Published online Dec 18, 2023. doi: 10.5500/wjt.v13.i6.290
Peer-review started: June 27, 2023
First decision: July 28, 2023
Revised: August 17, 2023
Accepted: October 17, 2023
Article in press: October 17, 2023
Published online: December 18, 2023
Processing time: 173 Days and 14.9 Hours
The shortage of deceased donor organs has prompted the development of alternative liver grafts for transplantation. Living-donor liver transplantation (LDLT) has emerged as a viable option, expanding the donor pool and enabling timely transplantation with favorable graft function and improved long-term outcomes. An accurate evaluation of the donor liver’s volumetry (LV) and anatomical study is crucial to ensure adequate future liver remnant, graft volume and precise liver resection. Thus, ensuring donor safety and an appropriate graft-to-recipient weight ratio. Manual LV (MLV) using computed tomography has traditionally been considered the gold standard for assessing liver volume. However, the method has been limited by cost, subjectivity, and variability. Automated LV techniques employing advanced segmentation algorithms offer improved reproducibility, reduced variability, and enhanced efficiency compared to manual measurements. However, the accuracy of automated LV requires further investigation. The study provides a comprehensive review of traditional and emerging LV methods, including semi-automated image processing, automated LV techniques, and machine learning-based approaches. Additionally, the study discusses the respective strengths and weaknesses of each of the aforementioned techniques. The use of artificial intelligence (AI) technologies, including machine learning and deep learning, is expected to become a routine part of surgical planning in the near future. The implementation of AI is expected to enable faster and more accurate image study interpretations, improve workflow efficiency, and enhance the safety, speed, and cost-effectiveness of the procedures. Accurate preoperative assessment of the liver plays a crucial role in ensuring safe donor selection and improved outcomes in LDLT. MLV has inherent limitations that have led to the adoption of semi-automated and automated software solutions. Moreover, AI has tremendous potential for LV and segmentation; however, its widespread use is hindered by cost and availability. Therefore, the integration of multiple specialties is necessary to embrace technology and explore its possibilities, ranging from patient counseling to intraoperative decision-making through automation and AI.
Core Tip: Accurate liver’s volumetry (LV) is imperative for successful living-donor liver transplantation to ensure adequate future liver remnant and graft volumes. Manual computed tomography scan delineation conventionally serves as the standard approach; however, it is constrained by factors such as cost, subjectivity, and variability. In contrast, automated LV techniques utilizing advanced segmentation algorithms present superior reproducibility, reduced variability, and enhanced efficiency compared with manual measurements. However, the accuracy of automated LV requires further investigation. The study comprehensively reviewed both traditional and emerging LV methods, including semi-automated image processing, automated LV techniques, and machine learning-based approaches, while analyzing their respective strengths and weaknesses.
- Citation: Machry M, Ferreira LF, Lucchese AM, Kalil AN, Feier FH. Liver volumetric and anatomic assessment in living donor liver transplantation: The role of modern imaging and artificial intelligence. World J Transplant 2023; 13(6): 290-298
- URL: https://www.wjgnet.com/2220-3230/full/v13/i6/290.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i6.290
Liver transplantation is the first-line treatment for patients with terminal liver disease. Deceased donor organ shortage and cultural barriers have led to the development of alternative graft types. Living-donor liver transplantation (LDLT) has emerged as an extension of the ex-situ graft transection concept, encompassing reduced-size and split-liver techniques. By enabling the expansion of the donor pool, LDLT offers the advantage of timely transplantation and holds the potential for excellent graft function and improved long-term outcomes[1-6]. Moreover, LDLT reduces waiting list mortality.
An adequate preoperative evaluation of the donor is essential for successful LDLT. Sufficient future liver remnant (FLR) and graft volume must be ensured through liver’s volumetry (LV) studies[7,8]. An FLR of 30% to 35% of the original liver volume is required for donor safety, whereas at least 4% of the standard liver volume or more than 0.8 and less than 3–3.5 of the graft recipient weight ratio (estimated before the surgery through imaging and confirmed after the graft is weighted) is required to meet the recipient’s needs[9,10]. Small grafts are associated with cellular damage due to excessive portal flow, leading to "small-for-size syndrome,” whereas large grafts may receive inadequate portal flow, resulting in "large-for-size syndrome"[11-17].
Manual liver volumetry (MLV) conducted on portal venous phase multidetector computed tomography (CT) scans with intravenous contrast is conventionally considered the standard method for measuring LV[7,18,19]. However, it can be costly, time-consuming, subjective, and prone to inter- and intra-observer variabilities. The process entails manual tracing of the liver borders using specialized software, necessitating the expertise of an experienced radiologist, often without the input of the surgeon. The percentage of error (PE) may vary significantly, ranging from 2% to 20%, which can have a dramatic effect on the final graft volume and transplantation outcomes[20-24].
Advancements in medical imaging, computational algorithms, and artificial intelligence (AI) have set the stage for the development and application of automated LV techniques. Automated LV holds significant promise in the evaluation of LDLT, as it utilizes sophisticated segmentation algorithms to delineate liver boundaries from CT or magnetic resonance imaging (MRI) scans. Therefore, enabling volumetric calculations and comprehensive volumetric analysis and allowing for the assessment of lobe-specific volumes, segmental volumes, and overall liver volume. Such automated approaches offer advantages over manual measurements, including enhanced reproducibility, reduced intra- and interobserver variability, and improved efficiency. However, the accuracy of automated LV techniques is yet to be conclusively determined[25-28].
The study aimed to provide a comprehensive review of the literature, presenting both traditional and emerging methods of LV and anatomical liver assessment, while discussing their respective strengths and weaknesses. By examining the current state of LV techniques, the review aimed to contribute to the advancement and optimization of liver transplantation outcomes.
The introduction of multiphasic CT and MRI techniques has led to the widespread adoption of MLV as the standard practice in liver transplant centers to estimate liver volume before accepting a living-donor as a suitable candidate. During the donor evaluation, a complete anatomical analysis of the hepatic veins, portal vein and hepatic arteries is provided by multiphasic CT and MRI. Bile duct anatomy is evaluated in cholangio MRI studies, specially, in left lobe and right lobe donors.
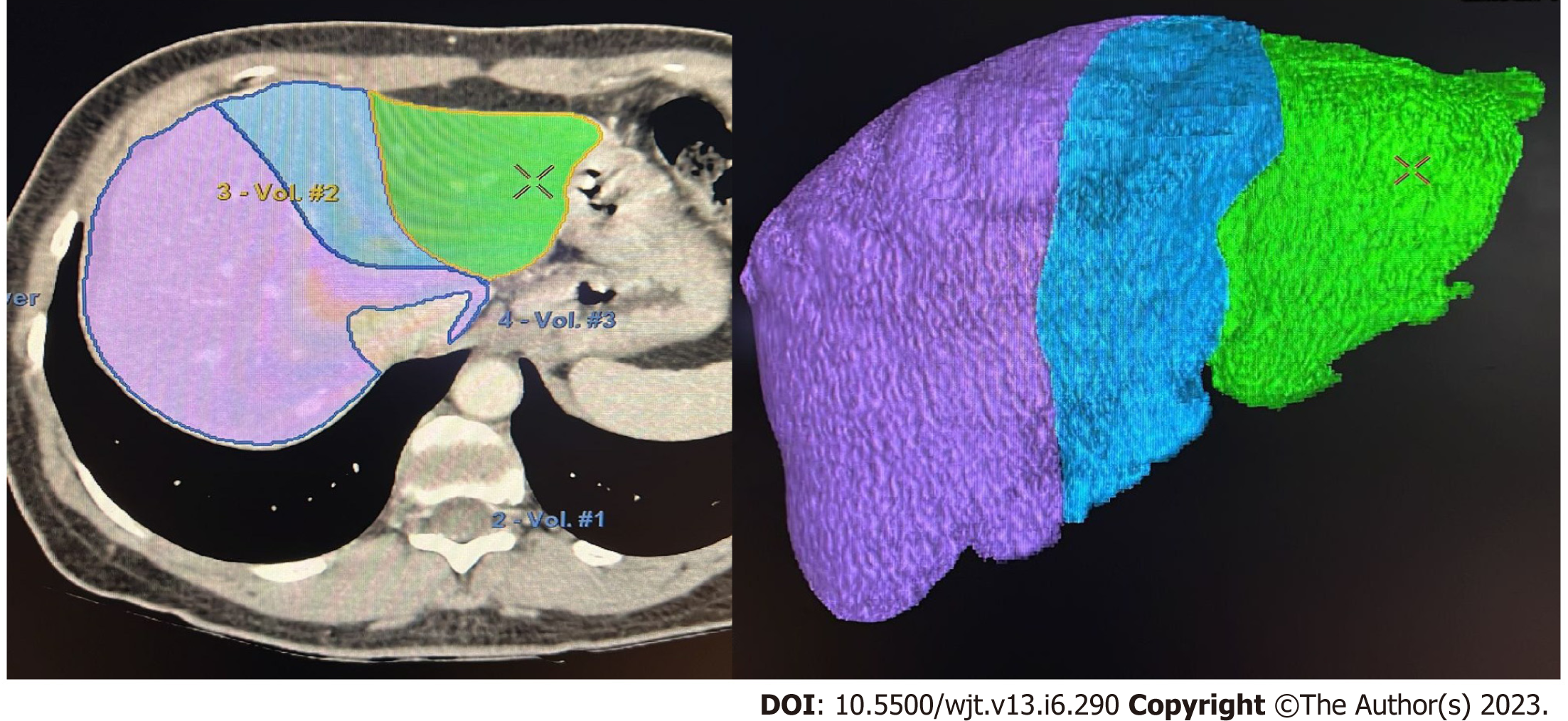
If the donor´s anatomy is suitable for the planned procedure, LV is carried out. The procedure involves manual delineation of the liver borders using sequential image slices to determine the overall liver volume. Subsequently, a transection plane is selected based on the specific type of liver graft and the inclusion of the middle hepatic vein (MHV)[25,29-31] (Figure 1).
Limitations include reliance on operator expertise and medical specialty, leading to discrepancies between the analyses performed by radiologists and surgeons, potentially related to the transection line. Furthermore, the inclusion of blood vessels and bile ducts in the final volume calculation can lead to overestimations[32]. Additionally, the LV procedure itself is time-consuming, typically requiring approximately 20-40 min to complete, which significantly affects the daily workflow of both radiologists and surgeons[19,33]. In terms of accuracy, PE ranges from 5% to 36% when comparing the estimated volume with the actual graft weight (AGW)[34]. It is important to note that errors can occur in both directions, resulting in overestimation and underestimations[8].
It is routinely considered that the density of the liver is equivalent to the density of water; therefore, the AGW is representative of the graft volume[35]. However, studies measuring AGW have identified the necessity of correction factors when estimating graft volume, as highlighted in Table 1. Recently, Lemke et al[36], measured the mean physical density of 16 transplanted liver lobes to be 1.1157 g/mL, asserting that the conversion factor was, on average, 12% higher than expected. Tongyoo et al[32] demonstrated that the AGW of a right lobe donor liver graft (RLDG) was approximately 91% of the estimated right lobe liver volume. The 9% volume reduction was attributed to intrahepatic blood flushed out of the liver by the preservation solution during back-table preparation[9,31,37]. Other inaccuracies may have been due to the inclusion of the MHV and/or the caudate lobe[38].
| Ref. | Formula | Research place |
| Poovathumkadavil et al[22], 2010 | LV = 12.26 × BW(kg) + 555.65 | Saudi Arabia |
| Noda et al[21], 1997 | LV = 0.05012 × BW0.78 | Japan |
| Johnson et al[20], 2005 | LV = 0.722 × BSA1.176 | North America |
| Yuan et al[24], 2008 | LV = 949.7 × BSA (m2) - 48.3 × age - 247.4 | China |
| Yoshizumi et al[23], 2003 | LV = (0.772 × BSA)/1.08 | North America |
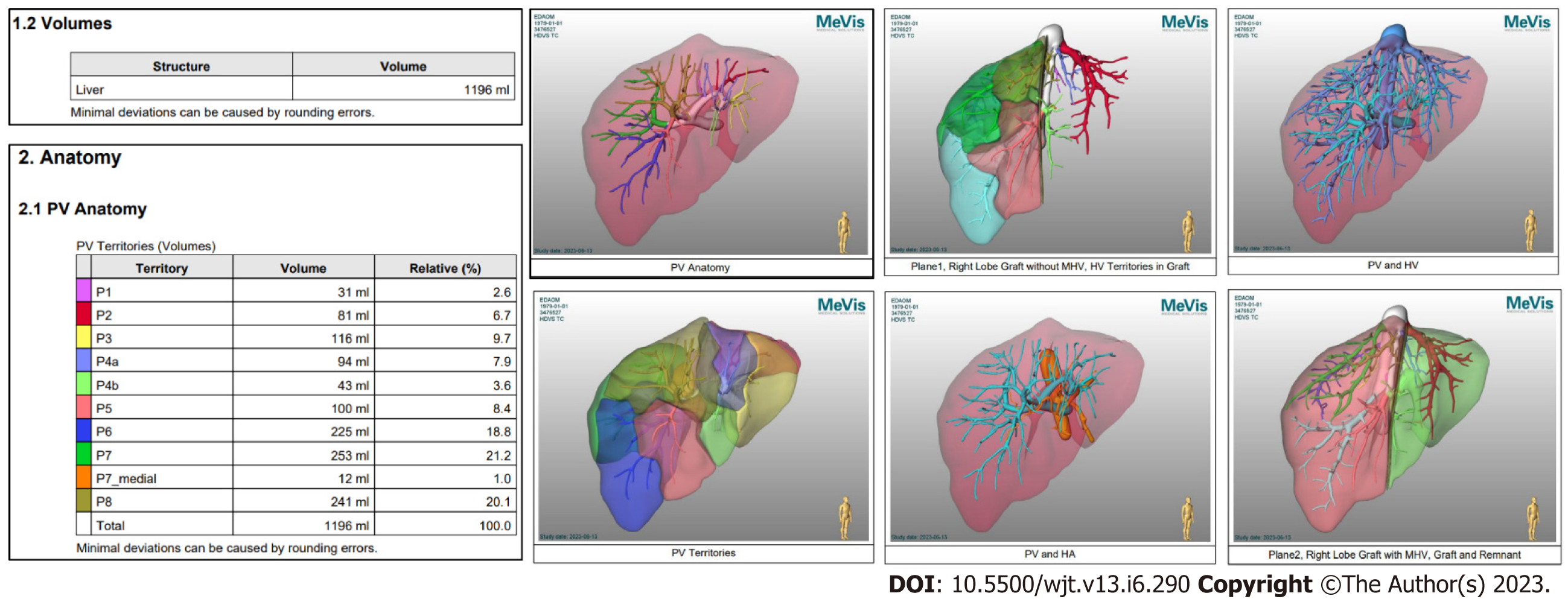
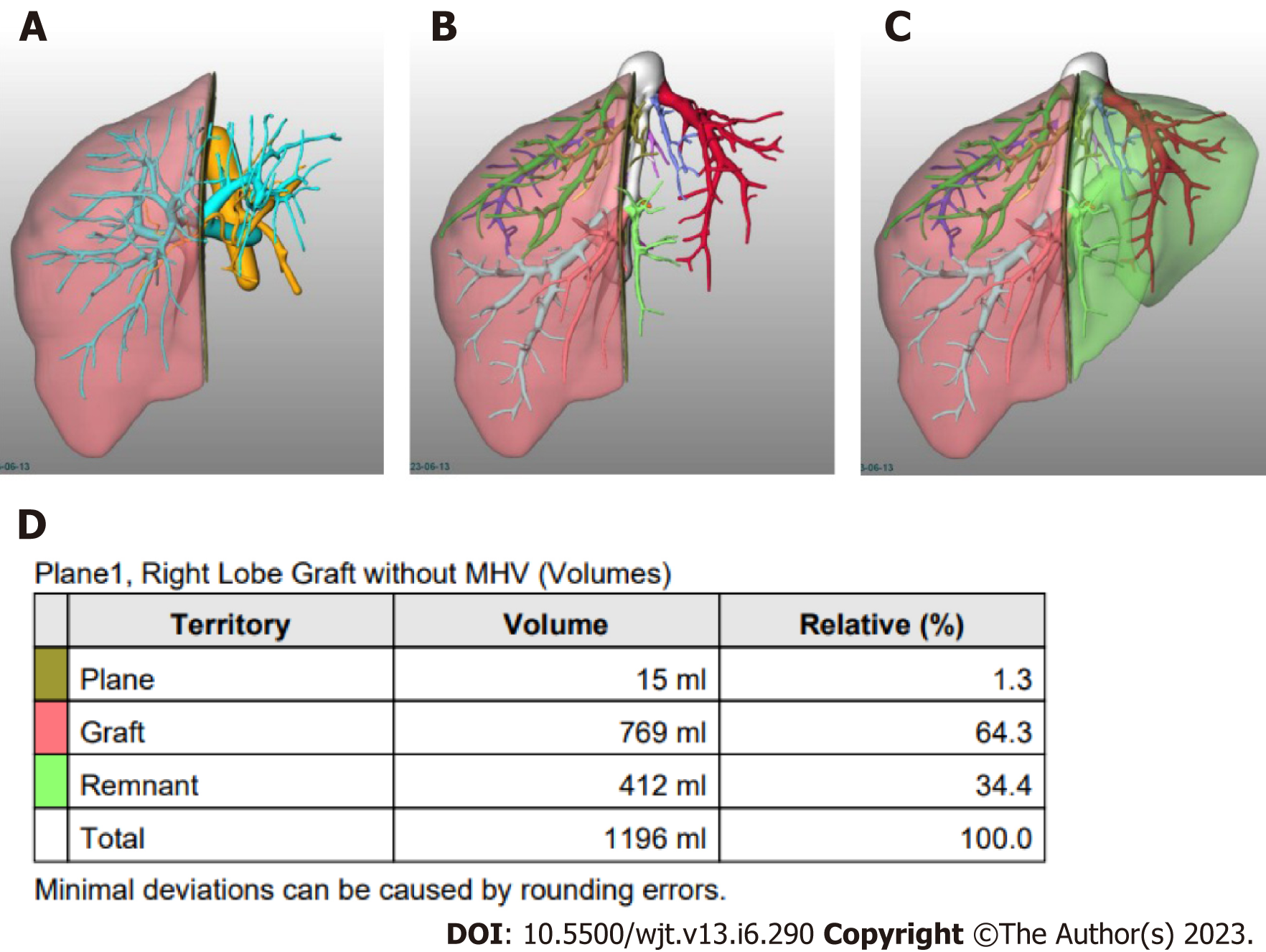
Semi-automated methods have been developed to address observer-related issues associated with manual measurements and to enhance the efficiency of LV and hepatic segmentation. An example of such a method is the MeVis Liver Analyzer (MeVis Medical Solutions AG, Bremen, Germany), which is a computer-assisted software that operates on CT images. Moreover, the software employs a modified live-wire algorithm to automatically determine the contours between user-defined boundary points based on the CT values and gradients. The algorithm parameters were tailored to each CT phase, including the venous (V), arterial (HA), and native (N) phases. To ensure accurate liver segmentation, manual correction of automatically delineated contours and manual drawing of the contour parts were performed. Live-wire contours were interactively determined on 3 mm axial two-dimensional (2D) CT slices. The software automatically interpolates and optimizes the contours of intermediate slices, with final adjustments made by the operator through manual corrections, if necessary (Figure 2).
Volumetric calculations, expressed in milliliters (mL), were performed by adding the areas of all segmented regions. Surrounding structures such as major extrahepatic vessels (portal vein, hepatic artery, and inferior vena cava) and the gallbladder fossa were excluded from the volume calculations (Figure 3).
Goja et al[39] discovered that semiautomated software tools exhibited the highest correlation (r = 0.82) for measuring right lobe grafts. However, left lobe grafts tend to be overestimated, whereas left lateral segment (LLS) grafts are often underestimated, with an underestimation of approximately 66% of the total LLS grafts. One possible explanation for the underestimation of LLS grafts is that CT scans typically underestimate the volume because the actual surgical plane of transection is approximately 1 cm to the right of the falciform ligament, whereas the radiological plane of transection is exactly at the falciform ligament. Other studies have addressed the accuracy of semi-automated image processing (SAIP), and their results are presented in Table 2.
| Ref. | Software and comparison | Reports |
| Pomposelli et al[47], 2012 | Software MeVis | A nonsignificant volume difference of approximately 17.5 mL and a low percentage error of approximately 2.8% |
| Compared right lobe graft volumes estimated by SAIP with actual graft weights measured during LDLT | ||
| Çelik et al[34], 2023 | CT Liver Analysis, Philips Healthcare-RLDG volumes by manual and SA were compared to AGW | Both manual and SA overestimated the graft weight (manual: 893 ± 155 mL vs AGW: 787 ± 128 g, P < 0.001, SA: 879 ± 143 mL vs AGW, P < 0.001). The mean interaction time was 27.3 ± 14.2 min for manual and 6.8 ± 1.4 min for SAIP (P < 0.001) |
| Mohapatra et al[31], 2020 | Myrian XP Liver 3D software (France)-RLDG, LLDG and LLSDG volumes by manual and SA were compared to AGW | Both manual and SA showed strong correlation with AGW (r = 0.834 and 0.856, respectively). The mean percentage error for manual and SA was 14.2 ± 12.5% and 12.2 ± 11.8%, respectively. The overall accuracy improved using SA (P = 0.015) |
| Kalshabay et al[25], 2023 | Vitrea software, including two different applications for manual segmentation (Volume analysis) and automated segmentation (CT liver analysis) | The manual method correlated better with AGW (r = 0.730) in comparison with the SA (r = 0.685) and the automated (r = 0.699) methods (P < 0.001). The mean error ratio in volume estimation by each application was 12.7 ± 16.6% for manual, 17.1 ± 17.3% for SA, 14.7 ± 16.8% for automated methods |
| SA software (OsiriX MD) | ||
| RLDG | ||
| Goja et al[39], 2018 | AW Volume share 6 (GE Healthcare; Chicago, Illinois, United States) | RLDGt: There was no statistically significant difference between mean SA and AGW in RL (722 ± 134 vs 717 ± 126 gm; P = 0.06). LLDG: Correlated strongly (r = 0.81, P < 0.001), mean SA was significantly high as compared to mean of AGW (460 ± 118 vs 433 ± 102 gm; P = 0.003). LLSDG: Mean SA was significantly low as compared to mean of AGW (203 ± 48 vs 254 ± 49 gm; P < 0.001) |
| RLDG, LLDG and LLSDG volumes by SA were compared to AGW |
Automated LV relies on advanced image-processing techniques and algorithms to accurately segment the liver from CT or MRI scans. The principles and algorithms vary depending on the approach employed. However, some common techniques and concepts are involved.
Before liver segmentation, image preprocessing techniques may be applied to enhance the image quality, reduce noise, and improve the contrast between the liver and surrounding structures. These techniques include filtering, intensity normalization, and image enhancement methods.
Segmentation algorithms were used to delineate the liver region of interest from the remaining images. Additionally, such algorithms aim to accurately identify the liver boundaries. Commonly used algorithms include threshold-based methods, region growing, active contours (or snakes), level sets, graph cuts, and machine-learning-based techniques.
Threshold-based methods involve setting intensity thresholds to separate the liver from the background or other organs. The liver is segmented based on predefined intensity ranges or statistical measures such as the mean intensity or intensity distribution.
Region-growing algorithms start from a seed point within the liver and iteratively develop the region by including pixels with similar characteristics (e.g., intensity, texture, or gradient) until a stopping criterion is met. The method is particularly useful when the liver has a distinct intensity pattern compared to the surrounding tissues.
Active contour models, also known as snakes, use an energy-optimization approach to iteratively deform a contour to fit the liver boundary. The contour was attracted to the image edges or intensity gradients, ensuring accurate delineation of the liver boundaries.
Level-set methods are mathematical techniques used to evolve a curve or surface over time to delineate the liver boundaries. The methods use the concept of level sets, which represent the evolving contour as a zero-level set of a higher-dimensional function.
Graph cut algorithms model the liver segmentation problem as an optimization task in a graph framework. The graph is constructed using image features, and the segmentation is achieved by identifying the minimum energy cut that separates the liver from the background.
Machine learning algorithms, such as random forests, support vector machines, and deep learning models, can be trained on annotated liver images to automatically segment the liver. Such algorithms learn the patterns and features that distinguish the liver from other structures and can provide accurate and robust segmentation results[40].
Most software tools employ a combination of techniques or advanced algorithms that are specific to their methodology. The choice of algorithm depends on factors such as image quality, complexity of liver structures, computational efficiency, and specific requirements of the application. Each algorithm has its advantages, limitations, and parameter settings, which must be carefully considered and optimized for accurate LV. A combination of techniques can be used to improve accuracy and robustness[41].
For example, the initial segmentation can be obtained using thresholding or region growth, followed by refinement using active contours or graph cuts. Hybrid approaches that combine multiple algorithms can leverage the strength of each technique to achieve more accurate LV. Additionally, the validation and evaluation of the automated LV results against the ground truth or manual segmentations are critical for assessing the algorithm's performance and reliability[42].
Most computer aided diagnostics used in clinical practice use conventional machine learning approaches, in which the effectiveness depends on the domain expertise of the developers. So, the limitations of conventional learning are linked to the limitations of the human developer. Manual and semi-automated volumetry is dependent on conventional machine learning. Deep learning has emerged as a state-of-the-art machine-learning method for many applications. Deep learning is a type of representation learning method in which a complex multilayer neural network architecture learns representations of data automatically by transforming the input information into multiple levels of abstraction[43].
Deep convolutional neural networks (DCNN) are widely used in image pattern recognition. They can automatically extract relevant features from training samples by adjusting their weights through backpropagation. In contrast to manual feature design, the DCNN learns feature representations during training. When trained with a large and representative dataset, the DCNN features outperformed the hand-engineered features by being highly selective and invariant. The automated deep learning process enables the analysis of numerous cases, surpassing human capabilities. Deep learning proves robust in handling variations across different classes, as long as the training set is diverse and extensive[40-43].
Automated LV and deep machine learning for LDLT has gained attention in recent years. There has been an increase in the number and quality of AI and machine learning studies in the medical field, mainly those focused on automating the interpretation of 2D image tests (MRI, CT, and radiographs), assembling three-dimensional models of organs and tissues, and volumetric calculations, including virtual segmentation of the liver. In liver resection and liver transplantation, most studies have a small number of cases, focusing on adult liver transplantation and RLDG, with very few studies on left lobe donor graft and left lateral segment donor graft[26-28,42-44]. The higher risk of the small-for-size syndrome in adult liver transplantation justifies the intense volumetric and anatomical studies on RLDG. Usually, for pediatric recipients (< 10 kg), an inaccurate volumetric assessment will rarely lead to insufficient liver volume; in contrast, the risk of the large-for-size syndrome is higher compared to the small-for-size syndrome. In such cases, the surgeon usually reduces the graft on the back table or converts it into a mono-segmental graft before implantation[45].
Automated software allows the surgeon to choose the transection plane; some studies have compared the correlation of these measurements for RLDG when performed by the surgeon using automated software with the manual measurements performed by radiologists. Moreover, both measurements had a good correlation with the AGW (r > 0.80), along with no significant difference between measurements by the surgeon and the radiologist[29].
As it is of paramount importance that the surgeon who is going to perform the procedure to perform the anatomical assessment and to choose the adequate liver segmentation plane, new softwares, focusing on the surgeon's interaction are being developed. A more user-friendly automated platform was developed by a group from the Republic of Korea[46], which they referred to as Dr. Liver. They validated the method in 50 RLDG and compared it to MLV. The correlation with AGW was better for the automated Dr. Liver (r = 0.98) than for the MLV (r = 0.92), although they were both good correlations. However, the percentage of absolute difference (%AD) from AGW of Dr. Liver (3.1% ± 2.8%) was significantly smaller than that of the MLV (10.2% ± 7.5%). None of the Dr. Liver measurements percentages of %AD was > 10%, while they were 46% for MLV measurements. Evaluation of %AD is very important in clinical practice because an error percentage of more than 10% can result in a small-for-size boundary graft volume. Also, the total time for task completion was shorter for Dr. Liver vs MLV (7.3 ± 1.4 min vs 37.9 ± 7.0 min).
Accurate preoperative assessment of the liver plays a critical role in ensuring the selection of suitable donors and improving recipient outcomes after LDLT. MLV initially emerged as the gold standard for accurate assessment. However, the time-consuming nature of the manual analysis, reliance on operator expertise, and high variability in PE have prompted the adoption of SAIP software tools, and more recently, automated software solutions. AI represents the future of LV and segmentation and offers immense potential in the field, leading to a future fully automated liver segmentation and volumetry based on deep-learning. However, the widespread adoption and daily application of AI are hindered by cost and accessibility limitations. We are responsible for embracing technology and fostering interdisciplinary collaborations in the fields of radiology, engineering, informatics, and surgery. The possibilities afforded by AI are limitless, ranging from patient counseling and education to intraoperative decision-making facilitated by automation and AI assistance.
We thank Dr. Renato Kist and Dr. Fernando Hexsel for kindly providing the manual volumetry images for this work.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yao W, China; Zheng H, China S-Editor: Fan JR L-Editor: A P-Editor: Zhang YL
| 1. | Lan X, Zhang H, Li HY, Chen KF, Liu F, Wei YG, Li B. Feasibility of using marginal liver grafts in living donor liver transplantation. World J Gastroenterol. 2018;24:2441-2456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Miller CM, Quintini C, Dhawan A, Durand F, Heimbach JK, Kim-Schluger HL, Kyrana E, Lee SG, Lerut J, Lo CM, Pomfret EA. The International Liver Transplantation Society Living Donor Liver Transplant Recipient Guideline. Transplantation. 2017;101:938-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Raghu VK, Carr-Boyd PD, Squires JE, Vockley J, Goldaracena N, Mazariegos GV. Domino transplantation for pediatric liver recipients: Obstacles, challenges, and successes. Pediatr Transplant. 2021;25:e14114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Siraj MS. Deceased Organ Transplantation in Bangladesh: The Dynamics of Bioethics, Religion and Culture. HEC Forum. 2022;34:139-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Vanholder R, Domínguez-Gil B, Busic M, Cortez-Pinto H, Craig JC, Jager KJ, Mahillo B, Stel VS, Valentin MO, Zoccali C, Oniscu GC. Organ donation and transplantation: a multi-stakeholder call to action. Nat Rev Nephrol. 2021;17:554-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 6. | Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation. 2011;91:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Kamel IR, Kruskal JB, Warmbrand G, Goldberg SN, Pomfret EA, Raptopoulos V. Accuracy of volumetric measurements after virtual right hepatectomy in potential donors undergoing living adult liver transplantation. AJR Am J Roentgenol. 2001;176:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Radtke A, Sotiropoulos GC, Nadalin S, Molmenti EP, Schroeder T, Lang H, Saner F, Valentin-Gamazo C, Frilling A, Schenk A, Broelsch CE, Malagó M. Preoperative volume prediction in adult living donor liver transplantation: how much can we rely on it? Am J Transplant. 2007;7:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Hiroshige S, Shimada M, Harada N, Shiotani S, Ninomiya M, Minagawa R, Soejima Y, Suehiro T, Honda H, Hashizume M, Sugimachi K. Accurate preoperative estimation of liver-graft volumetry using three-dimensional computed tomography. Transplantation. 2003;75:1561-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Lo CM, Fan ST, Liu CL, Wei WI, Lo RJ, Lai CL, Chan JK, Ng IO, Fung A, Wong J. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg. 1997;226:261-9; discussion 269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 419] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 11. | Martel G, Cieslak KP, Huang R, van Lienden KP, Wiggers JK, Belblidia A, Dagenais M, Lapointe R, van Gulik TM, Vandenbroucke-Menu F. Comparison of techniques for volumetric analysis of the future liver remnant: implications for major hepatic resections. HPB (Oxford). 2015;17:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18:40-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 582] [Article Influence: 145.5] [Reference Citation Analysis (1)] |
| 13. | Miki A, Sakuma Y, Ohzawa H, Saito A, Meguro Y, Watanabe J, Morishima K, Endo K, Sasanuma H, Shimizu A, Lefor AK, Yasuda Y, Sata N. Clearance of the liver remnant predicts short-term outcome in patients undergoing resection of hepatocellular carcinoma. World J Gastroenterol. 2022;28:5614-5625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Park S, Choi GS, Kim JM, Lee S, Joh JW, Rhu J. 3D Printing Model of Abdominal Cavity of Liver Transplantation Recipient to Prevent Large-for-Size Syndrome. Int J Bioprint. 2022;8:609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | Pu X, He D, Liao A, Yang J, Lv T, Yan L, Wu H, Jiang L. A Novel Strategy for Preventing Posttransplant Large-For-Size Syndrome in Adult Liver Transplant Recipients: A Pilot Study. Transpl Int. 2021;35:10177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Shoreem H, Gad EH, Soliman H, Hegazy O, Saleh S, Zakaria H, Ayoub E, Kamel Y, Abouelella K, Ibrahim T, Marawan I. Small for size syndrome difficult dilemma: Lessons from 10 years single centre experience in living donor liver transplantation. World J Hepatol. 2017;9:930-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Sparrelid E, Olthof PB, Dasari BVM, Erdmann JI, Santol J, Starlinger P, Gilg S. Current evidence on posthepatectomy liver failure: comprehensive review. BJS Open. 2022;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 18. | Higashiyama H, Yamaguchi T, Mori K, Nakano Y, Yokoyama T, Takeuchi T, Yamamoto N, Yamaoka Y, Tanaka K, Kumada K. Graft size assessment by preoperative computed tomography in living related partial liver transplantation. Br J Surg. 1993;80:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Suzuki K, Epstein ML, Kohlbrenner R, Garg S, Hori M, Oto A, Baron RL. Quantitative radiology: automated CT liver volumetry compared with interactive volumetry and manual volumetry. AJR Am J Roentgenol. 2011;197:W706-W712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl. 2005;11:1481-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 21. | Noda T, Todani T, Watanabe Y, Yamamoto S. Liver volume in children measured by computed tomography. Pediatr Radiol. 1997;27:250-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Poovathumkadavil A, Leung KF, Al Ghamdi HM, Othman Iel H, Meshikhes AW. Standard formula for liver volume in Middle Eastern Arabic adults. Transplant Proc. 2010;42:3600-3605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Yoshizumi T, Gondolesi GE, Bodian CA, Jeon H, Schwartz ME, Fishbein TM, Miller CM, Emre S. A simple new formula to assess liver weight. Transplant Proc. 2003;35:1415-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Yuan D, Lu T, Wei YG, Li B, Yan LN, Zeng Y, Wen TF, Zhao JC. Estimation of standard liver volume for liver transplantation in the Chinese population. Transplant Proc. 2008;40:3536-3540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Kalshabay Y, Zholdybay Z, Di Martino M, Medeubekov U, Baiguissova D, Ainakulova A, Doskhanov M, Baimakhanov B. CT volume analysis in living donor liver transplantation: accuracy of three different approaches. Insights Imaging. 2023;14:82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Lee S, Elton DC, Yang AH, Koh C, Kleiner DE, Lubner MG, Pickhardt PJ, Summers RM. Fully Automated and Explainable Liver Segmental Volume Ratio and Spleen Segmentation at CT for Diagnosing Cirrhosis. Radiol Artif Intell. 2022;4:e210268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Perez AA, Noe-Kim V, Lubner MG, Graffy PM, Garrett JW, Elton DC, Summers RM, Pickhardt PJ. Deep Learning CT-based Quantitative Visualization Tool for Liver Volume Estimation: Defining Normal and Hepatomegaly. Radiology. 2022;302:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Wang K, Mamidipalli A, Retson T, Bahrami N, Hasenstab K, Blansit K, Bass E, Delgado T, Cunha G, Middleton MS, Loomba R, Neuschwander-Tetri BA, Sirlin CB, Hsiao A; members of the NASH Clinical Research Network. Automated CT and MRI Liver Segmentation and Biometry Using a Generalized Convolutional Neural Network. Radiol Artif Intell. 2019;1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 29. | Bozkurt B, Emek E, Arikan T, Ceyhan O, Yazici P, Sahin T, Mammadov E, Serin A, Gurcan NI, Yuzer Y, Tokat Y. Liver Graft Volume Estimation by Manual Volumetry and Software-Aided Interactive Volumetry: Which is Better? Transplant Proc. 2019;51:2387-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Hagen F, Mair A, Bitzer M, Bösmüller H, Horger M. Fully automated whole-liver volume quantification on CT-image data: Comparison with manual volumetry using enhanced and unenhanced images as well as two different radiation dose levels and two reconstruction kernels. PLoS One. 2021;16:e0255374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Mohapatra N, Gurumoorthy Subramanya Bharathy K, Kumar Sinha P, Vasantrao Sasturkar S, Patidar Y, Pamecha V. Three-Dimensional Volumetric Assessment of Graft Volume in Living Donor Liver Transplantation: Does It Minimise Errors of Estimation? J Clin Exp Hepatol. 2020;10:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Tongyoo A, Pomfret EA, Pomposelli JJ. Accurate estimation of living donor right hemi-liver volume from portal vein diameter measurement and standard liver volume calculation. Am J Transplant. 2012;12:1229-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Mokry T, Bellemann N, Müller D, Lorenzo Bermejo J, Klauß M, Stampfl U, Radeleff B, Schemmer P, Kauczor HU, Sommer CM. Accuracy of estimation of graft size for living-related liver transplantation: first results of a semi-automated interactive software for CT-volumetry. PLoS One. 2014;9:e110201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Çelik H, Odaman H, Altay C, Ünek T, Özbilgin M, Egeli T, Ağalar C, Astarcıoğlu İK, Barlık F. Manual and semi-automated computed tomography volumetry significantly overestimates the right liver lobe graft weight: a single-center study with adult living liver donors. Diagn Interv Radiol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 35. | Fu-Gui L, Lu-Nan Y, Bo L, Yong Z, Tian-Fu W, Ming-Qing X, Wen-Tao W, Zhe-Yu C. Estimation of standard liver volume in Chinese adult living donors. Transplant Proc. 2009;41:4052-4056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Lemke AJ, Brinkmann MJ, Schott T, Niehues SM, Settmacher U, Neuhaus P, Felix R. Living donor right liver lobes: preoperative CT volumetric measurement for calculation of intraoperative weight and volume. Radiology. 2006;240:736-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Satou S, Sugawara Y, Tamura S, Yamashiki N, Kaneko J, Aoki T, Hasegawa K, Beck Y, Makuuchi M, Kokudo N. Discrepancy between estimated and actual weight of partial liver graft from living donors. J Hepatobiliary Pancreat Sci. 2011;18:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Yonemura Y, Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Gion T, Harada N, Ijichi H, Yoshimitsu K, Maehara Y. Validity of preoperative volumetric analysis of congestion volume in living donor liver transplantation using three-dimensional computed tomography. Liver Transpl. 2005;11:1556-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Goja S, Yadav SK, Yadav A, Piplani T, Rastogi A, Bhangui P, Saigal S, Soin AS. Accuracy of preoperative CT liver volumetry in living donor hepatectomy and its clinical implications. Hepatobiliary Surg Nutr. 2018;7:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Chartrand G, Cheng PM, Vorontsov E, Drozdzal M, Turcotte S, Pal CJ, Kadoury S, Tang A. Deep Learning: A Primer for Radiologists. Radiographics. 2017;37:2113-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 691] [Article Influence: 98.7] [Reference Citation Analysis (0)] |
| 41. | Lee JG, Jun S, Cho YW, Lee H, Kim GB, Seo JB, Kim N. Deep Learning in Medical Imaging: General Overview. Korean J Radiol. 2017;18:570-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 555] [Cited by in RCA: 597] [Article Influence: 74.6] [Reference Citation Analysis (2)] |
| 42. | Chan HP, Samala RK, Hadjiiski LM, Zhou C. Deep Learning in Medical Image Analysis. Adv Exp Med Biol. 2020;1213:3-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 328] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 43. | LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36149] [Cited by in RCA: 20049] [Article Influence: 2004.9] [Reference Citation Analysis (0)] |
| 44. | Ahn Y, Yoon JS, Lee SS, Suk HI, Son JH, Sung YS, Lee Y, Kang BK, Kim HS. Deep Learning Algorithm for Automated Segmentation and Volume Measurement of the Liver and Spleen Using Portal Venous Phase Computed Tomography Images. Korean J Radiol. 2020;21:987-997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Kasahara M, Kaihara S, Oike F, Ito T, Fujimoto Y, Ogura Y, Ogawa K, Ueda M, Rela M, D Heaton N, Tanaka K. Living-donor liver transplantation with monosegments. Transplantation. 2003;76:694-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Yang X, Yang JD, Hwang HP, Yu HC, Ahn S, Kim BW, You H. Segmentation of liver and vessels from CT images and classification of liver segments for preoperative liver surgical planning in living donor liver transplantation. Comput Methods Programs Biomed. 2018;158:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 47. | Pomposelli JJ, Tongyoo A, Wald C, Pomfret EA. Variability of standard liver volume estimation versus software-assisted total liver volume measurement. Liver Transpl. 2012;18:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |