Published online Jun 18, 2023. doi: 10.5500/wjt.v13.i4.201
Peer-review started: February 25, 2023
First decision: March 15, 2023
Revised: March 21, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: June 18, 2023
Processing time: 110 Days and 17.9 Hours
Warts are common in recipients of kidney transplantation (KT). Resistant warts which are not amenable to conventional therapies may lead to significant mor
We report a seven-year-old child who presented with recalcitrant plantar per
Stimulation of cell-mediated immunity against the human papilloma virus induced by the IL candida immunotherapy is thought to be a cause for wart resolution. With this therapy, whether it is necessary to augment the immunosuppression to prevent rejection is unclear as that may come with a risk of infectious complications. Larger, prospective studies in pediatric KT recipients are needed to explore these important issues.
Core Tip: Warts are common after pediatric kidney transplantation. Given immunosuppressed status, most children are unable to clear the warts with conventional anti-wart therapies. Local immunotherapy has emerged as an excellent treatment modality for treatment of resistant warts following kidney transplantation. However, the safety of such agents needs careful consideration with longitudinal studies. Here, we studied the efficacy and safety of local candida immunotherapy in an immunocompromised child with kidney transplantation and recalcitrant plantar warts.
- Citation: Acharya R, Bush R, Johns F, Upadhyay K. Efficacy and safety of local candida immunotherapy in recalcitrant warts in pediatric kidney transplantation: A case report. World J Transplant 2023; 13(4): 201-207
- URL: https://www.wjgnet.com/2220-3230/full/v13/i4/201.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i4.201
Cutaneous common warts (verruca vulgaris) are commonly seen after kidney transplantation (KT)[1-3]. In one study of 60 children and adolescents with KT, the incidence of warts was 28% with increased prevalence with time since KT. Plantar warts are the most common warts following KT[4]. The most common immunosuppressive regimen in these patients was tacrolimus and prednisone, in combination with either azathioprine or mycophenolate mofetil (MMF). The most commonly seen human papillomavirus (HPV) strains responsible for common warts are HPV-2, 27, 29, 34 and 57[5]. Painful warts can impair the quality of life and cause significant morbidity[6]. Unlike in non-immunocompromised individuals, warts in KT recipients may not undergo spontaneous resolution. Although the risk of cancerous conversion is primarily seen with genital warts, multiple verrucae (> 10 verrucae) may be associated with the development of actinic keratoses, invasive squamous cell carcinoma and basal cell carcinoma[7]. Some of the treatment options are topical keratolytics such as salicylate, cryotherapy with liquid nitrogen, electrofulguration or radiofrequency ablation, duct tape, pulsed dye or CO2 laser, intralesional (IL) bleomycin, surgical removal with curettage or cautery, and IL immunotherapy[8]. Here we describe a seven-year-old KT recipient with recalcitrant plantar warts who had an excellent response to the IL candida immunotherapy with no recurrence in the short-term follow-up of ten months.
A seven-year-old Caucasian male presented with multiple wart-like lesions in the plantar aspect of both foot after KT.
The patient had received a pre-emptive first living unrelated donor KT with bilateral native nephrectomies one month prior to the onset of the skin warts. He sustained early loss of renal allograft secondary to transplant renal artery thrombosis. Transplant kidney biopsy showed coagulative necrosis and he was transitioned to chronic hemodialysis until receiving a second deceased donor KT two months later with excellent allograft function. Induction immunosuppression (IS) for both first and second KT consisted of three doses of 1.5 mg/kg/dose ThymoglobulinR with steroid. Low dose tacrolimus and MMF were continued after the first failed KT. The first allograft was removed during the second KT. Maintenance IS for second KT consisted of tacrolimus, MMF and steroid. Serum trough tacrolimus level was maintained at the goal range.
The past medical history consisted of end stage renal disease secondary to posterior urethral valve. He however did not require dialysis given stable electrolytes and normal urine output. He had been immunized fully as per the routine childhood immunization schedule.
The patient’s personal and family history was otherwise unremarkable.
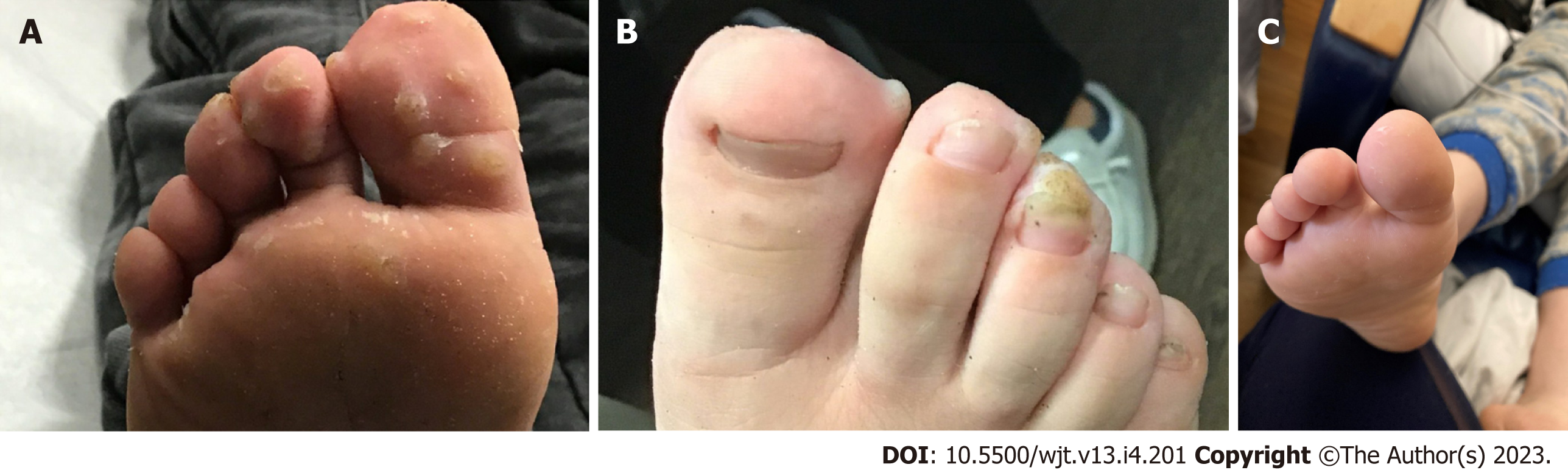
Physical examination of the patient showed normal vital signs and examination except for the abdomen with scar marks from prior surgeries and positive skin findings. Skin examination showed multiple verrucous papules and plaques on the anterior aspect of the plantar surface of foot bilaterally (Figures 1A and B), and some papular lesions in the left thumb. There were no warts seen in the genital region or the oropharynx region. The warts were extremely painful and would wake him throughout the night. The patient had difficulty ambulating and had to be carried to the clinic visits.
Complete blood count was normal. Renal allograft function remained stable after second KT with serum creatinine of 0.5-0.6 mg/dL. C-reactive protein was normal. Serum trough tacrolimus level was at the goal of 9-11 ng/mL in the first month post KT, then 8-10 ng/mL in the second month, followed by 6-8 ng/mL from three to six months post KT. Urinalysis showed no proteinuria, hematuria or urinary tract infections. Given cytomegalovirus (CMV) mismatch, he received oral valganciclovir for six months post-KT. CMV, Epstein-Barr virus and BK virus polymerase chain reaction (PCR)s were all negative until six months post KT. HPV genotyping of the warts was not done.
Chest X-ray was negative for pneumonia or other viral processes. Renal allograft sonogram was normal.
Plantar warts in a child with kidney transplantation.
He was evaluated by dermatologist and was treated with lidocaine ointment, WartPEEL (17% salicylic acid and 2% 5-fluorouracil), WartSTICK (40% salicylic acid), and Differin (0.3% Adapalene gel) under occlusion for several weeks without any clinical improvement of the signs and symptoms. Six months after the first KT, he was treated with a first dose of CandinR (IL Candida albicans antigen) to the largest wart paired with liquid nitrogen cryotherapy. Second dose of IL CandinR and liquid nitrogen was administered four weeks later.
