Published online Jul 18, 2022. doi: 10.5500/wjt.v12.i7.184
Peer-review started: January 12, 2022
First decision: March 16, 2022
Revised: April 20, 2022
Accepted: June 16, 2022
Article in press: June 16, 2022
Published online: July 18, 2022
Processing time: 183 Days and 7.1 Hours
Physical activity levels are significantly lower in kidney transplant (KT) recipients compared to the general population. The effects of exercise training in KT recipients with diabetes mellitus remain unclear, and so little is known about the role of increased exercise on cardiovascular risk and metabolic profile of KT patients.
To investigate the effects of a 6-mo home-based exercise training program on functional capacity, glucose levels and lipid profile of diabetic KT patients.
In total, 21 type II diabetic KT recipients were randomly assigned into two groups: Exercise (n = 11, aged 52.9 ± 10.1 years) and control (n = 10, aged 53.01 ± 9.5 years). All participants at baseline and the end of the study underwent bioche
At the end of the 6-mo study, the exercise group had significantly lower values in fasting plasma glucose by 13.4% (from 120.6 ± 28.9 mg/dL to 104.8 ± 21.9 mg/dL, P = 0.01), glycated hemoglobin by 1.5% (from 6.7% ± 0.4 to 6.6% ± 0.4, P = 0.01) and triglycerides by 8.5% (from 164.7 ± 14.8 mg/dL to 150.8 ± 11.6 mg/dL, P < 0.05) and higher values in high-density lipoprotein by 10.2% (from 51.4 ± 8.8 mg/dL to 57.2 ± 8.7 mg/dL, P < 0.05) and (VO2)peak by 4.7% (from 22.7 ± 3.3 to 23.8 ± 4.2, P = 0.02) than the control group. There were statistically significant differences between the two groups at the end of the study for fasting plasma glucose (decreased by 9.6%, P < 0.05), triglycerides (decreased by 4.5%, P = 0.04) and (VO2)peak (increased by 4.4%, P = 0.01). Finally, after training, there was a moderate, positive linear relationship between (VO2)peak and glycated hemoglobin in the exercise group (r = 0.408, P = 0.03).
The results demonstrated that a 6-mo home-based mixed type exercise training program can improve the functional capacity, levels of glucose and lipid profile of diabetic KT recipients.
Core Tip: Physical activity levels are significantly lower in kidney transplant (KT) recipients compared to the general population. The effects of exercise training in KT recipients with diabetes mellitus remain unclear, and so little is known about the role of increased exercise on cardiovascular risk and metabolic profile of KT patients. This randomized controlled trial aimed to investigate the effects of a 6-mo home-based exercise training program on functional capacity, glucose levels and lipid profile of diabetic KT patients. The results of the present study demonstrated that a long-term exercise training program is feasible and effective in diabetic KT recipients.
- Citation: Michou V, Nikodimopoulou M, Deligiannis A, Kouidi E. Metabolic and functional effects of exercise training in diabetic kidney transplant recipients. World J Transplant 2022; 12(7): 184-194
- URL: https://www.wjgnet.com/2220-3230/full/v12/i7/184.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i7.184
Renal transplantation is an effective treatment option for end-stage kidney disease patients and aims to improve quality of life and reduce mortality. Kidney transplant (KT) patients are dealing with many non- or modifiable risk factors after the transplantation surgery, especially due to the use of maintenance immunosuppression[1,2]. Dyslipidemia, abnormal glucose tolerance, hypertension, anemia and nephrotoxicity are common immunosuppressive therapy side effects in KT patients[2,3]. Unfavorable alterations in lipid and glucose profiles contribute to high cardiovascular risk[4], while low functional capacity due to comorbidities, corticosteroids and inactivity is common among these patients[5].
Diabetes mellitus incidence among the KT patient population is also high. Regular physical exercise can be an adjunct therapeutic modality for patients with diabetes mellitus, as it reduces the risk of cardiovascular disease, increases insulin sensitivity[5], leads to better glucose control and reduces lipid disorders[1]. High cardiovascular disease risk in KT patients is strongly associated with low physical activity levels[6-8]. Despite physical exercise benefiting KT patients’ general health, only a few patients include physical activity in their daily routine[9]. This may be due to the non-normalized physical fitness after transplantation and comorbidities[10,11].
Although most of the studies on KT recipients have previously evaluated functional capacity and metabolic profile compared to healthy individuals, only a few studies have investigated the effects of structured exercise programs on glucose levels and lipid profile. Results from the few studies on functional capacity in KT recipients have shown that physical inactivity is a risk factor contributing to a patient’s low physical fitness, which increases the risk of morbidity and mortality[5,9].
By increasing physical activity levels during their daily life KT recipients show favorable results, such as improvements in their cardiovascular fitness[6], even though the exact type, frequency or intensity recommended is not yet clear. Home-based exercise programs have previously largely been applied in hemodialysis and patients undergoing cardiovascular rehabilitation[4,12], while only two studies have so far provided home-based exercise rehabilitation programs for KT recipients[13,14]. This study aimed to examine the effects of a 6-mo home-based exercise training program on glycemic control, lipid profile and functional capacity of diabetic KT recipients.
Twenty-eight adult KT recipients with type 2 diabetes (T2D) mellitus were recruited from the Transplant Surgery Clinic of the Hippokration General Hospital of Thessaloniki, Greece. Exclusion criteria included age older than 70 years, body mass index over 40 kg/m2, presence of autoimmune disorders (such as systemic lupus erythematosus, multiple sclerosis, ulcerative colitis, Crohn’s disease or rheumatoid arthritis), history of recent coronary heart disease (CHD) (myocardial infarction, unstable angina) within the previous 6 mo, serious musculoskeletal problems that may limit the patient’s participation in this study, non-compliance with diabetes medication and previous participation in an exercise training program.
Initially, all patients who met the inclusion criteria underwent clinical examination {electrocardiography, hemodynamic [blood pressure and heart rate (HR)] and anthropometric (weight and height) measurements}, blood sampling and cardiorespiratory testing for their physical fitness estimation. After baseline measurements, patients were randomly assigned by simple randomization (drawing lots) to either an exercise group or a control group. Participants in the control group were asked to maintain their regular lifestyle and their current physical activity level during the study period. At the end of the 6-mo study, all patients underwent the same assessment. All tests were conducted by the same researcher, who was blinded to group allocation. Patients’ medications were asked to remain unchanged during the study period. This randomized controlled trial protocol was approved by the Ethics Committee of the Aristotle University of Thessaloniki (Protocol number: 117461/2019). All participants received all the necessary study information before the enrollment and provided written informed consent. The clinical trial started in September 2019 and ended in February 2020.
To assess patient functional capacity, patients underwent a symptom-limited cardiopulmonary exercise testing on a treadmill using a Bruce protocol[15] during morning hours (9:00-11:00 am). Breath-by-breath gas exchange was measured by the Med Graphics Breeze Suite CPX Ultima (Medical Graphics Corp, MN, United States). The electrocardiogram was continuously monitored throughout each test, and the blood pressure was measured at every stage. The endpoint was set as the respiratory exchange ratio ≥ 1.10. From each test, the peak oxygen uptake [(VO2)peak], pulmonary ventilation, ventilatory equivalents for oxygen (pulmonary ventilation/VO2), carbon dioxide (pulmonary ventilations/VCO2) and the ratio between VO2 and maximum HR (VO2/HRmax) were measured.
At baseline and the end of the study, blood samples were taken from the brachial artery between 7:00-9:00 am, after a 12-h fast by the same blinded microbiologist at the Hippokration General Hospital of Thessaloniki. Blood samples were drawn from each group to determine by photometric method hematocrit, by computational method hemoglobin, by ion-selective electrode method serum concentrations of sodium, potassium, calcium, magnesium and electrolytes (potassium, sodium, calcium, phosphorus, magnesium), by enzymatic colorimetric method fasting plasma glucose (FPG) (mg/Dl), serum triglycerides (TG) and hemoglobin A1c (HbA1c) and by enzymatic method serum total cholesterol (TC), high-density lipoprotein (HDL) and low-density lipoprotein (LDL). Results were analyzed through biochemical auto-analyzer devices.
Participants in the exercise group received a home-based exercise program for 6 mo. The exercise program included aerobic exercise and muscle strengthening exercises, 3 times per week for 60-90 min, with moderate intensity, i.e., 60%-80% of the maximum HR reached during cardiopulmonary exercise testing. Training intensity was increased gradually throughout the study according to each patient’s capacity and adaptations. Each exercise session started with a 10-min warm-up and finished with a 10-min recovery (upper and lower limp stretching).
The aerobic part of each exercise session consisted of walking through going up and down stairs or cycling on a stationary bike, initially for 15 min, with a consequent gradual increase of time by 5 min every 2 wk, reaching 40 min in the last 2 wk before the end of the program. After a 5 min break, patients continued with the strengthening part of the exercise program. Patients were asked to perform six dynamic muscle strengthening exercises using just their body weight at the beginning. During the first week, each patient had three familiarization sessions with a physical education teacher experienced in exercise rehabilitation for patients with chronic disease, who also gave him/her an information booklet with exercise instruction images and a detailed description of the strengthening part of the program. Strengthening exercises were performed in 2 sets of 8-10 repetitions (with a 1-min passive break between the sets), in a progressive sequence from sitting to standing position. The exercise prescription included three strengthening exercises for the upper limbs (such as shoulder press, bicep curl and triceps extension) and three for the lower limbs (such as leg flexion-extension). Progressively they were asked to perform the same exercises using rubber bands, balls and dumbbells (1 kg). Patients were advised to first perform 2 sets (8-10 repetitions) of upper limb strengthening exercise with balls and 2 sets with the 1 kg dumbbells (8-10 repetitions) in a sitting position. Second, patients were asked to place the rubber bands on their feet, tie them to the bottom of their bed or chair and do strengthening exercises in a sitting position (2 sets, 8-10 repetitions). Last, patients were asked to place the dumbbells on their feet in a standing position and move their legs back and forth, right and left of their torso, with hands placed in the middle of their body.
To ensure each patient’s autonomy, the interventional 6-mo home-based exercise program was individualized, while the progress and adherence to the program were monitored by telephone every week and a home visit every month to control improvement and possible modification of the program by the researcher. To enhance compliance, participants were asked to fill in individual diaries, describing the type, frequency and duration of each exercise session and significant notes, which were collected every week through telephone communications. Moreover, researchers contacted patients for possible modifications or recommendations for exercise prescription.
Furthermore, it was essential for patients to measure before each exercise session (at least 30 min before) their blood glucose, blood pressure and HR levels and note the results in their diary. If glucose concentration was below 70 mg/dL or above 130 mg/dL, patients were advised to avoid starting exercise. Moreover, intake of a small number of carbohydrates (10-15 g) before exercise or having a carbohydrate snack available, in case of signs of hypoglycemia, was an important preventive measure. Patients were also informed about the area of insulin injection that should be done in the abdominal cavity and not in the exercised limbs.
IBM Statistical Package for Social Sciences (SPSS 27.0 for Windows, Chicago, IL, United States) was used for the statistical analysis. The Kolmogorov-Smirnov test was used to evaluate variables’ normality of distribution. Mean differences within time and between the two groups were analyzed using two-way analysis of variance with repeated measures. Linear regression was used to study the association between variables that revealed statistically significant changes over time. Data were expressed as mean ± SD for normally distributed variables. The two-tailed P-values < 0.05 were considered statistically significant.
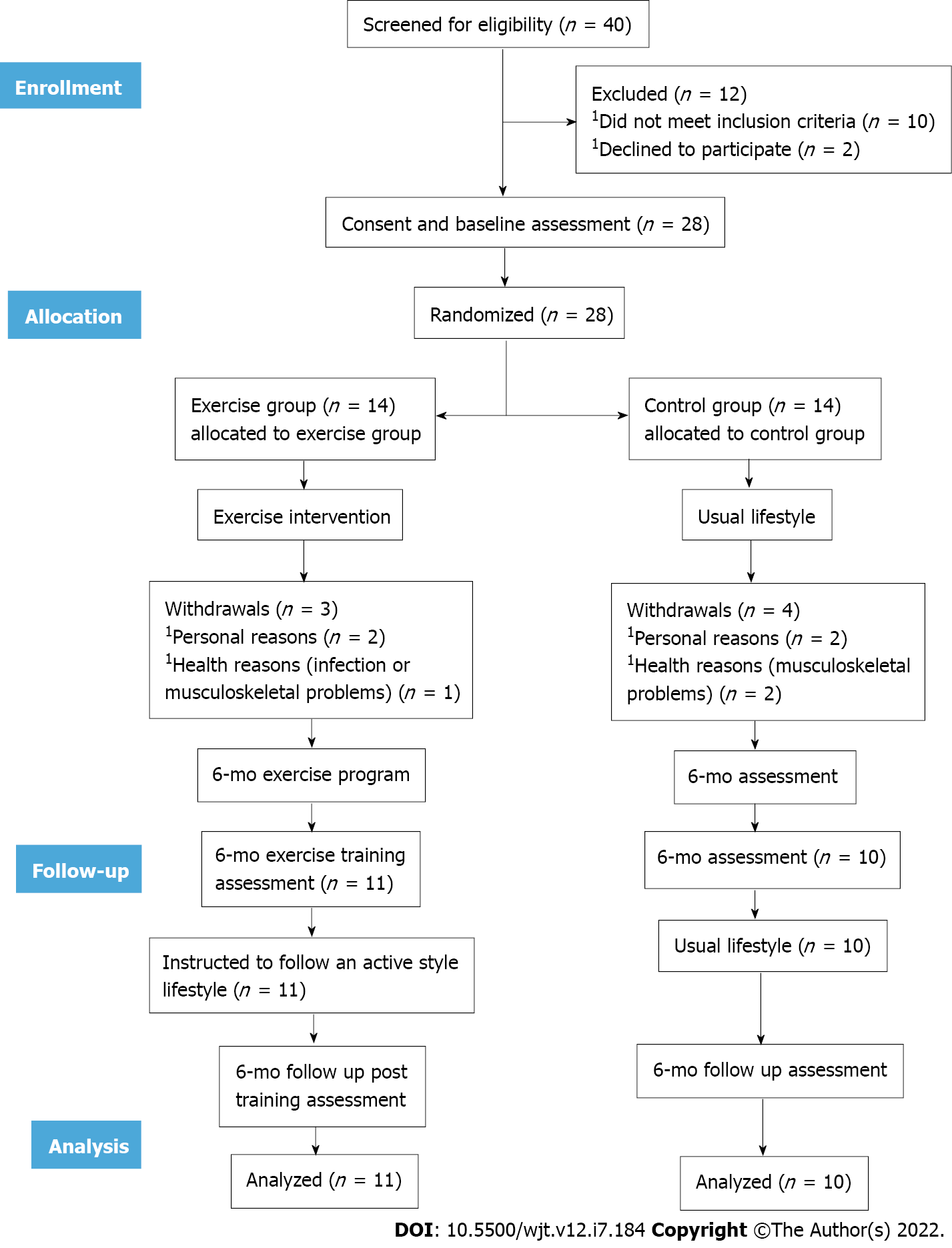
At baseline, 40 KT patients were screened for eligibility; 28 were included in our study and randomized to either exercise or control group. During the 6 mo, 3 patients from the exercise group and 4 patients from the control group withdrew from the study due to health reasons (such as infection or musculoskeletal problems) or personal reasons (such as lack of time). Therefore, 21 patients completed the study (exercise group: n = 11; control group: n = 10). The flowchart of participants was based on recommendations from the Consolidated Standards of Reporting Trials (Figure 1). There was no statistically significant difference between the two groups’ demographic and clinical data (Table 1). There were no exercise-induced musculoskeletal, cardiovascular, renal or other complications during the study.
| Exercise group | Control group | P value | |
| Sex (male/female) | 8/3 | 8/2 | 0.52 |
| Age (yr) | 52.9 ± 9.5 | 53.0 ± 13.1 | 0.51 |
| Height (cm) | 1.6 ± 0.5 | 1.6 ± 0.0 | 0.34 |
| Weight (kg) | 70.8 ± 12.2 | 72.1 ± 6.7 | 0.77 |
| BMI (kg/m2) | 24.4 ± 2.6 | 25.6 ± 2.0 | 0.23 |
| Place of residence | |||
| Rural area | 27.2% (3/11) | 40.0% (4/10) | 0.69 |
| Urban area | 72.7% (8/11) | 60.0% (6/10) | 0.42 |
| Education | |||
| Primary education | 54.5% (6/11) | 40.0% (4/10) | 0.33 |
| Secondary education | 18.1% (2/11) | 10.0% (1/10) | 0.68 |
| Higher education | 9.0% (1/11) | 20.0% (2/10) | 0.65 |
| No education | 18.1% (2/11) | 30.0% (3/10) | 0.70 |
| Employment status | |||
| Employed | 18.1% (2/11) | 10.0% (1/10) | 0.71 |
| Unemployed | 54.5% (6/11) | 40.0% (4/10) | 0.53 |
| Retired | 27.2% (3/11) | 50.0% (5/10) | 0.38 |
| Smoking | 18.1% (2/11) | 10.0% (1/10) | 0.74 |
| eGFR-CKD-EPI equation (mL/min) | 61.0 ± 7.3 | 59.5 ± 8.2 | 0.53 |
| Stage of diabetic nephropathy | |||
| Stage 3 | 81.8% (9/11) | 90.0% (9/10) | 0.77 |
| Stage 4 | 18.1% (2/11) | 10.0% (1/10) | 0.64 |
| Time after KTx (mo) | 47.4 ± 18.3 | 47.8 ± 18.1 | 0.68 |
| Primary causes of ESKD | |||
| Diabetes mellitus | 54.5% (6/11) | 50.0% (5/10) | 0.64 |
| Hypertension | 27.2% (3/11) | 20.0% (2/10) | 0.56 |
| Polycystic kidney disease | 18.1% (2/11) | 10.0% (1/10) | 0.56 |
| Glomerulonephritis | 9.0% (1/11) | 10.0% (1/10) | 0.72 |
| Nephrosclerosis | 9.0% (1/11) | 0.0% (0/10) | 0.55 |
| Reflux nephropathy | 0.0% (0/11) | 10.0% (1/10) | 0.61 |
| Others | 0.0% (0/11) | 10.0% (1/10) | 0.59 |
| Medication | |||
| Statins | 100.0% (11/11) | 100.0% (10/10) | 0.53 |
| Calcium channel blockers | 36.3% (4/11) | 50.0% (5/10) | 0.23 |
| Oral antidiabetic drugs | 18.1% (2/11) | 30.0% (3/10) | 0.51 |
| Angiotensin II receptor blockers/angiotensin converting enzyme blockers | 54.5% (6/11) | 50.0% (5/10) | 0.66 |
| Slow and/or intermediate acting insulin | 81.9% (9/11) | 70.0% (7/10) | 0.47 |
| Immunosuppression therapy (corticosteroid, tacrolimus, mycophenolate mofetil) | 100.0% (11/11) | 100.0% (10/10) | 0.74 |
| Adherence to medication | 90.9% (10/11) | 100.0% (10/10) | 0.82 |
| Hematocrit (%) | 42.1 ± 4.6 | 39.8 ± 4.5 | 0.63 |
| Hemoglobin (g/dL) | 14.1 ± 1.0 | 13.1 ± 1.6 | 0.16 |
| Na+ (mg/dL) | 139.8 ± 2.5 | 140.3 ± 4.3 | 0.90 |
| K+ (mg/dL) | 4.1 ± 0.3 | 4.3 ± 0.5 | 0.15 |
| Ca2+ (mg/dL) | 10.1 ± 0.5 | 9.7 ± 0.9 | 0.94 |
| P (mg/dL) | 2.9 ± 0.5 | 3.4 ± 0.4 | 0.09 |
| Mg+ (mg/dL) | 1.6 ± 0.1 | 1.6 ± 0.3 | 0.50 |
| Fe+ (mg/dL) | 89.8 ± 23.2 | 87.9 ± 16.6 | 0.54 |
| Urea (mg/dL) | 42.2 ± 8.7 | 48.1 ± 16.7 | 0.90 |
| Creatinine (mg/dL) | 1.1 ± 0.2 | 1.2 ± 0.5 | 0.16 |
| Alkaline phosphatase (mg/dL) | 72.1 ± 27.2 | 62.5 ± 10.4 | 0.17 |
| Uric acid (mg/dL) | 5.7 ± 1.1 | 5.9 ± 1.2 | 0.23 |
| 24-h urine albumin level (mg/dL) | 106.4 ± 25.1 | 115.6 ± 20.9 | 0.25 |
After the 6-mo home-based exercise program, a statistically significant reduction of FPG by 13.4% (P = 0.01), TG by 8.5% (P < 0.05) and HbA1c by 1.5% (P = 0.01) as well as a significant increase in HDL by 10.2% (P < 0.05) compared to the baseline values in the exercise group was noted. In contrast, there was no statistically significant difference in any biochemical parameter studied in the control group at the end of the study (Table 2). Concerning changes between groups at the end of the study, the mean concentrations of FPG and TG were decreased by 9.6% (P < 0.05) and 4.5% (P = 0.04), respectively.
| Exercise group | Control group | Exercise vs control group | ||||||
| Baseline | After 6-mo | P value | Baseline | After 6-mo | P value | Pre | Post | |
| FPG (mg/dL) | 120.6 ± 28.9 | 104.8 ± 21.9 | 0.01 | 116.1 ± 33.2 | 115.4 ± 33.9 | 0.38 | 0.47 | < 0.05 |
| TC (mg/dL) | 224.8 ± 30.4 | 224.0 ± 30.1 | 0.11 | 229.7 ± 28.8 | 230.8 ± 27.8 | 0.60 | 0.41 | 0.48 |
| TG (mg/dL) | 164.7 ± 14.8 | 150.8 ± 11.6 | < 0.05 | 165.4 ± 19.0 | 165.2 ± 20.5 | 0.67 | 0.11 | 0.04 |
| HDL (mg/dL) | 51.4 ± 8.8 | 57.2 ± 8.7 | < 0.05 | 51.1 ± 7.9 | 51.3 ± 12.6 | 0.43 | 0.56 | 0.06 |
| LDL (mg/dL) | 119.6 ± 11.4 | 119.4 ± 10.9 | 0.27 | 119.4 ± 17.0 | 119.5 ± 16.4 | 0.33 | 0.78 | 0.45 |
| HbA1c (%) | 6.7 ± 0.4 | 6.6 ± 0.4 | 0.01 | 6.5 ± 1.0 | 6.5 ± 1.1 | 0.25 | 0.20 | 0.36 |
Exercise group results from the cardiopulmonary exercise testing revealed a statistically significant increase in (VO2)peak by 4.7%, (P = 0.02) at the end of the study (Table 3). At baseline, there was no statistically significant difference between groups, but at the end of the study, there was only a significant intergroup difference in (VO2)peak, which was increased by 4.4% (P = 0.01) in the exercise group compared to controls.
| Exercise group | Control group | Exercise vs control group | ||||||
| Baseline | After 6-mo | P value | Baseline | After 6-mo | P value | Pre | Post | |
| (VO2)peak (mL/kg/min) | 22.7 ± 3.3 | 23.8 ± 4.2 | 0.02 | 21.9 ± 4.1 | 21.8 ± 3.2 | 0.34 | 0.43 | 0.01 |
| RERmax | 1.1 ± 0.0 | 1.2 ± 0.1 | 0.53 | 1.1 ± 0.0 | 1.1 ± 0.2 | 0.75 | 0.73 | 0.48 |
| VO2/HRmax | 12.6 ± 3.3 | 13.0 ± 3.0 | 0.23 | 12.7 ± 2.9 | 12.8 ± 2.6 | 0.69 | 0.63 | 0.51 |
| VE/(VO2)max | 37.2 ± 5.0 | 36.3 ± 2.2 | 0.54 | 37.4 ± 4.8 | 37.3 ± 4.5 | 0.56 | 0.54 | 0.62 |
| VE/V(CO2)max | 33.0 ± 4.4 | 32.4 ± 4.3 | 0.60 | 32.9 ± 4.1 | 33.2 ± 3.8 | 0.33 | 0.38 | 0.43 |
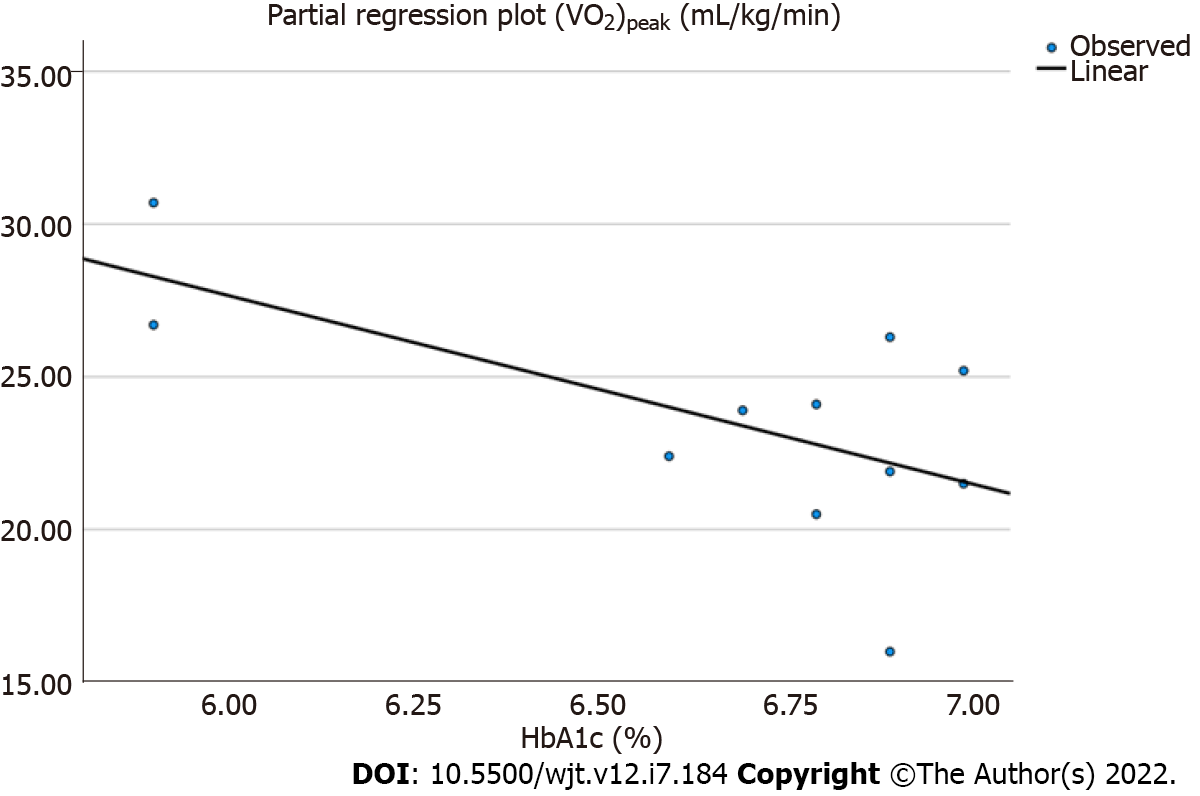
Lastly, linear regression analysis showed that there was a moderate, positive correlation only between (VO2)peak and HbA1c after training in the exercise group (r = 0.408, P = 0.03) (Figure 2).
The results of the present study demonstrated that a home-based aerobic and strengthening exercise training program improved serum lipids by lowering the TG and increasing the HDL levels and glucose metabolism, as reflected by fasting glucose and HbA1c levels in diabetic KT patients. Moreover, the improved cardiorespiratory fitness observed in the exercise patients was found to be linearly related to the improved HbA1c.
Randomized controlled trials on exercise training programs in KT patients are few, and so little is known about their positive or negative effects or the type, frequency or intensity of exercise in this population. Painter et al[13] was the first that studied the clinical effects of exercise training on CHD risk profile through the first year of renal transplantation. Results showed that even though exercise led to a statistically significant increase in HDL and decreased high TC-HDL ratio, which categorized patients at high CHD risk, exercise as the only modifiable parameter did not significantly reduce CHD risk in KT patients. Pooranfar et al[16] showed that a 10-wk, non-pharmaceutical, aerobic (at 45%-65% of maximum HR) and resistance exercise program statistically decreased TG, TC and LDL, while HDL remained unchanged.
A few years ago Juskowa et al[17] assessed the effects of early rehabilitation on the musculoskeletal system and blood atheromatic indices and found that after daily 30 min of exercise, FPG and HDL levels were statistically improved in the exercise group, while TC-HDL ratio was unchanged. There was also a positive correlation between improved graft function and muscle strength in the intervention group. The results of our study showed that a 6-mo mixed type exercise program led to a significant decrease in TG, FPG and HbA1c and an increase in HDL, without affecting the TC and LDL levels.
Interestingly, a 6-mo combined exercise program in our diabetic KT recipients led to a significantly improved lipid and glucose profile, while their functional capacity was enhanced, too. These results are very important, as cardiovascular mortality in KT patients is almost 10 times higher than in the general population[18], and the T2D prevalence according to global estimates will increase by 3.0%-6.0% at the end of 2025, with approximately 3 million T2D patients[19]. According to our results, combined exercise training in diabetic patients seems to be the most dominant choice.
De Feyter et al[20] showed that after a 5-mo progressive resistance training with high-intensity interval training, T2D patients under regular diabetes medication, had lower FPG and HbA1c levels, while HDL, LDL and TG did not statistically improve. Furthermore, Yavari et al[21] in a 52-wk aerobic, resistance or combined training program in 80 T2D patients found that aerobic or combined exercise statistically reduced TG, but the long-term combined exercise was associated with higher reductions both in HbA1c and TG levels compared to the aerobic or resistance training groups. Similarly, Cauza et al[22] compared the effects of short-term (4 mo) and long-term (8 mo) strength and endurance training on glucose and lipid control in 20 T2D patients. Results showed that HbA1c, TC, LDL and TG were statistically decreased in the group of the 8-mo combined training program, while the group in the 4-mo exercise program developed after the end of the exercise training an atherogenic lipid profile and did not improve glycemic control compared to those who continued exercising.
Our study revealed statistical differences between groups after a 6-mo combined exercise program in FPG and TG levels under stable diabetic medication for both groups, similarly to the above-mentioned studies. Maintenance of diabetic medication therapy is important to understand glycemic control and lipid profile relationship and to exact results towards effects of exercise on dyslipidemia without the impact of drugs[23].
According to a recent systematic review[24], structured exercise programs for KT patients have shown short-term improvements in aerobic capacity and muscular strength, while De Smet and Van Craenenbroeck[1] mentioned that exercise towards long-term effects is only slightly investigated. Improving functional capacity is very important for KT patients, with or without diabetes. According to Calella et al[24], exercise training improves the cardiovascular fitness of KT patients. However, (VO2)peak improvements were observed only after aerobic exercise training. In a recent randomized controlled trial, O’Connor et al[14] showed that (VO2)peak values have notably increased after a 12-wk non-supervised moderate-intensity aerobic exercise program and that after a 9-mo follow up there were statistically significant differences in the (VO2)peak values between the exercise and control groups.
On the contrary, Riess et al[25] showed that a supervised endurance and strength exercise program did not improve the cardiovascular disease score, although it improved the aerobic capacity and muscle strength of the KT recipients, who were taking statins and immunosuppression medication. Moreover, in a previous study of ours a 15.8% increase in (VO2)peak after a 6-mo aerobic exercise training on KT patients was also noted[26]. At the end of the study, we found a statistically significant increase of 4.7% in (VO2)peak of the exercise group and a significant intergroup difference in (VO2)peak.
This study has some limitations that need to be taken into consideration. Firstly, the sample size was small, which may decrease the power of our findings. However, this study included a 6-mo intervention, which is a considerably long period for patients. Secondly, the biochemical tests were performed only at baseline and after 6 mo. Unfortunately, there was neither an assessment in the middle of the study nor a follow-up. Thus, larger randomized controlled trials should be implemented in the specific population to confirm the favorable effects of exercise on their metabolic profile.
In conclusion, long-term aerobic and strengthening exercise training in diabetic KT patients was found to have many beneficial effects on patients’ metabolic profiles and functional capacities. The results of the present study demonstrated that a long-term exercise training program is feasible and effective in diabetic KT recipients. It is a major challenge to change their daily routine into active living to sustain their physical fitness and the benefits achieved by systemic exercise training.
According to the existing literature, kidney transplant (KT) recipients with diabetes mellitus seem to have low physical activity levels, while dyslipidemia and abnormal glucose profile are common cardiovascular risk factors.
As little is known about the effects of systematic exercise on the metabolic profile and cardiovascular risk of KT patients, we believe that this study will positively contribute to the literature gap.
This study aimed to investigate the effects of a mixed type 6-mo exercise program on functional capacity, glucose and lipid profile of KT patients with diabetes mellitus.
KT patients were randomly divided into two groups. Both exercise and control groups underwent biochemical blood analysis, in order to determine lipid and glucose levels, at baseline and at the end of the study. Cardiopulmonary exercise testing was also done to assess functional capacity.
At the end of the 6-mo study, fasting plasma glucose, glycated hemoglobin, triglycerides, high-density lipoprotein and the peak oxygen uptake [(VO2)peak] were statistically improved in the exercise group, while a positive linear relationship between peak oxygen uptake and glycated hemoglobin was also found (r = 0.408, P = 0.03).
According to the results, a 6-mo home-based mixed type exercise training program can significantly improve the metabolic profile and functional capacity of diabetic KT recipients.
It is crucial for future larger randomized controlled trials to explore the side effects of exercise on the metabolic profile and respiratory responses of diabetic KT recipients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lee KS, South Korea; Long P, China; Shalaby MN, Egypt S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | De Smet S, Van Craenenbroeck AH. Exercise training in patients after kidney transplantation. Clin Kidney J. 2021;14:ii15-ii24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Rysz J, Franczyk B, Radek M, Ciałkowska-Rysz A, Gluba-Brzózka A. Diabetes and Cardiovascular Risk in Renal Transplant Patients. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1087] [Cited by in RCA: 1043] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 4. | Imran HM, Baig M, Erqou S, Taveira TH, Shah NR, Morrison A, Choudhary G, Wu WC. Home-Based Cardiac Rehabilitation Alone and Hybrid With Center-Based Cardiac Rehabilitation in Heart Failure: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2019;8:e012779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Ponticelli C, Favi E. Physical Inactivity: A Modifiable Risk Factor for Morbidity and Mortality in Kidney Transplantation. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Armstrong K, Rakhit D, Jeffriess L, Johnson D, Leano R, Prins J, Garske L, Marwick T, Isbel N. Cardiorespiratory fitness is related to physical inactivity, metabolic risk factors, and atherosclerotic burden in glucose-intolerant renal transplant recipients. Clin J Am Soc Nephrol. 2006;1:1275-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Zelle DM, Corpeleijn E, Stolk RP, de Greef MH, Gans RO, van der Heide JJ, Navis G, Bakker SJ. Low physical activity and risk of cardiovascular and all-cause mortality in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6:898-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Kang AW, Garber CE, Eaton CB, Risica PM, Bostom AG. Physical Activity and Cardiovascular Risk among Kidney Transplant Patients. Med Sci Sports Exerc. 2019;51:1154-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Bellizzi V, Cupisti A, Capitanini A, Calella P, D'Alessandro C. Physical activity and renal transplantation. Kidney Blood Press Res. 2014;39:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Williams TJ, McKenna MJ. Exercise limitation following transplantation. Compr Physiol. 2012;2:1937-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Zanotto T, Gobbo S, Bullo V, Vendramin B, Roma E, Duregon F, Bocalini DS, Di Blasio A, Cugusi L, Furian L, Di Bella C, Neunhaeuserer D, Battista F, Bergamin M, Ermolao A. Postural balance, muscle strength, and history of falls in end-stage renal disease patients living with a kidney transplant: A cross-sectional study. Gait Posture. 2020;76:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Baggetta R, D'Arrigo G, Torino C, ElHafeez SA, Manfredini F, Mallamaci F, Zoccali C, Tripepi G; EXCITE Working group. Effect of a home based, low intensity, physical exercise program in older adults dialysis patients: a secondary analysis of the EXCITE trial. BMC Geriatr. 2018;18:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Painter PL, Hector L, Ray K, Lynes L, Paul SM, Dodd M, Tomlanovich SL, Ascher NL. Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am J Kidney Dis. 2003;42:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | O'Connor EM, Koufaki P, Mercer TH, Lindup H, Nugent E, Goldsmith D, Macdougall IC, Greenwood SA. Long-term pulse wave velocity outcomes with aerobic and resistance training in kidney transplant recipients - A pilot randomised controlled trial. PLoS One. 2017;12:e0171063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Nerlekar N, Mulley W, Rehmani H, Ramkumar S, Cheng K, Vasanthakumar SA, Rashid H, Barton T, Nasis A, Meredith IT, Moir S, Mottram PM. Feasibility of exercise stress echocardiography for cardiac risk assessment in chronic kidney disease patients prior to renal transplantation. Clin Transplant. 2016;30:1209-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Pooranfar S, Shakoor E, Shafahi M, Salesi M, Karimi M, Roozbeh J, Hasheminasab M. The effect of exercise training on quality and quantity of sleep and lipid profile in renal transplant patients: a randomized clinical trial. Int J Organ Transplant Med. 2014;5:157-165. [PubMed] |
| 17. | Juskowa J, Lewandowska M, Bartłomiejczyk I, Foroncewicz B, Korabiewska I, Niewczas M, Sierdziński J. Physical rehabilitation and risk of atherosclerosis after successful kidney transplantation. Transplant Proc. 2006;38:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Neale J, Smith AC. Cardiovascular risk factors following renal transplant. World J Transplant. 2015;5:183-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 19. | Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9344] [Cited by in RCA: 8964] [Article Influence: 426.9] [Reference Citation Analysis (1)] |
| 20. | De Feyter HM, Praet SF, van den Broek NM, Kuipers H, Stehouwer CD, Nicolay K, Prompers JJ, van Loon LJ. Exercise training improves glycemic control in long-standing insulin-treated type 2 diabetic patients. Diabetes Care. 2007;30:2511-2513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Yavari A, Najafipoor F, Aliasgharzadeh A, Niafar M, Mobasseri M. Effect of aerobic exercise, resistance training or combined training on glycaemic control and cardiovascular risk factors in patients with type 2 diabetes. Biology Sport. 2012;29:135-143. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Cauza E, Hanusch-Enserer U, Strasser B, Kostner K, Dunky A, Haber P. The metabolic effects of long term exercise in Type 2 Diabetes patients. Wien Med Wochenschr. 2006;156:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Wang S, Ji X, Zhang Z, Xue F. Relationship between Lipid Profiles and Glycemic Control Among Patients with Type 2 Diabetes in Qingdao, China. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Calella P, Hernández-Sánchez S, Garofalo C, Ruiz JR, Carrero JJ, Bellizzi V. Exercise training in kidney transplant recipients: a systematic review. J Nephrol. 2019;32:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Riess KJ, Haykowsky M, Lawrance R, Tomczak CR, Welsh R, Lewanczuk R, Tymchak W, Haennel RG, Gourishankar S. Exercise training improves aerobic capacity, muscle strength, and quality of life in renal transplant recipients. Appl Physiol Nutr Metab. 2014;39:566-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Kouidi E, Vergoulas G, Anifanti M, Deligiannis A. A randomized controlled trial of exercise training on cardiovascular and autonomic function among renal transplant recipients. Nephrol Dial Transplant. 2013;28:1294-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |