Published online Sep 18, 2023. doi: 10.5500/wjt.v13.i5.239
Peer-review started: February 10, 2023
First decision: March 15, 2023
Revised: April 19, 2023
Accepted: June 14, 2023
Article in press: June 14, 2023
Published online: September 18, 2023
Processing time: 216 Days and 6.7 Hours
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) are novel oral hypoglycemic agents garnering much attention for their substantial benefits. These recent data have positioned SGLT2i at the forefront of diabetic chronic kidney disease (CKD) and heart failure management. SGLT2i use post-kidney transplant is an emerging area of research. Highlights from this mini review include the following: Empagliflozin is the most prescribed SGLT2i in kidney transplant recipients (KTRs), median time from transplant to initiation was 3 years (range: 0.88-9.6 years). Median baseline estimated glomerular filtration rate (eGFR) was 66.7 mL/min/1.73 m2 (range: 50.4-75.8). Median glycohemoglobin (HgbA1c) at initiation was 7.7% (range: 6.9-9.3). SGLT2i were demonstrated to be effective short-term impacting HgbA1c, eGFR, hemoglobin/hematocrit, serum uric acid, and serum magnesium levels. They are shown to be safe in KTRs with low rates of infections, hypoglycemia, euglycemic diabetic ketoacidosis, and stable tacrolimus levels. More data is needed to demonstrate long-term outcomes. SGLT2i appear to be safe, effective medications for select KTRs. Our present literature, though limited, is founded on precedent robust research in CKD patients with diabetes. Concurrent research/utilization of SGLT2i is vital to not only identify long-term patient, graft and cardiovascular outcomes of these agents, but also to augment management in KTRs.
Core Tip: Multiple large trials have demonstrated sodium-glucose cotransporter-2 inhibitors (SGLT2i) associated kidney and cardiovascular benefits for chronic kidney disease patients with diabetes. Important considerations are critical to determine safety and efficacy of these medications after kidney transplantation. While evidence is limited, SGLT2i appear to be both safe and effective short-term. More robust research is needed to determine the long-term impacts of their use in kidney transplant recipients. Appropriate patient selection and monitoring are vital to clinical use and future research efforts of SGLT2i in kidney transplantation.
- Citation: Ramakrishnan P, Garg N, Pabich S, Mandelbrot DA, Swanson KJ. Sodium-glucose cotransporter-2 inhibitor use in kidney transplant recipients. World J Transplant 2023; 13(5): 239-249
- URL: https://www.wjgnet.com/2220-3230/full/v13/i5/239.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i5.239
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) or "gliflozins", are oral hypoglycemics that work by inducing glucosuria. They are derived from phlorizin, a glucosuric compound found in apple tree root bark. As described by van Bommel et al[1], there are 2 clinically significant sodium-glucose transporters found in humans: SGLT1 Low-affinity high-capacity transport in the distal convoluted tubule and SGLT1 high-affinity low-capacity transporter in proximal convoluted tubule. As noted by Salvatore et al[2] and Sawaf et al[3], SGLT2i reduces the glucose excretion threshold to 2.2 mmol/L (40 mg/dL) from 10 mmol/L (180 mg/dL).Consequently, they have been shown to reduce glycohemoglobin (HgbA1c) by 0.6%-0.9% with glomerular filtration rate (GFR) > 60 mL/min and 0.3%-0.4% with GFR 30-59 mL/min[1]. SGLT2i also block the sodium/glucose symport channel in the proximal convoluted tubule leading to osmotic diuresis and natriuresis[3]. This excess sodium excretion is thought to induce afferent vasoconstriction through glomerular feedback thereby reducing hyperfiltration[1].
Data on SGLT2i have demonstrated great promise for their use in chronic kidney disease (CKD) patients with diabetes. In a recent meta-analysis, Zelniker et al[4] synthesized the findings of multiple landmark trials (EMPA-REG OUTCOME, CANVAS and DECLARE-TIMI). They showed that SGLT2i reduced the risk of renal disease progression by 45% [hazard ratio (HR) = 0.55, 95% confidence interval (CI): 0.48-0.64, P < 0.0001], and cardiovascular death or heart failure hospitalization by 23% (HR = 0.77, 95%CI: 0.71-0.84, P < 0.0001) in patients with and without atherosclerotic heart disease.
These renoprotective benefits have been observed in patients with, as well as without, diabetes. Heerspink et al[5], via dapagliflozin (DAPA)-CKD, showed that DAPA in CKD patients with or without diabetes reduced the risk of a composite outcome of estimated GFR (eGFR) decline of at least 50%, end stage kidney disease (ESKD), or death from renal/cardiovascular cause (HR = 0.56, 95%CI: 0.45-0.68, P < 0.001) with a number needed to treat of 19 (95%CI: 15-27).
As shown by adoption of SGLT2i as first line therapies for CKD patients with diabetes by the 2022 Kidney Disease Improving Global Outcomes guidelines by Rossing et al[6], evidence for these agents is promising. Favorable outcomes owing to SGLT2is have led the transplant community to investigate their broader application.
Many dependent diabetic kidney transplant recipients (KTR) appear to be likely beneficiaries of SGLT2i therapy. As described by Chewcharat et al[7], 40% of waitlisted patients have diabetes mellitus (DM) and 15%-30% of non-diabetic patients develop post-transplant DM (PTDM)[7]. PTDM is associated with high rates of graft loss, cardiovascular disease, infectious complications, and mortality[7]. Inherent risks of kidney transplantation e.g., urinary tract infection, concern for drug interactions i.e., immunosuppression, and acute kidney injury/CKD risk, have raised safety and efficacy concerns of SGLT2i.
In this mini review, we aim to summarize recent literature describing SGLT2i usage in KTRs to: (1) Provide guidance for clinical use; (2) identify current limitations; and (3) highlight future directions. We hope this mini review will act as a reference for clinicians and researchers alike to advance clinical/translational research and characterize SGLT2i’s role in diabetes management after kidney transplantation.
We conducted literature searches in PubMed, Cochrane, Google Scholar from January 2019 to January 2023 and reference lists of relevant studies and reviews. Key words utilized in our search included the following: “SGLT2 inhibitors, SGLT2i, KTRs, type 2 diabetes mellitus, post-transplant diabetes mellitus.”
We limited our search to studies with available full text and English language. In this mini review, we selected studies of SGLT2i use in type 2 DM (T2DM) and/or PTDM in KTRs that were either: (1) Prospective randomized control trials; (2) prospective case series; and/or (3) retrospective case series with comparison groups. We limited study inclusion to those occurring in the last 4 years to highlight recent research.
For our analysis of the following outcomes: HgbA1c, eGFR, weight, blood pressure, Immunosuppression drug interactions, adverse events, we pooled studies that reported these data together. As descriptions of cost, novel findings, and long-term outcomes were limited to one or a few studies, these were simply discussed in context of specific studies.
Nine studies met our search criteria: 1 randomized controlled trial, 2 prospective observational studies, and 5 retrospective analyses, of which 2 had comparison groups. All nine studies occurred in the last 4 years.
In this sample, empagliflozin (n = 241) was the most prescribed SGLT2i followed by DAPA (n = 85) and canagliflozin (n = 74). The median time from transplant for initiating SGLT2i was 3 years (range: 0.88-9.6 years post-transplant). Median baseline eGFR was 66.7 mL/min/1.73 m2 (range: 50.4-75.8). Median HgbA1c at initiation was 7.7% (range: 6.9-9.3). The following results were seen and are summarized in Table 1.
| Ref. | Type, location | Follow up | Treatment arms | Inclusion/exclusion criteria | Baseline eGFR (mL/min/1.73 | Time from transplant | Result | Adverse events/treatment discontinuation | Comments |
| Lemke et al[10], 2022 | Retrospective, United States | 12 mo; 27 pts ≥ 12 mo | Cana (n = 12); Dapa (n = 3); Empa (n = 24) | T2DM or PTDM; SGLT2i; 4/2013 to 10/2020; care solely in health system | eGFR, median (IQR) 69 (54-76); HgbA1c Median (IQR) 8.4 (7.8-9.2) | Median (IQR) 28 mo (16-60) | HbA1c↓ (8.4-7.5 at 3 mo; 7.5 at 12 mo; eGFR ↔/Cr ↔ at 3/12 mo; Wt ↓1.6 kg | UTI (n = 6; 3 required hospital stay; 1 ICU). Diabetic foot ulcer (n = 2). Hypoglycemia (n = 2; insulin n = 1, glipizide n = 1). No DKA, AKI dehydration requiring IVF, Fournier gangrene, Genital infection, fractures. Discontinued tx: n = 17 [d/c after a median (IQR) 244d (117-401)], n = 6 for cost, n = 4, eGFR, n = 3 infection, n = 1 poor wound healing, n = 1, hypoglycemia, n = 1 self d/c, n = 1, death unrelated to SGLT2i | 38% on ACEi/ARB at initiation. Insufficient proteinuria data; Tac levels stable. 5/6 had prior UTI, 4/6 continued SGLT2i w/o recurrence |
| Lim et al[13], 2022 | Retrospective, South Korea | 62 mo ± 42 mo | Empa (n = 150). Dapa (n = 76) vs non-SGLT2i (n = 1857) | T2DM or PTDM Pancreas Transplant Prescribed SGLT2i < 90 from transplant | eGFR at 3 mo post-transplant 66.9 ± 17.7 vs 68.4 ± 20.1. HgbA1c at 3 mo post-transplant. Both 7.3 ± 1.4 | Mean 3.8 yr ± 4.5 | A risk primary outcome = composite outcome of all-cause mortality, DCGF, and SCr doubling: Multivariate [aHR (0.43; 95%CI = 0.24-0.78, P = 0.006) propensity score-matched; aHR (0.45; 95%CI = 0.24-0.85, P = 0.013)]. HbA1c = NR. eGFR stable at 8 mo. ↓SCr doubling significantly in unadjusted and adjusted models. Wt = NR | UTI/genital mycotic infection: (SGLT2i 4.5 events/100 patient-year vs non-SGLT2i 6.2/100 patient-year). No DKA. Discontinued txt: NR | 15.6% eGFR dip over 10% during first month. eGFR recovered thereafter. 48.7% of the SGLT2i cohort was on ACEi/ARB. Composite all-cause mortality, DCGF, or SCr doubling in KTRs |
| Hisadome et al[12], 2021 | Retrospective observational study, Japan | 48 wk | SGLT2i (n = 29); Cana (n = 9); Empa (n = 4); Dapa (n = 3); Luseo (n = 5); Ipra (n = 7); Tofo (n = 1) vs Other oral glycemic agent (n = 60); DDP4i (n = 42); meglitinides (n = 9); metformin (n = 4); SU (n = 4) α-glucosidase | ESRD patients w/T2DM nephropathy pre-transplant PO hypoglycemic. Follow up at outside centers < 1 yr follow up. Missing data | eGFR mean ± SD: 50.4 ± 13.9; 47.5 ± 13.1. HgbA1c mean ± SD: 7.7 ± 0.9; 7.6 ± 1.1 | NR | HgbA1c 7.7 -> 7.6 (same) vs 7.6 to 7.5. Wt -0.7 ± 5.1 kg vs 1.6 ± 4.5 kg. eGFR 50.4 -> 51.4 vs 47.5 to 46.3. BP went up (7 mmHg ± 20 vs -3 ± 24) | UTI 2:0; CV disease 0:2. BPAR 1:1. Discontinued txt: NR | 71.2% of the SGLT2i group was also on ACEi/ARB. Stable tac levels (P = 0.755) |
| Song et al[14], 2021 | Retrospective, United States | 101 d | Empa (n = 43); Cana (n =6); Dapa (n = 1) | PTDM eGFR ≥ 30. AKI in prior ≤ 30 d. UTI in prior 6 mo | eGFR at initiation: Mean 66.7; 30-45 (n = 7; 14%) HbA1c mean ± SD: 7.1 ± 0.1 | Median (IQR): 319.5 d (122-696). 40% within 200 d | ΔeGFR 3 mo: -1 mL/min; 6 mo: 1 mL/min. ΔHgbA1c 0.53%. Treated UTI 7 (14%). Approximately 20% typical rate. Change in insulin (-3.7 units ± 22.8). Wt (-2.95 kg ± 3.54, 95%CI 3.53-1.5). HgbA1c ↔; eGFR ↔; Wt↓. ΔMag2+ ↑ by 0.13 | UTI (n = 7). D/C txt: n = 9 (5, UTI; 1 genital infection, 1; native disease; recurrence, 1 PTDM; resolution, 1; physician preference) | 80% T2DM; 98% on prednisone; UTI rate comparable to KT population (14%) |
| AlKindi et al[8], 2020 | Retrospective case series, United Arab Emirates | Range: 3 mo to 2 yr | Empa 10 mg n = 5; 25 mg n = 1. Dapa 25 mg n = 2 | Diabetic KTRs; SGLT2i between 06/16-01/19 | eGFR mean ± SD: 75.8 ± 13.4; HbA1c mean ± SD: 9.3 ± 1.4 | mean ± SD: 9.6 yr ± 6.41 | HgbA1c↓ (9.0 at 3 mo; 8.6 at 6 mo; 7.7 at 9 mo; 7.4 at 12 mo; eGFR ↔ median eGFR 72 (range 62-76) at 12 mo. Wt (mean wt 84.82 kg -> 82.87 at 3 mo -> 82.75 at 6 mo). BP not statistically significant though 9 pt difference | UTI + hospitalization (n = 1); pt w hx of UTI no UTI w ppx. Discontinuation rate: NR | 2/8 T2DM; 6/8 PTDM; all LURKTx |
| Halden et al[9], 2019 | Prospective, double blind, RCT Norway | 6 mo | Cana 10 mg (n = 22). Placebo (n = 22) | ≥ 18 yr; ≥ 1 yr post-transplant. PTDM only < 20% SCr deviation in last 2 mo. ≥ 3 mo stable immunosuppression. eGFR ≤ 30; Pregnant or nursing | eGFR Median (IQR) 66(57-68): 59 (52-72). HbA1c Median (IQR) 6.9 (6.5-8.2): 6.8 (6.1-7.2) | Median (IQR) 3 yr (1-16): 3 yr (1-15) | HbA1c↓ (6.9 to 6.7 vs 6.6 to 6.9); eGFR↓ (2 mo), ↔ (6 mo)-66 to 61 vs 59 to 59. Weight↓ (92 kg to 88.8 kg vs 84 to 85 kg). No real impact on BP. Hgb increase 13.9 to 14.5. ↓Uric acid. Tac/CSA/Siro levels stable | Urosepsis 1:0 (hx of recurrent UTI), UTI 3:3, genital infection 1:0, dizziness 2:0, hematuria 1:0. Discontinued txt: n = 2 (recurrent UTI, urosepsis): 3 (withdrew consent, colon cancer, no longer PTDM) | High DPP-4i use; most were not on additional therapy |
| Schwaiger et al[16], 2019 | Prospective | 1 mo (n = 14); 12 mo (n = 8) | Empagliflozin (n = 14). Reference (n = 24) | eGFR > 30; < 40 IU/d insulin. HgbA1c < 8.5. Adequately diagnosed PTDM | Baseline. eGFR 55.6. Baseline HgbA1c 6.5 | Median 69.4 ± 57.2 mo | HgbA1c 6.5-> 6.6 at 4 wk (P > 0.05). eGFR 55.6 -> 47.5 at 4 wk (P > 0.05). Average TBW↓ 1.6 kg; Waist circ ↓4 cm. ECV/TBFV decreased | UTIs: 5:9. Genital infection: 1. AKI, DKA, Fournier’s-NR | 100% on steroids. Median onset of PTDM was 0.5 months. 100% on insulin |
| Shah et al[11], 2019 | Prospective | 6 mo | Canagliflozin (n = 25) | ≥ 18 years old. CrCl (ml/min) > 60; HgbA1c > 6.5; T2DM; PTDM. Unstable Cr. ALT > 2 × ULN; TBili > 2 × ULN; Recent UTI/genital mycotic infection | Baseline Cr (mg/dL): 1.1 ± 0.2; Baseline HgbA1c: 8.5 ± 1.5 | Mean duration of transplant = 2.7 yr (0.2-13.2) | HgbA1c: 8.5 ± 1.5 -> 7.6 ± 1. Cr: 1.1 ± 0.2 -> 1.1 ± 0.3. Weight: 78.6 ± 12.1 -> 76.1 ± 11.2 (P < 0.05). SBP (mmHg): 142 ± 21 -> 134 ± 17 (P < 0.05) | No increase in UTI/genital infections. No hypoglycemia or DKA. Fatigue (n = 3). Discontinued treatment (n = 1) | 20 T2DM; 5 PTDM. Reduction of other hypoglycemics needed. 1 KTR self d/ced. Used fixed 100 mg dose. Stable tac doses |
HgbA1c generally improved with changes between 0.2%-1% in the reported studies. Notably, in the study by AlKindi et al[8], which included a cohort with a mean HgbA1c of 9.3% at initiation as well as excellent allograft function, HgbA1c decreased by 2.3% at 12 mo. As is described by Halden et al[9], more robust impacts on glycemic control were observed in those with higher HgbA1c and eGFR.
eGFR was preserved in most studies over a period of 6-12 mo[8-13]. Lim et al[13] observed a 10% eGFR dip in 15.6% of their cohort with SGLT2i initiation, though eGFR did recover from this and stabilize. After month 5, there was no significant difference in eGFR between dippers and non-dippers. At last follow up (8 mo post-SGLT2i initiation), eGFR in the dippers (67.9 ± 13.9, n = 24) was comparable to that of the non-dippers [67.9 ± 13.9 mL/min/1.73 m2 (n = 24) vs 69.8 ± 19.0 mL/min/1.73 m2 (n = 106), P = 0.358].
Though specific data on long term eGFR are lacking, Lim et al[13] did report a significant reduction in terms of SCr doubling in the SGLT2i cohort vs non-SGLT2i users in both unadjusted (HR = 0.49, 95%CI: 0.29-0.85) , adjusted (across multiple models: Adjusted HR (aHR) = 0.37-0.41, 95%CI: 0.22-0.90, all P < 0.05) , and propensity-score matching (aHR = 0.45, 95%CI: 0.23-0.88, P = 0.019) at 72 mo of follow up.
Proteinuria was not assessed in these studies. As proteinuria is a major risk factor for and driver of progressive CKD, this is certainly an area that needs further studying.
Weight decreased in 8 studies with a median weight decrease of 1.95 kg (range: 0.7-3.2 kg)[8-10,12,14].
Blood pressure changes were reported in 4 studies, with mixed results[8,9,11,12]. The magnitude of these changes was on the order of 7-9 mmHg, which are likely clinically significant. This is reaffirmed by findings in the ADVANCE trial, whereby Heerspink et al[15] showed that randomization to perindopril-indapamide compared to placebo in CKD ≥ 3 patients with diabetes for 5 years prevented 12 cardiovascular events with reductions in systolic blood pressure on the order of 4.5 mmHg.
Though data on drug interactions with immunosuppression were limited, four studies did not observe clinically nor statistically significantly differences in drug trough levels after SGLT2i initiation[9-12].
Urinary tract infections (UTI) were the most common adverse event observed across the various studies. When reported, these ranged from none observed up to 36%. 4 studies reported rates between 13%-15%[8-10,14].
Genital infections (GI) occurred but less commonly than UTI in KTRs with only a few GI occurring in studies where it was distinctly described[9,14,16].
Graft function remained stable throughout these studies despite high angiotensin converting enzyme inhibitors (ACEi)/aldosterone receptor blockers (ARB) utilization and the observed eGFR dip at the 4-6 wk mark[10,12,13].
Leg amputation was not observed in any of the studies described. Schwaiger et al[16] reported on this in their study with empagliflozin. As is aptly described by Heyward et al[17] in their systematic review and meta-analysis, the risk for lower extremity amputation for SGLT2i use in the non-transplant population has only been observed with canagliflozin.
In these small studies, no episodes of euglycemic diabetic ketoacidosis were reported. Song et al[14] noted a wide range of insulin dose reductions post-SGLT2i incorporation. Hypoglycemia was noted infrequently in these studies (n = 2 per Lemke et al[10]). This risk for hypoglycemia is highest for those with well controlled diabetes (HgbA1c < 8) as well as those on insulin and/or sulfonylurea-class medications, as was the case in the Lemke et al’s study[10].
Lemke et al[10] identified cost as the highest reported reason for SGLT2i discontinuation (35%, n = 6). Over time, SGLT2i have become more affordable. Aggarwal et al[18] recently described out-of-pocket expenses for SGLT2i, noting that for most insured patients, median cost for 30 d of SGLT2i therapy cost around $38.43 (range: $3.87-$49.42)[18].
In their comprehensive randomized controlled trial, Halden et al[9] observed increased hemoglobin/hematocrit and decreased uric acid levels with SGLT2i use. Song et al[14] observed an improvement in serum magnesium levels after SGLT2i initiation.
Lim et al[13] showed a significant reduction at five years in their composite outcome of all-cause mortality, death-censored graft failure (DCGF), and serum creatinine doubling with SGLT2i use in both multivariate (aHR = 0.43; 95%CI: 0.24-0.78, P = 0.006] and propensity score-matched aHR (0.45; 95%CI: 0.24-0.85, P = 0.013). Otherwise, these studies lacked long term outcome data.
Though these studies are heterogenous and limited in terms of design and size, short term safety and efficacy outcomes of SGLT2i use in diabetic KTRs appear comparable to those observed in CKD patients with diabetes.
Glycemic control paralleled that seen in the non-transplant DM population with modest HgbA1c improvements. Though most studies included KTRs with adequate allograft function, Hisadome et al[12] and Song et al[14] included a substantial number of individuals with eGFR in the 30-45 range, which approximates to CKD stage 3b. Though there are potential differences between CKD and CKD after transplant as described by Djamali et al[19], evidence supporting safe, effective use of SGLT2i in KTRs with decreased allograft function is encouraging.
Remarkably, the eGFR decline, recovery and stabilization of kidney function that occurs in non-transplant diabetics was also observed in some KTRs without significant unfavorable impacts on long term graft function. This was in the setting of high reported concurrent ACEi/ARB use which are recommended as first line agents in diabetic kidney disease. This is exciting, as renin-angiotensin-aldosterone system (RAAS) blockade plays a vital role in diabetic CKD/cardiovascular management. Moreover, the major trials for SGLT2i, such as EMPA-REG OUTCOME by Wanner et al[20] reported RAAS blockade use between 80%-85% of those studied. In summary, the illustration of eGFR stability with simultaneous use of SGLT2i/RAAS blockade in KTRs across multiple studies will hopefully quell clinician fears regarding their concurrent use.
It is unfortunate that proteinuria was not an endpoint in any of the included studies. Results of studies in the general population, namely those by Jongs et al[21], Cherney et al[22], and Trujillo et al[23], in terms of SGLT2i effect on proteinuria, are both limited and mixed[21-23]. EMPA-REG OUTCOME by Wanner et al[20], CANVAS by Neal et al[24] and CREDENCE by Perkovic et al[25] and DAPA-CKD by Heerspink et al[5], suggested utility for these agents in reducing the geometric mean urinary albumin creatinine ratio, increasing the likelihood of regression in albuminuria stage and reducing the risk of macroalbuminuria progression[20,21]. Further investigations into whether or not SGLT2i impact proteinuria in KTRs will be important not only to better understand these medications, but also to help with agent selection if a difference e.g., empagliflozin and canagliflozin vs DAPA, is observed between them in KTRs.
Weight loss occurred in almost every study, likely due to the osmotic diuresis caused by SGLT2i use. This is also occurring due to fat loss from caloric wasting via glucose. As the weight loss demonstrated for most patients is less than 5% total body weight, this likely has little bearing clinically.
That being said, perhaps weight loss can underscore future studies examining impacts on truncal obesity, waist size (as Schwaiger et al[16] remarked), cholesterol, uric acid levels, and other markers of obesity/metabolic syndrome and their impacts on kidney and cardiovascular outcomes.
Blood pressure outcomes were less clear across these studies, which is at least partly explained by the different mechanisms and influences on blood pressure in KTRs compared to CKD patients as described by Kasiske et al[26]. It is unlikely due to weight loss alone given the magnitude of weight loss as previously noted. As these medications are studied further in KTRs, perhaps novel mechanisms for how SGLT2i influence blood pressure will be elucidated.
While UTIs were observed in these studies, they did not appear to occur significantly more than in KTRs not on SGTL2i. As described by Brune et al[27], the prevalence of UTI after transplant varies significantly based on several factors (namely via definition, study, population, length of follow up). However, they state that a reasonable benchmark based on larger studies is a 1-year incidence rate around 30%. Lemke et al[10] reported continued use of SGLT2i after UTI, with one of those patients requiring hospitalization for treatment, but without recurrent disease. Long term impacts of SGLT2i use/glucosuria not only on UTI risk, but also asymptomatic bacteriuria, antibiotic use and associated complications are ongoing uncertainties. GI were observed but at a fairly low rate compared to UTI as described above.
Though limited, drug level data suggest that SGLT2i have little to no impact on calcineurin inhibitor (CNI) trough levels, nor were increased episodes of rejection observed. As Scheen[28] describes in his excellent review on the subject, SGLT2i metabolism minimally involves cytochrome CYP3A4, making SGLT2i-CNI drug interactions slight at best.
As described by Song et al[14], hypomagnesemia was improved in KTRs on SGLT2i. As hypomagnesemia is associated with increased risk of cardiovascular and infection-related mortality as noted by Panthofer et al[29] and Odler et al[30] and this is an important management target. Per Huang et al[31], there may be a role for pre-emptive SGLT2i use as hypomagnesemia itself has been shown to increase the risk of PTDM in KTRs. Additionally, hypomagnesemia treatment can be challenging as most magnesium formulations cause diarrhea. Therefore, SGLT2i may play a role obviate/minimize high magnesium supplementation needs.
Though not discussed thoroughly in the literature, Bilezikian et al[32] and Kohan et al[33] note that there are some concerns for SGLT2i and their impacts on bone health. Lemke et al[10] reported on fractures in their study, noting none occurred. Though this is a nascent area of research, Blau and Taylor[34] demonstrated a possible mechanism via the FGF23–1,25-dihydroxyvitamin D–parathyroid hormone axis. As impaired bone health is common in KTRs, seeing how this relationship bears out in longer, more robust studies will be important for patient selection and ongoing management. At the moment, there is insufficient data to attribute substantial fracture risk to SGLT2i use.
With SGLT2i being relatively new agents, there is a paucity of data on long-term kidney, cardiovascular, and survival outcomes. Determination of their impact on long-term outcomes will require larger, protracted investigations. This is illustrated in the EMPA-REG trial by Wanner et al[35], in which SGLT2i-mediated eGFR preservation was first seen around 80 wk of therapy vs placebo.
Ideally, future retrospective studies, like that by Lim et al[13] and/or prospective analyses can describe these relationships going forward. These would be best achieved by multi-center, large trials analogous to their landmark predecessors.
In their comprehensive study review on the management of PTDM, Hecking et al[36] aptly summarize direct and indirect potential benefits of SGLT2i in kidney transplantation. Though some of this is extrapolated from non-KT research, novel impacts such as reduction in vascular rigidity as well as hypoxia-inducible factor-1, could be impactful in the kidney transplant population regarding cardio-/reno-/vascular health, anti-inflammatory properties and perhaps anemia management as described by Hecking et al[36], Gupta and Wish[37].
A key limitation of these studies is that they evaluated KTRs with diabetes alone. As this is a logical starting place for investigating the efficacy of SGLT2i, to suggest that these medications are limited only to KTRs with diabetes is too narrow a view. With kidney transplantation existing as a state of CKD as well as ESKD itself portending significant cardiovascular risk, we surmise (and hope) that the benefits of SGLT2i will extend to KTRs without diabetes as well. As was demonstrated in DAPA-CKD, a multicenter randomized controlled trial of 4304 patients in which 32.5% of the patients were non-diabetic, SGLT2i increased the likelihood of albuminuria regression and reduced the likelihood of progression to more severe stages of albuminuria in CKD patients with and without diabetes[5]. Therefore, KTRs without diabetes warrant investigation into the utility of SGLT2i use.
Though more research is needed, there appears to be a subset of non-insulin dependent diabetic KTRs who ought to benefit from SGLT2i therapy.
Identifying appropriate candidates is a critical step for implementing SGLT2i therapy routinely. Though questions remain presently regarding long term safety, the stalwart evidence from the CKD literature is compelling for the transplant community to press forward.
In their recent review, Patel et al[38] proposed an “ideal” KTR SGLT2i candidate. While this provides a nice general framework, we have additional characteristics to build on this model for identifying SGLT2i candidates.
At present, there does not appear to be substantial evidence on when post-transplant to initiate SGLT2i therapy. Earlier initiation i.e., prior to 6- or 12-mo post-transplant may be beneficial for at least 3 reasons: (1) PTDM appears to be an early post-transplant complication. This is shown by Jenssen and Hartmann[39], in their review on PTDM, where they cite Porrini et al[40]. In their study, 32% of the cohort developed PTDM (215/672). Of these 215, 187 (87%) of these KTRs developed PTDM prior to 12 mo; (2) major benefits of SGLT2i therapy such as eGFR preservation may require long term medication use, as EMPA-REG showed[35]; and (3) as is aptly described by Wolfe et al[41] in their seminal study on mortality after deceased donor kidney transplant (DDKT), there is increased risk of death in the early post-transplant time period. Perhaps this will promote studies of initiating SGLT2i at the time of transplantation in select patients e.g., DDKT with immediate graft function.
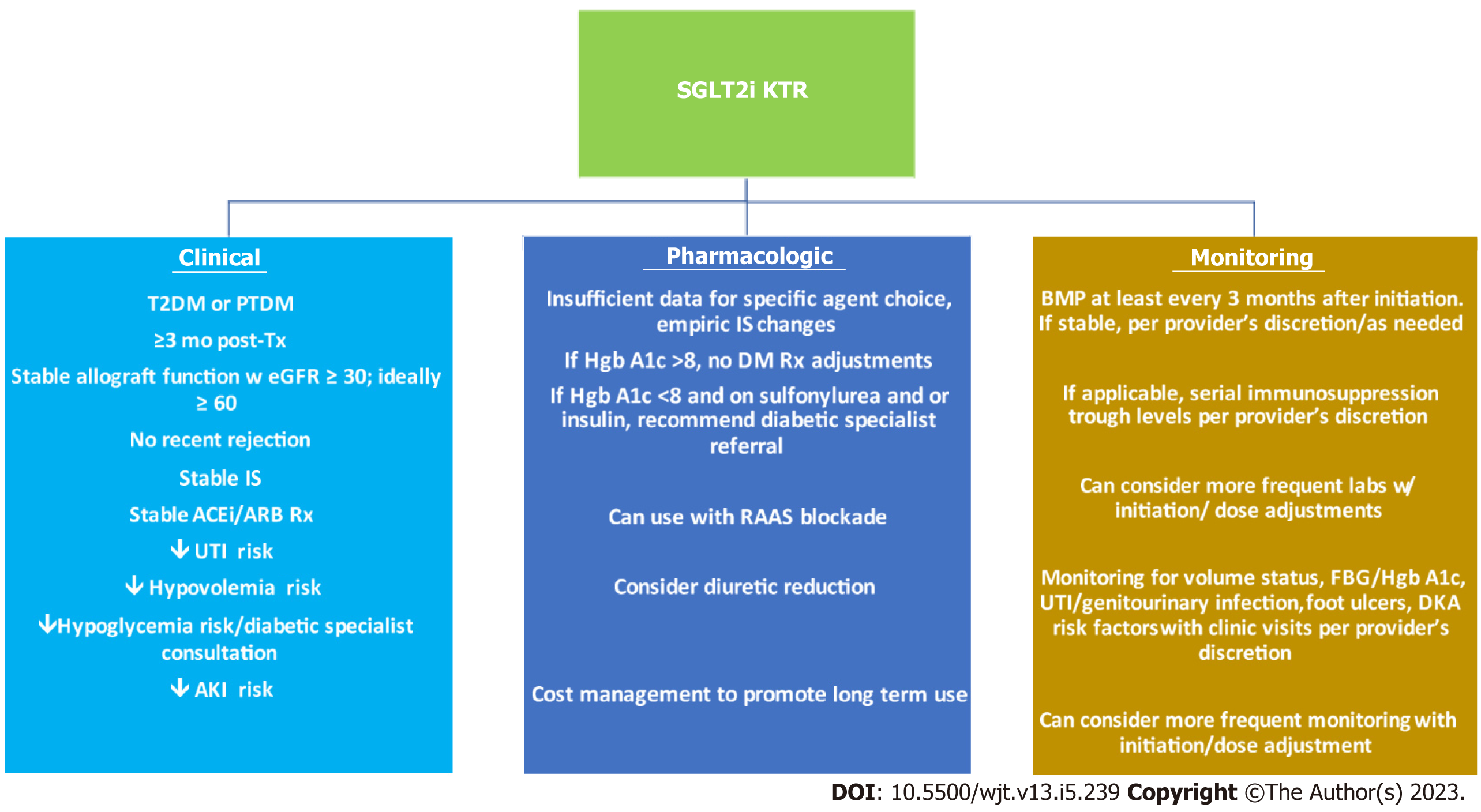
In the following section, we will put forth clinical recommendations for SGLT2i use in KTRs. These are based on the aforementioned results as well as inclusion/exclusion criteria in the studies reviewed. As this is an evolving science, these are solely recommendations i.e., provider discretion remains crucial to using these medications. These are also summarized in Figure 1.
Based on the literature reviewed, we propose the following as good candidates for SGLT2i use: (1) KTRs with pre-transplant T2DM or PTDM; (2) at least 3 mo post-transplant; (3) stable allograft function preferably with eGFR of at ≥ 30 mL/min/1.73 m2, ideally ≥ 60 mL/min/1.73 m2 for the past 2 mo; (4) no rejection episodes within the past 3 mo; (5) at least 3 mo of stable immunosuppression; (6) stable ACEi/ARB doses; (7) patients at low risk for volume depletion e.g., low risk for unstable diarrhea, vomiting; (8) patients at low risk for hypoglycemia e.g., HgbA1c > 8 or < 8 and not on a sulfonylurea or insulin. If at risk for hypoglycemia, would consult diabetic specialist for regimen titration; (9) patients without significant UTI history or diabetic foot ulcers; (10) patients with low risk for acute kidney injury; and (11) patients who may benefit from novel aspects of SGLT2i: Hypomagnesemia, hyperuricemia, anemia.
In terms of pharmacologic therapy titration in the context of SGLT2i initiation, we recommend the following: (1) Insufficient data to support empiric adjustments to maintenance immunosuppression or to diabetic prescriptions; (2) can consider reducing diuretic doses; (3) advise diabetic specialist consultation for KTRs with well controlled diabetes (HgbA1c < 8) and other diabetic agents, particularly insulin or sulfonylureas, to help titrate their diabetic regimen to minimize the risk of hypo
In terms of monitoring parameters, we recommend the following: (1) Renal function assessment at least every 3 mo at a minimum. Can consider more frequent monitoring with initiation/dose adjustments; (2) if applicable (on CNI or mammalian target of rapamycin inhibitor therapy), serial immunosuppression trough levels per provider’s discretion. Can consider more frequent monitoring with initiation/dose adjustments; (3) routine monitoring for volume status, risk factors for diabetic ketoacidosis, hypoglycemia; and (4) routine monitoring for signs and symptoms of UTI, diabetic foot ulcers.
It is somewhat challenging to put forth contraindications to use at this time, particularly when the evidence for use is so persuasive. Assuredly there are patients in whom SGLT2i use poses greater risk of harm than benefit e.g., a KTR with a history of DKA, at risk for or experiencing recurrent transplant pyelonephritis, and/or chronic osteomyelitis and/or active diabetic foot wounds. Ultimately, the determination of benefit vs risk requires clinical reasoning, evaluation and patient-provider dialogue on whether SGLT2i use is in the patient’s best interest.
Notably, guidance exists in the literature regarding patient handout communications when initiating SGLT2i therapy. Lam et al[42] provide an excellent version that is generally applicable to KTRs.
Though early data on SGLT2i implementation in KTRs is promising, it is albeit limited.
There are 3 main limitations in the data on the use of SGLT2i in KTRs. Longitudinal studies with large enrollment volume are absent. The longest follow up was around 8.5 years with most having far less. This leaves cardiovascular, graft and mortality outcomes unexplored. Rare adverse events like euglycemic DKA or osteoporosis are also not explored. RCTs are necessary to establish causality and bolster clinical practice recommendations. Most of the studies in SGLT2i are limited to retrospective, observational design or case series.
The SGLT2i story is one that is well underway. There appears to be substantial evidence supporting their use in terms of safety and short-term efficacy based on the studies we described and their antecedents. What lies ahead regarding long-term SGLT2i therapy is unknown. With SGLT2i, we are not working ab initio (from the beginning). Rather, as is precedent in some of the greatest epics and sagas (i.e., the Mahābhārata, Homer’s Iliad and Odyssey, Virgil’s Aeneid, Dante’s Divine Comedy) as noted by Lochtefeld[43], Murray[44] and Raffa[45], we can and ought to forge ahead into the unknown in medias res-into the middle of things.
While the current literature gives insight into short-term outcomes of SGLT2i use in KTRs, more research is needed to identify the long-term impacts of SGLT2i use in this population.
Currently, there are 2 actively recruiting clinical trials (NCT04965935 aka INFINITI2019 and NCT04906213 aka CREST-KT).
INFINITI2019 is a double-blind, placebo-controlled trial aimed at comparing DAPA to placebo in 52 KTRs. The primary outcome is blood pressure reduction in addition to fasting blood glucose, HgbA1c, continuous home glucose monitoring, arterial stiffness, systemic vascular resistance, change in baseline measured GFR, change in eGFR, proximal tubular natriuresis, albuminuria, change in baseline urinary and plasma oxidative stress markers, change in tubule interstitial hypoxia, CNI levels, and adverse events.
CREST-KT is a single-center, double-blinded randomized controlled trial of empagliflozin therapy in 72 KTRS with (36) and without (36) diabetes. After dividing by diabetes diagnosis, the groups will be randomized 2:1 to empagliflozin 10 mg vs placebo i.e., 48 KTRs will be on empagliflozin and 24 KTRS will be on placebo. Study time is planned to be 18 mo.
Primary outcomes include: Change in eGFR, change in albuminuria, change in cardiac structure by 3D echocardiogram, change in blood insulin level, change in fasting blood sugar, # of UTIs and # of GI.
Secondary outcomes include: Change in kidney biopsy from time zero to 6 mo and change in HgbA1c as well as AEs.
In addition to these studies, hopefully future randomized controlled trials examining long term renal outcomes as well as cardiovascular outcomes, particularly in patients with known heart failure, will help guide appropriate SGLT2i use and influence guidelines and practice patterns for SGLT2i in KTRs.
SGLT2i appear to be safe, effective medications in the arsenal of post-transplant therapies for select KTRs. Our present literature, though somewhat limited, is founded on preceding strong research in CKD patients with diabetes. Concurrent research and utilization of SGLT2i is vital to not only identify long-term patient, graft and cardiovascular outcomes of these agents, but also to augment diabetic, CKD, and cardiovascular management in KTRs in media res.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shuang W, China; Tentolouris N, Greece S-Editor: Fan JR L-Editor: A P-Editor: Zhang YL
| 1. | van Bommel EJ, Muskiet MH, Tonneijck L, Kramer MH, Nieuwdorp M, van Raalte DH. SGLT2 Inhibition in the Diabetic Kidney-From Mechanisms to Clinical Outcome. Clin J Am Soc Nephrol. 2017;12:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 2. | Salvatore T, Galiero R, Caturano A, Rinaldi L, Di Martino A, Albanese G, Di Salvo J, Epifani R, Marfella R, Docimo G, Lettieri M, Sardu C, Sasso FC. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 125] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 3. | Sawaf H, Thomas G, Taliercio JJ, Nakhoul G, Vachharajani TJ, Mehdi A. Therapeutic Advances in Diabetic Nephropathy. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 4. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation. 2019;139:2022-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 531] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 5. | Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 3039] [Article Influence: 607.8] [Reference Citation Analysis (1)] |
| 6. | Rossing P, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, Khunti K, Liew A, Michos ED, Navaneethan SD, Olowu WA, Sadusky T, Tandon N, Tuttle KR, Wanner C, Wilkens KG, Zoungas S, Craig JC, Tunnicliffe DJ, Tonelli MA, Cheung M, Earley A, de Boer IH. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: an update based on rapidly emerging new evidence. Kidney Int. 2022;102:990-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 155] [Article Influence: 51.7] [Reference Citation Analysis (1)] |
| 7. | Chewcharat A, Prasitlumkum N, Thongprayoon C, Bathini T, Medaura J, Vallabhajosyula S, Cheungpasitporn W. Efficacy and Safety of SGLT-2 Inhibitors for Treatment of Diabetes Mellitus among Kidney Transplant Patients: A Systematic Review and Meta-Analysis. Med Sci (Basel). 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | AlKindi F, Al-Omary HL, Hussain Q, Al Hakim M, Chaaban A, Boobes Y. Outcomes of SGLT2 Inhibitors Use in Diabetic Renal Transplant Patients. Transplant Proc. 2020;52:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 9. | Halden TAS, Kvitne KE, Midtvedt K, Rajakumar L, Robertsen I, Brox J, Bollerslev J, Hartmann A, Åsberg A, Jenssen T. Efficacy and Safety of Empagliflozin in Renal Transplant Recipients With Posttransplant Diabetes Mellitus. Diabetes Care. 2019;42:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 10. | Lemke A, Brokmeier HM, Leung SB, Mara KC, Mour GK, Wadei HM, Hill JM, Stegall M, Kudva YC, Shah P, Kukla A. Sodium-glucose cotransporter 2 inhibitors for treatment of diabetes mellitus after kidney transplantation. Clin Transplant. 2022;36:e14718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Shah M, Virani Z, Rajput P, Shah B. Efficacy and Safety of Canagliflozin in Kidney Transplant Patients. Indian J Nephrol. 2019;29:278-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Hisadome Y, Mei T, Noguchi H, Ohkuma T, Sato Y, Kaku K, Okabe Y, Nakamura M. Safety and Efficacy of Sodium-glucose Cotransporter 2 Inhibitors in Kidney Transplant Recipients With Pretransplant Type 2 Diabetes Mellitus: A Retrospective, Single-center, Inverse Probability of Treatment Weighting Analysis of 85 Transplant Patients. Transplant Direct. 2021;7:e772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Lim JH, Kwon S, Jeon Y, Kim YH, Kwon H, Kim YS, Lee H, Kim YL, Kim CD, Park SH, Lee JS, Yoo KD, Son HE, Jeong JC, Lee J, Lee JP, Cho JH. The Efficacy and Safety of SGLT2 Inhibitor in Diabetic Kidney Transplant Recipients. Transplantation. 2022;106:e404-e412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 14. | Song CC, Brown A, Winstead R, Yakubu I, Demehin M, Kumar D, Gupta G. Early initiation of sodium-glucose linked transporter inhibitors (SGLT-2i) and associated metabolic and electrolyte outcomes in diabetic kidney transplant recipients. Endocrinol Diabetes Metab. 2021;4:e00185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Heerspink HJ, Ninomiya T, Perkovic V, Woodward M, Zoungas S, Cass A, Cooper M, Grobbee DE, Mancia G, Mogensen CE, Neal B, Chalmers J; ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide in patients with type 2 diabetes and chronic kidney disease. Eur Heart J. 2010;31:2888-2896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Schwaiger E, Burghart L, Signorini L, Ristl R, Kopecky C, Tura A, Pacini G, Wrba T, Antlanger M, Schmaldienst S, Werzowa J, Säemann MD, Hecking M. Empagliflozin in posttransplantation diabetes mellitus: A prospective, interventional pilot study on glucose metabolism, fluid volume, and patient safety. Am J Transplant. 2019;19:907-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 17. | Heyward J, Mansour O, Olson L, Singh S, Alexander GC. Association between sodium-glucose cotransporter 2 (SGLT2) inhibitors and lower extremity amputation: A systematic review and meta-analysis. PLoS One. 2020;15:e0234065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 18. | Aggarwal R, Vaduganathan M, Chiu N, Bhatt DL. Out-of-Pocket Costs for SGLT-2 (Sodium-Glucose Transport Protein-2) Inhibitors in the United States. Circ Heart Fail. 2022;15:e009099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 19. | Djamali A, Kendziorski C, Brazy PC, Becker BN. Disease progression and outcomes in chronic kidney disease and renal transplantation. Kidney Int. 2003;64:1800-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2303] [Cited by in RCA: 2516] [Article Influence: 279.6] [Reference Citation Analysis (0)] |
| 21. | Jongs N, Greene T, Chertow GM, McMurray JJV, Langkilde AM, Correa-Rotter R, Rossing P, Sjöström CD, Stefansson BV, Toto RD, Wheeler DC, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9:755-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 22. | Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Abdul Gafor AH, Greasley PJ, Laverman GD, Lim SK, Di Tanna GL, Reich HN, Vervloet MG, Wong MG, Gansevoort RT, Heerspink HJL; DIAMOND investigators. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol. 2020;8:582-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 23. | Trujillo H, Caravaca-Fontán F, Caro J, Morales E, Praga M. The Forgotten Antiproteinuric Properties of Diuretics. Am J Nephrol. 2021;52:435-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Neal B, Perkovic V, Matthews DR. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 25. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3913] [Article Influence: 652.2] [Reference Citation Analysis (0)] |
| 26. | Kasiske BL, Anjum S, Shah R, Skogen J, Kandaswamy C, Danielson B, O'Shaughnessy EA, Dahl DC, Silkensen JR, Sahadevan M, Snyder JJ. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 27. | Brune JE, Dickenmann M, Wehmeier C, Sidler D, Walti L, Golshayan D, Manuel O, Hadaya K, Neofytos D, Schnyder A, Boggian K, Müller T, Schachtner T, Khanna N, Schaub S; Swiss Transplant Cohort Study. Impact of different urinary tract infection phenotypes within the first year post-transplant on renal allograft outcomes. Am J Transplant. 2022;22:1823-1833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 389] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 29. | Panthofer AM, Lyu B, Astor BC, Singh T, Aziz F, Mandelbrot D, Parajuli S, Mohamed M, Djamali A, Garg N. Post-kidney transplant serum magnesium exhibits a U-shaped association with subsequent mortality: an observational cohort study. Transpl Int. 2021;34:1853-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Odler B, Deak AT, Pregartner G, Riedl R, Bozic J, Trummer C, Prenner A, Söllinger L, Krall M, Höflechner L, Hebesberger C, Boxler MS, Berghold A, Schemmer P, Pilz S, Rosenkranz AR. Hypomagnesemia Is a Risk Factor for Infections after Kidney Transplantation: A Retrospective Cohort Analysis. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Huang JW, Famure O, Li Y, Kim SJ. Hypomagnesemia and the Risk of New-Onset Diabetes Mellitus after Kidney Transplantation. J Am Soc Nephrol. 2016;27:1793-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Bilezikian JP, Watts NB, Usiskin K, Polidori D, Fung A, Sullivan D, Rosenthal N. Evaluation of Bone Mineral Density and Bone Biomarkers in Patients With Type 2 Diabetes Treated With Canagliflozin. J Clin Endocrinol Metab. 2016;101:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 33. | Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 497] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 34. | Blau JE, Taylor SI. Adverse effects of SGLT2 inhibitors on bone health. Nat Rev Nephrol. 2018;14:473-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Wanner C, Heerspink HJL, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, Hantel S, Woerle HJ, Broedl UC, von Eynatten M, Groop PH; EMPA-REG OUTCOME Investigators. Empagliflozin and Kidney Function Decline in Patients with Type 2 Diabetes: A Slope Analysis from the EMPA-REG OUTCOME Trial. J Am Soc Nephrol. 2018;29:2755-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 36. | Hecking M, Sharif A, Eller K, Jenssen T. Management of post-transplant diabetes: immunosuppression, early prevention, and novel antidiabetics. Transpl Int. 2021;34:27-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 37. | Gupta N, Wish JB. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD. Am J Kidney Dis. 2017;69:815-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 317] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 38. | Patel N, Hindi J, Farouk SS. Sodium-Glucose Cotransporter 2 Inhibitors and Kidney Transplantation: What Are We Waiting For? Kidney360. 2021;2:1174-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Jenssen T, Hartmann A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat Rev Endocrinol. 2019;15:172-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 40. | Porrini EL, Díaz JM, Moreso F, Delgado Mallén PI, Silva Torres I, Ibernon M, Bayés-Genís B, Benitez-Ruiz R, Lampreabe I, Lauzurrica R, Osorio JM, Osuna A, Domínguez-Rollán R, Ruiz JC, Jiménez-Sosa A, González-Rinne A, Marrero-Miranda D, Macía M, García J, Torres A. Clinical evolution of post-transplant diabetes mellitus. Nephrol Dial Transplant. 2016;31:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3684] [Cited by in RCA: 3836] [Article Influence: 147.5] [Reference Citation Analysis (1)] |
| 42. | Lam D, Shaikh A. Real-Life Prescribing of SGLT2 Inhibitors: How to Handle the Other Medications, Including Glucose-Lowering Drugs and Diuretics. Kidney360. 2021;2:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Lochtefeld JG. The illustrated encyclopedia of Hinduism. 1st ed. New York: Rosen, 2002. |
| 44. | Murray CJ. Encyclopedia of the romantic era, 1760-1850. New York: Fitzroy Dearborn, 2004. |
| 45. | Raffa GP. The complete Danteworlds: a reader's guide to the Divine Comedy. Chicago, London: The University of Chicago Press, 2009. |