The patient had a significant improvement with almost complete resolution of the warts after two CandinR paired with liquid nitrogen cryotherapy. Complete resolution was indicated by complete disappearance of the hyperkeratosis and thickening of the skin. Due to the presence of few scattered lesions only, a third dose of liquid nitrogen cryotherapy was administered two months later without IL CandinR. There were no side effects observed such as blister, infection, post-inflammatory altered pigmentation, scarring or anaphylaxis. During a ten-month follow-up period since the second and last CandinR therapy, there have been no recurrences of the warts (Figure 1C).
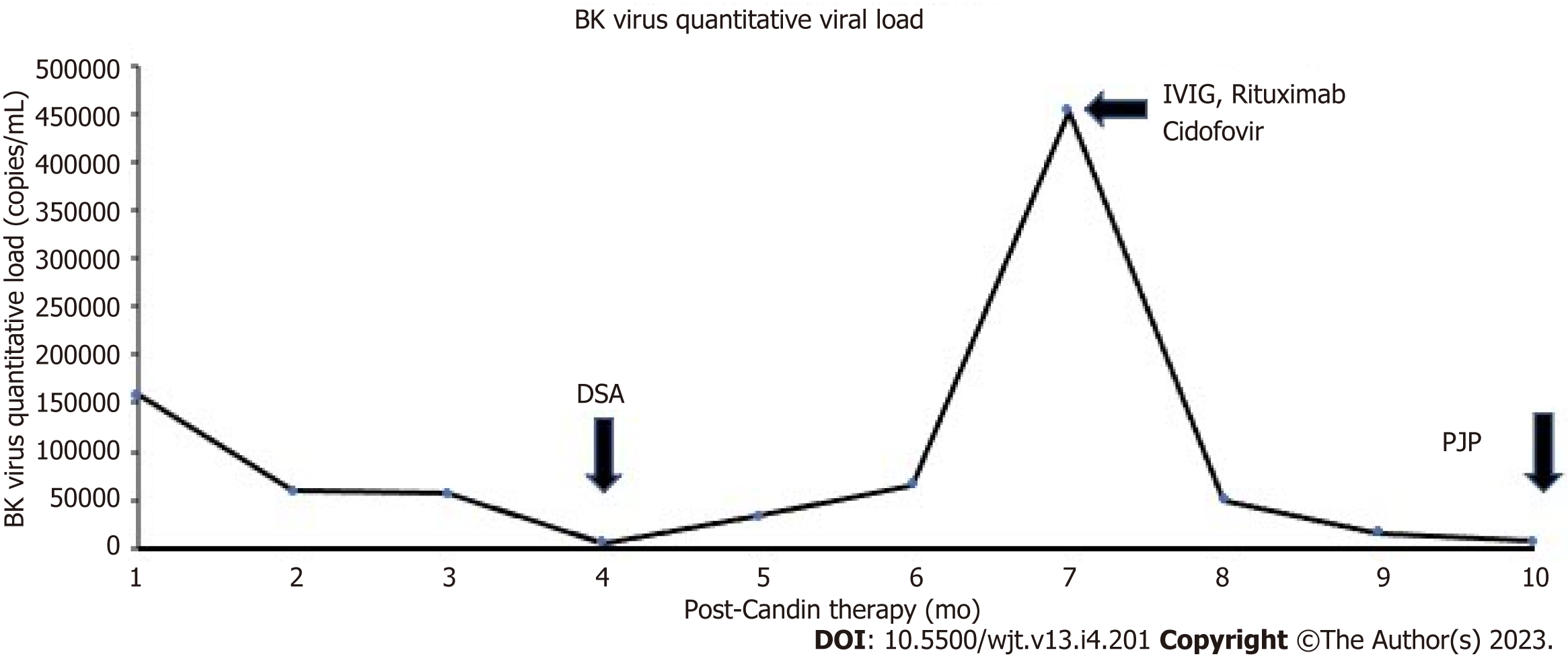
Three weeks after the first CandinR injection, a follow-up whole blood BK virus deoxyribonuclease (DNA) PCR showed BK viral load of 159000 copies/mL (ARUP laboratories, Salt Lake City, UT, United States). His immunosuppression regimen had been same as before and his allograft function was stable. Following this, his tacrolimus dose was reduced with a lower trough goal level of 3-5 ng/mL, mycophenolate was discontinued and leflunomide was started. Over the next one year, his BK virus DNA PCR showed persistent positivity with a peak viral load of 453000 copies/mL seven months post last CandinR therapy. He then received two monthly intravenous (IV) immunoglobulin therapies and a course of IV cidofovir with the most recent BK virus load of 6280 copies/mL ten months after the last CandinR therapy (Figure 2). The most recent immunosuppression regimen consists of tacrolimus 2 mg twice daily, prednisone 10 mg daily and leflunomide 20 mg daily.
Donor specific antibodies (DSA) were obtained monthly as a part of the transplant center’s protocol. Four months after the last CandinR injection, weak DSA to class I antigens [B58, C12; 2500 mean fluorescent intensity (MFI) for both] were observed. Subsequently, strong DSAs against class II antigen (DQ6, 10000 MFI) also started appearing a month later. However, the serum creatinine remained stable around 0.6-0.7 mg/dL. He was treated with intravenous immunoglobulin and a dose of Rituximab 375 mg/m2. A kidney transplant biopsy could not be obtained due to parental hesitation. However, a plasma donor-derived cell-free DNA (dd-cfDNA) test showed elevated value of 1.21% (reference range: < 0.7% dd-cfDNA, Viracor TRAC kidney dd-cfDNA, Eurofins Transplant Genomics, Framingham, MA, United States). A decision was made to serially follow the dd-cfDNA and DSA closely given stable serum creatinine.
Ten months after the last CandinR therapy, he presented with hypoxia and respiratory distress with chest X-ray showing ground-glass opacities in the lungs. He underwent bronchoscopy; the PCR of the bronchoalveolar lavage was positive for pneumocystis jirovecii and diagnosed with pneumocystis jirovecii pneumonia (PJP). He was treated with trimethoprim-sulfamethoxazole with complete resolution of respiratory symptoms. Immunosuppression was kept the same with a goal trough tacrolimus level of 3-5 ng/mL.
In patients with cutaneous warts with suboptimal or no response to conventional anti-wart therapies, IL immunotherapy may be useful[9,10]. Various IL immunotherapy regimen have been described such as candida antigen, mumps antigen, measles mumps rubella vaccine, purified protein derivative and bacilli calmette-guerin vaccine[10]. One systematic review showed 68% cure-rate of local immunotherapy in plantar warts, as opposed to low cure rate with topical salicylic acid and cryotherapy[11]. A randomized placebo-controlled trial by Horn et al[12] showed excellent efficacy of IL candida, mumps or trichophyton skin test antigens. The possible mechanism of action of immunotherapy is the proliferation of HPV-specific peripheral blood mononuclear cells that possibly mediate an immunologic attack against the wart tissue[13].
Data on efficacy of IL candida in children is scarce[14]. Alikhan et al[15] reported a retrospective study of 100 adults and children with verruca vulgaris who were treated with IL purified candida antigen therapy with 39% complete response and 41% partial response rate. In their study, six out of seven patients who were immunocompromised demonstrated partial or complete response rate. The proposed mechanism is via stimulation of a cell-mediated immune response. Phillips et al[13] retrospectively reviewed adults and children who received monthly IL candida antigen with 72% complete resolution rate. Another retrospective study of 220 children with multiple and recalcitrant warts who received IL candida injections showed 71% and 17% complete and partial response rates respectively. There were no side effects reported except for some discomfort at the time of injection[16]. IL Candida immunotherapy has also been shown to be efficacious in treating the distant non-injected warts[17]. Whether the similar results are expected in immunocompromised individuals needs to be studied on a larger scale.
With regards to immunosuppressed patients, the prevalence of warts corresponds with the duration of immunosuppressive therapy, increasing to 50%-92% in patients who are more than 4-5 years after transplantation[3]. Our report is unique in that the onset of warts was fairly rapid following KT. In most HPV infections in immunocompetent individuals, the cellular and cytotoxic immunity provided by T cells and natural killer cells are sufficient to control the warts[18]; however, in immunocompromised patients, due to lack of cell-mediated immunity, the proliferation of virus occurs causing warts, sometimes in unusual locations such as bladder[19,20]. Indeed, a few studies have reported clearance of the warts with reduction or cessation of immunosuppression only in KT recipients[21,22]. Conversion to another anti-rejection agent may be useful as well. Nguyen et al[23] reported 4 children with warts and molluscum contagiosum who benefited from conversion from tacrolimus/mycophenolate to tacrolimus/Leflunomide. Conversion to sirolimus has been shown to be effective for recalcitrant cutaneous viral warts in liver transplant recipients[24,25]. There is not much data on the efficacy of the IL immunotherapy. In patients who do undergo local immunotherapy, it is not known whether immunosuppression decreases the efficacy of local immunotherapy such as IL candida. Immunosuppression usually is at the maximal level during the first few months of KT and it will be interesting to study the efficacy of these immunotherapies during this period of maximal immunosuppression. On the other hand, there are also potential safety concerns with stimulating cell-mediated immunity with IL Candida therapy leading to rejection[26]. Few studies done in the children on warts have not looked at these longitudinal issues[4]. In our patient, there was a temporal relationship between the onset of BK viremia and CandinR therapy along with observation of DSA, elevated dd-cfDNA, and PJP a few months following the completion of CandinR. However, we could not establish a direct cause and relationship between IL candida and these findings. Since dd-cfDNA has been shown to diagnose subclinical rejection even in the absence of deranged renal function, this test may be important in children who have underwent IL candida therapy for establishing an early diagnosis of rejection and possibly close monitoring and treatment[27]. In those with concurrent viral infections and elevated dd-cfDNA, it is challenging to decide the amount of immunosuppression, as in our case. Also, since children with current BK viremia have been shown to have significantly higher median plasma dd-cfDNA, the importance of elevated dd-cfDNA in this subset of children is uncertain[28]. Also, as seen in this report, there may be as association between BK virus and HPV as both belong to the human papovavirus family. These are important topics of discussion that will need to be studied in further larger studies.
A decision of whether to treat with immunotherapy such as IL candida in immunocompromised transplant recipients is challenging due to concerns with efficacy and the possibility of rejection, and perhaps infections. Well-designed prospective studies are needed in the future to determine the efficacy and safety of this potentially curative treatment for the recalcitrant warts.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bellini MI, Italy; Sarier M, Turkey S-Editor: Liu JH L-Editor: A P-Editor: Zhang YL
| 1. | Oliveira WRP, Tirico MCCP, Souza AAV, Codarin FR, Silva LLC, Festa Neto C. Skin lesions in organ transplant recipients: a study of 177 consecutive Brazilian patients. Int J Dermatol. 2019;58:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Oh CC, Lee HY, Tan BK, Assam PN, Kee TYS, Pang SM. Dermatological conditions seen in renal transplant recipients in a Singapore tertiary hospital. Singapore Med J. 2018;59:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Lunn A, Ravenscroft J, Watson AR. Cutaneous warts in children before and after renal transplantation. Pediatr Nephrol. 2010;25:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Martelli-Marzagão F, Yamashiro AS, Ogawa MM, Santos GF Jr, Tomimori J, Porro AM. Clinical and histopathological characterization and typing of the human papillomavirus in common warts of kidney transplant recipients. An Bras Dermatol. 2010;85:743-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Moloney FJ, Keane S, O'Kelly P, Conlon PJ, Murphy GM. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Cho CY, Lo YC, Hung MC, Lai CC, Chen CJ, Wu KG. Risk of cancer in patients with genital warts: A nationwide, population-based cohort study in Taiwan. PLoS One. 2017;12:e0183183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Gibbs S, Harvey I. Topical treatments for cutaneous warts. Cochrane Database Syst Rev. 2006;CD001781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Bhalala KB, Poojary S, Shah KS. Comparative Study of Efficacy of Intralesional Purified Protein Derivative (PPD) Versus Intralesional Measles, Mumps, and Rubella (MMR) Vaccine in Management of Multiple Viral Warts. J Cutan Aesthet Surg. 2021;14:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Fields JR, Saikaly SK, Schoch JJ. Intralesional immunotherapy for pediatric warts: A review. Pediatr Dermatol. 2020;37:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | García-Oreja S, Álvaro-Afonso FJ, García-Álvarez Y, García-Morales E, Sanz-Corbalán I, Lázaro Martínez JL. Topical treatment for plantar warts: A systematic review. Dermatol Ther. 2021;34:e14621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, Candida, and Trichophyton skin test antigens: a single-blinded, randomized, and controlled trial. Arch Dermatol. 2005;141:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Phillips RC, Ruhl TS, Pfenninger JL, Garber MR. Treatment of warts with Candida antigen injection. Arch Dermatol. 2000;136:1274-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Majid I, Imran S. Immunotherapy with intralesional Candida albicans antigen in resistant or recurrent warts: a study. Indian J Dermatol. 2013;58:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Alikhan A, Griffin JR, Newman CC. Use of Candida antigen injections for the treatment of verruca vulgaris: A two-year mayo clinic experience. J Dermatolog Treat. 2016;27:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, Aguilera Peirò P, Baltà Cruz S, Hernández Ruiz ME, Baselga Torres E. Intralesional Candida Antigen Immunotherapy for the Treatment of Recalcitrant and Multiple Warts in Children. Pediatr Dermatol. 2015;32:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Maronn M, Salm C, Lyon V, Galbraith S. One-year experience with candida antigen immunotherapy for warts and molluscum. Pediatr Dermatol. 2008;25:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | McBride AA. Human papillomaviruses: diversity, infection and host interactions. Nat Rev Microbiol. 2022;20:95-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 212] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 19. | Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Sarier M, Ozel E, Duman I, Yuksel Y, Demirbas A. HPV type 45-positive condyloma acuminata of the bladder in a renal transplant recipient. Transpl Infect Dis. 2017;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Maor D, Brennand S, Goh MSY, Chong AH. Recalcitrant hyperkeratotic verrucae in a renal transplant recipient clearing with cessation of immunosuppression. JAAD Case Rep. 2018;4:471-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Ash MM, Jolly PS. A Case Report of the Resolution of Multiple Recalcitrant Verrucae in a Renal Transplant Recipient After a Mycophenolate Mofetil Dose Reduction. Transplant Proc. 2017;49:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Nguyen L, McClellan RB, Chaudhuri A, Alexander SR, Chen SF, Concepcion W, Grimm P. Conversion from tacrolimus/mycophenolic acid to tacrolimus/Leflunomide to treat cutaneous warts in a series of four pediatric renal allograft recipients. Transplantation. 2012;94:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Dharancy S, Catteau B, Mortier L, Boleslawski E, Declerck N, Canva V, Piette F, Mathurin P, Pruvot FR. Conversion to sirolimus: a useful strategy for recalcitrant cutaneous viral warts in liver transplant recipient. Liver Transpl. 2006;12:1883-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Shahidi S, Moeinzadeh F, Mohammadi M, Gholamrezaei A. Sirolimus-based immunosuppression for treatment of cutaneous warts in kidney transplant recipients. Iran J Kidney Dis. 2011;5:351-353. [PubMed] |
| 26. | Kawashima S, Joachim K, Abdelrahim M, Abudayyeh A, Jhaveri KD, Murakami N. Immune checkpoint inhibitors for solid organ transplant recipients: clinical updates. Korean J Transplant. 2022;36:82-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 27. | Park S, Guo K, Heilman RL, Poggio ED, Taber DJ, Marsh CL, Kurian SM, Kleiboeker S, Weems J, Holman J, Zhao L, Sinha R, Brietigam S, Rebello C, Abecassis MM, Friedewald JJ. Combining Blood Gene Expression and Cellfree DNA to Diagnose Subclinical Rejection in Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2021;16:1539-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 28. | Dandamudi R, Gu H, Goss CW, Walther L, Dharnidharka VR. Longitudinal Evaluation of Donor-Derived Cellfree DNA in Pediatric Kidney Transplantation. Clin J Am Soc Nephrol. 2022;17:1646-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |