Published online Jul 18, 2022. doi: 10.5500/wjt.v12.i7.195
Peer-review started: June 28, 2021
First decision: July 14, 2021
Revised: August 4, 2022
Accepted: June 20, 2022
Article in press: June 20, 2022
Published online: July 18, 2022
Processing time: 381 Days and 5.6 Hours
Enhanced recovery after surgery (ERAS) started a revolution that changed age-old surgical stereotypical practices regarding the overall management of the surgical patient. In the last decade, ERAS has gained significant acceptance in the community of general surgery, in addition to several other surgical specialties, as the evidence of its advantages continues to grow. One of the last remaining fields, given its significant complexity and intricate nature, is liver transplantation (LT).
To investigate the existing efforts at implementing ERAS in LT.
We conducted a systematic review of the existing studies that evaluate ERAS in orthotopic LT, with a multimodal approach and focusing on measurable clinical primary endpoints, namely length of hospital stay.
All studies demonstrated a considerable decrease in length of hospital stay, with no readmission or negative impact of the ERAS protocol applied to the postoperative course.
ERAS is a well-validated multimodal approach for almost all types of surgical procedures, and its future in selected LT patients seems promising, as the preliminary results advocate for the safety and efficacy of ERAS in the field of LT.
Core Tip: Enhanced recovery after surgery (ERAS) is a multimodal perioperative care pathway designed to achieve early recovery for patients undergoing major surgery. The benefits of ERAS in liver transplantation seem promising, and further studies should be conducted to validate its application in properly selected patients.
- Citation: Katsanos G, Karakasi KE, Antoniadis N, Vasileiadou S, Kofinas A, Morsi-Yeroyannis A, Michailidou E, Goulis I, Sinakos E, Giouleme O, Oikonomou IM, Evlavis G, Tsakiris G, Massa E, Mouloudi E, Tsoulfas G. Enhanced recovery after surgery in liver transplantation: Challenges and feasibility. World J Transplant 2022; 12(7): 195-203
- URL: https://www.wjgnet.com/2220-3230/full/v12/i7/195.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i7.195
Enhanced recovery after surgery (ERAS) is a multimodal perioperative care pathway designed to achieve early recovery for patients undergoing major surgery[1]. Since its introduction in 1997 by Kehlet et al[2], initially destined for and subsequently established in colorectal surgery, the concept of ERAS was validated and has since evolved and spread to a multitude of surgical disciplines[3] including solid organ transplantation[4].
Although the concept of enhanced recovery was explored in liver transplantation (LT) before its official introduction by Kehlet et al[2] as early as 1990 in the form of early extubation yielding encouraging results[5], it was done so without the classic multimodal approach, focusing and highlighting on the importance of anesthesia management in these patients[6]. Over the years, independent studies have validated the significance and efficiency of other classic ERAS parameters such as preoperative nutrition, early mobilization, early feeding, and optimal analgesia of patients undergoing LT. Nevertheless, the medical literature is scarce in studies that combine all of the above parameters in a classic large-scale ERAS approach specific for LT. This narrative review paper will investigate existing efforts at implementing ERAS in LT, as well as try to identify the existing challenges and future potential developments in the field.
This review paper investigates existing efforts at implementing ERAS in LT and identifies the existing challenges and future potential developments in the field.
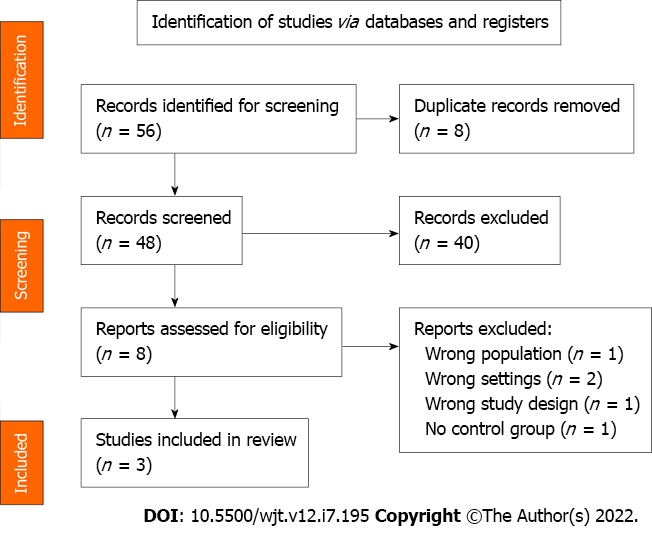
Our goal was to identify the existing studies that evaluate ERAS in orthotopic LT, with a multimodal approach and focusing on measurable clinical primary endpoints, namely length of hospital stay. Medline, Embase, OVID, and the Cochrane library were searched in the English language using the search terms (ERAS OR “enhanced recovery” OR “fast track” AND “liver transplantation”) from years 1990 to 2021 and after independent assessment from three reviewers, three articles were selected. PRISMA flow chart is presented in Figure 1.
There was a small number of studies identified, which were limited scale non-randomized single-center observational studies, with the exception of the work of Rao et al[7], who presented a prospective single-blinded randomized study including 128 patients divided in two groups: ERAS (n = 54) and control (n = 74). The ERAS group was analyzed by logistic stepwise regression analysis and displayed a decreased intensive care unit and hospital stay, without significant difference in the postoperative complication rate between the two groups and no readmissions or postoperative mortality during the follow-up period. Brustia et al[8] conducted a small-scale feasibility study with 10 patients treated prospectively with an ERAS protocol who were compared with 20 matched patients treated by the same team in previous years. They designed an elaborate 26-point ERAS protocol and observed a 47% reduction in the total length of stay compared to the control arm. There were no readmissions or postoperative mortality during the follow-up period.
Xu et al[9] reported a cohort of 93 patients, 40 in the ERAS group and 53 in the control group, and found a significant reduction of postoperative hospital stay in favor of the ERAS group (14.5 vs 16 d; P < 0.001). No difference in postoperative complication rate between the two groups and no readmissions or postoperative mortality were noted.
Common inclusion criteria used in the aforementioned studies are presented in Table 1. As expected, patients’ Model for End-Stage Liver Disease (MELD) scores were low in all four studies, as they reflect patient status[10]. All studies included patients with a MELD score well below 25. Patients with no previous history of LT were also selected for the ERAS group in all three studies. A considerable number of patients for ERAS LT had a hepatocellular carcinoma (HCC)-related indication in all three studies (Brustia 90%, Xu 42.5%, Rao 33.3%).
Given the lack of a standardized ERAS protocol, each team designed its own protocols, based on previous experience from existing literature on other surgical fields. Table 2 depicts a comparison of the preoperative, intraoperative and post-operative characteristics between the three studies. All of the studies applied multimodal measures in the three distinct phases of classic ERAS protocols: preoperative, intraoperative and postoperative phase. In Table 3, measures applied by all three authors are depicted in capital letters. Of the 26 points proposed by Brustia et al[8], 11 (42.3%) were observed by all three authors.
| Preoperative | Brustia et al[8] | Xu et al[9] | Rao et al[7] | |||
| ERAS group, n = 10 | CONTROL group, n = 20 | ERAS group, n = 40 | CONTROL group, n = 53 | ERAS group, n = 54 | CONTROL group, n = 74 | |
| Gender | ||||||
| Male | 8 | 17 | 35 | 46 | 40 | 58 |
| Female | 2 | 3 | 5 | 7 | 1 | 16 |
| Age, yr | 60.1 (52.5-66.1) | 58.2 (52.6-65.3) | 49.5 (40-56.8) | 53 (47-59) | 52.4 + 15.2 | 55.8 + 14.3 |
| Primary cause | ||||||
| Alcohol | 7 (70%) | 9 (45%) | 7 | 3 | 6 (11.1) | 10 (13.5) |
| Viral cirrhosis | 7 (70%) | 10 (50%) | 11 | 16 | 30 (55.6) | 40 (54.1) |
| HBV | 2 (20%) | 4 (20%) | NA | NA | NA | NA |
| HCV | 6 (60%) | 8 (40%) | NA | NA | NA | NA |
| Metabolic syndrome | 2 (20%) | 4 (20%) | NA | NA | NA | NA |
| Biliary disease | 0 | 3 (15%) | NA | NA | NA | NA |
| HCC | 9 (90%) | 9 (45%) | 17 | 24 | 18 (33.3) | 24 (32.4) |
| MELD score | 7 (6-10) | 7 (6-9) | 14 (9-22) | 17 (14-19) | 7.7 + 3.2 | 7.9 + 4.6 |
| Intraoperative | ||||||
| Operative time | 6.0 (5.9-8.4) h | 6.7 (5.7-8.2) h | 443.7 + 85.3min | 453.5 + 62.3min | 265 (215-360) min | 325 (275-455) min |
| Anhepatic period | NA | NA | 44.3 + 5.2 min | 42.7 + 4.2 min | 45 (35-70) min | 60 (50-75) min |
| Blood loss | NA | NA | 775 (525-1000) mL | 800 (600-1000) mL | 1100 (300-4200) mL | 2900 (1600-7000) mL |
| Hypothermia during the operation (n, %) | NA | NA | 0 | 12% | 0 | 0 |
| Postoperative | ||||||
| Early extubation (h) | 2 (0-2) | 7.5 (4.5-13.0) | 0 | 6 (5.5-8) | NA | NA |
| ICU stay (d) | 3 (2-4) | 4.5 (3.0-8.3) | 2 (2-3) | 4 (4-5) | 2 (1-7) | 5 (3-15) |
| Complications (n, %) | 5 (50%) | 16 (80%) | 9 (22.5%) | 26 (49.1%) | 10 (18.5%) | 20 (27%) |
| Pain score after operation | 3 (1.0-4.0) POD | 4.5 (2.7-6.) POD | 2.45+ 0.54 | 3.02+0.44 | NA | NA |
| Postoperative hospital stay (d) | 9.5 (9.0-10.5) | 18 (14.3-24.3) | 14.5 (12-17) | 16 (15-18) | 18 (15-32) | 28 (23-35) |
| Readmission within 30 d after discharge | NA | NA | 0 | 0 | 0 | 0 |
| Preoperative | Brustia et al[8] | Xu et al[9] | Rao et al[7] |
| 1 Outpatient counseling and information | √ | √ | √ |
| 2 Preoperative carbohydrate loading | √ | √ | √ |
| 3 Absence of preanesthetic medication (anxiolytic) | √ | ||
| Intraoperative | |||
| 4 Antimicrobial prophylaxis and skin preparation | √ | ||
| 5 Prevention of intraoperative hypothermia | √ | √ | |
| 6 Incision | √ | ||
| 7 Adapted IV filling | √ | √ | √ |
| 8 Temporary portocaval anastomosis | √ | ||
| 9 No prophylactic nasogastric intubation | √ | √ | |
| 10 No prophylactic abdominal drainage | √ | √ | |
| 11 Prevention of postoperative nausea and vomiting | √ | ||
| 12 Antithrombotic prophylaxis and/oranti-aggregation | √ | √ | |
| 13 Early extubation (< 6 h after the endof lt) | √ | √ | √ |
| Postoperative | |||
| 14 Early mobilization (POD1) | √ | √ | √ |
| 15 Patient-controlled analgesia | √ | √ | |
| 16 Gastric probe removal POD1 | √ | √ | |
| 17 Clear liquid per OS POD1 | √ | √ | √ |
| 18 Enteral feeding per OS POD1 | √ | √ | √ |
| 19 Stop IV fluids POD1 | √ | √ | |
| 20 Per OS analgesia (POD2) | √ | √ | |
| 21 Abdominal drain removal POD2 | √ | ||
| 22 Urinary probe removal POD2 | √ | √ | √ |
| 23 Stop IV analgesia POD3 | √ | √ | |
| 24 Independent mobilization POD3 | √ | √ | √ |
| 25 Daily revision of discharge criteria | √ | √ | √ |
| 26 Audit | √ | √ | √ |
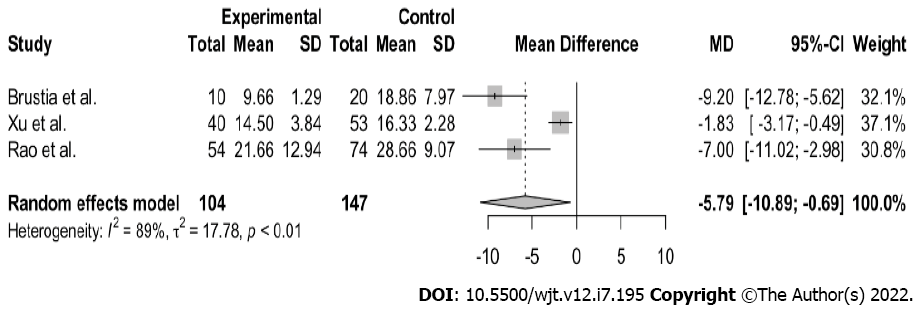
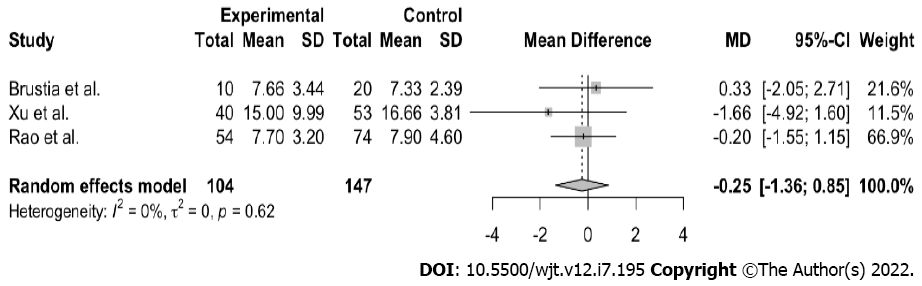
All three studies demonstrated a considerable decrease in length of hospital stay, with no readmissions or negative impact of the ERAS protocol applied in the postoperative course (Table 2). From the above-mentioned publications, we meta-analyzed the primary endpoint, postoperative hospital stay. The variable was continuous, and the results were summarized using median and 25%-75% values (because the data were skewed). The sample mean and standard deviations were calculated using the formula of Wan et al[11]. The random-effects model was applied for the meta-analysis, as high heterogeneity was expected among the studies with regard to study populations and diagnostic procedures. The presence of between-study heterogeneity was quantitatively reflected with the I2 index, considering I2 of > 50%, indicative of statistically significant heterogeneity. R studio version 4.0.2 software was used to perform all of the statistical analyses, employing the packages “meta” and “metaphor.” A comparison of total hospital stay showed a statistically significant difference in both groups (n = 251; MD- 5.79; 95% confidence interval (CI), 10.89 to 0.69; I2 = 89%; P < 0.01). Nevertheless, great heterogeneity was observed between the samples (Figure 2). A similar meta-analysis of the MELD score showed that there was no statistically significant difference in both groups (n = 251, MD -0.25, 95%CI, -1.36 to 0.85; I2 0%; P = 0.62) (Figure 3). As aforementioned, all patients were low MELD patients with a mean MELD well below 20.
The scarcity of strong evidence in the widespread application of ERAS programs in LT may reflect the reluctance of teams to implicate such protocols in a cohort of patients that are generally perceived as a frail, high-risk group, undergoing a major surgical procedure of a life-threatening nature. The evolution of LT on the other hand, is a successful story, evolving from an experimental and innovative procedure to a more “standard” one over the last several decades, and especially when performed in high volume centers with experienced multidisciplinary teams. Throughout the years, LT has proved its life saving nature as an operation and the morbidity and mortality plummeted, offering patients excellent survival and quality of life[12]. The major incentives in applying ERAS in LT came from the successful application of Enhanced Recovery Programs in Liver Surgery[13] and the subsequent publication of suggested guidelines for ERAS in Liver Surgery[14]. Although ERAS with its multimodal approach pattern did not appear in the literature until recently, the concept of multimodal clinical pathways in LT was raised as early as 2011 by Pavlakis et al of the Beth Israel Deaconess Medical Center team[15], characterizing the transplantation domain as an “ideal forum for successful implementation of clinical pathways” and highlighting their importance and potential in reducing length of stay, morbidity, costs, as well as improving patient satisfaction. Piñero et al[16] introduced in 2015 the concept of the early discharge from hospital following LT focusing on healthcare costs and proposed an early discharge prediction model based on MELD points (exception MELD points were deemed a favorable prognostic factor), length of surgery (time < 4 h), transfusion of less than 5 units of packed red blood cells, and early respirator weaning. The author concluded that early discharge from the hospital following LT is feasible, without a negative impact on patient or graft survival, nor did it increase short-term rehospitalization. A recent publication of Brustia et al[18] in Paris reinforced the basis for further developing ERAS in LT. Although it is a small-scale single-center observational study, the authors reported a 47% reduction of length of hospital stay with no safety issues in a small but well-designed protocol. This conclusion was corroborated by all three publications mentioned above, demonstrating that ERAS in LT could be possible in a larger scale and should be further studied. Rodríguez-Laiz et al[17] presented a cohort of 236 patients who were treated with a comprehensive multistep ERAS protocol that is the product of lessons and experiences emanating from liver surgery and other disciplines aiming to evaluate its value as a proof-of-concept. In this study, the authors identified 133 patients who were discharged early and they retrospectively defined them as the ERAS group. However, their study, with extremely short lengths of stay, was inherently flawed, as the authors pointed out, by a lack of a traditional control group; for this reason, their article was not included in our final selection. In 2021 Brustia et al[18] drafted the “Guidelines for Perioperative Care for Liver Transplantation: Enhanced Recovery After Surgery (ERAS) Society Recommendations,” after a systematic review by a wide international panel of experts and the application of the Delphi method. The authors of the manuscript recognized the lack of current strong evidence in ERAS in LT but laid a solid foundation and precious scaffold, which can serve as the basis for large studies in the definitive validation of ERAS in LT.
ERAS is a well-validated multimodal approach for almost all types of surgical procedures, and its future in selected LT patients seems promising, as the preliminary results advocate for the safety and efficacy of ERAS in the field of LT. The majority of studies analyzing ERAS in LT use a cohort of low MELD highly selected patients that might not represent the majority of patients that benefit from LT; an issue that has to be addressed. The overall majority of patients in the three studies analyzed were low MELD HCC patients, and this type of selection might harbor an inherent bias in evaluating ERAS in LT. However it is a first step and understandably first steps must be careful. The encouraging results presented, along with the observed benefit of a well-designed ERAS protocol in these patients mandates further exploration and expansion of inclusion criteria in these types of protocols. After all, an earlier discharge might be the result of a better overall patient management in all aspects of their journey through the hospital and not necessarily the primary endpoint.
One of the key factors in implementing ERAS protocols is the understanding of the philosophy behind ERAS by both patients and caregivers and although this might seem simple or a given, studies indicate that this might not be the case[19,20]. As ERAS is new to the field of LT, similar issues are expected to occur. In the first years of the implementation of ERAS in colorectal surgery, many issues arose concerning patient and physician capability of correctly implementing and accepting what proved to be a validated protocol for better patient recovery[21,22] including the complexity of these multimodal pathways[23], the need for teamwork along with the difficulty of eradicating old surgical stereotypes of traditional care. Agrafiotis et al[24], along with the first author of the present review, have explored in 2013 the efficacy of a “soft” non-strict fast-track protocol in a cohort of 92 patients undergoing colorectal surgery. The conclusion was that even without a strict ERAS protocol, enhanced recovery and accelerated safe patient discharge are possible, pointing out among others[25] that “length of stay should not be an aim in itself within an enhanced recovery protocol. The main object of these programs ought to be the enhancement of patient recovery and not earlier discharge.” This statement is endorsed by our team, in the Transplantation Department of a public Medical School part of a public healthcare system with significant challenges, who tried to evaluate the implementation of a non-strict ERAS protocol in selected LT patients in a small cohort of patients trying to replicate the results of Brustia et al[8]. In a small feasibility and safety study, we observed a 56% decrease in hospital stay in the ERAS group without any safety issues (unpublished data). These encouraging results might indicate that ERAS, when implemented in the right way, can be beneficial to patients even in small volume transplant centers and their implementation should be encouraged. We also noted the lack of estimation of the importance of every point in the proposed ERAS protocols towards the final endpoint, which hinders the simplification of these protocols, as we do not currently know which one of the steps – if any - could be omitted without a significant compromise in the outcome.
Henric Kehlet pointed out the delay of the development of ERAS: “there is an urgent need for better implementation of the current established scientific evidence for ERAS practices in order to fill the still very present gap between knowing and doing” and has been advocating for many years the concept of “stress free, pain free” operations[26], which might seem an impossible task for operations of the magnitude of a LT. However, as the term “fast-track” was gradually replaced by the more correct term “enhanced recovery,” the concept of “first better, then faster” had to be reappraised[27,28].
Enhanced recovery means better recovery and its value should be further exploited for liver transplant patients. After all, ERAS is not about the type of operation; ERAS is about the patient.
Enhanced recovery after surgery (ERAS) is a multimodal perioperative care pathway designed to achieve early recovery for patients undergoing major surgery.
In the last decade, ERAS has gained significant acceptance in the community of general surgery, in addition to several other surgical specialties, as the evidence of its advantages continues to grow. Orthotopic Liver Transplantation (LT) remains one of the last frontiers in the application of ERAS.
To evaluate existing data on the use of ERAS in orthotopic LT.
We conducted a systematic review of the existing studies that evaluate ERAS in orthotopic LT with a multimodal approach and focusing on measurable clinical primary endpoints, namely length of hospital stay.
All studies demonstrated a considerable decrease in length of hospital stay, with no readmissions or negative impact of the ERAS protocols in the postoperative period.
Enhanced recovery can be safely applied in selected LT patients and its value should be further exploited.
The future widespread use of ERAS in selected LT patients seems promising.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Surgeons; American Association for the Study of Liver Diseases; American Gastroenterological Association; American Society of Transplant Surgeons; American Society of Transplantation.
Specialty type: Transplantation
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Feier F, Brazil; Ferrarese A, Italy; Kaido T, Japan S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | ERAS Society. [cited 2 June 2021]. Available from: https://erassociety.org/. [DOI] [Full Text] |
| 2. | Kehlet H, Mogensen T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg. 1999;86:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 352] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Zhang X, Yang J, Chen X, Du L, Li K, Zhou Y. Enhanced recovery after surgery on multiple clinical outcomes: Umbrella review of systematic reviews and meta-analyses. Medicine (Baltimore). 2020;99:e20983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Golder HJ, Papalois V. Enhanced Recovery after Surgery: History, Key Advancements and Developments in Transplant Surgery. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Rossaint R, Slama K, Jaeger M, Konrad M, Pappert D, Bechstein W, Blumhardt G, Neuhaus P, Falke KJ. Fluid restriction and early extubation for successful liver transplantation. Transplant Proc. 1990;22:1533-1534. [PubMed] |
| 6. | Biancofiore G, Bindi ML, Romanelli AM, Boldrini A, Bisà M, Esposito M, Urbani L, Catalano G, Mosca F, Filipponi F. Fast track in liver transplantation: 5 years' experience. Eur J Anaesthesiol. 2005;22:584-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Rao JH, Zhang F, Lu H, Dai X Z, Zhang CY, Qian XF, Wang XH, Lu L. Effects of multimodal fast-track surgery on liver transplantation outcomes. Hepatobiliary & pancreatic diseases international: HBPD INT 2017; 16: 364–369. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Brustia R, Monsel A, Conti F, Savier E, Rousseau G, Perdigao F, Bernard D, Eyraud D, Loncar Y, Langeron O, Scatton O. Enhanced Recovery in Liver Transplantation: A Feasibility Study. World J Surg. 2019;43:230-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Xu Q, Zhu M, Li Z, Zhu J, Xiao F, Liu F, Wang Y, Liu C. Enhanced recovery after surgery protocols in patients undergoing liver transplantation: A retrospective comparative cohort study. Int J Surg. 2020;78:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Samuel D, Coilly A. Management of patients with liver diseases on the waiting list for transplantation: a major impact to the success of liver transplantation. BMC Med. 2018;16:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3433] [Cited by in RCA: 7017] [Article Influence: 637.9] [Reference Citation Analysis (0)] |
| 12. | Adam R, McMaster P, O'Grady JG, Castaing D, Klempnauer JL, Jamieson N, Neuhaus P, Lerut J, Salizzoni M, Pollard S, Muhlbacher F, Rogiers X, Garcia Valdecasas JC, Berenguer J, Jaeck D, Moreno Gonzalez E; European Liver Transplant Association. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transpl. 2003;9:1231-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 414] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 13. | Coolsen MM, Wong-Lun-Hing EM, van Dam RM, van der Wilt AA, Slim K, Lassen K, Dejong CH. A systematic review of outcomes in patients undergoing liver surgery in an enhanced recovery after surgery pathways. HPB (Oxford). 2013;15:245-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Melloul E, Hübner M, Scott M, Snowden C, Prentis J, Dejong CH, Garden OJ, Farges O, Kokudo N, Vauthey JN, Clavien PA, Demartines N. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg. 2016;40:2425-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 407] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 15. | Pavlakis M, Hanto DW. Clinical pathways in transplantation: a review and examples from Beth Israel Deaconess Medical Center. Clin Transplant. 2012;26:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Piñero F, Fauda M, Quiros R, Mendizabal M, González-Campaña A, Czerwonko D, Barreiro M, Montal S, Silberman E, Coronel M, Cacheiro F, Raffa P, Andriani O, Silva M, Podestá LG. Predicting early discharge from hospital after liver transplantation (ERDALT) at a single center: a new model. Ann Hepatol. 2015;14:845-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Rodríguez-Laiz GP, Melgar-Requena P, Alcázar-López CF, Franco-Campello M, Villodre-Tudela C, Pascual-Bartolomé S, Bellot-García P, Rodríguez-Soler M, Miralles-Maciá CF, Más-Serrano P, Navarro-Martínez JA, Martínez-Adsuar FJ, Gómez-Salinas L, Jaime-Sánchez FA, Perdiguero-Gil M, Díaz-Cuevas M, Palazón-Azorín JM, Such-Ronda J, Lluís-Casajuana F, Ramia-Ángel JM. Fast-Track Liver Transplantation: Six-year Prospective Cohort Study with an Enhanced Recovery After Surgery (ERAS) Protocol. World J Surg. 2021;45:1262-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Brustia R, Monsel A, Skurzak S, Schiffer E, Carrier FM, Patrono D, Kaba A, Detry O, Malbouisson L, Andraus W, Vandenbroucke-Menu F, Biancofiore G, Kaido T, Compagnon P, Uemoto S, Rodriguez Laiz G, De Boer M, Orloff S, Melgar P, Buis C, Zeillemaker-Hoekstra M, Usher H, Reyntjens K, Baird E, Demartines N, Wigmore S, Scatton O. Guidelines for Perioperative Care for Liver Transplantation: Enhanced Recovery After Surgery (ERAS) Recommendations. Transplantation. 2022;106:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 19. | Herbert G, Sutton E, Burden S, Lewis S, Thomas S, Ness A, Atkinson C. Healthcare professionals' views of the enhanced recovery after surgery programme: a qualitative investigation. BMC Health Serv Res. 2017;17:617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Alawadi ZM, Leal I, Phatak UR, Flores-Gonzalez JR, Holihan JL, Karanjawala BE, Millas SG, Kao LS. Facilitators and barriers of implementing enhanced recovery in colorectal surgery at a safety net hospital: A provider and patient perspective. Surgery. 2016;159:700-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Maessen J, Dejong CH, Hausel J, Nygren J, Lassen K, Andersen J, Kessels AG, Revhaug A, Kehlet H, Ljungqvist O, Fearon KC, von Meyenfeldt MF. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg. 2007;94:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 371] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 22. | Kahokehr A, Sammour T, Zargar-Shoshtari K, Thompson L, Hill AG. Implementation of ERAS and how to overcome the barriers. Int J Surg. 2009;7:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Feroci F, Lenzi E, Baraghini M, Garzi A, Vannucchi A, Cantafio S, Scatizzi M. Fast-track colorectal surgery: protocol adherence influences postoperative outcomes. Int J Colorectal Dis. 2013;28:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Agrafiotis AC, Corbeau M, Buggenhout A, Katsanos G, Ickx B, Van de Stadt J. Enhanced recovery after elective colorectal resection outside a strict fast-track protocol. A single centre experience. Int J Colorectal Dis. 2014;29:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, von Meyenfeldt MF, Ubbink DT, Gouma DJ, Bemelman WA; Laparoscopy and/or Fast Track Multimodal Management Versus Standard Care (LAFA) Study Group; Enhanced Recovery after Surgery (ERAS) Group. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 472] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 26. | Kehlet H. Enhanced Recovery After Surgery (ERAS): good for now, but what about the future? Can J Anaesth. 2015;62:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 27. | Vehmeijer SBW, Husted H, Kehlet H. Outpatient total hip and knee arthroplasty. Acta Orthop. 2018;89:141-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Kehlet H. Enhanced postoperative recovery: good from afar, but far from good? Anaesthesia. 2020;75 Suppl 1:e54-e61. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |