Published online Apr 18, 2022. doi: 10.5500/wjt.v12.i4.72
Peer-review started: June 1, 2021
First decision: September 2, 2021
Revised: September 13, 2021
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: April 18, 2022
Processing time: 315 Days and 21 Hours
Predispositions for severe coronavirus disease 2019 (COVID-19) are age, immunosuppression, and co-morbidity. High levels of maintenance immunosuppression render intestinal transplant (ITx) patients vulnerable for severe COVID-19. COVID-19 also provokes several gastroenterological pathologies which have not been discussed in ITx, so far.
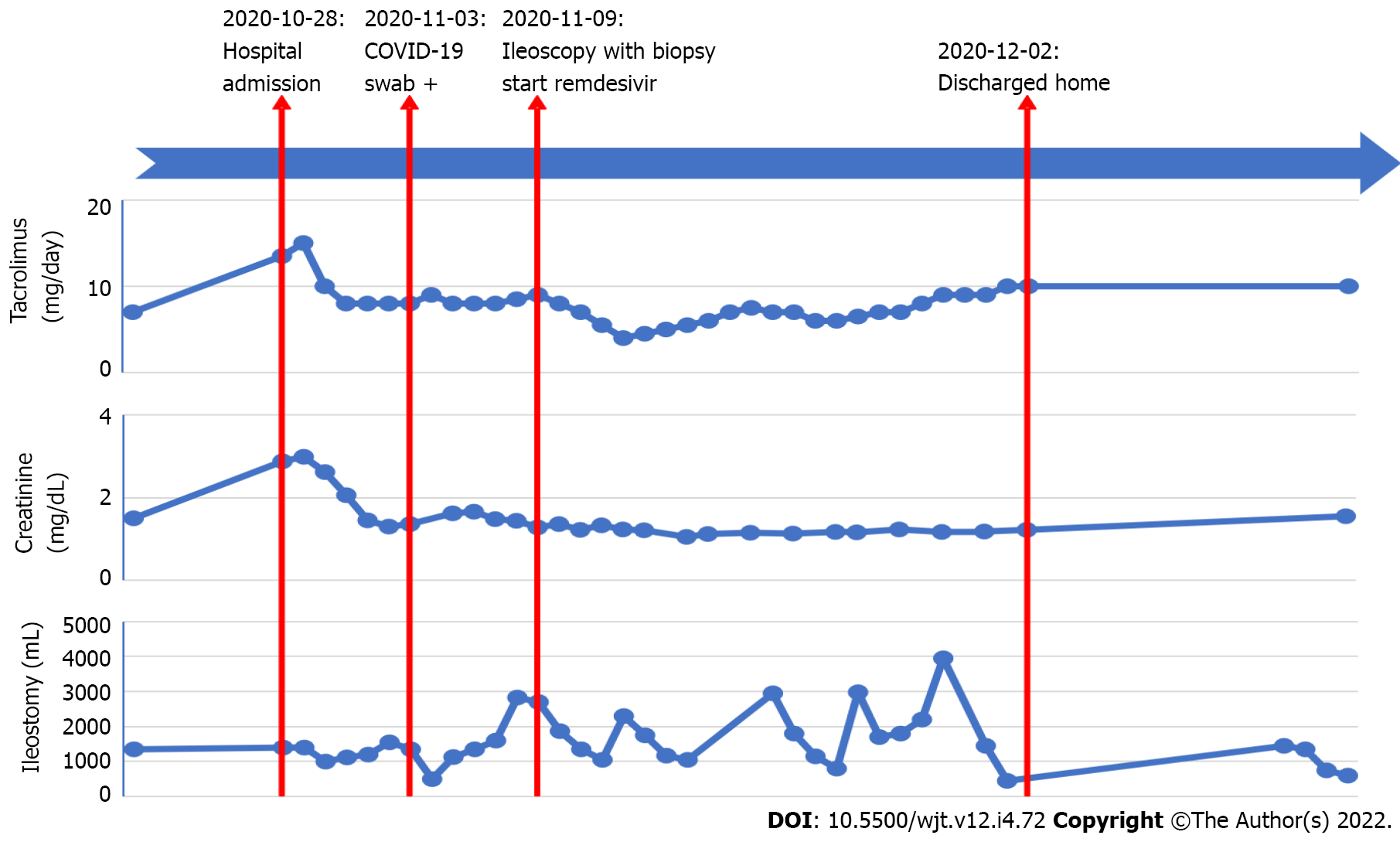
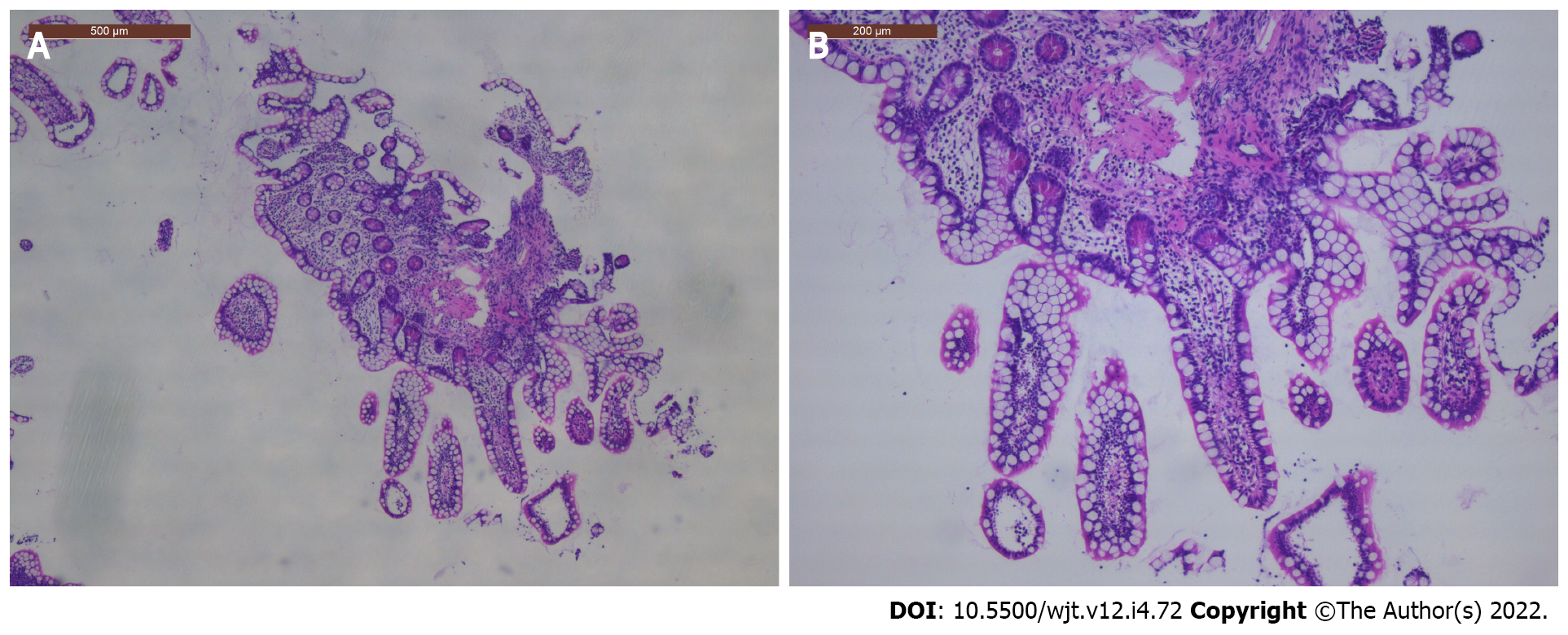
During the second European COVID-19 wave in November 2020, an ITx recipient was admitted to the hospital because of electrolyte disturbances due to dehydration. Immunosuppression consisted of tacrolimus, azathioprine, and low-dose corticosteroids. During hospitalization, she tested positive on screening COVID-19 nasopharyngeal polymerase chain reaction swab, while her initial test was negative. She was initially asymptomatic and had normal inflammatory markers. Tacrolimus levels were slightly raised, as Azathioprine was temporarily halted. Due to elevated D-dimers at that time, prophylactic low-molecular weight heparin was started. Seven days after the positive test, dyspnea, anosmia, and C-reactive protein increase (25 mg/L) were noted. Remdesivir was administered during 5 d in total. High stomal output was noted in two consecutive days and several days thereafter. To exclude infection or rejection, an ileoscopy and biopsy were performed and excluded these. Four weeks later, she was discharged from the hospital and remains in good health since then.
Early eradication of severe acute respiratory syndrome coronavirus 2 in ITx recipients may be warranted to prevent acute rejection provocation by it.
Core Tip: Acute rejection is often seen in intestinal transplant (ITx) recipients due to the high immunogenicity of the intestinal graft. However, it might also be provoked by latent presence of viruses, due to the high immunosuppression needs. Recently, chronic latency of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the intestine has been shown. Hence, early recognition, eradication, and follow-up on intestinal biopsies in ITx recipients might be warranted to prevent the potential acute rejection provocation of the intestinal graft by SARS-CoV-2.
- Citation: Clarysse M, Ceulemans LJ, Wauters L, Gilbo N, Capiau V, De Hertogh G, Laleman W, Verslype C, Monbaliu D, Pirenne J, Vanuytsel T. Potential importance of early treatment of SARS-CoV-2 infection in intestinal transplant patient: A case report. World J Transplant 2022; 12(4): 72-78
- URL: https://www.wjgnet.com/2220-3230/full/v12/i4/72.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i4.72
Coronavirus disease 2019 (COVID-19), provoked by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), poses a major challenge in intestinal transplantation (ITx) due to the high immunogenicity of the graft, requiring high levels of immunosuppression. In the early phase of the pandemic, patients were treated with hydroxychloroquine[1]. The treatment of SARS-CoV-2 in transplant patients was altered over time in favor of dexamethasone, antivirals, or only supportive therapy[2-4]. Next to this, it is known that SARS-CoV-2 provokes gastroenterological manifestations, due to its invasion of the enterocytes[5]. It has recently been shown that SARS-CoV-2 remained latent present in the upper gastrointestinal tract, as well as in the small intestine, until at least 3 mo post-COVID-19 positivity[6]. Several other latent gastrointestinal tract viruses are known to be able to provoke acute rejection of the intestinal graft, due to the high immunosuppression needs in these ITx recipients[7,8]. To our knowledge, the influence of SARS-CoV-2-related gastroenterological manifestations in ITx patients or the provoked risk for rejection have not been elucidated so far.
We recently encountered a SARS-CoV-2 infection in a 41-year-old female ITx-recipient, acquired during hospitalization for dehydration and electrolyte disturbances, during the second European COVID-19 wave in November 2020.
She underwent an isolated intestinal re-transplantation, combined with a kidney, in August 2019 for chronic allograft enteropathy. After her re-ITx, she underwent a conversion of her terminal ileostomy to a low ileorectal anastomosis with protective loopileostomy on September 29, 2020.
Her first isolated ITx was in December 2004 for chronic intestinal pseudo-obstruction with recurrent catheter sepsis. In between the two ITx procedures, she was in good health and never encountered an acute rejection, until she developed chronic allograft enteropathy for which she was back on parenteral nutrition since February 2019.
Negative.
On admission, on October 28, 2020, she was on tacrolimus (3.5 mg bidaily, target trough level: 7-8 μg/L), azathioprine (50 mg/d), and methylprednisolone (4 mg/d). She had no fever, respiratory issues, nor recent contact with a potential COVID-19 positive patient.
She tested negative on SARS-CoV-2 on a nasopharyngeal polymerase chain reaction (PCR)-test (Figure 1). Her lab values revealed an acute deterioration of kidney function and electrolyte disturbances. Six days after admission, on November 3, 2020, she tested positive for SARS-CoV-2 on a screening PCR-test.
There were no clinical nor biochemical signs of infection or chest X-ray alterations.
The final diagnosis of this presented case is mild COVID-19.
Azathioprine was temporarily halted, and tacrolimus levels slightly raised towards target trough levels of 8-9 μg/L. Prophylactic low-molecular weight heparin was started as D-dimers measured 4110 ng/mL (normal ≤ 500 ng/mL). She was transferred to the COVID-19 low-care ward of our hospital. Five days later, on November 8, 2020, her stomal output increased with 227% up to 2830 mL/24 h. As rejection was suspected, ileoscopy via the stoma was performed on November 9, 2020, and ileal biopsies were taken (Figure 2). These excluded inflammation or rejection. That same day, anosmia and mild dyspnea with normal oxygen saturation developed. Body temperature increased until 37.8 °C and C-reactive protein level was 25 mg/L (normal < 5 mg/L). Remdesivir was intravenously administered for 5 d with 200 mg as loading dose and 100 mg daily thereafter. After the remdesivir treatment was finished, azathioprine was restarted, and tacrolimus trough levels lowered to standard levels.
Weekly SARS-CoV-2 PCR remained positive, until a cycle threshold (Ct)-value of 39.22 was found, 4 wk after her first positive test, on November 30, 2020, and she was removed from the COVID-19 ward as the internal hospital protocol states when the Ct-value is > 29. Stomal output kept fluctuating for 1 mo, with several days of high output (> 1200 mL/24 h). With adequate fluid replacement, renal function remained stable, and the patient could be discharged on December 2, 2020 remaining in good health since then. SARS-CoV-2 PCR remained negative since then, and 3 mo after discharge from the hospital SARS-CoV-2 immunoglobulin G (IgG) nucleocapsid antigen was negative. The patient gave informed consent, and ethical approval from the institutional review board was obtained (S64844).
We present the first report, to our knowledge, of mild COVID-19 in an ITx-patient treated with remdesivir, prophylactic low-molecular weight heparin, and temporary interruption of azathioprine. As according to the currently available evidence in transplant recipients, azathioprine was halted and tacrolimus slightly raised in return[9,10]. However, it has recently been shown that solid organ transplant recipients can also be successfully treated without adjustment of immunosuppressive therapy and without any antiviral treatment[4]. Our patient was preemptively treated with remdesivir as antiviral treatment. Up till now, there is not much yet known about remdesivir treatment in solid organ transplant recipients[11]. Recent reports have shown its tolerability and safety in kidney transplant recipients, without effects on kidney or liver function[12,13]. However, it is strongly advised to monitor regularly liver biochemistry in patients treated with remdesivir, as hepatotoxic side effects have been described[11,14].
Although gastroenterological manifestations, including diarrhea, nausea, vomiting, and loss of appetite, are commonly seen in COVID-19 patients, symptomatology was mild in our case and limited to high stomal output[5,15,16]. These clinical symptoms might also be suggestive for an acute rejection in ITx recipients, which should be treated with an increase of immunosuppression or pulse corticosteroids, which is opposite in the case of an gastroenterological infectious process[8]. This symptomatic overlap renders the cause of the gastroenterological manifestations more difficult and hence influences the treatment strategy. If not treated promptly, acute rejection might eventually lead to intestinal graft loss[17]. Only endoscopic evaluation with histopathologic confirmation of acute rejection on biopsy can make a clear differentiation. A recent study showed that D-dimers > 1850 ng/mL, which was the case in our patient (up to 4110 ng/mL), is the best discriminator to find major intestinal mucosal abnormalities at endoscopy in COVID-19 positive patients[18].
It is known that viral entrance of SARS-CoV-2, by the angiotensin-converting enzyme 2 receptor, which is abundantly present in the enterocytes of the gastrointestinal tract, plays a major role[5,6,18]. This viral entrance provokes an acute inflammatory response, which coincides with ischemic damage due to the procoagulant state and endothelialitis, which has also been observed in ITx rejection[17,18]. Several other viruses have already been shown to mimic intestinal graft rejection by crypt apoptosis, such as cytomegalovirus, Epstein-Barr virus, adenovirus, and norovirus[7,8]. Close monitoring, during the postinfectious period of these viruses, is also important as the infection might provoke acute rejection of the intestinal graft[8]. For SARS-CoV-2, such a correlation has not been shown so far. However, as shown by Gaebler et al[6], SARS-CoV-2 can remain latent present in even asymptomatic patients at least 3 mo post-COVID-19[6]. As SARS-CoV-2 is able to enter the enterocytes by the angiotensin-converting enzyme 2-receptor and provoke an acute inflammatory response, it is hypothetically possible that SARS-CoV-2 might mimic or provoke acute rejection of the intestinal graft in ITx recipients as well. As such, follow-up of SARS-CoV-2 antigen on routine or screening , re
SARS-CoV-2 nucleocapsid (N) antibodies assay, on the Abbott Architect system, was negative in our patient, despite SARS-CoV-2 positive PCR 3 mo earlier. However, it has been shown that SARS-CoV-2 IgG anti-N are positive in only 62% of SARS-CoV-2 PCR positive transplant recipients 1-2 mo post-infection, whilst these are decreasing towards only 55% at 3-4 mo and even 38% at 5-7 mo post-infection. This decline in anti-N is mainly seen in mild disease form[19]. SARS-CoV-2 spike (S) antibodies, on the contrary, are more durable with IgG anti-S present in 92% at 1-2 mo, 84% at 3-4 mo, and even 76% at 6-7 mo post-infection in transplant recipients[4]. Next to this, the analysis was run on the Abbott Architect system, of which it has been shown that it is less sensitive in transplant recipients, in comparison to non-transplant recipients and in comparison to other assets, due to a different targeting antigen[20]. It is proposed that the spike antigen is more immunogenic than the nucleocapsid antigen in immunosuppressed patients[20]. On top of that, there is evidence that spike antibodies may provide functional immunity information, as there is a correlation between spike antibodies and neutralizing antibodies[21,22]. As such, analyzing the anti-S might be clinically more relevant than the anti-N in immunosuppressed patients[20].
Early treatment of SARS-CoV-2 should be considered in ITx recipients in order to eradicate the virus and to prevent acute rejection mimicry or provocation and potential graft loss. SARS-CoV-2 antigen determination on ileal biopsies of ITx recipients might be routinely performed to screen for the hypothesis of SARS-CoV-2 acute rejection mimicry or provocation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Transplantation Society, No. 27551; Intestinal Rehabilitation and Transplant Association, No. 27551; International Pediatric Transplant Association, No. 27551; The International Society of Experimental Microsurgery; The European Society for Organ Transplantation, No. 12825.
Specialty type: Transplantation
Country/Territory of origin: Belgium
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oltean M, Sweden S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Papa-Gobbi R, Bueno A, Serradilla J, Talayero P, Stringa P, Pascual-Miguel B, Alcolea-Sánchez A, González-Sacristan R, Andrés AM, López-Santamaría M, Rumbo M, Ramos-Boluda E, Hernández-Oliveros F. Novel coronavirus (SARS-CoV-2) infection in a patient with multivisceral transplant. Transpl Infect Dis. 2021;23:e13430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Sharma P, Chen V, Fung CM, Troost JP, Patel VN, Combs M, Norman S, Garg P, Colvin M, Aaronson K, Sonnenday CJ, Golob JL, Somers EC, Doshi MM. COVID-19 Outcomes Among Solid Organ Transplant Recipients: A Case-control Study. Transplantation. 2021;105:128-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Avery RK. COVID-19 Therapeutics for Solid Organ Transplant Recipients; 6 Months Into the Pandemic: Where Are We Now? Transplantation. 2021;105:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Søfteland JM, Friman G, von Zur-Mühlen B, Ericzon BG, Wallquist C, Karason K, Friman V, Ekelund J, Felldin M, Magnusson J, Haugen Löfman I, Schult A, de Coursey E, Leach S, Jacobsson H, Liljeqvist JÅ, Biglarnia AR, Lindnér P, Oltean M. COVID-19 in solid organ transplant recipients: A national cohort study from Sweden. Am J Transplant. 2021;21:2762-2773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 583] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 6. | Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, Cipolla M, Viant C, Barnes CO, Bram Y, Breton G, Hägglöf T, Mendoza P, Hurley A, Turroja M, Gordon K, Millard KG, Ramos V, Schmidt F, Weisblum Y, Jha D, Tankelevich M, Martinez-Delgado G, Yee J, Patel R, Dizon J, Unson-O'Brien C, Shimeliovich I, Robbiani DF, Zhao Z, Gazumyan A, Schwartz RE, Hatziioannou T, Bjorkman PJ, Mehandru S, Bieniasz PD, Caskey M, Nussenzweig MC. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1246] [Article Influence: 311.5] [Reference Citation Analysis (0)] |
| 7. | Talmon GA. Histologic features of cytomegalovirus enteritis in small bowel allografts. Transplant Proc. 2010;42:2671-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Soltys KA, Reyes JD, Green M. Risks and epidemiology of infections after intestinal transplantation. In: Transplant Infections. 4th edition. Ljungman P, Snydman D, Boeckh M, editors. Cham: Springer International Publishing, 2016: 235-248. |
| 9. | Yi SG, Rogers AW, Saharia A, Aoun M, Faour R, Abdelrahim M, Knight RJ, Grimes K, Bullock S, Hobeika M, McMillan R, Mobley C, Moaddab M, Huang HJ, Bhimaraj A, Ghobrial RM, Gaber AO. Early Experience With COVID-19 and Solid Organ Transplantation at a US High-volume Transplant Center. Transplantation. 2020;104:2208-2214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 10. | Azzi Y, Bartash R, Scalea J, Loarte-Campos P, Akalin E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation. 2021;105:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 236] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 11. | Elens L, Langman LJ, Hesselink DA, Bergan S, Moes DJAR, Molinaro M, Venkataramanan R, Lemaitre F. Pharmacologic Treatment of Transplant Recipients Infected With SARS-CoV-2: Considerations Regarding Therapeutic Drug Monitoring and Drug-Drug Interactions. Ther Drug Monit. 2020;42:360-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Elec AD, Oltean M, Goldis P, Cismaru C, Lupse M, Muntean A, Elec FI. COVID-19 after kidney transplantation: Early outcomes and renal function following antiviral treatment. Int J Infect Dis. 2021;104:426-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Buxeda A, Arias-Cabrales C, Pérez-Sáez MJ, Cacho J, Cabello Pelegrin S, Melilli E, Aladrén MJ, Galeano C, Lorenzo I, Mazuecos A, Saura IM, Franco A, Ruiz-Fuentes MDC, Sánchez-Cámara LA, Siverio O, Martin ML, González-García E, López V, Martin-Moreno PL, Moina I, Moral Berrio E, Moreso F, Portolés JM, Santana-Estupiñán R, Zárraga S, Canal C, Sánchez-Álvarez E, Pascual J, Crespo M. Use and Safety of Remdesivir in Kidney Transplant Recipients With COVID-19. Kidney Int Rep. 2021;6:2305-2315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 15. | Zhong P, Xu J, Yang D, Shen Y, Wang L, Feng Y, Du C, Song Y, Wu C, Hu X, Sun Y. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (1)] |
| 16. | Lei HY, Ding YH, Nie K, Dong YM, Xu JH, Yang ML, Liu MQ, Wei L, Nasser MI, Xu LY, Zhu P, Zhao MY. Potential effects of SARS-CoV-2 on the gastrointestinal tract and liver. Biomed Pharmacother. 2021;133:111064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (3)] |
| 17. | Rabant M, Racapé M, Petit LM, Taupin JL, Aubert O, Bruneau J, Barbet P, Goulet O, Chardot C, Suberbielle C, Lacaille F, Canioni D, Duong Van Huyen JP. Antibody-mediated rejection in pediatric small bowel transplantation: Capillaritis is a major determinant of C4d positivity in intestinal transplant biopsies. Am J Transplant. 2018;18:2250-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Vanella G, Capurso G, Burti C, Fanti L, Ricciardiello L, Souza Lino A, Boskoski I, Bronswijk M, Tyberg A, Krishna Kumar Nair G, Angeletti S, Mauro A, Zingone F, Oppong KW, de la Iglesia-Garcia D, Pouillon L, Papanikolaou IS, Fracasso P, Ciceri F, Rovere-Querini P, Tomba C, Viale E, Eusebi LH, Riccioni ME, van der Merwe S, Shahid H, Sarkar A, Yoo JWG, Dilaghi E, Speight RA, Azzolini F, Buttitta F, Porcari S, Petrone MC, Iglesias-Garcia J, Savarino EV, Di Sabatino A, Di Giulio E, Farrell JJ, Kahaleh M, Roelandt P, Costamagna G, Artifon ELA, Bazzoli F, Testoni PA, Greco S, Arcidiacono PG. Gastrointestinal mucosal damage in patients with COVID-19 undergoing endoscopy: an international multicentre study. BMJ Open Gastroenterol. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Chavarot N, Leruez-Ville M, Scemla A, Burger C, Amrouche L, Rouzaud C, Lebreton X, Martinez F, Sberro-Soussan R, Legendre C, Zuber J, Anglicheau D. Decline and loss of anti-SARS-CoV-2 antibodies in kidney transplant recipients in the 6 mo following SARS-CoV-2 infection. Kidney Int. 2021;99:486-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Prendecki M, Clarke C, Gleeson S, Greathead L, Santos E, McLean A, Randell P, Moore LSP, Mughal N, Guckian M, Kelleher P, Mcadoo SP, Willicombe M. Detection of SARS-CoV-2 Antibodies in Kidney Transplant Recipients. J Am Soc Nephrol. 2020;31:2753-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, Jiang K, Arunkumar GA, Jurczyszak D, Polanco J, Bermudez-Gonzalez M, Kleiner G, Aydillo T, Miorin L, Fierer DS, Lugo LA, Kojic EM, Stoever J, Liu STH, Cunningham-Rundles C, Felgner PL, Moran T, García-Sastre A, Caplivski D, Cheng AC, Kedzierska K, Vapalahti O, Hepojoki JM, Simon V, Krammer F. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1452] [Cited by in RCA: 1377] [Article Influence: 275.4] [Reference Citation Analysis (2)] |
| 22. | Premkumar L, Segovia-Chumbez B, Jadi R, Martinez DR, Raut R, Markmann A, Cornaby C, Bartelt L, Weiss S, Park Y, Edwards CE, Weimer E, Scherer EM, Rouphael N, Edupuganti S, Weiskopf D, Tse LV, Hou YJ, Margolis D, Sette A, Collins MH, Schmitz J, Baric RS, de Silva AM. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 689] [Article Influence: 137.8] [Reference Citation Analysis (0)] |