Published online Sep 18, 2023. doi: 10.5500/wjt.v13.i5.221
Peer-review started: April 10, 2023
First decision: June 1, 2023
Revised: June 5, 2023
Accepted: June 12, 2023
Article in press: June 12, 2023
Published online: September 18, 2023
Processing time: 157 Days and 16.1 Hours
The second half of the previous century witnessed a tremendous rise in the number of clinical kidney transplants worldwide. This activity was, however, accompanied by many issues and challenges. An accurate diagnosis and appropriate management of causes of graft dysfunction were and still are, a big challenge. Kidney allograft biopsy played a vital role in addressing the above challenge. However, its interpretation was not standardized for many years until, in 1991, the Banff process was started to fill this void. Thereafter, regular Banff meetings took place every 2 years for the past 30 years. Marked changes have taken place in the interpretation of kidney allograft biopsies, diagnosis, and classification of rejection and other non-rejection pathologies from the original Banff 93 classification. This review attempts to summarize those changes for increasing the awareness and understanding of kidney allograft pathology through the eyes of the Banff process. It will interest the transplant surgeons, physicians, pathologists, and allied professionals associated with the care of kidney transplant patients.
Core Tip: The efforts to standardize the nomenclature, classification, and reporting of kidney allograft biopsies were initiated in 1991 by a small group of renal pathologists, transplant physicians, and surgeons at a meeting in Banff, Alberta, Canada. Thereafter, regular meetings of the now ever-expanding, multidisciplinary, and international Banff community have been held every 2 years at different places around the world to revise, update and refine the classification. Major and frequent changes have occurred in the classification over the three decades of its evolution, making it extremely complex and difficult to comprehend, particularly for beginners in the field. The classification has essentially changed from pathology-based to pathogenesis-based classification and has become clinician-friendly and treatment-friendly. This review is an attempt to summarize the changes in the classification in an easily understandable manner and describe the rationale behind these changes for easy assimilation by both neophytes and practicing renal pathologists, transplant physicians, surgeons, and other relevant stakeholders.
- Citation: Mubarak M, Raza A, Rashid R, Shakeel S. Evolution of human kidney allograft pathology diagnostics through 30 years of the Banff classification process. World J Transplant 2023; 13(5): 221-238
- URL: https://www.wjgnet.com/2220-3230/full/v13/i5/221.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i5.221
Kidney transplantation is the preferred type of treatment for end-stage kidney disease patients throughout the world. Advancements in surgical techniques, immunosuppressive regimens, and infection control during the second half of the previous century markedly improved the short- and medium-term outcomes of kidney allografts. However, the long-term results are still poor and little progress has been made in this area[1,2]. Although highly effective, kidney trans
The Banff classification schema for kidney allograft pathology was developed in 1991 and is an ongoing process with an international approach[4-9]. The first Banff meeting was held at the small town of Banff in Alberta, Canada in August 1991, which was attended by a group of 12 nephropathologists and transplant clinicians with a common interest in kidney transplantation with the goal of standardizing the reporting of kidney allograft biopsies[10]. The objectives were two-fold: To guide therapy and to establish an objective endpoint for clinical trials. The first report in full paper form was published in 1993 after several cross-consultations and follow-up discussions[10]. These meetings have subsequently taken place biennially, initially at Banff and later on, in other parts of the world but have been named the Banff meetings after their original place of meeting to revisit, revise and update the Banff classification. Our understanding of kidney transplant pathology, and particularly, rejections has grown and improved with discourses and publications emerging out of these meetings[4-6,9]. At the same time, the classification has become more complex and difficult to assimilate, particularly for novices in the field.
The Banff schema is distinctive as the classification criteria are developed based on a consensus approach. Although the original Banff classification was developed based on expert opinion, its subsequent revisions and refinements have been made based on published sound scientific studies, multicenter studies by the Banff working groups (BWGs) on problematic areas, and consensus among the experts[6]. In this review, we summarize the major changes in the Banff classification and the rationale behind these changes. This will help the neophytes and practicing nephropathologists, nephrologists, and other stakeholders to better understand this classification.
For a kidney allograft biopsy to fulfill its role as the gold standard for accurate diagnosis, it needs to be adequate and as representative as possible of the whole kidney allograft and be prepared according to recommended protocols. With the evolution in the diagnostic criteria of the Banff categories, changes have also taken place in the tissue adequacy criteria, technical preparation guidelines, and the extent of the workup of kidney allograft biopsies (Table 1). In the first Banff meeting, the biopsy adequacy criteria were less stringent, particularly with regard to the number of blood vessels required. The first major change in adequacy criteria was made in 1997 when two cylinders of kidney allograft parenchyma including both cortex and medulla with 10 glomeruli and two arteries were recommended to fulfill adequacy criteria[11]. As the process of rejection is often focal and patchy in distribution, particularly during the early phase, a generous sampling of the kidney cortex from different areas is desirable for an accurate diagnosis, necessitating the recommendation for two cores of allograft tissue. During the first decade of the Banff process, the study of kidney allograft biopsy was based only on morphologic evaluation, i.e. light microscopy (LM). From the 2001 meeting onwards, a piece of frozen tissue for C4d was made mandatory for the complete evaluation of allograft pathologic lesions[12,13]. C4d staining should be done on all kidney allograft biopsies, preferably by immunofluorescence (IF), or by immunohistochemistry (IHC) technique, if the former is not available[13]. The threshold of C4d staining intensity is one grade level lower with the IHC technique. In the Banff 2013 meeting, conditional use of electron microscopy (EM) was recommended in certain situations[14-16]. EM study is not done routinely on all kidney allograft biopsies at all centers, but is performed in the case of suspected recurrent or de novo glomerulonephritis, persistent significant but unexplained proteinuria, and for the diagnosis of early chronic changes of allograft rejection such as transplant glomerulopathy and sometimes peritubular capillaries (PTCs). Tissue fixed in 2.5% glutaraldehyde provides optimum results for transmission EM. Standard techniques are employed for the preparation and interpretation of EM. For the above-mentioned ancillary investigations, the biopsy needs to be divided in such a manner that a minimum number of glomeruli and/or PTCs be present in specimens apportioned for IF and EM to maximize their utility. Ideally, this should be done in the biopsy suite under the dissection microscope while the specimen is fresh. Tissue samples should then be placed in appropriate fixatives for respective studies. Although not mandatory, IHC for polyomavirus is recommended on all renal allograft biopsies to help detect early viral lesions. In brief, the pathologic evaluation of kidney allograft biopsies has evolved from pure LM to ancillary techniques of IHC, IF, EM, and more recently, molecular studies to accurately diagnose transplant pathology lesions.
| Parameters/investigations | Requirements |
| Number of cores | Two (these should be divided to procure tissue for IF and EM studies, if necessary) |
| Components of graft parenchyma | Both cortex and medulla |
| For the light microscopic study | A significant amount of cortex containing up to: (1) 10 glomeruli; and (2) 2 arteries |
| For the immunofluorescence study | Cortex with up to 3 glomeruli |
| For the electron microscopic study | Cortex with 1 glomerulus |
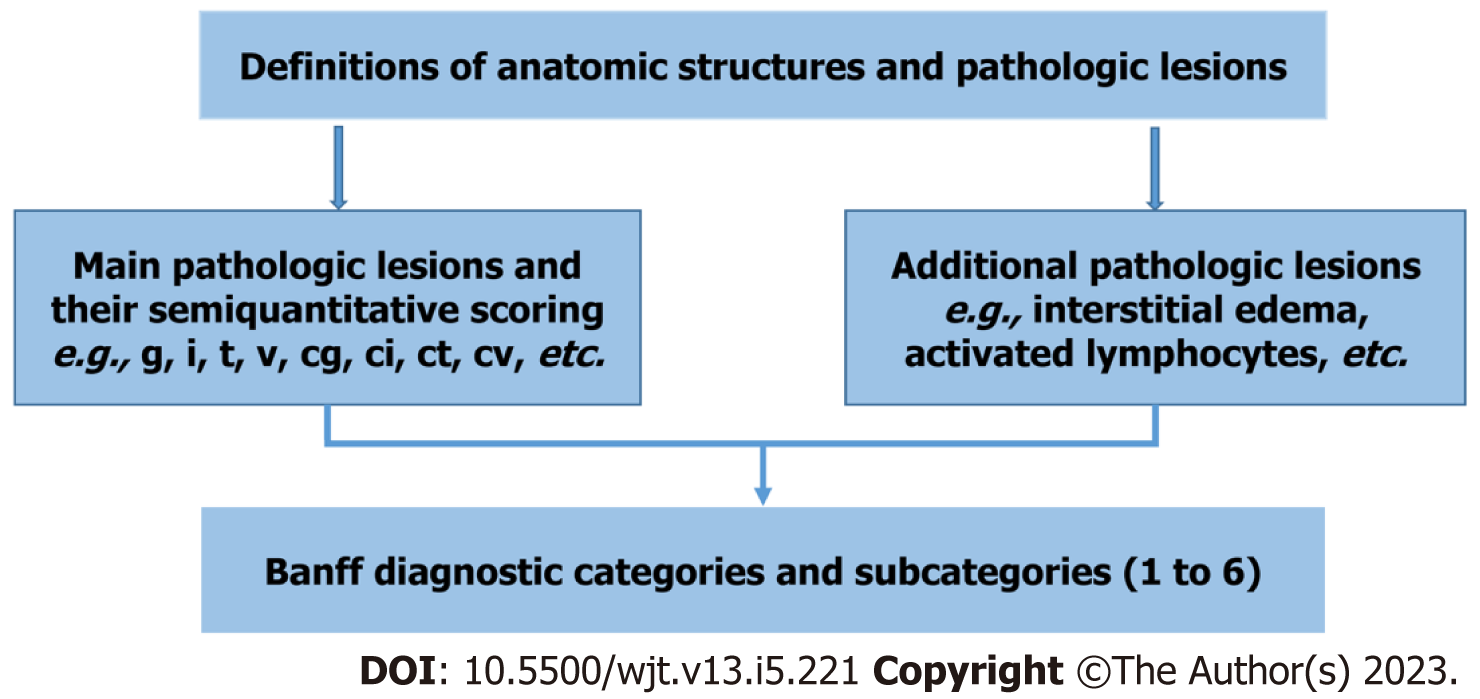
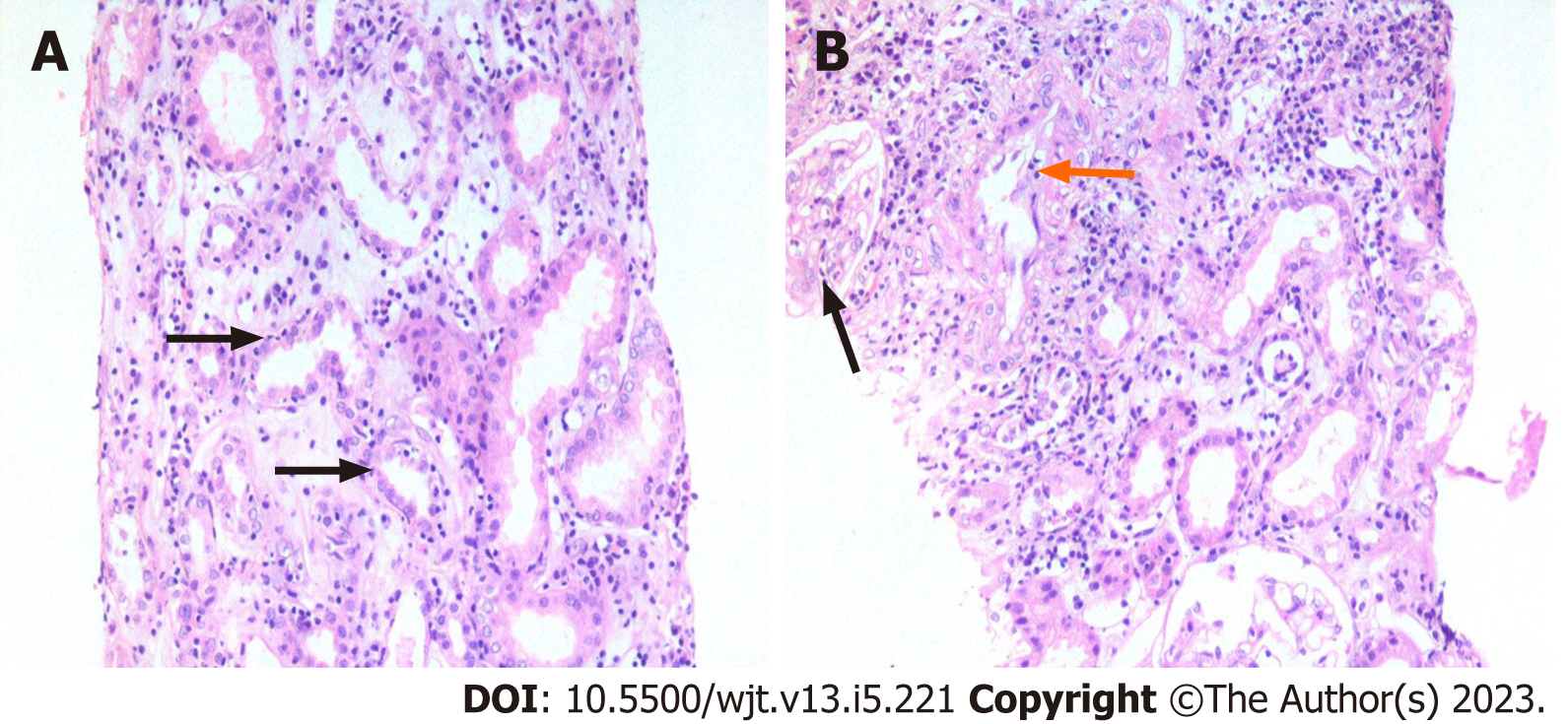
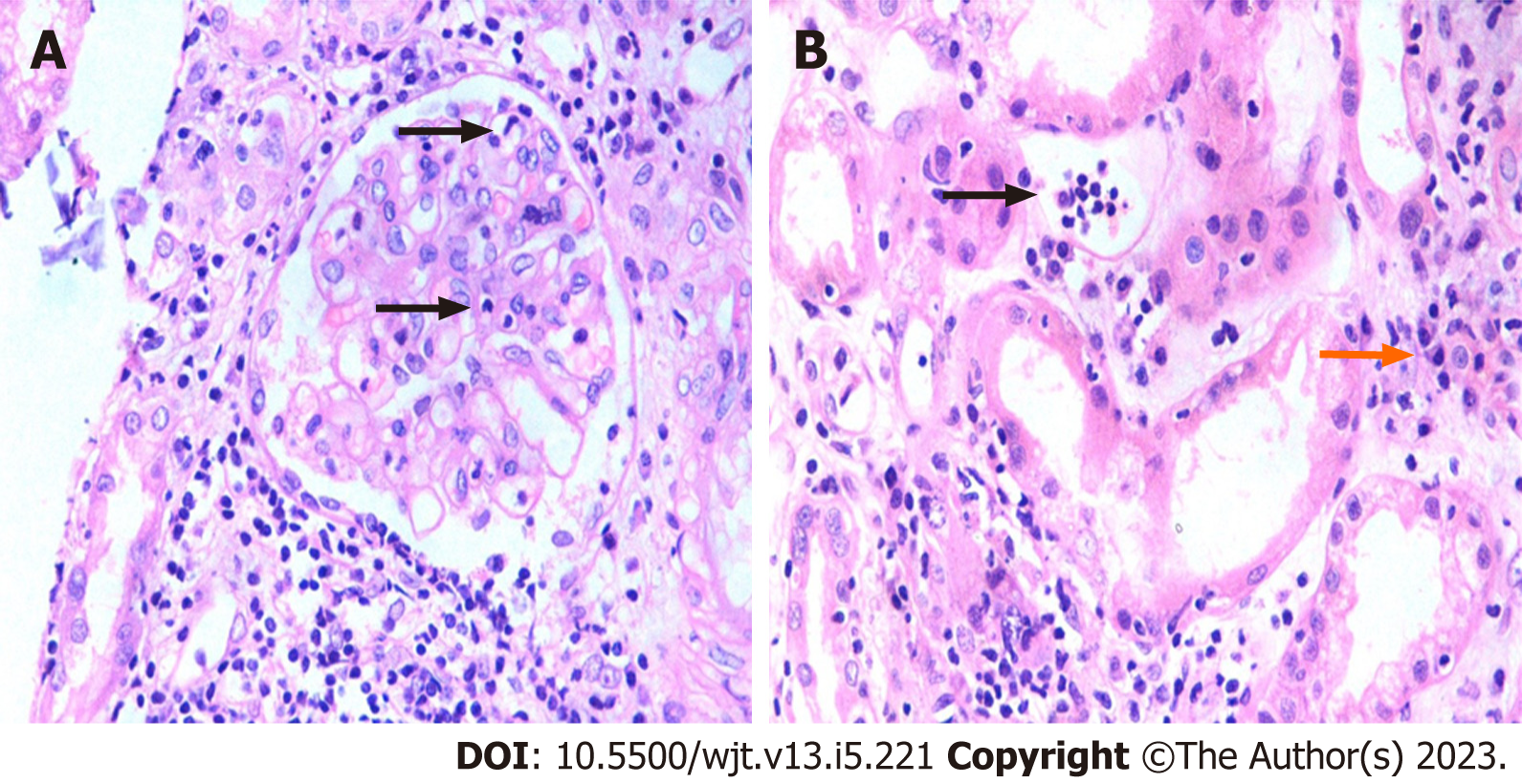
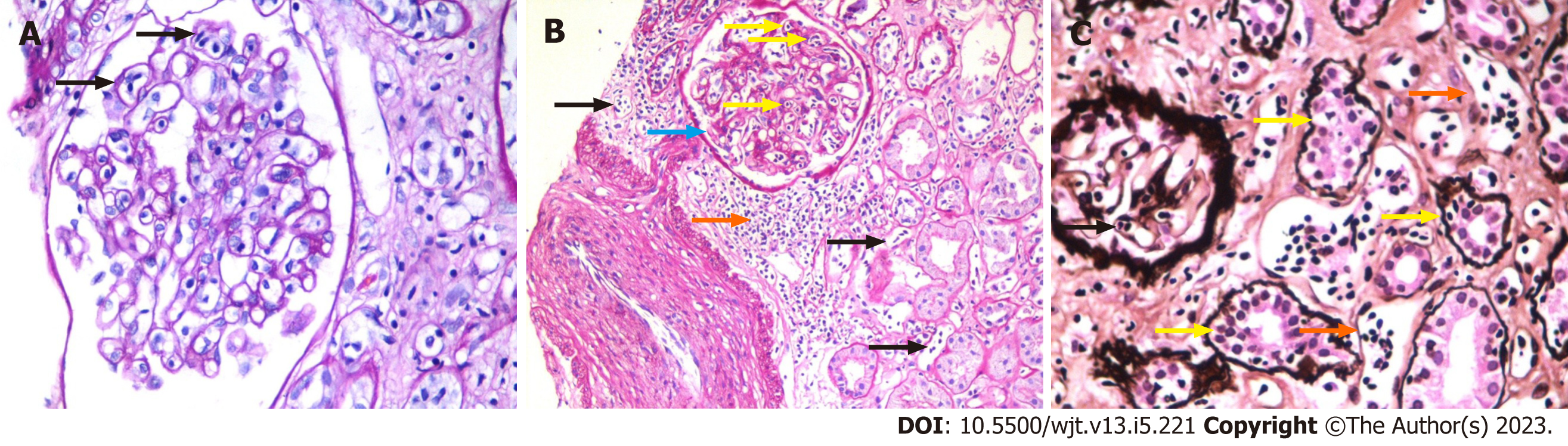
The foundation of the Banff classification system is centered on the morphological evaluation of kidney allograft tissue comprising four basic components. Each of these components can be involved in either the acute or chronic disease processes, particularly the rejection process. The Banff process identified and defined the lesions of the allograft parenchyma in a systemic and semi-quantitative way (Table 2). Thus, the components of the Banff classification system can conveniently be grouped as: (1) Definitions of various components or lesions; (2) Diagnostic lesions; (3) Semi-quantitative scoring of the lesions; (4) Additional diagnostic features; and (5) The diagnostic categories (Figure 1). None of the individual lesions, in isolation, except possibly for intimal arteritis, is diagnostic of the rejection process. A combination of the lesions along with scores above some threshold value is needed to make a specific diagnosis. It is important to be thoroughly familiar with all these components of the Banff classification in order to reliably and precisely apply it in clinical or research settings[9]. Some important diagnostic lesions are illustrated in Figures 2-4.
| Components of the allograft | Acute lesions | Scoring as 0, 1, 2, 3 | Chronic lesions | Scoring as 0, 1, 2, 3 | Acute & chronic lesions | Scoring as 0, 1, 2, 3 |
| Glomeruli | g | - | cg | - | ||
| Interstitium | i | - | ci | - | ti, i-IFTA | - |
| Tubules | t | - | ct | - | t-IFTA | - |
| Vessels | v | - | cv | - | ||
| Peritubular capillaries | ptc | - | ptcml | - | ||
| C4d | C4d | - |
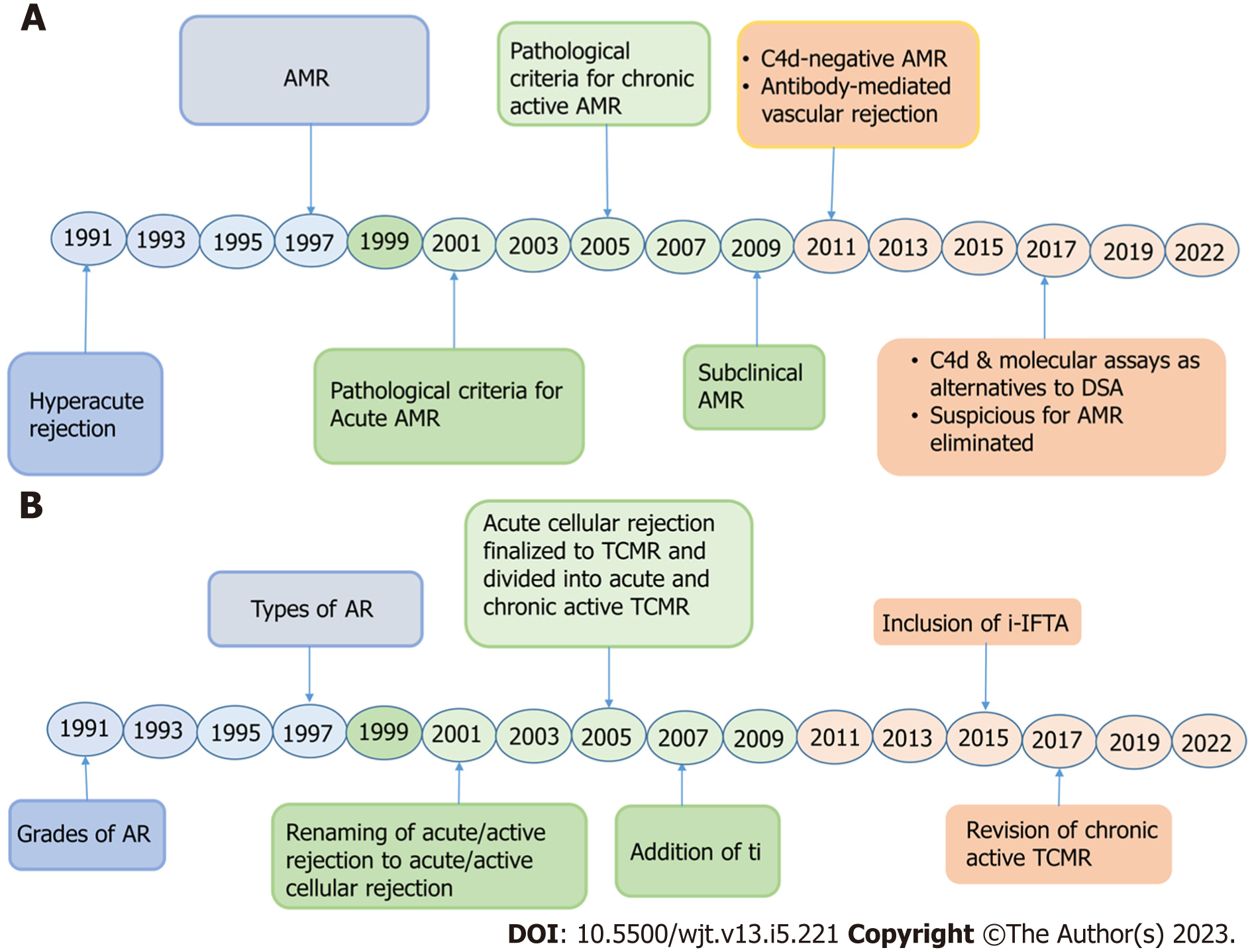
The year 2021 marked the 30th anniversary of the beginning of the Banff classification process for standardized reporting of renal allograft pathological lesions. However, XVth biennial Banff meeting that was to be held that year was postponed and held in 2022 due to the coronavirus disease 2019 situation[6]. It is befitting to review the evolution of the Banff classification, its strengths and limitations, future prospects, and opportunities. It will be more convenient if we divide the evolution of the Banff classification into three decade-wise eras, as below.
The first decade may be called the decade of development and reconciliation/integration of the Banff classification, the latter with a rival classification developed in the United States called the Collaborative Clinical Trials in Transplantation (CCTT) modification of the classification[17]. This merger was the most significant achievement during the first decade as it paved the way for a single uniformly agreed-upon international classification for renal allograft pathology[6]. Three main and full papers on the classification were published during this decade, two of which became popular, the first was published in 1993 and the second in 1999. During this decade, the main focus of classification schema was on the cellular part of the rejection[18-20]. In fact, this category was named acute rejection without further qualification. Later on, it was changed to acute/active cellular and still later, acute/active T-cell mediated rejection (TCMR). The role of antibodies beyond the immediate post-transplant period was not formally recognized during this decade, although studies were going on this subject. The workup of renal allograft biopsy was based on morphologic examination alone. The adequacy criteria for renal allograft biopsy were made more stringent in Banff 1997 classification but were still limited to the morphological study of the biopsy material.
During this decade, the focus of the Banff classification was shifted to the development of the pathologic criteria and their refinement for a conclusive diagnosis of antibody-mediated rejection (AMR) beyond the immediate post-transplant period. Starting from the 2001 Banff meeting, the attention of the transplant community was shifted to the increasing recognition of the role of alloantibodies in causing allograft rejection. During the 2001 Banff meeting, pathologic criteria were formulated for the first time for a definitive diagnosis of AMR in the acute setting, i.e. beyond the immediate post-transplant period[12]. These included not only the morphologic criteria but also immunopathologic and serologic criteria. This led to the evolution of the Banff classification to a multidisciplinary schema. The adequacy of tissue was expanded to a portion of tissue for C4d staining and an optional tissue sample for EM study. In the 2005 Banff meeting, criteria for the diagnosis of chronic active AMR were formulated, thus recognizing the full spectrum of antibody-mediated injury[21]. With the widespread application of these criteria in clinical practice, it became obvious that not all forms of AMR are C4d positive. Hence, the need arose for a subtype of AMR to be introduced in the Banff classification, i.e. C4d-negative AMR, which was introduced in the Banff 2009 meeting[22]. From Banff 2001 to Banff 2011 meetings, only diffuse C4d staining (C4d3), involving more than 50% of PTCs, was recognized as fulfilling the immunopathologic criterion for AMR[23]. In the Banff 2013 meeting, focal (c4d2: 10%-50%) staining of PTCs for C4d was also accepted, as it was also associated with donor-specific antibodies (DSAs) and reduced allograft survival[14]. BWGs were also created during the 2009 Banff meeting for addressing the problematic areas of the Banff classification for evidence-based investigations and solutions[22].
During this decade, further clarifications and refinements were made in the diagnostic criteria and classification of chronic active AMR and TCMR[14,24-27]. The thresholds of some of the lesions were re-defined[14]. Integrations of ancillary techniques of IHC, EM, and molecular studies were recommended for an accurate diagnosis and classification of rejection processes. Digital pathology (DP) and artificial intelligence (AI) tools were explored for their possible role in predicting, diagnosing, and prognosticating kidney allograft pathology[25,26].
Following a brief overview of the decade-wise evolution of the overall classification schema, it is now appropriate to discuss the evolutionary changes of the Banff classification according to individual diagnostic categories in some detail. The names of categories have changed many times. Here, we use the most representative name of the category, which may not be necessarily the same as in the latest Banff classification, the 2019 Banff classification.
This is the first category of the original Banff construct and is still so. The definition of this category is self-explanatory. In fact, this category is a diagnosis of exclusion, which implies that not only the graft parenchyma should be free of inflammatory cell infiltration, but it should also show no features of acute tubular injury (ATI) or acute tubular necrosis. Taken at its face value, this category appears superfluous. The rationale behind using and retaining this category seems to be the unavoidable consequence of an overenthusiastic biopsy approach. In some centers, allograft biopsies are performed too quickly or for transient rises in serum creatinine, such that the morphological lesions are either not fully expressed or there is a cause of allograft dysfunction outside of the graft parenchyma. In some instances, sampling error may account for the normal appearances of the sampled cores, especially when the rise in serum creatinine is of an appreciable degree. This is the only category of the Banff classification that cannot be diagnosed in concurrence with any other category.
No significant changes have occurred in this category except for a name change from the “Normal” in the 1993 report through to the 2013 paper to the “Normal biopsy or nonspecific changes” in the 2015 Banff report[10,23,24]. The prevalence of this category in renal allograft biopsies should ideally be low.
Although the founders of the Banff classification recognized the importance of alloantibodies in causing allograft injury right from the beginning of the development of the classification and category 2 was devoted to alloantibodies in the original classification, their full scope was not fully known at that time[28-31]. The role of antibodies was initially thought to be limited only to the immediate post-transplant period[10,11]. With increasing experience and the resulting accumulating evidence, this notion proved to be incorrect and this category has undergone dramatic changes including its nomenclature during the three decades of the evolution of the Banff classification (Figure 5A).
A number of factors contributed to this increased focus on AMR: (1) Increased recognition of the condition as a result of identification of a highly sensitive and equally specific biomarker of AMR, i.e. C4d and subsequently some relatively specific pathological features of AMR; (2) A better understanding of the roles and techniques for the detection of anti-donor antibodies; (3) Increasing numbers of re-transplants, i.e. second or third transplants; and (4) Increased rates of transplantations across ABO and other immunologic barriers as a result of a shortage of donor organs.
Before discussing the changes in the Banff category of AMR at length, it seems prudent to discuss briefly the pathophysiology of AMR. The main target of AMR is the endothelial cell lining the vascular system, most notably those lining the microcirculation (glomeruli and PTCs in the kidney), but larger arteries may also be targeted. This is comprehensible as the alloantibodies are found in the bloodstream and they first encounter the endothelium of the blood vessels and interact with it. The intensity and nature of the subsequent pathological lesions are variable and depend on many factors including antibody type[32].
The terminology of the category has changed many times, as depicted in Table 3. These changes were necessitated by changing knowledge and accumulating evidence of the role of antibodies in causing allograft injury. In the Banff 93 classification, the category was named hyperacute rejection (HAR). The reason behind this was that, at that time, antibodies were thought to be responsible for causing only this particular type of rejection. In the Banff 97 classification, the category was renamed “antibody-mediated rejection” and divided on clinical grounds into two categories depending on their presentation, as shown in Table 3. HAR and accelerated acute rejections have fortunately become exceedingly rare nowadays. As a result, the Banff classification dropped these terms from the subsequent revised versions. In contrast to cellular rejection, antibodies themselves are not easily visualized in the kidney allograft biopsy tissue. Hence, identification of their role beyond the immediate post-transplant period required the discovery and use of some biomarkers of antibody action. The discovery of C4d in 1993 was such a transformative marker, which prompted research into the role of alloantibodies in allograft rejection[33-39]. In the Banff 2001 meeting, the role of antibodies was recognized in acute settings also, i.e. beyond the immediate post-transplant period[12]. In this meeting, for the first time, the pathological criteria were developed for the diagnosis of acute AMR and types of AMR were described. This marked the debut of not only the ancillary technique of C4d staining but also incorporated serology in the Banff classification system, making it a multidisciplinary classification[12]. In the Banff 2005 meeting, diagnostic criteria were developed for chronic AMR including the lesions of transplant glomerulopathy (cg), PTC basement membrane multilayering (ptcml), and transplant arteriopathy (cv)[21]. From the Banff 2007 meeting onwards, the name of this category was changed to antibody-mediated changes, instead of AMR, as it had been shown in experimental studies that not all antibodies cause rejection but some of the antibodies may be involved in accommodation and the full spectrum of antibody actions and outcome on graft tissue was being explored[40,41]. Thus, the present terminology reflects the broad spectrum of actions that can be mediated by antibodies in the graft, many of which still remain to be explored.
| Meeting reports, year | Category 2: Antibody-mediated rejection1 |
| Banff, 1993 | Hyperacute rejection |
| Banff, 1997 | AMR3 |
| Hyperacute | |
| Accelerated acute3 | |
| Banff, 1997 update (2001) | Diagnostic criteria for acute antibody-mediated rejection were developed. Three types were described as: (1) Types I: ATN-like3; (2) Types II: Capillary3; and (3) Type III: Arterial3 |
| Banff, 2005 | Diagnostic criteria for chronic antibody-mediated rejection were developed |
| Banff, 2007 | Antibody-mediated changes2,3 |
| C4d deposition without rejection3 | |
| Acute antibody-mediated rejection | |
| Chronic active3 antibody-mediated rejection | |
| Banff, 2013 | Antibody-mediated changes |
| Acute/active antibody-mediated rejection | |
| Chronic active antibody-mediated rejection | |
| C4d-negative antibody3-mediated rejection | |
| Banff, 2015 | Antibody-mediated changes |
| Acute/active3 antibody-mediated rejection | |
| Chronic active antibody-mediated rejection | |
| C4d staining without evidence of rejection | |
| Transplant arteriopathy may be seen in chronic AMR | |
| Banff, 2017 | Antibody-mediated changes |
| Active3 AMR | |
| Chronic active AMR | |
| C4d staining without evidence of rejection | |
| 3 criteria for AMR diagnosis remain but C4d can substitute for DSA | |
| DSA testing still advised | |
| Suspicious for AMR eliminated | |
| Banff, 2019 | Category 2: Antibody-mediated changes |
| Active AMR | |
| Chronic active AMR | |
| Chronic (inactive) AMR3 | |
| C4d staining without evidence of rejection |
With extensive use of c4d in clinical practice, particularly in the protocol biopsies, it was observed that a small number of graft biopsies show c4d positivity in the absence of morphological or clinical features of acute or chronic AMR. Thus, a new subcategory of “C4d deposition without morphologic evidence of active rejection“ was added to the Banff classification in 2007[40,41]. Two new lesions of C4d and PTC were also introduced and presented at the 2007 Banff meeting for validation and future incorporation[42].
From Banff 2001 to Banff 2011, only diffuse C4d staining (c4d3) on IF, involving more than 50% of PTCs, was recognized as fulfilling the immunopathologic requirement for AMR, which was amended in the Banff 2013 meeting to accept focal (c4d2: Staining 10%-50% of PTCs) PTC staining as it was shown to correlate with DSA and decreased allograft survival[14].
The second major modification in the AMR category took place in the Banff 2013 meeting[13]. Any degree of v (v > 0) was incorporated in the morphologic criteria for active AMR in contrast to the previous requirement of v3 in light of new studies[43,44]. The absolute condition (criterion 2) for PTC C4d staining for the definite diagnosis of active and chronic active AMR, was supplanted by the criterion of “Evidence of the recent/current interaction of alloantibody with the microvascular endothelium”, the latter including C4d but alternatively a minimum of moderate microvascular inflammation (g + ptc ≥ 2) or amplified expression of validated gene transcripts in the biopsy material suggestive of endothelial injury[45-50]. This signaled the first incorporation of molecular diagnostics into the Banff classification[13]. With these additional criteria, many studies showed that a substantial number of AMR cases were C4d negative[51,52]. Thus, the subcategory of C4d-negative AMR was included in the classification at this meeting. The significance of this change can be judged by the fact that this inclusion was incorporated in the title of the paper of the 2013 Banff meeting[13]. In addition, at the Banff 2013 meeting, the minimum thresholds for glomerulitis score (g1) and chronic glomerulopathy by LM (cg1) were revisited based on results of BWG data showing better interobserver reproducibility with the revised criteria. The potential role of EM in the diagnostic evaluation of kidney allograft biopsies was also emphasized, with the inclusion of precise criteria for ptcml to augment the specificity of this pattern for chronic AMR and the inclusion of a new subtype of cg by EM exclusively (cg1a)[53,54].
In the Banff 2019 meeting, a subtype of chronic AMR, i.e. chronic inactive AMR was also introduced, as it could be diagnosed on kidney allograft biopsies[26]. Of note, there is no category of chronic (inactive) in TCMR. There is no provision for borderline changes (BCs) in the AMR category, but diagnostic thresholds do apply. The diagnosis of “suspicious for AMR (sAMR)” was finally removed from the Banff 2017 classification.
In summary, considerable progress has been made in the category of AMR. Molecular studies have been officially incorporated in the Banff classification for diagnosis of this category only. The absolute requirement for C4d its diagnosis has been eliminated. The requirement of DSA is also not absolute. sAMR has been removed from the classification.
The original Banff classification recognized a category of BCs in reporting the renal allograft pathological lesions and the category is still there even after 30 years of refinements and evolution of the classification. This category encompasses only the tubulointerstitial type of rejection and is used when there is mild (i1) to severe (i3) interstitial inflammation but the tubulitis is of mild degree only (t1) or vice versa and no arteritis is present (v0). The true clinical significance of this lesion is still contentious and is largely determined by the clinical context of the case[55-62]. Some of the factors that may determine its clinical relevance include the source of the donor organ, time post-transplant, and the indication and timing of kidney allograft biopsies. Most of the studies on this category have been carried out in deceased donor transplantation programs[56-62]. Only a few studies are available on the clinical relevance of this finding in the live-related renal transplant setting[63,64].
The criteria for the diagnosis of BCs have changed little over time and essentially consist of any combination of interstitial inflammation (i) and tubulitis (t) scores below the threshold level required for the diagnosis of rejection, i.e. “i2, t2”. The original criteria for the borderline category were developed at the Banff 1991 meeting, describing it as a “very mild” form of acute rejection lacking intimal arteritis (v0) and having only mild to moderate patchy mononuclear interstitial inflammatory cell infiltration (i1 or i2) with foci of mild tubulitis (t1; 1 to 4 mononuclear cells per tubular cross-section). In this initial classification, “no treatment” was suggested[10]. In 2005, the Banff group suggested the expansion of the “BC” category to include lesions with minimal interstitial inflammatory cell infiltration (i0) to i3 with t0 or t1 or t2, t3 with i0, i1[21]. This was further confirmed in the Banff 2007 meeting[42]. However, the minimum threshold of BC was restored to “i1, t1” in the Banff 2019 meeting, as longitudinal studies by Nankivell et al[65] showed no effect of isolated “t” on allograft survival.
The reported frequency of this category varies widely and ranges from 46% to 74 % on dysfunctional kidney allograft biopsies performed at different time points after transplantation[66,67]. This category is also most problematic from the point of view of the treatment. In reality, this category does not represent a true pathophysiological phenomenon. It represents a heterogeneous category of lesions encompassing pathophysiological processes with minimal, nonspecific, insignificant inflammation to clinically important TCMR, capable of immune-mediated allograft injury resulting in poor immunological, functional, and histological outcomes if not treated[55-58]. This category has been created primarily because of varying clinical criteria and the timing of kidney allograft biopsies. In an ideal world, an allograft biopsy should be categorized as rejection or no rejection, but in practice, it is not always so clear-cut. As the biological process of rejection is a gradual and patchy process, it takes time to develop full-blown features of rejection. If a biopsy is performed very early during the development of rejection, the pathological lesions will not have reached the threshold of diagnosis of rejection, and will inevitably be classified as a BC category. It is hoped that in near future this category will be eliminated with the incorporation of better diagnostic markers, particularly, molecular markers.
The spectrum of TCMR, as it is now officially called, was defined from the origin of the classification as the Banff diagnostic category 4 and now includes acute TCMR, grades IA, IB, IIA, IIB, and III, and chronic active TCMR (CA TCMR), grades IA, IB and II. There are no subgrades of grade II and no grade III in CA TCMR[26]. In addition, there is no subtype of chronic (inactive) in the category of TCMR, as is true for AMR. Category 4 is separated from category 3 by a threshold of i2, t2 and any v. Banff diagnostic categories 3 and 4 are mutually exclusive but can be diagnosed concurrently with other lesions from categories 2, 5, and 6.
In the previous iterations of the Banff schema, the main emphasis was on the diagnosis and classification of acute rejection, which essentially implied TCMR. This focus on cellular rejection dominated the transplant pathology field and subdued the recognition and characterization of AMR for quite some time during the earlier period of the Banff classification. On the other contrary, the category of cellular rejection lagged behind AMR in undergoing modifications till the very recent past. The most important changes in this category took place during the 1997 Banff meeting (Table 4). Thereafter, this category remained fairly stable and immune to changes except for minor nomenclature changes. More recent Banff updates have made some modifications to the morphological criteria and subdivision of this category (Figure 5B).
| Meeting reports, year | Category 4: T cell-mediated rejection1 |
| Banff, 1993 | Acute rejection, grades I, II, III |
| Banff, 1997 | Acute/ active cellular rejection |
| Types2 I A/B, II A/B, III | |
| Banff, 1997 update (2001) | Acute/ active cellular rejection |
| Types I A/B, II A/B, III | |
| Banff, 2005 | TCMR2 |
| Acute, types2 I A/B, II A/B, III | |
| Chronic active2 (includes only transplant arteriopathy) | |
| Banff, 2007 | TCMR |
| Acute | |
| Chronic active (includes only transplant arteriopathy) | |
| Banff, 2013 | TCMR |
| Acute | |
| Chronic active (includes only transplant arteriopathy) | |
| Banff, 2015 | TCMR |
| Acute | |
| Chronic active TCMR may have tubulointerstitial changes2 | |
| Banff, 2017 | TCMR |
| Acute | |
| Chronic active TCMR grades I A/B and II defined | |
| Banff, 2019 | i-IF/TA and t-IF/TA included in criteria2 (inflammation and tubulitis in areas of scarring) |
| In chronic active TCMR with i >1, diagnosis to be combined chronic active and acute TCMR2 |
As alluded to earlier, the main emphasis of the first Banff classification was on the accurate diagnosis and classification of acute rejection, which essentially included acute cellular rejection. As this type of rejection is characterized by the infiltration of mononuclear cells into the allograft parenchyma, its diagnosis was relatively easy and obvious in most cases. The main targets of cellular rejection are the tubules, interstitium, and larger blood vessels. Microcirculation inflammation is rarely observed in this type of rejection[10,11,68-75]. The Banff classification introduced for the first time a minimum threshold (i.e. i2, t2) for the definitive diagnosis of acute cellular rejection of tubulointerstitial type or type I rejection. This separated it from the category of BCs in the cellular alloimmune lesions. There is no BC category for vascular or type II and type III rejections and the presence of even one lymphocyte in the intima is sufficient to diagnose vascular rejection. Early Banff classifications also emphasized the importance of the topographic location of the inflammatory cell infiltrate in the biopsy[10,11].
The nomenclature of this category has changed from acute rejection to acute cellular rejection to TCMR over the years reciprocating the changes in category 2 of AMR, as shown in Table 4 and Figure 3. With regard to the classification of TCMR, the first Banff classification divided acute rejection into three “grades” based on the increasing severity of the allograft damage, as shown in Table 4. These were: Grade I (mild), grade II (moderate), and grade III (severe) (Table 4). This classification lumped together both the tubulointerstitial and vascular rejections in grade II. It may be noted that this subdivision was based purely on a morphological or pathological basis with little consideration of the underlying pathogenetic basis of rejection. As mentioned earlier, the first main change in this category was effected in the Banff 97 meeting, when pathologists using the original Banff 93 classification and its CCTT modification got together and merged the two classifications to develop a unified and international consensus-based Banff 97 classification with substantial input from the CCTT classification, which stressed the pathogenetic foundation for the categorization of cellular rejection[4,5,10,11]. The Banff 97 classification divided acute/active rejection into “types” and subtypes rather than grades (Table 4). The main modifications in the Banff 97 classification included the segregation of type I (tubulointerstitial) rejection from vascular (type II) rejection. Type III rejection was categorized separately as in the Banff 93 classification (Table 4). This merger signified the adaptability of the Banff group to incorporate the observations of pathologists using CCTT modification and also embrace the emerging evidence from newer investigations showing that vasculitis per se has significant consequences for the response to treatment and/or graft survival[35,36].
In the Banff 97-update published in 2003 (from the Banff 2001 meeting), with profound and far-reaching changes in the category of AMR, the category of acute/active rejection was rechristened as acute/active cellular rejection (ACR), to underscore its distinction from AMR. Its types and subtypes, however, remained unchanged from the Banff 97 classification. The name of the category was again changed to TCMR in the Banff 2005 update (Table 4). On this occasion, it was further categorized into acute TCMR, which now encompassed all the subtypes of ACR of the Banff 97-update, and CA TCMR. The latter was diagnosed by the presence of chronic allograft arteriopathy only till the 2017 Banff meeting, characterized by new-onset arterial fibrointimal thickening with mononuclear cell infiltration in fibrosis, and the creation of neo-intima. Compared to previous iterations of the Banff classification, the Banff 2007 classification added a new “i” lesion score, labeled as “ti” (total interstitial inflammation score) to the schema[34,76-81]. Not much was changed in the criteria for diagnosis or classification of cellular rejection during the Banff 2009, Banff 2011, and Banff 2013 meetings. In the Banff meeting of 2015, it was realized and acknowledged that CA TCMR may manifest itself with tubulointerstitial inflammation in the form of inflammation in areas of scarred cortex (i-IFTA) in addition to arterial lesions[24]. However, detailed criteria and formal inclusion of CA TCMR were not affected till the 2017 meeting[25]. In the 2019 meeting, tubulitis in mildly to moderately atrophic tubules within the scarred cortex (t-IFTA) was also added as the diagnostic criterion for CA TCMR in addition to i-IFTA and ti scores. Thus, CA TCMR was divided into two types, in contrast to three types in acute TCMR. These types were: Types IA and IB and type II. A provision was also made for the diagnosis of borderline CA TCMR if the diagnostic criteria of CA TCMR types IA and IB were not fulfilled[26]. Banff 2019 also allows concurrent diagnosis of acute and CA TCMR, especially in the presence of intimal arteritis in association with acute TCMR types IA and IB[6,26].
It is obvious from the above deliberations that the mainstay for the diagnosis and classification of cellular rejection has remained and is still so the LM evaluation of allograft biopsy material with little help from the ancillary techniques of IHC or EM. More recently, molecular markers have shown promising results for a conclusive diagnosis of acute cellular rejection in single-center studies[82-84]. These need to be validated in large, multicenter trials and the methodology needs to be standardized before they can be used widely in clinical practice. These represent the current challenges for the Banff classification system. The molecular information may be integrated with the histomorphological findings in the Banff classification in the future to improve the accuracy of diagnosis and classification of rejection, particularly borderline and CA TCMR cases[85-93].
This category of Banff classification of kidney allograft pathology was created to diagnose, classify, and grade (or stage) chronic fibrosing changes of kidney allografts resulting from a variety of causes[94]. Historically, many important changes took place in this category over the past three decades of the evolution of the Banff classification process. In the Banff 2019 meeting, this category has been replaced by polyomavirus nephropathy[26]. The rationale behind this change is that this category seems to be redundant in the absence of a plausible cause. The Banff schema stresses the need to determine the etiology of chronic changes whenever possible[21]. Moreover, chronic subtypes have been added to both types of rejection over the last two decades of evolution.
It seems appropriate to briefly summarize the evolution of the nomenclature and classification of chronic sclerosing lesions in the Banff schema, which are reflective of a continued and improved understanding of the pathophysiology of these lesions (Table 5). During the pre-Banff era, the term “chronic rejection” was utilized for all forms of chronic allograft dysfunction. This approach was an oversimplification, in that chronic changes in the allograft parenchyma are caused not only by alloimmune factors but also by non-immune factors and a distinction among these is important for tailored treatment. The Banff system put forward the nomenclature of “chronic allograft nephropathy (CAN)” in 1991 as a non-committal alternative to the then prevalent and confusing designation of “chronic rejection”. The Banff 93 schema categorized CAN into three grades of increasing severity based on the degree of interstitial fibrosis and tubular atrophy (IFTA)[10]. No subdivision of the CAN grades was made and all etiologies of chronic changes were combined together in this unified category. In addition, many histomorphological patterns of, for example, chronic AMR, were not discovered at that time. In the Banff 1995 meeting, the chronic allograft damage index was merged with the CAN group to grade the severity of chronic changes. However, no subdivision of CAN was made in this update of the classification. In the Banff 97 meeting, an effort was made toward subclassifying chronic changes in the allograft and identifying and documenting the changes caused by the rejection processes. A subclassification of each of the grades of chronic changes into “a” and “b” categories was attempted depending on the absence or presence of obvious features associated with “chronic rejection”, respectively. The 3-tier grading of the CAN entity remained the same as in previous classifications[11]. No modifications in the terminology or grading of CAN were attempted in the 97-update classification (Banff 2001 meeting) or the Banff 2003 meeting reports[12].
| Meeting reports, year | Category 5: Chronic allograft nephropathy |
| Banff, 1993 | CAN, grades, I, II, III |
| Banff, 1997 | CAN, grades, I, II, III, each divided into a and b subcategories1 |
| Banff, 1997 update (2001) | CAN, grades, I, II, III, a and b |
| Banff, 2005 | IFTA, of no specific etiology1, grades I, II, III |
| Banff, 2007 | IFTA of no specific etiology, grades I, II, III |
| Banff, 2013 | IFTA of no specific etiology, grades I, II, III |
| Banff, 2015 | IFTA of no specific etiology, grades I, II, III |
| Banff, 2017 | IFTA of no specific etiology, grades I, II, III |
| Banff, 2019 | Grading of polyoma viral nephropathy into classes 1, 2 and 3 (adequate sampling for scoring should include 2 cores with medulla)1 |
A significant modification in the category of chronic sclerosing changes was effected in the Banff 2005 meeting when the terminology of CAN was abolished and substituted by the term “interstitial fibrosis/tubular atrophy (IFTA)”, with no evidence of specific etiology[21]. The etiologic groups of “a” subcategory of CAN in the previous classifications were relocated to the “other” category, while the chronic allo-immune causes were moved to the respective categories of AMR and TCMR as chronic active or chronic (inactive) types. Thus, category 5 in the Banff 2005 report and all succeeding updates (till 2019), included only those forms of chronic changes for which no specific etiological findings were found on the biopsy (Table 5). In the 2019 Banff classification, the category of IFTA has been eliminated and replaced by polyomavirus nephropathy, as shown in Table 5.
It is worth highlighting here that category 5 of the Banff classification can co-exist with any other types of renal allograft pathology, except category 1, which is normal. It is important to record and report both the acute and chronic pathological changes on the biopsies to guide therapy and determine the prognosis.
In summary, the nomenclature, categorization, and subcategorization of chronic changes have undergone substantial alterations over the past three decades of the Banff consensus classification. Of late, the focus was on identifying the early specific features relevant to causes of chronic allograft damage, so that their progression may be halted with intervention. This was somewhat facilitated by the ancillary techniques of IHC and EM. In the latest published 2019 Banff meeting report, this category has been eliminated altogether.
Renal allograft biopsy is a valuable diagnostic and prognostic tool in the hands of clinicians for evaluating the structural and functional status of the kidney allograft. It can provide important information about the underlying cause of graft dysfunction or rejection. Studies have shown that renal allograft biopsies change the clinical diagnosis in an average of 36% (24%-47%) of cases and treatment in 59% of cases. However, because only a tiny amount of kidney tissue is obtained during the biopsy, several limitations and shortcomings exist in the extent to which renal allograft biopsies can prove useful. A close liaison between the clinicians and nephropathology laboratory will go a long way in overcoming many of these shortcomings[13]. According to studies, the sensitivity of diagnosis of rejection is 90% with one core of tissue and it increases to 99% when two cores are obtained[95-102]. Apart from the above-mentioned inherent sampling error, it is well known that there can be interobserver variability among nephropathologists in interpreting renal allograft biopsies. It can arise due to variations in individual expertise, experience, training, and personal judgment. Many studies have documented the occurrence of interobserver variability in renal pathology, including the interpretation of allograft biopsies[103-112]. Sometimes, the biopsy has to be repeated in view of the above considerations, particularly when the biopsy findings do not match the clinical picture. Furthermore, it is to be noted that the interobserver variability is not uniform for all the pathological lesions observed on renal allograft biopsies. For some lesions, the interobserver agreement is good, e.g., ATI, interstitial fibrosis, and vascular changes are often more easily recognized and agreed upon by pathologists, leading to higher concordance in their interpretation. On the other hand, certain pathological patterns may be more challenging and prone to interobserver variability. For instance, the diagnosis and grading of TCMR, AMR, or BCs can sometimes be subjective and require expert judgment. These categories of pathological lesions often involve evaluating subtle changes in cellular infiltrates, glomerular lesions, or peritubular capillaritis, which can lead to differences in interpretation among pathologists.
It is important to recognize that considerable efforts have been made to minimize this variability. Quality control measures such as consensus conferences, panel discussions, and standardized reporting systems have been implemented to improve agreement among pathologists. For instance, the Banff classification system is widely used to grade and classify various pathological findings in renal allograft biopsies. This standardized approach helps in enhancing the reproducibility and consistency of pathological interpretations. In addition, advancements in ancillary diagnostic tests, morphometry, DP, and telepathology have allowed for remote consultation and collaboration among experts, further reducing the impact of interobserver variability[113,114].
While interobserver variability remains a consideration, renal allograft biopsy continues to be an essential diagnostic tool in clinical practice. It provides valuable information for guiding treatment decisions, assessing the severity of graft dysfunction, monitoring for rejection, and identifying other causes of kidney dysfunction. Collaborative efforts, standa
The turnaround time for reporting a renal allograft biopsy varies depending on several factors, including the urgency of the clinical situation, the workload of the pathology laboratory, and the complexity of the biopsy specimen. In general, the time frame for reporting renal allograft biopsies can range from a few hours to a few days. However, it’s important to note that this is a general estimate and the actual turnaround time varies among different institutions and laboratories. In urgent or critical cases, such as suspected acute rejection or severe graft dysfunction, the pathology laboratory may prioritize the processing and reporting of the biopsy to expedite the diagnosis and facilitate prompt clinical decision-making. In such situations, the reporting time is typically shorter, ranging from a few hours (as short as 2-3 h) to 24-48 h, and is based on LM interpretation alone. In the authors’ laboratory, LM findings of all renal allograft biopsies are reported within 4-8 h’ time. For those cases, where there is no immediate need for urgent intervention, the reporting time is typically longer. This allows pathologists to carefully evaluate the biopsy specimen, conduct any necessary additional testing or staining such as IF, IHC, or EM, and provide a detailed and accurate report.
It is important to note that the clinical decision-making process based on renal allograft biopsy requires a multidisciplinary approach, involving nephrologists, transplant surgeons, pathologists, and other healthcare professionals. In particular, making a clinical decision based on renal allograft biopsy involves a comprehensive evaluation that integrates the pathological findings with clinical information and other relevant diagnostic tests such as imaging studies, drug levels, urine culture, and other laboratory tests. Collaboration and communication among the healthcare team members is the key to providing optimal patient care.
Although major advances have been made in understanding and categorizing kidney allograft pathology during the last three decades, there are still many problematic areas that remain to be addressed. Issues of sampling errors, reproducibility, borderline category, subclinical rejection, global training and competence, and lack of sensitivity and specificity of morphological features are some such areas[115-118]. Many gaps remain from the diagnostic accuracy and precision point of view, e.g., diagnostic thresholds and BCs. Focused research on molecular diagnostics, DP, AI, machine learning, and deep learning are some of the tools being actively investigated to address these problems[119-123]. Non-invasive serum and urinary molecular markers are also being actively sought for early and reliable identification of the rejection process right from the inception of the injury[124-127]. Integration of these with clinical, laboratory, and biopsy-based investigations may lead to a multimodal diagnostic algorithm for quick and accurate diagnosis for optimal treatment outcomes. Translation of the advancements in pathology diagnostics has yet not been realized into improved long-term graft outcomes.
The first 30 years of the Banff classification system have witnessed tremendous progress in understanding, streamlining, and classifying kidney allograft pathology, particularly the rejection. Essentially, the classification has changed from pathology-based to pathogenesis-based and has become clinician-friendly and treatment-friendly. However, many gaps remain from the diagnostic sensitivity point of view, e.g., diagnostic thresholds and BCs. More sophisticated techniques and digital and computational approaches are being actively researched at present to further improve diagnostic accuracy and precision of the classification.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alkhatib AJ, Jordan; Gonzalez FM, Chile S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Anyszek A, Wyzgał J, Czyżewski Ł. Long-term Results of Kidney Transplantation: Analysis of Selected Factors. Transplant Proc. 2020;52:2305-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Wang JH, Skeans MA, Israni AK. Current Status of Kidney Transplant Outcomes: Dying to Survive. Adv Chronic Kidney Dis. 2016;23:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 3. | Zhang J, Qiu J, Chen GD, Wang CX, Wang C, Yu SJ, Chen LZ. Etiological analysis of graft dysfunction following living kidney transplantation: a report of 366 biopsies. Ren Fail. 2018;40:219-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Solez K. History of the Banff classification of allograft pathology as it approaches its 20th year. Curr Opin Organ Transplant. 2010;15:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Solez K, Racusen LC. The Banff classification revisited. Kidney Int. 2013;83:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Loupy A, Mengel M, Haas M. Thirty years of the International Banff Classification for Allograft Pathology: the past, present, and future of kidney transplant diagnostics. Kidney Int. 2022;101:678-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 7. | Gowrishankar S. Banff Classification from 1991 to 2019. A Significant Contribution to Our Understanding and Reporting of Allograft Renal Biopsies. Indian J Nephrol. 2022;32:1-7. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Dhrolia M, Ahmad A. Development of Banff Classification from 1991 to 2019 for identifying renal allograft rejection: A narrative review for nephrologists. J Pak Med Assoc. 2022;72:1615-1621. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, Horsfield C, Loupy A, Mengel M, Perkowska-Ptasińska A, Rabant M, Racusen LC, Solez K, Becker JU. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation. 2018;102:1795-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 541] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 10. | Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, Croker BP, Droz D, Dunnill MS, Halloran PF. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993;44:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1111] [Cited by in RCA: 1075] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 11. | Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2522] [Cited by in RCA: 2496] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 12. | Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 821] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 13. | Cimen S, Geldenhuys L, Guler S, Imamoglu A, Molinari M. Impact of specimen adequacy on the assessment of renal allograft biopsy specimens. Braz J Med Biol Res. 2016;49:e5301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M; Banff meeting report writing committee. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1103] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 15. | Haas M. The Revised (2013) Banff Classification for Antibody-Mediated Rejection of Renal Allografts: Update, Difficulties, and Future Considerations. Am J Transplant. 2016;16:1352-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Hara S. Banff 2013 update: Pearls and pitfalls in transplant renal pathology. Nephrology (Carlton). 2015;20 Suppl 2:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Colvin RB, Cohen AH, Saiontz C, Bonsib S, Buick M, Burke B, Carter S, Cavallo T, Haas M, Lindblad A, Manivel JC, Nast CC, Salomon D, Weaver C, Weiss M. Evaluation of pathologic criteria for acute renal allograft rejection: reproducibility, sensitivity, and clinical correlation. J Am Soc Nephrol. 1997;8:1930-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 199] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Hara S. Cell mediated rejection revisited: Past, current, and future directions. Nephrology (Carlton). 2018;23 Suppl 2:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Jeong HJ. Diagnosis of renal transplant rejection: Banff classification and beyond. Kidney Res Clin Pract. 2020;39:17-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Metter C, Torrealba JR. Pathology of the kidney allograft. Semin Diagn Pathol. 2020;37:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ. Banff '05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy ('CAN'). Am J Transplant. 2007;7:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 830] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 22. | Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A. Banff '09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 601] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 23. | Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, Farver CF, Rodriguez ER, Wallace WD, Glotz D; Banff meeting report writing committee. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 327] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 24. | Loupy A, Haas M, Solez K, Racusen L, Glotz D, Seron D, Nankivell BJ, Colvin RB, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell L, Drachenberg C, Dragun D, de Kort H, Gibson IW, Kraus ES, Lefaucheur C, Legendre C, Liapis H, Muthukumar T, Nickeleit V, Orandi B, Park W, Rabant M, Randhawa P, Reed EF, Roufosse C, Seshan SV, Sis B, Singh HK, Schinstock C, Tambur A, Zeevi A, Mengel M. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am J Transplant. 2017;17:28-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 506] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 25. | Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, Nankivell BJ, Halloran PF, Colvin RB, Akalin E, Alachkar N, Bagnasco S, Bouatou Y, Becker JU, Cornell LD, Duong van Huyen JP, Gibson IW, Kraus ES, Mannon RB, Naesens M, Nickeleit V, Nickerson P, Segev DL, Singh HK, Stegall M, Randhawa P, Racusen L, Solez K, Mengel M. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18:293-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 795] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 26. | Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell LD, Clahsen-van Groningen MC, Demetris AJ, Dragun D, Duong van Huyen JP, Farris AB, Fogo AB, Gibson IW, Glotz D, Gueguen J, Kikic Z, Kozakowski N, Kraus E, Lefaucheur C, Liapis H, Mannon RB, Montgomery RA, Nankivell BJ, Nickeleit V, Nickerson P, Rabant M, Racusen L, Randhawa P, Robin B, Rosales IA, Sapir-Pichhadze R, Schinstock CA, Seron D, Singh HK, Smith RN, Stegall MD, Zeevi A, Solez K, Colvin RB, Mengel M. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318-2331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 583] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 27. | Bissonnette MLZ, Riazy M, Cunningham AM, Gill JS. A Step toward Understanding the Story Behind the Pictures: Molecular Diagnostics and the Banff Classification of Renal Allograft Pathology. J Am Soc Nephrol. 2022;33:2131-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 28. | Haas M. Evolving criteria for the diagnosis of antibody-mediated rejection in renal allografts. Curr Opin Nephrol Hypertens. 2018;27:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Jeong HS, Kim DG, Lee ST, Huh KH, Kim YS, Jeong HJ, Lim BJ. Clinical Significance of Revised Banff Criteria in the Diagnosis of Antibody-Mediated Rejection. Transplant Proc. 2019;51:1488-1490. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Cornell LD. Histopathologic Features of Antibody Mediated Rejection: The Banff Classification and Beyond. Front Immunol. 2021;12:718122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Mauiyyedi S, Crespo M, Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Tolkoff-Rubin NE, Williams WW, Delmonico FL, Cosimi AB, Colvin RB. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol. 2002;13:779-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 352] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 32. | Wang S, Zhang C, Wang J, Yang C, Xu M, Rong R, Zhu T, Zhu D. Endothelial Cells in Antibody-Mediated Rejection of Kidney Transplantation: Pathogenesis Mechanisms and Therapeutic Implications. J Immunol Res. 2017;2017:8746303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Riethmüller G, Land W, Albert E. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int. 1993;43:1333-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 394] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Böhmig GA, Exner M, Habicht A, Schillinger M, Lang U, Kletzmayr J, Säemann MD, Hörl WH, Watschinger B, Regele H. Capillary C4d deposition in kidney allografts: a specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol. 2002;13:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 35. | Nickeleit V, Zeiler M, Gudat F, Thiel G, Mihatsch MJ. Detection of the complement degradation product C4d in renal allografts: diagnostic and therapeutic implications. J Am Soc Nephrol. 2002;13:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 36. | Kedainis RL, Koch MJ, Brennan DC, Liapis H. Focal C4d+ in renal allografts is associated with the presence of donor-specific antibodies and decreased allograft survival. Am J Transplant. 2009;9:812-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Seemayer CA, Gaspert A, Nickeleit V, Mihatsch MJ. C4d staining of renal allograft biopsies: a comparative analysis of different staining techniques. Nephrol Dial Transplant. 2007;22:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Mauiyyedi S, Pelle PD, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, Williams WW, Cosimi AB, Schneeberger EE, Colvin RB. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12:574-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 39. | Regele H, Böhmig GA, Habicht A, Gollowitzer D, Schillinger M, Rockenschaub S, Watschinger B, Kerjaschki D, Exner M. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002;13:2371-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 325] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 40. | Haas M. The significance of C4d staining with minimal histologic abnormalities. Curr Opin Organ Transplant. 2010;15:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Dominy KM, Willicombe M, Al Johani T, Beckwith H, Goodall D, Brookes P, Cook HT, Cairns T, McLean A, Roufosse C. Molecular Assessment of C4d-Positive Renal Transplant Biopsies Without Evidence of Rejection. Kidney Int Rep. 2019;4:148-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1514] [Cited by in RCA: 1496] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 43. | Lefaucheur C, Loupy A, Vernerey D, Duong-Van-Huyen JP, Suberbielle C, Anglicheau D, Vérine J, Beuscart T, Nochy D, Bruneval P, Charron D, Delahousse M, Empana JP, Hill GS, Glotz D, Legendre C, Jouven X. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet. 2013;381:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 44. | Salazar ID, Merino López M, Chang J, Halloran PF. Reassessing the Significance of Intimal Arteritis in Kidney Transplant Biopsy Specimens. J Am Soc Nephrol. 2015;26:3190-3198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Sellarés J, Reeve J, Loupy A, Mengel M, Sis B, Skene A, de Freitas DG, Kreepala C, Hidalgo LG, Famulski KS, Halloran PF. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant. 2013;13:971-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 46. | Loupy A, Lefaucheur C, Vernerey D, Chang J, Hidalgo LG, Beuscart T, Verine J, Aubert O, Dubleumortier S, Duong van Huyen JP, Jouven X, Glotz D, Legendre C, Halloran PF. Molecular microscope strategy to improve risk stratification in early antibody-mediated kidney allograft rejection. J Am Soc Nephrol. 2014;25:2267-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 47. | Adam B, Afzali B, Dominy KM, Chapman E, Gill R, Hidalgo LG, Roufosse C, Sis B, Mengel M. Multiplexed color-coded probe-based gene expression assessment for clinical molecular diagnostics in formalin-fixed paraffin-embedded human renal allograft tissue. Clin Transplant. 2016;30:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Adam BA, Smith RN, Rosales IA, Matsunami M, Afzali B, Oura T, Cosimi AB, Kawai T, Colvin RB, Mengel M. Chronic Antibody-Mediated Rejection in Nonhuman Primate Renal Allografts: Validation of Human Histological and Molecular Phenotypes. Am J Transplant. 2017;17:2841-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Halloran PF, Pereira AB, Chang J, Matas A, Picton M, De Freitas D, Bromberg J, Serón D, Sellarés J, Einecke G, Reeve J. Microarray diagnosis of antibody-mediated rejection in kidney transplant biopsies: an international prospective study (INTERCOM). Am J Transplant. 2013;13:2865-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 50. | Loupy A, Lefaucheur C. Antibody-Mediated Rejection of Solid-Organ Allografts. N Engl J Med. 2018;379:1150-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 357] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 51. | Orandi BJ, Alachkar N, Kraus ES, Naqvi F, Lonze BE, Lees L, Van Arendonk KJ, Wickliffe C, Bagnasco SM, Zachary AA, Segev DL, Montgomery RA. Presentation and Outcomes of C4d-Negative Antibody-Mediated Rejection After Kidney Transplantation. Am J Transplant. 2016;16:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Haas M. Pathology of C4d-negative antibody-mediated rejection in renal allografts. Curr Opin Organ Transplant. 2013;18:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Haas M, Mirocha J. Early ultrastructural changes in renal allografts: correlation with antibody-mediated rejection and transplant glomerulopathy. Am J Transplant. 2011;11:2123-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Wavamunno MD, O'Connell PJ, Vitalone M, Fung CL, Allen RD, Chapman JR, Nankivell BJ. Transplant glomerulopathy: ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant. 2007;7:2757-2768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 55. | Nankivell BJ. The meaning of borderline rejection in kidney transplantation. Kidney Int. 2020;98:278-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | de Freitas DG, Sellarés J, Mengel M, Chang J, Hidalgo LG, Famulski KS, Sis B, Einecke G, Halloran PF. The nature of biopsies with "borderline rejection" and prospects for eliminating this category. Am J Transplant. 2012;12:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Becker JU, Chang A, Nickeleit V, Randhawa P, Roufosse C. Banff Borderline Changes Suspicious for Acute T Cell-Mediated Rejection: Where Do We Stand? Am J Transplant. 2016;16:2654-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 58. | Nankivell BJ, Agrawal N, Sharma A, Taverniti A, P'Ng CH, Shingde M, Wong G, Chapman JR. The clinical and pathological significance of borderline T cell-mediated rejection. Am J Transplant. 2019;19:1452-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 59. | McRae M, Bouchard-Boivin F, Béland S, Noël R, Côté I, Lapointe I, Lesage J, Latulippe E, Riopel J, Santoriello D, Husain SA, Désy O, Houde I, Batal I, De Serres SA. Impact of the Current Versus the Previous Diagnostic Threshold on the Outcome of Patients With Borderline Changes Suspicious for T Cell-mediated Rejection Diagnosed on Indication Biopsies. Transplantation. 2018;102:2120-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Stites E, Kumar D, Olaitan O, John Swanson S, Leca N, Weir M, Bromberg J, Melancon J, Agha I, Fattah H, Alhamad T, Qazi Y, Wiseman A, Gupta G. High levels of dd-cfDNA identify patients with TCMR 1A and borderline allograft rejection at elevated risk of graft injury. Am J Transplant. 2020;20:2491-2498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 61. | Schweitzer EJ, Drachenberg CB, Anderson L, Papadimetriou JC, Kuo PC, Johnson LB, Klassen DK, Hoehn-Saric E, Weir MR, Bartlett ST. Significance of the Banff borderline biopsy. Am J Kidney Dis. 1996;28:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Meehan SM, Siegel CT, Aronson AJ, Bartosh SM, Thistlethwaite JR, Woodle ES, Haas M. The relationship of untreated borderline infiltrates by the Banff criteria to acute rejection in renal allograft biopsies. J Am Soc Nephrol. 1999;10:1806-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | El-Agroudy AE, Wafa EW, Abbas TM, El-Husseini A, Gheith OA, El-Baz M, Ghoneim MA. Characteristics of patients with Banff borderline changes in renal allograft biopsies. Exp Clin Transplant. 2009;7:228-232. [PubMed] |
| 64. | Mubarak M, Shakeel S, Abbas K, Aziz T, Zafar MN, Naqvi SA, Rizvi SA. Borderline Changes on Dysfunctional Renal Allograft Biopsies: Clinical Relevance in a Living Related Renal Transplant Setting. Exp Clin Transplant. 2017;15:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 65. | Nankivell BJ, P'Ng CH, Chapman JR. Does tubulitis without interstitial inflammation represent borderline acute T cell mediated rejection? Am J Transplant. 2019;19:132-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Fernández-Camargo DA, Marino L, Muñiz-Cuervo E, Aceves-Rodríguez EM, Vargas NJ, Cohen-Bucay A, Alberú J, Uribe-Uribe NO, Morales-Buenrostro LE. Sub-classification of borderline changes into diffuse or focal and its impact on long-term renal transplant outcomes. Transpl Immunol. 2022;72:101594. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 67. | Wiebe C, Rush DN, Gibson IW, Pochinco D, Birk PE, Goldberg A, Blydt-Hansen T, Karpinski M, Shaw J, Ho J, Nickerson PW. Evidence for the alloimmune basis and prognostic significance of Borderline T cell-mediated rejection. Am J Transplant. 2020;20:2499-2508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 68. | Minervini MI, Torbenson M, Scantlebury V, Vivas C, Jordan M, Shapiro R, Randhawa PS. Acute renal allograft rejection with severe tubulitis (Banff 1997 grade IB). Am J Surg Pathol. 2000;24:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Filippone EJ, Farber JL. The Histological Spectrum and Clinical Significance of T Cell-mediated Rejection of Kidney Allografts. Transplantation. 2023;107:1042-1055. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 70. | Hoffman W, Mehta R, Jorgensen DR, Sood P, Randhawa P, Wu CM, Puttarajappa C, Shah NA, Tevar AD, Hariharan S. The Impact of Early Clinical and Subclinical T Cell-mediated Rejection After Kidney Transplantation. Transplantation. 2019;103:1457-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 71. | Helanterä I, Mengel M. Revisiting acute T cell-mediated rejection in kidney allografts. Am J Transplant. 2022;22:681-682. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 72. | Naesens M, Haas M, Loupy A, Roufosse C, Mengel M. Does the definition of chronic active T cell-mediated rejection need revisiting? Am J Transplant. 2021;21:1689-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Yamamoto I, Kawabe M, Hayashi A, Kobayashi A, Yamamoto H, Yokoo T. Challenges Posed by the Banff Classification: Diagnosis and Treatment of Chronic active T-cell mediated Rejection. Nephron. 2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 74. | Gaber LW, Moore LW, Alloway RR, Flax SD, Shokouh-Amiri MH, Schroder T, Gaber AO. Correlation between Banff classification, acute renal rejection scores and reversal of rejection. Kidney Int. 1996;49:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Seron D, Rabant M, Becker JU, Roufosse C, Bellini MI, Böhmig GA, Budde K, Diekmann F, Glotz D, Hilbrands L, Loupy A, Oberbauer R, Pengel L, Schneeberger S, Naesens M. Proposed Definitions of T Cell-Mediated Rejection and Tubulointerstitial Inflammation as Clinical Trial Endpoints in Kidney Transplantation. Transpl Int. 2022;35:10135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 76. | Mengel M, Reeve J, Bunnag S, Einecke G, Jhangri GS, Sis B, Famulski K, Guembes-Hidalgo L, Halloran PF. Scoring total inflammation is superior to the current Banff inflammation score in predicting outcome and the degree of molecular disturbance in renal allografts. Am J Transplant. 2009;9:1859-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 77. | Mannon RB, Matas AJ, Grande J, Leduc R, Connett J, Kasiske B, Cecka JM, Gaston RS, Cosio F, Gourishankar S, Halloran PF, Hunsicker L, Rush D; DeKAF Investigators. Inflammation in areas of tubular atrophy in kidney allograft biopsies: a potent predictor of allograft failure. Am J Transplant. 2010;10:2066-2073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 78. | Lefaucheur C, Gosset C, Rabant M, Viglietti D, Verine J, Aubert O, Louis K, Glotz D, Legendre C, Duong Van Huyen JP, Loupy A. T cell-mediated rejection is a major determinant of inflammation in scarred areas in kidney allografts. Am J Transplant. 2018;18:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 79. | Nankivell BJ, Shingde M, Keung KL, Fung CL, Borrows RJ, O'Connell PJ, Chapman JR. The causes, significance and consequences of inflammatory fibrosis in kidney transplantation: The Banff i-IFTA lesion. Am J Transplant. 2018;18:364-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 80. | Halloran PF, Matas A, Kasiske BL, Madill-Thomsen KS, Mackova M, Famulski KS. Molecular phenotype of kidney transplant indication biopsies with inflammation in scarred areas. Am J Transplant. 2019;19:1356-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 81. | Matas AJ, Helgeson ES, Gaston R, Cosio F, Mannon R, Kasiske BL, Hunsicker L, Gourishankar S, Rush D, Michael Cecka J, Connett J, Grande JP. Inflammation in areas of fibrosis: The DeKAF prospective cohort. Am J Transplant. 2020;20:2509-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Halloran PF, Pereira AB, Chang J, Matas A, Picton M, De Freitas D, Bromberg J, Serón D, Sellarés J, Einecke G, Reeve J. Potential impact of microarray diagnosis of T cell-mediated rejection in kidney transplants: The INTERCOM study. Am J Transplant. 2013;13:2352-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 83. | Halloran PF, Reeve J, Akalin E, Aubert O, Bohmig GA, Brennan D, Bromberg J, Einecke G, Eskandary F, Gosset C, Duong Van Huyen JP, Gupta G, Lefaucheur C, Malone A, Mannon RB, Seron D, Sellares J, Weir M, Loupy A. Real Time Central Assessment of Kidney Transplant Indication Biopsies by Microarrays: The INTERCOMEX Study. Am J Transplant. 2017;17:2851-2862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 84. | Reeve J, Böhmig GA, Eskandary F, Einecke G, Gupta G, Madill-Thomsen K, Mackova M, Halloran PF; INTERCOMEX MMDx-Kidney Study Group. Generating automated kidney transplant biopsy reports combining molecular measurements with ensembles of machine learning classifiers. Am J Transplant. 2019;19:2719-2731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 85. | Madill-Thomsen K, Perkowska-Ptasińska A, Böhmig GA, Eskandary F, Einecke G, Gupta G, Halloran PF; MMDx-Kidney Study Group. Discrepancy analysis comparing molecular and histology diagnoses in kidney transplant biopsies. Am J Transplant. 2020;20:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 86. | Matsunami M, Rosales IA, Adam BA, Oura T, Mengel M, Smith RN, Lee H, Cosimi AB, Colvin RB, Kawai T. Long-term Kinetics of Intragraft Gene Signatures in Renal Allograft Tolerance Induced by Transient Mixed Chimerism. Transplantation. 2019;103:e334-e344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | Smith RN. In-silico performance, validation, and modeling of the Nanostring Banff Human Organ transplant gene panel using archival data from human kidney transplants. BMC Med Genomics. 2021;14:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 88. | Eikmans M, Gielis EM, Ledeganck KJ, Yang J, Abramowicz D, Claas FFJ. Non-invasive Biomarkers of Acute Rejection in Kidney Transplantation: Novel Targets and Strategies. Front Med (Lausanne). 2018;5:358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 89. | Oellerich M, Sherwood K, Keown P, Schütz E, Beck J, Stegbauer J, Rump LC, Walson PD. Liquid biopsies: donor-derived cell-free DNA for the detection of kidney allograft injury. Nat Rev Nephrol. 2021;17:591-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 90. | Filippone EJ, Farber JL. The Monitoring of Donor-derived Cell-free DNA in Kidney Transplantation. Transplantation. 2021;105:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 91. | Mengel M, Loupy A, Haas M, Roufosse C, Naesens M, Akalin E, Clahsen-van Groningen MC, Dagobert J, Demetris AJ, Duong van Huyen JP, Gueguen J, Issa F, Robin B, Rosales I, Von der Thüsen JH, Sanchez-Fueyo A, Smith RN, Wood K, Adam B, Colvin RB. Banff 2019 Meeting Report: Molecular diagnostics in solid organ transplantation-Consensus for the Banff Human Organ Transplant (B-HOT) gene panel and open source multicenter validation. Am J Transplant. 2020;20:2305-2317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 92. | Halloran PF, Venner JM, Madill-Thomsen KS, Einecke G, Parkes MD, Hidalgo LG, Famulski KS. Review: The transcripts associated with organ allograft rejection. Am J Transplant. 2018;18:785-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 93. | Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, Salvatierra O Jr. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349:125-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 546] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 94. | Haas M. Chronic allograft nephropathy or interstitial fibrosis and tubular atrophy: what is in a name? Curr Opin Nephrol Hypertens. 2014;23:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Parfrey PS, Kuo YL, Hanley JA, Knaack J, Xue Z, Lisbona R, Guttmann RD. The diagnostic and prognostic value of renal allograft biopsy. Transplantation. 1984;38:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Matas AJ, Tellis VA, Sablay L, Quinn T, Soberman R, Veith FJ. The value of needle renal allograft biopsy. III. A prospective study. Surgery. 1985;98:922-926. [PubMed] |
| 97. | Waltzer WC, Miller F, Arnold A, Jao S, Anaise D, Rapaport FT. Value of percutaneous core needle biopsy in the differential diagnosis of renal transplant dysfunction. J Urol. 1987;137:1117-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Kiss D, Landmann J, Mihatsch M, Huser B, Brunner FP, Thiel G. Risks and benefits of graft biopsy in renal transplantation under cyclosporin-A. Clin Nephrol. 1992;38:132-134. [PubMed] |
| 99. | Manfro RC, Lee JY, Lewgoy J, Edelweiss MI, Gonçalves LF, Prompt CA. [The role of percutaneous renal biopsy in kidney transplant]. Rev Assoc Med Bras (1992). 1994;40:108-112. [PubMed] |
| 100. | Kon SP, Templar J, Dodd SM, Rudge CJ, Raftery MJ. Diagnostic contribution of renal allograft biopsies at various intervals after transplantation. Transplantation. 1997;63:547-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Al-Awwa IA, Hariharan S, First MR. Importance of allograft biopsy in renal transplant recipients: correlation between clinical and histological diagnosis. Am J Kidney Dis. 1998;31:S15-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 102. | Pascual M, Vallhonrat H, Cosimi AB, Tolkoff-Rubin N, Colvin RB, Delmonico FL, Ko DS, Schoenfeld DA, Williams WW Jr. The clinical usefulness of the renal allograft biopsy in the cyclosporine era: a prospective study. Transplantation. 1999;67:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Solez K, Hansen HE, Kornerup HJ, Madsen S, Sørensen AW, Pedersen EB, Marcussen N, Benediktsson H, Racusen LC, Olsen S. Clinical validation and reproducibility of the Banff schema for renal allograft pathology. Transplant Proc. 1995;27:1009-1011. [PubMed] |
| 104. | Furness PN, Taub N, Assmann KJ, Banfi G, Cosyns JP, Dorman AM, Hill CM, Kapper SK, Waldherr R, Laurinavicius A, Marcussen N, Martins AP, Nogueira M, Regele H, Seron D, Carrera M, Sund S, Taskinen EI, Paavonen T, Tihomirova T, Rosenthal R. International variation in histologic grading is large, and persistent feedback does not improve reproducibility. Am J Surg Pathol. 2003;27:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 105. | Sar A, Worawichawong S, Benediktsson H, Zhang J, Yilmaz S, Trpkov K. Interobserver agreement for Polyomavirus nephropathy grading in renal allografts using the working proposal from the 10th Banff Conference on Allograft Pathology. Hum Pathol. 2011;42:2018-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 106. | Marcussen N, Olsen TS, Benediktsson H, Racusen L, Solez K. Reproducibility of the Banff classification of renal allograft pathology. Inter- and intraobserver variation. Transplantation. 1995;60:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 107. | Joh K, Morozumi K, Kitamura H. Symposium: evaluating the reproducibility of pathological diagnosis using the 1997 Banff classification update. Clin Transplant. 2006;20 Suppl 15:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 108. | Huang SC, Lin YJ, Wen MC, Lin WC, Fang PW, Liang PI, Chuang HW, Chien HP, Chen TD. Unsatisfactory reproducibility of interstitial inflammation scoring in allograft kidney biopsy. Sci Rep. 2023;13:7095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 109. | Mengel M, Sis B, Halloran PF. SWOT analysis of Banff: strengths, weaknesses, opportunities and threats of the international Banff consensus process and classification system for renal allograft pathology. Am J Transplant. 2007;7:2221-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 110. | Furness PN, Taub N; Convergence of European Renal Transplant Pathology Assessment Procedures (CERTPAP) Project. International variation in the interpretation of renal transplant biopsies: report of the CERTPAP Project. Kidney Int. 2001;60:1998-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 240] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 111. | Gough J, Rush D, Jeffery J, Nickerson P, McKenna R, Solez K, Trpkov K. Reproducibility of the Banff schema in reporting protocol biopsies of stable renal allografts. Nephrol Dial Transplant. 2002;17:1081-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 112. | Veronese FV, Manfro RC, Roman FR, Edelweiss MI, Rush DN, Dancea S, Goldberg J, Gonçalves LF. Reproducibility of the Banff classification in subclinical kidney transplant rejection. Clin Transplant. 2005;19:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 113. | Brazdziute E, Laurinavicius A. Digital pathology evaluation of complement C4d component deposition in the kidney allograft biopsies is a useful tool to improve reproducibility of the scoring. Diagn Pathol. 2011;6 Suppl 1:S5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 114. | Jen KY, Olson JL, Brodsky S, Zhou XJ, Nadasdy T, Laszik ZG. Reliability of whole slide images as a diagnostic modality for renal allograft biopsies. Hum Pathol. 2013;44:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 115. | Farris AB, Moghe I, Wu S, Hogan J, Cornell LD, Alexander MP, Kers J, Demetris AJ, Levenson RM, Tomaszewski J, Barisoni L, Yagi Y, Solez K. Banff Digital Pathology Working Group: Going digital in transplant pathology. Am J Transplant. 2020;20:2392-2399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 116. | Farris AB, Vizcarra J, Amgad M, Cooper LAD, Gutman D, Hogan J. Artificial intelligence and algorithmic computational pathology: an introduction with renal allograft examples. Histopathology. 2021;78:791-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 117. | Aubert O, Higgins S, Bouatou Y, Yoo D, Raynaud M, Viglietti D, Rabant M, Hidalgo L, Glotz D, Legendre C, Delahousse M, Shah N, Sis B, Campbell P, Mengel M, Jouven X, Duong Van Huyen JP, Lefaucheur C, Loupy A. Archetype Analysis Identifies Distinct Profiles in Renal Transplant Recipients with Transplant Glomerulopathy Associated with Allograft Survival. J Am Soc Nephrol. 2019;30:625-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 118. | Marsh JN, Matlock MK, Kudose S, Liu TC, Stappenbeck TS, Gaut JP, Swamidass SJ. Deep Learning Global Glomerulosclerosis in Transplant Kidney Frozen Sections. IEEE Trans Med Imaging. 2018;37:2718-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 119. | Hermsen M, de Bel T, den Boer M, Steenbergen EJ, Kers J, Florquin S, Roelofs JJTH, Stegall MD, Alexander MP, Smith BH, Smeets B, Hilbrands LB, van der Laak JAWM. Deep Learning-Based Histopathologic Assessment of Kidney Tissue. J Am Soc Nephrol. 2019;30:1968-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 226] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 120. | Yatim KM, Azzi JR. Novel Biomarkers in Kidney Transplantation. Semin Nephrol. 2022;42:2-13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 121. | Merhi B, Bayliss G, Gohh RY. Role for urinary biomarkers in diagnosis of acute rejection in the transplanted kidney. World J Transplant. 2015;5:251-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 122. | Seo JW, Lee YH, Tae DH, Park SH, Moon JY, Jeong KH, Kim CD, Chung BH, Park JB, Kim YH, Seok J, Joo SH, Lee SH, Lee JS. Non-Invasive Diagnosis for Acute Rejection Using Urinary mRNA Signature Reflecting Allograft Status in Kidney Transplantation. Front Immunol. 2021;12:656632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 123. | Van Loon E, Naesens M. Blood transcriptomics as non-invasive marker for kidney transplant rejection. Nephrol Ther. 2021;17S:S78-S82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 124. | Sigdel TK, Yang JYC, Bestard O, Schroeder A, Hsieh SC, Liberto JM, Damm I, Geraedts ACM, Sarwal MM. A urinary Common Rejection Module (uCRM) score for non-invasive kidney transplant monitoring. PLoS One. 2019;14:e0220052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 125. | Al Jurdi A, Gassen RB, Borges TJ, Solhjou Z, Hullekes FE, Lape IT, Efe O, Alghamdi A, Patel P, Choi JY, Mohammed MT, Bohan B, Pattanayak V, Rosales I, Cravedi P, Kotton CN, Azzi JR, Riella LV. Non-Invasive Monitoring for Rejection in Kidney Transplant Recipients After SARS-CoV-2 mRNA Vaccination. Front Immunol. 2022;13:838985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 126. | Kant S, Brennan DC. Donor Derived Cell Free DNA in Kidney Transplantation: The Circa 2020-2021 Update. Transpl Int. 2022;35:10448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 127. | Mannon RB, Morris RE, Abecassis M, Axelrod D, Bala S, Friedman GS, Heeger PS, Lentine KL, Loupy A, Murphy B, Nickerson P, Sarwal M, O'Doherty I, Spear N, Karpen SR. Use of biomarkers to improve immunosuppressive drug development and outcomes in renal organ transplantation: A meeting report. Am J Transplant. 2020;20:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |