Published online Sep 18, 2022. doi: 10.5500/wjt.v12.i9.299
Peer-review started: April 29, 2022
First decision: May 12, 2022
Revised: June 1, 2022
Accepted: September 8, 2022
Article in press: September 8, 2022
Published online: September 18, 2022
Processing time: 136 Days and 16.3 Hours
Vitamin D deficiency occurs in more than 80% of kidney transplant recipients. Its immunomodulatory effects can predispose transplant recipients to rejection and chronic allograft nephropathy (CAN). This study determined the association be
To determine the relationship between serum 25 (OH) vitamin D level and bio
Adult renal transplant recipients followed at the clinic between January 2013 and 2018 were included. Recipients requiring graft biopsy due to declined function, hematuria, and proteinuria were reviewed. The two groups were compared re
Fifty-two recipients who underwent graft biopsy met the inclusion criteria. In all, 14 recipients had a vitamin D level > 15 ng/mL (group 1) vs ≤ 15 ng/mL (group 2) in 38. In total, 27 patients had biopsy-proven rejection, and 19 had CAN. There was only 1 recipient with biopsy-proven rejection in group 1, whereas there were 24 patients with rejection in group 2. The rejection rate was significantly higher in group 2 than in group 1 (P < 0.001). Four patients were diagnosed with CAN in group 1 vs fifteen in group 2. There was no significant difference in the CAN rate between the two groups. PTH was higher at the time of graft biopsy (P = 0.009, P = 0.022) in group 1 with a mean of 268 pg/mL. Donor-specific antibodies were detected in 14 (56.0%) of the re
The serum 25 (OH) vitamin D level in kidney transplant recipients remained low. Although low serum vitamin D level emerged as a risk factor for rejection in univariate analysis, this finding was not confirmed by multivariate analysis. Prospective studies are required to determine the effect of serum vitamin D levels on allograft rejection.
Core Tip: This study analyzed the results of 130 kidney transplant recipients. Of the 52 recipients who underwent graft biopsy and met the study inclusion criteria, 14 had a vitamin D level > 15 ng/mL vs ≤ 15 ng/mL in 38. Although low serum vitamin D level emerged as a risk factor for rejection in univariate analysis, this finding was not confirmed by multivariate analysis. Nonetheless, diagnostic and predictive accuracy is limited when a single test is used, and larger-scale prospective clinical studies are needed to clearly discern the effects of serum vitamin D level on the renal allograft rejection rate.
- Citation: Buyukdemirci S, Oguz EG, Cimen SG, Sahin H, Cimen S, Ayli MD. Vitamin D deficiency may predispose patients to increased risk of kidney transplant rejection. World J Transplant 2022; 12(9): 299-309
- URL: https://www.wjgnet.com/2220-3230/full/v12/i9/299.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i9.299
Kidney transplantation is the best treatment option for patients with terminal kidney failure. Successful transplantation prolongs longevity and significantly improves quality of life. In addition, following kidney transplantation, 75% of recipients return to work, and approximately 1 in 50 females can get pregnant[1]. For recipients to experience these benefits, close follow-up and optimization of modifiable risk factors are crucial. One of the modifiable risk factors is the serum vitamin D level[2].
It is known that 25 (OH) vitamin D plays a significant role in calcium and phosphate balance. Furthermore, a low vitamin D level can have deleterious effects on renal allografts[3,4]. A large prospective clinical study on kidney transplant recipients reported that a low 25 (OH) vitamin D level was associated with a reduced glomerular filtration rate (GFR) at 9 mo post-transplantation[5]. More
The immunomodulatory features of vitamin D have been observed in autoimmune diseases such as psoriasis and rheumatoid arthritis and in experimental transplant models showing that vitamin D analogs amplified cyclosporin A’s inhibitory effects on acute and chronic allograft rejection[8,9]. Like
This single-center retrospective cohort study was performed at the Health Sciences University of Turkey, Diskapi Research and Training Hospital, Department of Nephrology and Transplantation, Ankara, Turkey. All adult renal transplant recipients followed at the transplant clinic between January 2013, and July 2018 were reviewed. Among these patients, recipients requiring allograft biopsy due to progressive graft function decline, new-onset hematuria, and proteinuria were included in the study.
Allograft biopsies were performed as per Kidney Disease Improving Global Outcomes (KDIGO) practice guidelines[11]. Banff 97 criteria were used to evaluate biopsy specimens[12]. Biopsy specimens were considered adequate if they had ≥ 10 glomeruli and two arteries; patients with inadequate biopsy specimens were excluded from the study. Additionally, patients with post-transplant follow-up < 1 year were excluded from the study to establish a homogeneous cohort. The serum vitamin D level was mea
Demographic characteristics, medical history, prior type and duration of dialysis, donor type, human leukocyte antigen (HLA) mismatches, maintenance immunosuppression, biopsy results, and serum vitamin D level at the time of graft biopsy were obtained from hospital records by a research nurse. In addition, as this study determined the relationship between serum vitamin D level and allograft biopsy results, other biochemical parameters associated with rejection and CAN, such as the GFR, and serum creatinine, albumin, calcium, phosphate, and parathyroid hormone (PTH) levels at the time of graft biopsy, were also recorded. The study protocol was approved by the hospital’s ethical review committee (06.08.2018-no. 53/20) and was carried out in accordance with the Declaration of Helsinki and the Declaration of Istanbul. All patients provided written informed consent.
Recipients of live donor kidneys were induced with interleukin 2 receptor blockers and steroids, whereas recipients of deceased donor kidneys were induced with anti-thymocyte globulin and steroids. Maintenance immunosuppression was based on mycophenolate mofetil (MMF), prednisone, and calc
The serum vitamin D level was measured using the chemiluminescence method (Kit No: A98856; Beckman Coulter Inc., Sykesville, MD, United States). A serum vitamin D level > 30 ng/mL (i.e., > 75 nmol/L) was considered adequate. Concentrations between 15 and 30 ng/mL (40-75 nmol/L) were considered vitamin D insufficiency, whereas < 15 ng/ mL (< 37.5 nmol/L) was considered vitamin D deficiency according to KDIGO guidelines[13].
The serum PTH concentration was measured via immunochemiluminescent assay (Kit No: A16972; Beckman Coulter). Total calcium, phosphate, glucose, blood cell count, albumin, uric acid, total chol
Data were analyzed using IBM SPSS Statistics for Windows v.22.0 (IBM Corp., Armonk, NY, United States). The distribution of data was analyzed using the Kolmogorov-Smirnov test. Mean ± SD was used for descriptive analysis of parametric quantitative data, whereas number and percentage were used to analyze the qualitative data. The student’s t-test was used for parametric data analysis, and the Mann-Whitney U test was used for non-parametric data analysis. Pearson’s chi-square test was used to analyze qualitative data. The level of statistical significance was set at P < 0.05. Binary logistic regression analysis was used to determine the independent factors related to rejection. After excluding multicollinear variables, clinically relevant variables and parameters presenting statistical significance were subject to the binary logistic regression analysis. The odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were used to show the factors affecting the outcomes.
Among 130 kidney transplant recipients, 52 met the study inclusion criteria. The mean age of the recipients was 41 ± 11.9 years, of which 38 (73.1%) were male and 14 (26.9%) were female. During the post-transplantation period, 25 (48.1%) patients had hypertension and 15 (28.8%) had diabetes mellitus. Pre-transplantation duration of dialysis was 5.8 ± 4.71 years, and hemodialysis was the most common therapy (82.7%). The majority (65.4%) of the study population received live donor kidney transplants, of which 3 (5.8%) were transplanted preemptively. Of the 34 live donors, 20 were spousal donations, 10 were first-degree relatives, and 4 were second-degree relatives.
The average age of the donors was 49.6 ± 9.7 years, and the majority of them were 29 (55.8%) male. The mean post-transplant duration of follow-up was 5.91 ± 1.83 years. The mean number of HLA mismatches was 3 ± 1. Delayed graft function developed in 9 (17.6%) patients. Fourteen (27.5%) patients were donor-specific antibody (DSA)-positive at the time of renal biopsy. Kidney failure had occurred due to hypertension in 25 (48.1%), diabetes mellitus in 15 (28.8%), glomerulonephritis in 7 (13.5%), post-renal kidney disease in 3 (5.8), and unknown reasons in 2 (3.8%) of the recipients (Table 1).
| Parameter | Patients, n = 52 |
| Mean age, yr | 41 ± 11.9 |
| Male, n (%)/female, n (%) | 38 (73.1)/14 (26.9) |
| DM, n (%)/HT, n (%) | 15 (28.8)/25 (48.1) |
| Hemodialysis, n (%)/peritoneal dialysis, n (%) | 43 (82.7)/6 (11.5) |
| Mean dialysis duration, yr | 5.8 ± 4.71 |
| Pre-emptive, n (%) | 3 (5.8) |
| Donor type: Living, n (%)/Cadaver, n (%) | 34 (65.4)/18 (34.6) |
| Donor sex: Male/female | 29 (55.8)/23 (44.2) |
| Donor age in yr | 49.6 ± 9.7 |
| Time since transplantation, yr | 5.91 ± 1.83 |
| Number of HLA mismatches | 3 ± 1 |
| DGF, n (%) | 9 (17.6) |
| DSA, n (%) | 14 (27.5) |
| Cyclosporine/tacrolimus serum levels, ng/mL | 545 ± 89/4.8 ± 0.8 |
| MMF, gr/d | 1.7 ± 0.3 |
| Pre-transplant kidney failure etiology | |
| DM, n (%) | 15 (28.8) |
| HT, n (%) | 25 (48.1) |
| Glomerulonephritis, n (%) | 7 (13.5) |
| Post-renal kidney failure, n (%) | 3 (5.8) |
| Unknown, n (%) | 2 (3.8) |
Maintenance immunosuppressive regimens at the time of graft biopsy were as follows: 38 (73.4%) patients were on a combination of MMF, tacrolimus, and prednisone, whereas 11 (20.9%) were receiving a combination of MMF, cyclosporine, and prednisone. Only 3 (5.7%) of the recipients used mechanistic target of rapamycin inhibitor-based regimens. At the time of allograft biopsy, the average serum trough calcineurin level was 4.8 ± 0.8, cyclosporine serum level ng/mL was 545 ± 89, and the mean daily intake of MMF was 1.7 ± 0.3 gr/d. Within the study cohort 20 patients were receiving vitamin D treatments according to the KDIGO guidelines. Among the 52 allograft biopsies, 25 (48%) showed rejection. Acute T cell-mediated rejection, acute antibody-mediated rejection (ABMR), and chronic active ABMR were observed in 6 (11.5%), 10 (19.2%), and 9 (17.3%) of the recipients, respectively. CAN was noted in 19 (36.5%) of the recipients. Calcineurin toxicity was observed in 3 (5.8%) patients, whereas BK virus nephropathy and recurrent nephritis were noted in 4 (7.7%) and 1 (1.9%), respectively.
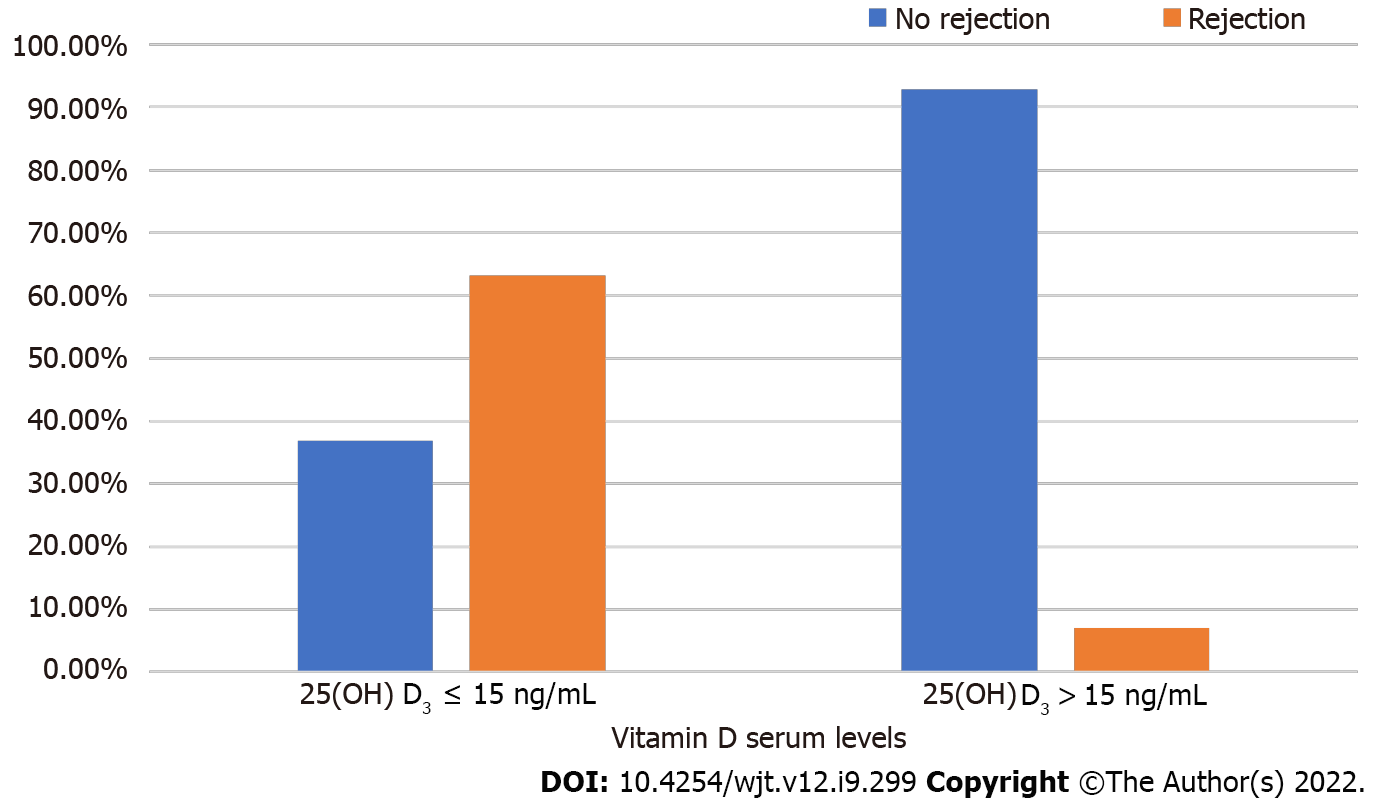
The study population was divided into two groups based on the serum vitamin D level (Table 2). Patients with a vitamin D level > 15 ng/mL constituted group 1, and those with a level ≤ 15 ng/mL constituted group 2. The two groups were compared concerning graft function, HLA mismatches, biochemical parameters, GFR, and rejection status. Group 1 included 14 (27%) patients, and group 2 included 38 (73%). There were no significant differences concerning age, comorbidities, or HLA mismatches between the groups (P > 0.05). Males were predominant in group 2 (P = 0.035). Four (28.6%) recipients in group 1 and 15 (39.5%) recipients in group 2 were diagnosed with CAN. There was no significant difference in the CAN rate between the two groups (P > 0.05). Only 1 (7.1%) recipient was diagnosed with rejection in group 1 and 24 (63.2%) recipients in group 2. The biopsy-proven rejection rate was significantly higher in group 2 compared to group 1 (P < 0.001) (Figure 1).
| Vitamin D level | Group 1 (> 15 ng/mL), n = 14 | Group 2 (≤ 15 ng/mL), n = 38 | P value |
| Age, yr | 40 ± 11.9 | 41 ± 12.0 | 0.856 |
| Male, n (%) | 7 (50) | 31 (81.6) | 0.035 |
| DM/HT, n (%) | 2 (14.3)/6 (42.9) | 13 (34.2)/19 (50) | 0.300/0.759 |
| Hemodialysis/peritoneal dialysis | 12 (92.3)/1 (7.7) | 31 (86.1)/5 (13.9) | 1.00 |
| Mean dialysis duration, yr | 5.9 ± 4.5 | 5.6 ± 3.7 | 0.839 |
| Preemptive, n (%) | 1 (7.1) | 2 (5.3) | 1.00 |
| Rejection, n (%) | 1 (7.1) | 24 (63.2) | < 0.001 |
| CAN, n (%) | 4 (28.6) | 15 (39.5) | 0.534 |
| Number of HLA mismatches | 3 ± 1 | 3 ± 1 | 1.00 |
| ESRD actual, n (%) | 7 (58.3) | 10 (27) | 0.80 |
| Hemoglobin, g/dL | 11.5 ± 2.0 | 10.7 ± 2.4 | 0.266 |
| Glucose, mg/dL | 106 ± 60.7 | 98 ± 33.9 | 0.433 |
| Albumin, g/dL | 4.0 ± 0.4 | 3.7 ± 0.6 | 0.063 |
| Uric acid, mg/dL | 7.1 ± 1.8 | 7.7 ± 1.5 | 0.276 |
| Urea, mg/dL | 68 ± 35.3 | 77 ± 38.6 | 0.416 |
| Creatinine, mg/dL | 2.08 ± 0.61 | 2.21 ± 1.22 | 0.702 |
| eGFR, mL/min/1.73 m2 | 38 ± 18.3 | 41 ± 19.7 | 0.609 |
| Proteinuria, g/d | 1.0 ± 0.9 | 2.5 ± 3.1 | 0.261 |
| Cholesterol, mg/dL | 186 ± 36.9 | 177 ± 46.2 | 0.515 |
| Triglyceride, mg/dL | 178 ± 82.9 | 191 ± 110.1 | 0.877 |
| Calcium, mg/dL | 8.9 ± 0.99 | 8.7 ± 0.80 | 0.400 |
| Phosphorus, mg/dL | 4.8 ± 1.84 | 4.5 ± 1.86 | 0.657 |
| PTH, pg/mL (range) | 205 (78-927) | 268 (59-955) | 0.007 |
| CRP, mg/dL | 24 ± 48.2 | 21 ± 29.9 | 0.483 |
The estimated GFR (eGFR) was 38 ± 18.3 in group 1 and 41 ± 19.7 in group 2. There was no significant difference between these groups regarding eGFR (P > 0.05). In addition, hemoglobin, serum glucose, albumin, CRP, calcium, phosphate, uric acid, total cholesterol, triglyceride, blood urea nitrogen, and creatinine did not significantly differ between the two groups (P > 0.05). The mean PTH level was 205 pg/mL in group 1 and 268 pg/mL in group 2. PTH level was higher in group 2 than in group 1 (P = 0.007).
The study cohort was also divided into two groups based on the presence or absence of biopsy-proven rejection (Table 3). The mean age was 39 ± 12.9 in the rejection group and 42 ± 10.9 in the no-rejection group. In the rejection group females were predominant [22 (88%) vs 16 (59.3%); P = 0.020]. The comorbid status, previous dialysis vintage, and donor characteristics did not differ between these two groups (P > 0.05). Hemoglobin, glucose, CRP, calcium, uric acid, lipid profile, and the number of HLA mismatches did not differ between groups (P > 0.05). Nevertheless, there were significant differences in the serum albumin, phosphorus, PTH, vitamin D, and DSA levels. The albumin was 4.0 ± 0.5 g/dL in the no-rejection group vs 3.5 ± 0.6 g/dL in the rejection group (P = 0.001). Phosphorus, PTH, and vitamin D levels in the no-rejection group were 3.9 ± 1.52 mg/dL, 197 pg/mL, and 17.4 ± 7.2 ng/mL, respectively. The results of these parameters in the rejection group were 5.3 ± 1.96 mg/dL for phosphorus, 310 pg/mL for PTH, and 9.7 ± 3.4 ng/dL for vitamin D serum levels. The P values of these comparisons showed a statistically significant difference between the two groups (P = 0.009, P = 0.022, and P = 0.003, respectively). DSA positivity was present in 14 (56%) of those with rejection (56%), whereas no patients in the non-rejection group had DSA positivity (P < 0.001). There was no significant difference between the two groups regarding serum cutaneous neurogenic inflammation levels and daily MMF dose (P > 0.05). Kidney failure with a GFR < 15 mL/min was observed in 5 (18.5%) patients in the non-rejection group and 12 (48%) in the rejection group. The kidney failure rate was significantly higher in the rejection group (P = 0.024); patients in the rejection group had lower GFRs and higher serum creatinine levels (P = 0.012 and P = 0.016, respectively). The serum vitamin D level was significantly lower, and the PTH level was significantly higher in the rejection group than in the non-rejection group (P = 0.003 and P = 0.022). A regression analysis was performed using rejection risk factors (Table 4). In univariate regression analysis, female sex, serum vitamin D level, phosphorus, and albumin were found to be effective in the development of rejection (P = 0.027, P = 0.007, P = 0.023, P = 0.008). However, these risk factors did not demonstrate a significant effect (P > 0.05).
| Rejection | No | Yes | P value |
| Patients, n | 27 | 25 | |
| Mean age, yr | 42 ± 10.9 | 39 ± 12.9 | 0.316 |
| Female, n (%) | 16 (59.3) | 22 (88.0) | 0.020 |
| DM, n (%)/HT, n (%) | 7 (25.9)/13 (48) | 8 (32.0)/12 (48) | 0.629/0.991 |
| Donor type Cadaver, n (%) | 7 (25.9) | 11 (44.0) | 0.171 |
| Donor age, yr | 47.7 ± 9.6 | 51.8 ± 9.6 | 0.133 |
| Time since transplantation, yr | 4.4 ± 1.4 | 5.3 ± 3.1 | 0.236 |
| Number of HLA mismatches | 2.2 ± 1.2 | 2.6 ± 1.2 | 0.263 |
| DSA, n (%) | 0 | 14 (56.0) | < 0.001 |
| Cyclosporine/tacrolimus serum levels, ng/mL | 576 ± 98/4.7 ± 0.9 | 490 ± 29/4.9 ± 0.7 | 0.063/0.352 |
| MMF, gr/d | 1.7 ± 0.3 | 1.7 ± 0.3 | 0.601 |
| ESRD actual, n (%) | 5 (18.5) | 12 (48) | 0.024 |
| Hemoglobin, g/dL | 11.5 ± 2.2 | 10.4 ± 2.3 | 0.095 |
| Glucose, mg/dL | 95 ± 37.7 | 107 ± 46.7 | 0.399 |
| Albumin, g/dL | 4.0 ± 0.5 | 3.5 ± 0.6 | 0.001 |
| Uric acid, mg/dL | 7.3 ± 1.6 | 7.7 ± 1.6 | 0.364 |
| Creatinine, mg/dL | 1.78 ± 0.44 | 2.59 ± 1.40 | 0.016 |
| eGFR, mL/min/1.73 m² | 45 ± 19.3 | 36 ± 18.3 | 0.012 |
| Cholesterol, mg/dL | 177 ± 37.2 | 181 ± 49.8 | 0.810 |
| Triglyceride, mg/dL | 180 ± 103.4 | 196 ± 104.1 | 0.379 |
| Calcium, mg/dL | 8.8 ± 0.79 | 8.7 ± 0.92 | 0.562 |
| Phosphorus, mg/dL | 3.9 ± 1.51 | 5.3 ± 1.96 | 0.009 |
| PTH, pg/mL (range) | 197 (59-440) | 310 (106-955) | 0.022 |
| Vitamin D, ng/mL | 14.7 ± 7.2 | 9.7 ± 3.4 | 0.003 |
| CRP, mg/mL | 20 ± 24.9 | 23 ± 43.2 | 0.05 |
| Univariate regressions | Multivariate regression | |||||||
| B | OR | 95%CI | P value | B | OR | 95%CI | P value | |
| Age | -0.02 | 0.97 | 0.93-1.02 | 0.381 | -0.04 | 0.95 | 0.87-1.03 | 0.265 |
| Sex, female | 1.61 | 5.04 | 1.20-21.06 | 0.027 | 1.12 | 3.08 | 0.31-30.45 | 0.336 |
| Donor type Cadaver, n (%) | 0.46 | 1.58 | 0.50-5.00 | 0.434 | 1.52 | 4.60 | 0.71-29.77 | 0.109 |
| Donor sex, female, n (%) | -0.33 | 0.71 | 0.23-2.15 | 0.555 | 0.40 | 1.50 | 0.26-8.38 | 0.643 |
| Donor age, yr | 0.03 | 1.04 | 0.98-1.10 | 0.192 | 0.40 | 1.04 | 0.95-1.13 | 0.340 |
| DGF, n (%) | -0.22 | 0.80 | 0.18-3.40 | 0.763 | -1.41 | 0.24 | 0.01-4.36 | 0.337 |
| MMF, gr/d | 0.78 | 2.19 | 0.69-6.97 | 0.183 | 0.84 | 2.32 | 0.33-16.37 | 0.397 |
| Serum fosfor (mg/dL) | 0.43 | 1.53 | 1.06-2.22 | 0.023 | 0.63 | 1.87 | 1.01-3.48 | 0.05 |
| Vitamin D, ng/mL | -0.153 | 0.85 | 0.76-0.96 | 0.007 | -0.12 | 0.88 | 0.72-1.06 | 0.196 |
| PTH | 0.01 | 1.00 | 1.00-1.01 | 0.052 | 0.01 | 1.00 | 0.99-1.00 | 0.516 |
| Albumin (g/dL) | -1.49 | 0.22 | 0.07-0.68 | 0.008 | -1.17 | 0.30 | 0.05-1.69 | 0.177 |
| CSA serum level, ng/mL | -0.01 | 0.99 | 0.99-1.00 | 0.265 | 0.01 | 1.00 | 0.99-1.00 | 0.983 |
| TAC serum level, ng/mL | 0.167 | 1.18 | 0.92-1.51 | 0.189 | 0.02 | 1.01 | 0.54-1.89 | 0.955 |
Vitamin D deficiency is associated with a broad spectrum of diseases, including autoimmune conditions such as inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and type 1 diabetes. In addition, vitamin D deficiency is associated with a severe decrease in the GFR and shorter life expectancy in patients with chronic kidney diseases[14-16].
Epidemiological studies conducted with kidney transplant recipients reported that the prevalence of vitamin D deficiency is as high as 90%, possibly due to the side effects of immunosuppressive regimens and a reduction in sun exposure related to the recommendation that these patients avoid sunlight[2,3,17,18]. Falkiewicz et al[19] reported severe 1.25-dihydroxyvitamin D deficiency in 83% of kidney transplant recipients and that these patients had a high graft failure rate, which is in agreement with the present finding that the mean serum vitamin D level was 12.3 ± 6.2 ng/mL, indicating severe vitamin D deficiency. Findings regarding the relationship between vitamin D and organ rejection are inconsistent. For example, Zimmerman et al[5] reported no relationship between the vitamin D level and acute allograft rejection. By contrast, Kim et al[20] who conducted a prospective clinical trial that considered 25 nmol/L as the threshold for vitamin D deficiency, observed a correlation between a low vitamin D level and the acute rejection rate. Similarly, Lee et al[21] reported that kidney transplant recipients with a vitamin D level < 50 nmol/L within 30 d of transplantation had a higher risk of acute rejection during the 1st year post-transplant. Additionally, Bienaimé et al[22] showed that vitamin D deficiency led to interstitial fibrosis and tubular atrophy within the kidney parenchyma in kidney transplant recipients.
Vitamin D deficiency is associated with glomerular disease in native and transplanted kidneys, and this finding has been attributed to endothelial cell dysfunction. Therefore, it was proposed that a low serum vitamin D level and an elevated fibroblast growth factor-23 level hinder endothelial cell function and lead to endothelial injury[23-25]. Although normal endothelium expresses major histocompatibility complex (MHC) class I antigens only, in endothelial injury and inflammation cases, MHC class II antigens are also expressed on the cell surface. These MHC class II antigens increase the recruitment and adhesion of CD4+ T cells and initiate allorecognition. Alloantigen recognition subsequently triggers the production of inflammatory mediators and activates the complement cascade[26-28]. The present study could not evaluate endothelial dysfunction or MHC class II antigen expression due to its retrospective design; however, a correlation between a low serum vitamin D level and the kidney rejection rate was observed (P < 0.001).
On the other hand, as graft rejection and CAN share some immunological pathways, we suggest that the serum vitamin D level might play a role in CAN risk[29]. To the best of our knowledge, the present study is the first to examine the relationship between vitamin D deficiency and CAN. In the present study, the CAN rate did not differ according to the vitamin D level (P = 0.534).
The present findings indicate that the long-term graft survival rate remains moderate, even with meticulous management of risk factors, including vitamin D replacement. In this study, patients with rejection had higher phosphorus and PTH measurements at the time of graft biopsy (P = 0.009, P = 0.022), and vitamin D and albumin levels were significantly lower in this group (P = 0.003, P = 0.001). Univariate regression analysis elucidated that female sex, serum vitamin D, phosphorus, and albumin were significant risk factors affecting rejection. However, in the multivariate regression analysis, these risk factors did not affect the rejection status (P > 0.05).
The present study had some limitations, including a retrospective single-center design; the retrospective design might have led to selection and recall biases, and its single-center nature precludes generalization of the findings. In addition, the study population was small and might have been insufficient for establishing the existence of cause and effect relations.
In conclusion, the serum 25 (OH) vitamin D level of kidney transplant recipients remained low despite vitamin D replacement recommended by KDIGO guidelines. However, the multivariate regression analysis did not find the same variables effective on rejection. Nonetheless, diagnostic and predictive accuracy is limited when a single test is used, and larger-scale prospective clinical studies are needed to more clearly discern the effects of the serum vitamin D level on the renal allograft rejection rate.
Vitamin D deficiency is commonly diagnosed in patients with kidney transplantation. Deficiency rate remains high despite replacement therapies as per the Kidney Disease Improving Global Outcomes guidelines.
Vitamin D has immunomodulatory effects and vitamin D receptors can be found in various types of cells including T cells and dendritic cells. Its deficiency may predispose transplant recipients to rejection and chronic allograft nephropathy (CAN).
This study determined the association between the serum 25 (OH) vitamin D, biopsy-proven allograft rejection, and CAN rates.
Retrospective clinical study involving adult kidney transplant recipients requiring graft biopsy due to declined function, hematuria, and proteinuria.
Vitamin D level was 9.7 ± 3.4 ng/mL in the rejection group vs 14.7 ± 7.2 in the non-rejection group; this difference was statistically significant (P = 0.003). In univariate regression analysis of risk factors affecting rejection, sex, serum vitamin D, phosphorus and albumin were found to have impact (P = 0.027, P = 0.007, P = 0.023, P = 0.008). In multivariate regression analysis, the same factors did not affect rejection.
The serum 25 (OH) vitamin D level in kidney transplant recipients remained low. Although low serum vitamin D level emerged as a risk factor for rejection in univariate analysis, this finding was not confirmed by multivariate analysis. Prospective studies are required to appreciate the effect of serum vitamin D levels on allograft rejection.
Kidney transplantation is the best treatment option for patients with terminal kidney failure. Successful transplantation prolongs longevity and significantly improves the quality of life. However, the long term success of kidney transplantation depends on preventing the chronic allograft dysfunction. Chr
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Shamseldeen AA, Egypt; Sureshkumar KK, United States S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | van Walraven C, Austin PC, Knoll G. Predicting potential survival benefit of renal transplantation in patients with chronic kidney disease. CMAJ. 2010;182:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Querings K, Girndt M, Geisel J, Georg T, Tilgen W, Reichrath J. 25-hydroxyvitamin D deficiency in renal transplant recipients. J Clin Endocrinol Metab. 2006;91:526-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Stavroulopoulos A, Cassidy MJ, Porter CJ, Hosking DJ, Roe SD. Vitamin D status in renal transplant recipients. Am J Transplant. 2007;7:2546-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Keyzer CA, Riphagen IJ, Joosten MM, Navis G, Muller Kobold AC, Kema IP, Bakker SJ, de Borst MH; NIGRAM consortium. Associations of 25(OH) and 1,25(OH)2 vitamin D with long-term outcomes in stable renal transplant recipients. J Clin Endocrinol Metab. 2015;100:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Zimmerman D, House AA, Kim SJ, Booth RA, Zhang T, Ramsay T, Knoll G. The Risk of Acute Rejection Following Kidney Transplant by 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D Status: A Prospective Cohort Study. Can J Kidney Health Dis. 2017;4:2054358117699822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9399] [Cited by in RCA: 9398] [Article Influence: 522.1] [Reference Citation Analysis (1)] |
| 7. | Rech MA, Fleming JN, Moore CL. 25-hydroxyvitamin D deficiency and opportunistic viral infections after kidney transplant. Exp Clin Transplant. 2014;12:95-100. [PubMed] |
| 8. | Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Redaelli CA, Wagner M, Günter-Duwe D, Tian YH, Stahel PF, Mazzucchelli L, Schmid RA, Schilling MK. 1α,25-Dihydroxyvitamin D3 shows strong and additive immunomodulatory effects with cyclosporine A in rat renal allotransplants. Kidney Int. 2002;61:288-296. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Li F, Liu P, Zhang X, Zhang Q, Tang S, Zhu M, Qiu M. 1,25(OH)2D3-mediated amelioration of aortic injury in streptozotocin-induced diabetic rats. Inflammation. 2013;36:1334-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9 Suppl 3:S1-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 1086] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 12. | Cimen S, Geldenhuys L, Guler S, Imamoglu A, Molinari M. Impact of specimen adequacy on the assessment of renal allograft biopsy specimens. Braz J Med Biol Res. 2016;49:e5301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;S1-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 1069] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 14. | Wöbke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol. 2014;5:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832-2838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1248] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 16. | González EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol. 2004;24:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 291] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 17. | Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 736] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 18. | Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1106] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 19. | Falkiewicz K, Boratynska M, Speichert-Bidzińska B, Magott-Procelewska M, Biecek P, Patrzalek D, Klinger M. 1,25 Dihydroxyvitamin D deficiency posttransplant predicts poorer outcome after renal transplantation. Transplant Proc. 2009;41:3002-3005. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Kim H, Kang SW, Yoo TH, Kim MS, Kim SI, Kim YS, Choi KH. The impact of pretransplant 25-hydroxy vitamin D deficiency on subsequent graft function: an observational study. BMC Nephrol. 2012;13:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Lee JR, Dadhania D, August P, Lee JB, Suthanthiran M, Muthukumar T. Circulating levels of 25-hydroxyvitamin D and acute cellular rejection in kidney allograft recipients. Transplantation. 2014;98:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Bienaimé F, Girard D, Anglicheau D, Canaud G, Souberbielle JC, Kreis H, Noël LH, Friedlander G, Elie C, Legendre C, Prié D. Vitamin D status and outcomes after renal transplantation. J Am Soc Nephrol. 2013;24:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Al-Ishaq RK, Kubatka P, Brozmanova M, Gazdikova K, Caprnda M, Büsselberg D. Health implication of vitamin D on the cardiovascular and the renal system. Arch Physiol Biochem. 2021;127:195-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Yildirim T, Yilmaz R, Altindal M, Turkmen E, Arici M, Altun B, Erdem Y. Endothelial dysfunction in renal transplant recipients: role of vitamin D and fibroblast growth factor-23. Transplant Proc. 2015;47:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Silswal N, Touchberry CD, Daniel DR, McCarthy DL, Zhang S, Andresen J, Stubbs JR, Wacker MJ. FGF23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am J Physiol Endocrinol Metab. 2014;307:E426-E436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 26. | Sudfeld CR, Giovannucci EL, Isanaka S, Aboud S, Mugusi FM, Wang M, Chalamilla G, Fawzi WW. Vitamin D status and incidence of pulmonary tuberculosis, opportunistic infections, and wasting among HIV-infected Tanzanian adults initiating antiretroviral therapy. J Infect Dis. 2013;207:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Ban TH, Kim JH, Jang HB, Lee YS, Choi BS, Park CW, Yang CW, Kim YS, Chung BH. Clinical effects of pre-transplant serum 25-hydroxyvitamin D level on post-transplant immunologic and non-immunologic outcomes in kidney transplant recipients. Transpl Immunol. 2017;40:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Westhorpe CLV, Norman MU, Hall P, Snelgrove SL, Finsterbusch M, Li A, Lo C, Tan ZH, Li S, Nilsson SK, Kitching AR, Hickey MJ. Effector CD4+ T cells recognize intravascular antigen presented by patrolling monocytes. Nat Commun. 2018;9:747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1480] [Article Influence: 67.3] [Reference Citation Analysis (0)] |