability is maintained for long follow-up periods[57-65]. Most patients are typically affected by LSCD for chemical injury, but this technology is used also to treat LSCD due to autoimmune and congenital pathologies.
Another technique is autologous conjunctival epithelial cells cultivated ex vivo, in which the cultivated tissue is fornical conjunctiva. The specimen is placed on a denuded human AM and submerged in a culture medium with grow factors and antibiotics. The cultivated tissue is then shaped and transplanted to the prepared corneal surface. A study with 12 eyes reported a success rate of 66.6% and 16.6% of partial success (conjunctival epithelial ingrowth recurred in 2 corneal quadrants), but we have no other data about the clinical outcomes in humans[66].
SLET is a recent procedure for unilateral disease and seeds donor stem cells directly on an AM placed on the recipient's ocular surface, completely obviating any need for laboratory conditions of expansion[67]. Although CLET reduced the complications of CLAU, cell expansion required a clinical-grade lab with regulatory approvals, which was and continues to be very expensive to build and maintain. SLET, introduced by Sangwan et al[67] in 2012, combining the benefits of CLAU and CLET while avoiding the limitations of both strategies.
Unilateral LSCD is the primary indication for autologous SLET. Ocular burns are the most common cause of unilateral LSCD, so it is not surprising that this indication is covered by almost all of the published literature on autologous SLET. More recently, the first case reports of allogenic SLET in cases of bilateral LSCD have been proposed and involved patients with severe chemical burns and dry eyes, respectively[68,69]. In the SLET technique, in the superior limbal district of the unaffected contralateral eye, a portion of 2 mm × 2 mm of limbal tissue is removed under topical anesthesia and placed in a balanced saline solution.
The corneal surface is exposed by removing the fibrovascular corneal pannus after 360-degree conjunctival peritomy and peribulbar anesthesia is induced. With the epithelial side up, human AM is grafted over the cornea and secured with fibrin glue, and the margins are trimmed to fit the external conjunctival borders. Eight to ten tiny pieces of the limbal sclerocorneal tissue are cut into pieces and adhered to the AM in a circular pattern using fibrin glue, sparing the optical zone[67]. In a study involving six patients with total unilateral LSCD, visual acuity improved in four of the recipients' eyes (66.6%), going from 20/200 or worse before SLET surgery to 20/60 or better afterward. None of the donor eyes experienced any complications. It took 9.2 mo on average to follow up[67].
Patients with severe ocular surface disease need to be treated in a methodical, step-by-step manner. To achieve the best results in the rehabilitation of the ocular surface, it is crucial to select the patient's most appropriate strategy of treatment. The underlying pathology, the extent, and severity of ocular surface disease, including the degree of stem cell damage, unilaterality or bilateralism of the condition, the presence or absence of conjunctival inflammation, and whether tear production is normal (significantly altered or absent), the patient's age, and systemic co-morbidities are important factors in the choice of regimen among the various surgical procedures proposed for the treatment of LSCD.
The development of xenobiotic-free culture systems and the standardization of culture conditions are two improvements that must be made in order to advance the therapeutic approach. Additionally, to guarantee the functionality and long-term regeneration of the transplants, tissue engineering strategies must incorporate a kind of quality control, verifying the preservation of stem cells during the culture process.
Clarifying the signaling pathways that control stem cell function and fate in vivo and in vitro is one of the remaining challenges. Future trends include the creation of biomimetic scaffolds that can deliver drugs, growth factors, or signaling molecules to help further promote cell function and tissue regeneration in addition to acting as structural supports for living cells.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Long P, China S-Editor: Qu XL L-Editor: Wang TQ P-Editor: Qu XL
| 1. | Sun TT, Lavker RM. Corneal epithelial stem cells: past, present, and future. J Investig Dermatol Symp Proc. 2004;9:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Gonzalez G, Sasamoto Y, Ksander BR, Frank MH, Frank NY. Limbal stem cells: identity, developmental origin, and therapeutic potential. Wiley Interdiscip Rev Dev Biol. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Joe AW, Yeung SN. Concise review: identifying limbal stem cells: classical concepts and new challenges. Stem Cells Transl Med. 2014;3:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Chee KY, Kicic A, Wiffen SJ. Limbal stem cells: the search for a marker. Clin Exp Ophthalmol. 2006;34:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Sherwin T, McGhee CN. Corneal epithelial homeostasis. Ophthalmology. 2010;117:190-1; author reply 191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Chang CY, Green CR, McGhee CN, Sherwin T. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci. 2008;49:5279-5286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Majo F, Rochat A, Nicolas M, Jaoudé GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 8. | Deng SX, Borderie V, Chan CC, Dana R, Figueiredo FC, Gomes JAP, Pellegrini G, Shimmura S, Kruse FE; and The International Limbal Stem Cell Deficiency Working Group. Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea. 2019;38:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 238] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 9. | Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. Ocul Surf. 2018;16:58-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 10. | Calonge M, Diebold Y, Sáez V, Enríquez de Salamanca A, García-Vázquez C, Corrales RM, Herreras JM. Impression cytology of the ocular surface: a review. Exp Eye Res. 2004;78:457-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Kate A, Basu S. A Review of the Diagnosis and Treatment of Limbal Stem Cell Deficiency. Front Med (Lausanne). 2022;9:836009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Barbaro V, Ferrari S, Fasolo A, Pedrotti E, Marchini G, Sbabo A, Nettis N, Ponzin D, Di Iorio E. Evaluation of ocular surface disorders: a new diagnostic tool based on impression cytology and confocal laser scanning microscopy. Br J Ophthalmol. 2010;94:926-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Araújo AL, Ricardo JR, Sakai VN, Barros JN, Gomes JÁ. Impression cytology and in vivo confocal microscopy in corneas with total limbal stem cell deficiency. Arq Bras Oftalmol. 2013;76:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Wang LY, Wei ZY, Cao K, Su GY, Liang QF. [In vivo confocal microscopic characteristics of limbal stem cell deficiency]. Zhonghua Yan Ke Za Zhi. 2020;56:447-455. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Chan EH, Chen L, Rao JY, Yu F, Deng SX. Limbal Basal Cell Density Decreases in Limbal Stem Cell Deficiency. Am J Ophthalmol. 2015;160:678-84.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Bhattacharya P, Edwards K, Harkin D, Schmid KL. Central corneal basal cell density and nerve parameters in ocular surface disease and limbal stem cell deficiency: a review and meta-analysis. Br J Ophthalmol. 2020;104:1633-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Varma S, Shanbhag SS, Donthineni PR, Mishra DK, Singh V, Basu S. High-Resolution Optical Coherence Tomography Angiography Characteristics of Limbal Stem Cell Deficiency. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Dilly PN. Structure and function of the tear film. Adv Exp Med Biol. 1994;350:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Maguire MG, Stark WJ, Gottsch JD, Stulting RD, Sugar A, Fink NE, Schwartz A. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology. 1994;101:1536-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 314] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Thoft RA. Conjunctival transplantation. Arch Ophthalmol. 1977;95:1425-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 117] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Vastine DW, Stewart WB, Schwab IR. Reconstruction of the periocular mucous membrane by autologous conjunctival transplantation. Ophthalmology. 1982;89:1072-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Kwitko S, Marinho D, Barcaro S, Bocaccio F, Rymer S, Fernandes S, Neumann J. Allograft conjunctival transplantation for bilateral ocular surface disorders. Ophthalmology. 1995;102:1020-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Thoft RA. Keratoepithelioplasty. Am J Ophthalmol. 1984;97:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 91] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Pfister RR. Corneal stem cell disease: concepts, categorization, and treatment by auto- and homotransplantation of limbal stem cells. CLAO J. 1994;20:64-72. [PubMed] |
| 25. | Jenkins C, Tuft S, Liu C, Buckley R. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye (Lond). 1993;7 ( Pt 5):629-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709-22; discussion 722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 678] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 27. | Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 246] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Tsubota K, Toda I, Saito H, Shinozaki N, Shimazaki J. Reconstruction of the corneal epithelium by limbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995;102:1486-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 161] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996;94:677-743. [PubMed] |
| 30. | Croasdale CR, Schwartz GS, Malling JV, Holland EJ. Keratolimbal allograft: recommendations for tissue procurement and preparation by eye banks, and standard surgical technique. Cornea. 1999;18:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442-1443. [PubMed] |
| 32. | Cheung AY, Sarnicola E, Holland EJ. Long-Term Ocular Surface Stability in Conjunctival Limbal Autograft Donor Eyes. Cornea. 2017;36:1031-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Kreimei M, Sorkin N, Einan-Lifshitz A, Rootman DS, Chan CC. Long-term outcomes of donor eyes after conjunctival limbal autograft and allograft harvesting. Can J Ophthalmol. 2019;54:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Fernandes M, Sangwan VS, Rao SK, Basti S, Sridhar MS, Bansal AK, Dua HS. Limbal stem cell transplantation. Indian J Ophthalmol. 2004;52:5-22. [PubMed] |
| 35. | Kate A, Basu S. Mini-conjunctival autograft combined with deep anterior lamellar keratoplasty for chronic sequelae of severe unilateral chemical burn: A case report. Int J Surg Case Rep. 2021;88:106508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Dua HS, Azuara-Blanco A. Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br J Ophthalmol. 2000;84:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Baradaran-Rafii A, Eslani M, Jamali H, Karimian F, Tailor UA, Djalilian AR. Postoperative complications of conjunctival limbal autograft surgery. Cornea. 2012;31:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Sabater AL, Perez VL. Amniotic membrane use for management of corneal limbal stem cell deficiency. Curr Opin Ophthalmol. 2017;28:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Meallet MA, Espana EM, Grueterich M, Ti SE, Goto E, Tseng SC. Amniotic membrane transplantation with conjunctival limbal autograft for total limbal stem cell deficiency. Ophthalmology. 2003;110:1585-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Giachos I, Angelidis CD, Doumazos S, Tzavara C, Palioura S. Outcomes of Combined Penetrating Keratoplasty and Limbal Stem Cell Transplantation: A Meta-Analysis on Simultaneous Versus Sequential Surgery. Cornea. 2023;42:787-796. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Santos MS, Gomes JA, Hofling-Lima AL, Rizzo LV, Romano AC, Belfort R Jr. Survival analysis of conjunctival limbal grafts and amniotic membrane transplantation in eyes with total limbal stem cell deficiency. Am J Ophthalmol. 2005;140:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Barreiro TP, Santos MS, Vieira AC, de Nadai Barros J, Hazarbassanov RM, Gomes JÁ. Comparative study of conjunctival limbal transplantation not associated with the use of amniotic membrane transplantation for treatment of total limbal deficiency secondary to chemical injury. Cornea. 2014;33:716-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Tan DT, Ficker LA, Buckley RJ. Limbal transplantation. Ophthalmology. 1996;103:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Le Q, Chauhan T, Yung M, Tseng CH, Deng SX. Outcomes of Limbal Stem Cell Transplant: A Meta-analysis. JAMA Ophthalmol. 2020;138:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 45. | Movahedan A, Cheung AY, Eslani M, Mogilishetty G, Govil A, Holland EJ. Long-term Outcomes of Ocular Surface Stem Cell Allograft Transplantation. Am J Ophthalmol. 2017;184:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | El-Hofi AH, Helaly HA. Evaluation of limbal transplantation in eyes with bilateral severe ocular surface damage secondary to chemical injury. Clin Ophthalmol. 2019;13:383-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Tsubota K, Satake Y, Kaido M, Shinozaki N, Shimmura S, Bissen-Miyajima H, Shimazaki J. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340:1697-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 317] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 48. | Maruyama-Hosoi F, Shimazaki J, Shimmura S, Tsubota K. Changes observed in keratolimbal allograft. Cornea. 2006;25:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Tsubota K, Satake Y, Ohyama M, Toda I, Takano Y, Ono M, Shinozaki N, Shimazaki J. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol. 1996;122:38-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 311] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 50. | Parihar JKS, Parihar AS, Jain VK, Kaushik J, Nath P. Allogenic cultivated limbal stem cell transplantation versus cadaveric keratolimbal allograft in ocular surface disorder: 1-year outcome. Int Ophthalmol. 2017;37:1323-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Kethiri AR, Basu S, Shukla S, Sangwan VS, Singh V. Optimizing the role of limbal explant size and source in determining the outcomes of limbal transplantation: An in vitro study. PLoS One. 2017;12:e0185623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Vemuganti GK, Kashyap S, Sangwan VS, Singh S. Ex-vivo potential of cadaveric and fresh limbal tissues to regenerate cultured epithelium. Indian J Ophthalmol. 2004;52:113-120. [PubMed] |
| 53. | Notara M, Shortt AJ, O'Callaghan AR, Daniels JT. The impact of age on the physical and cellular properties of the human limbal stem cell niche. Age (Dordr). 2013;35:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Biber JM, Skeens HM, Neff KD, Holland EJ. The cincinnati procedure: technique and outcomes of combined living-related conjunctival limbal allografts and keratolimbal allografts in severe ocular surface failure. Cornea. 2011;30:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Chan CC, Biber JM, Holland EJ. The modified Cincinnati procedure: combined conjunctival limbal autografts and keratolimbal allografts for severe unilateral ocular surface failure. Cornea. 2012;31:1264-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Pellegrini G, Ardigò D, Milazzo G, Iotti G, Guatelli P, Pelosi D, De Luca M. Navigating Market Authorization: The Path Holoclar Took to Become the First Stem Cell Product Approved in the European Union. Stem Cells Transl Med. 2018;7:146-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 57. | Pauklin M, Fuchsluger TA, Westekemper H, Steuhl KP, Meller D. Midterm results of cultivated autologous and allogeneic limbal epithelial transplantation in limbal stem cell deficiency. Dev Ophthalmol. 2010;45:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 58. | Sangwan VS, Basu S, Vemuganti GK, Sejpal K, Subramaniam SV, Bandyopadhyay S, Krishnaiah S, Gaddipati S, Tiwari S, Balasubramanian D. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95:1525-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 59. | Pellegrini G, Rama P, Matuska S, Lambiase A, Bonini S, Pocobelli A, Colabelli RG, Spadea L, Fasciani R, Balestrazzi E, Vinciguerra P, Rosetta P, Tortori A, Nardi M, Gabbriellini G, Traverso CE, Macaluso C, Losi L, Percesepe A, Venturi B, Corradini F, Panaras A, Di Rocco A, Guatelli P, De Luca M. Biological parameters determining the clinical outcome of autologous cultures of limbal stem cells. Regen Med. 2013;8:553-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 60. | Ramírez BE, Sánchez A, Herreras JM, Fernández I, García-Sancho J, Nieto-Miguel T, Calonge M. Stem Cell Therapy for Corneal Epithelium Regeneration following Good Manufacturing and Clinical Procedures. Biomed Res Int. 2015;2015:408495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 61. | Ganger A, Vanathi M, Mohanty S, Tandon R. Long-Term Outcomes of Cultivated Limbal Epithelial Transplantation: Evaluation and Comparison of Results in Children and Adults. Biomed Res Int. 2015;2015:480983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Scholz SL, Thomasen H, Hestermann K, Dekowski D, Steuhl KP, Meller D. [Long-term results of autologous transplantation of limbal epithelium cultivated ex vivo for limbal stem cell deficiency]. Ophthalmologe. 2016;113:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 834] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 64. | Schwab IR. Cultured corneal epithelia for ocular surface disease. Trans Am Ophthalmol Soc. 1999;97:891-986. [PubMed] |
| 65. | Shimazaki J, Higa K, Morito F, Dogru M, Kawakita T, Satake Y, Shimmura S, Tsubota K. Factors influencing outcomes in cultivated limbal epithelial transplantation for chronic cicatricial ocular surface disorders. Am J Ophthalmol. 2007;143:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 66. | Ricardo JR, Cristovam PC, Filho PA, Farias CC, de Araujo AL, Loureiro RR, Covre JL, de Barros JN, Barreiro TP, dos Santos MS, Gomes JA. Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea. 2013;32:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:931-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 310] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 68. | Arya SK, Bhatti A, Raj A, Bamotra RK. Simple Limbal Epithelial Transplantation in Acid Injury and Severe Dry Eye. J Clin Diagn Res. 2016;10:ND06-ND07. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Bhalekar S, Basu S, Sangwan VS. Successful management of immunological rejection following allogeneic simple limbal epithelial transplantation (SLET) for bilateral ocular burns. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |