Published online Mar 18, 2022. doi: 10.5500/wjt.v12.i3.59
Peer-review started: September 22, 2021
First decision: October 27, 2021
Revised: November 15, 2021
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: March 18, 2022
Processing time: 176 Days and 2.6 Hours
Autoimmune hepatitis is a chronic inflammatory disease of the liver that is characterized by circulating autoantibodies and elevated serum globulin levels. Liver transplantation may be required for patients with acute liver failure, decompensated cirrhosis, and hepatocellular carcinoma. Recurrence is defined as development of the same disease in the allograft following liver transplantation. Autoimmune hepatitis recurs in 36%-68% of the recipients 5 years after liver transplantation. De novo autoimmune hepatitis is the development of autoimmune hepatitis like clinical and laboratory characteristics in patients who had undergone liver transplantation for causes other than autoimmune hepatitis. Diagnostic work up for recurrent and de novo autoimmune hepatitis is similar to the diagnosis of the original disease, and it is usually difficult. Predniso(lo)ne with or without azathioprine is the main treatment for recurrent and de novo autoimmune hepatitis. Early diagnosis and treatment are vital for patient prognosis because de novo autoimmune hepatitis and recurrent autoimmune hepatitis cause graft loss and result in subsequent retransplantation if medical treatment fails.
Core Tip: Autoimmune hepatitis is a chronic inflammatory disease of the liver that is characterized by circulating autoantibodies and elevated serum globulin levels. Liver transplantation may be required for patients with acute liver failure, decompensated cirrhosis, and hepatocellular carcinoma. De novo autoimmune hepatitis and recurrent autoimmune hepatitis are known causes of late graft dysfunction following liver transplantation which should be included in the differential diagnosis.
- Citation: Harputluoglu M, Caliskan AR, Akbulut S. Autoimmune hepatitis and liver transplantation: Indications, and recurrent and de novo autoimmune hepatitis. World J Transplant 2022; 12(3): 59-64
- URL: https://www.wjgnet.com/2220-3230/full/v12/i3/59.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i3.59
Autoimmune hepatitis is a chronic inflammatory disease of the liver that is characterized by circulating autoantibodies and elevated serum globulin levels. This disease may manifest as elevated liver transaminases, acute hepatitis, cirrhosis or acute liver failure[1]. Autoimmune hepatitis is classified into types 1 and 2. Patients with positive antinuclear antibody (ANA) and/or anti-smooth muscle antibody (anti-SMA) are classified as type 1, whereas type 2 is defined by the presence of anti-liver-kidney microsomal type 1 antibody (anti-LKM-1) or anti-liver cytosol type 1 antibody (anti-LC-1) positivity. Autoimmune hepatitis is mainly treated with immunosuppressive drugs such as glucocorticoids and azathioprine (AZA). In this review, indications for liver transplantation in patients with autoimmune hepatitis and the diagnosis and treatment of recurrent autoimmune hepatitis after liver transplantation are discussed. Additionally, de novo autoimmune hepatitis, which can be seen in patients who have received liver transplantation for indications other than autoimmune hepatitis, are discussed.
Liver transplantation may be indicated for patients with autoimmune hepatitis if one of the following conditions are present: (1) Acute liver failure; (2) Decompensated cirrhosis (Model for End-Stage Liver Disease score ≥ 15); or (3) Hepatocellular carcinoma. Liver transplantation may be required if there is a failure to diagnose and treat autoimmune hepatitis, inadequate response or intolerance to immunosuppressive therapy, or if the patients are not compliant with the treatment. Ultimately, 10%-20% of patients with autoimmune hepatitis eventually need liver transplantation[2,3].
Autoimmune hepatitis accounts for approximately 5% and 2%-3% of liver transplants in the United States and Europe, respectively[4,5]. The frequency of acute and chronic rejection after liver trans-plantation for autoimmune hepatitis is more frequent compared to other liver diseases[6]. Five-year patient and graft survivals for autoimmune hepatitis are reported to be 80%-90% and 72%-74%, respectively[7].
Recurrence of autoimmune hepatitis after liver transplantation: Recurrence is defined as reappearance of the disease in the liver allograft. Autoimmune hepatitis recurs in 8%-12% of patients within the first year and 36%-68% within 5 years following liver transplantation[6]. Recurrent autoimmune hepatitis frequency is not significantly affected by the graft type (either living related or cadaveric)[8]. Diagnostic workup of recurrent autoimmune hepatitis is similar to diagnosing the original disease and it is equally challenging. The main reason for the complexity in diagnosis is the absence of a specific marker for diagnosis. In addition, immunosuppressive therapy may mask some features of the original disease. The disease progression may differ and may lead to an atypical presentation. Transplant recipients with recurrence of autoimmune hepatitis usually have elevated transaminases, fever, fatigue, jaundice, abdominal pain, skin rash, and joint pain upon presentation[9]. Nevertheless, the presentation of recurrence of autoimmune hepatitis is not specific and can be seen in other complications of liver transplantation. Hypergammaglobulinemia is defined as increased serum IgG levels, and together with positivity of ANA and SMA, make up the serological findings of the disease. The pathophysiology of recurrent autoimmune hepatitis is not comprehensively understood and is similar to the mechanisms involved in the development of classical autoimmune hepatitis. The main histopathological feature of recurrent autoimmune hepatitis is prominent lymphocytic interface activity with or without plasma cell infiltration. Other pathological findings are acute lobular hepatitis with focal hepatocyte necrosis, acidophil bodies with lymphoplasmacytic cells, pseudo-rosetting of hepatocytes, perivenular lymphoplasmacytic inflammation, and confluent and bridging necrosis with lymphoplasmacytic infiltration (severe inflammatory activity)[10]. Cellular and antibody-mediated forms of cytotoxicity are involved in the pathogenesis of the disease. These features may be less evident or absent in certain instances. The differential diagnoses include rejection, drug hepatotoxicity, de novo steatohepatitis, and viral hepatitis, including hepatitis E. The diagnosis is performed by excluding other possible etiologies.
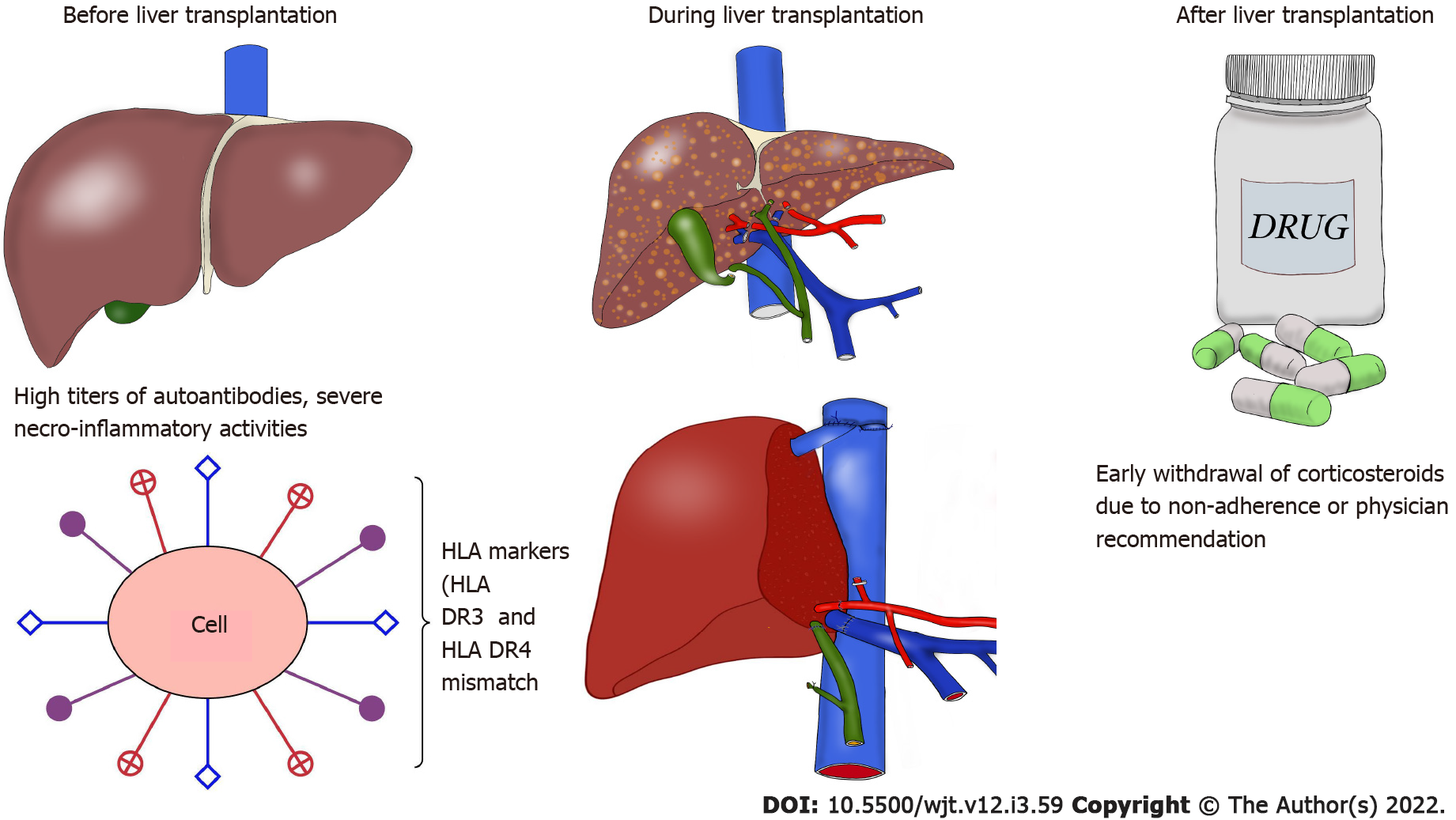
Many risk factors such as the effects of immunosuppressive therapy as well as recipient- and donor-related factors play an important part in the recurrence of autoimmune hepatitis in the liver allograft. Early corticosteroid withdrawal for reasons such as nonadherence or physician recommendation, high titers of autoantibodies at the time of liver transplantation, coexisting autoimmune disorders, association of human leukocyte antigen (HLA)-DR3 and HLA-DR4 mismatch, and severe necroinflammatory activities in the explant liver at the time of liver transplantation are some of the reported risk factors of recurrence[9]. Figure 1 summarizes the factors implicated in the development of recurrent autoimmune hepatitis.
Recurrent autoimmune hepatitis needs prompt treatment because nearly half of cases are resistant to therapy and result in graft failure. Treatment is usually empirical. In mild cases, only increasing compliance with immunosuppressive therapy and increasing immunosuppressive doses are sufficient. In severe cases, predniso(lo)ne (30 mg/d) and AZA (1-2 mg/kg/d) are required. The combination of corticosteroids and mycophenolate mofetil (MMF) may also be the initial therapeutic approach[6]. When laboratory values improve, the dose of corticosteroids is tapered to 5-10 mg within 1-2 mo[9,11]. Patients who do not respond to this combination are considered for other immunosuppressive agents such as calcineurin inhibitors or inhibitors of mammalian target of rapamycin. In cases with severe liver failure, retransplantation may be required. It has been reported that retransplantation is required in 33%-60% of patients with recurrent autoimmune hepatitis[6,12,13].
De novo autoimmune hepatitis: De novo autoimmune hepatitis is the development of autoimmune hepatitis in patients who underwent liver transplantation for reasons other than autoimmune hepatitis. In its latest update, the Banff Working Group for liver allograft pathology proposed replacing the term de novo autoimmune hepatitis with plasma cell-rich rejection[14]. De novo autoimmune hepatitis is more common in children than in adults (5%-10% vs 1%-3%)[6,11]. Clinical findings in de novo autoimmune hepatitis are similar to those observed in recurrent autoimmune hepatitis and autoimmune hepatitis. Serum aspartate aminotransferase, alanine aminotransferase, and IgG levels are high. One of the most striking features of de novo autoimmune hepatitis is detection of newly developed autoantibodies. Patients with de novo autoimmune hepatitis may have ANA, antimitochondrial antibody, anti-SMA antibodies and also anti-LKM-1, anti-LC, antibodies to gastric parietal cells, and atypical anti-liver/kidney cytosolic antibody targeting the antigen glutathione-S-transferase T1 (GSTT1) may be positive. The main histological feature in de novo autoimmune hepatitis is interface hepatitis with lymphocytes and plasma cells. Other histopathological features are spotty necrosis, portal fibrosis, and bile duct injury[15].
Older donors, the mismatch of GSTT1 genotype of donor and recipient, the use of antilymphocyte antibodies, treatment with tacrolimus or MMF are associated with a higher risk of de novo autoimmune hepatitis[16]. Cyclosporine A and granulocyte colony-stimulating factor treatment is reported to be protective against de novo autoimmune hepatitis. The pathogenesis of de novo autoimmune hepatitis is still unknown. Although it has been suggested that antibodies against GSST1 antigen may play a role in the development, it may also develop in the absence of these antibodies. Therefore, the role of antibodies against GSST1 antigens in pathogenesis is not fully established. One of the possible mechanisms for the development of de novo autoimmune hepatitis is the release of autoantigens from the damaged tissue during reperfusion which exacerbates the autoimmune response after liver transplantation. Other possibilities are due to molecular similarities; in other words, exposure to microorganisms that share amino acid sequences with autoantigens causing crossreactive immunity. In fact, viral infections (which are common after transplantation) can cause autoimmunity by various mechanisms[17]. In addition, interferons used for hepatitis C have potent immunomodulatory effects and can trigger autoimmune disorders in immunosuppressive patients. Today, since interferon-free treatment regimens are used in the treatment of hepatitis C after liver transplantation, hepatitis C patients are now safer in terms of the risks of interferon after transplantation.
While the results of treatment of de novo autoimmune hepatitis are promising, poor outcomes such as cirrhosis and graft loss can be seen if these patients are not treated properly. Therefore, early diagnosis and treatment of this disease has paramount importance. Predniso(lo)ne with or without AZA continues to be the mainstay of treatment for de novo autoimmune hepatitis. If there is no response to these agents, then MMF can be given instead of AZA[11].
The risk of acute and chronic rejection in patients undergoing liver transplantation for autoimmune hepatitis is higher than in patients who are transplanted for other indications. Corticosteroids may prevent development of rejection or relapse on the long term however, usually they are tapered to reduce the risk of infections and adverse effects of steroids. Corticosteroids have many side effects, including infection, depression, osteoporosis, diabetes, hypertension and adrenal suppression, which significantly affect the quality of life in recipients following liver transplantation[18]. The issue of how long corticosteroids should be given to prevent rejection and relapse in patients with autoimmune hepatitis remains a controversial issue. There have been few studies on the long-term administration of corticosteroids after transplantation in autoimmune hepatitis patients. In a study involving 73 patients with autoimmune hepatitis who underwent liver transplantation, it has been shown that long-term treatment with low-dose corticosteroid in combination with other immunosuppressive medication reduced recurrence rates of autoimmune hepatitis[19]. The recent American Association for the Study of Liver Diseases (AASLD) guidelines emphasize that the data supporting the long-term administration of corticosteroids to prevent post-transplant rejection, graft loss and recurrent autoimmune hepatitis are limited and the treatment is not justified. Therefore, AASLD suggested corticosteroids should be gradually tapered in following liver transplantation[6]. The latest European Association for the Study of the Liver guidelines regarding autoimmune hepatitis do not provide a clear recommendation on how long corticosteroids should be given after transplantation[20].
Another alternative approach is meticulous selection of patients that are at high risk of recurrence and who may benefit from intensified immunosuppression. This group of patients should receive long-term steroids. Steroids should be tapered gradually with close follow-up, if the risk of recurrence is low and long-term steroid administration would cause additional problems in the patients such in patients with diabetes, hypertension, hyperlipidemia and osteoporosis[21].
Until a specific marker is developed or standardization of the diagnosis of recurrent or de novo autoimmune hepatitis is developed, steroids will always be an important part of treatment and duration of steroid use will always be a matter of debate.
De novo autoimmune hepatitis and recurrent autoimmune hepatitis are known causes of late graft dysfunction in pediatric and adult liver transplantation. In liver transplant recipients with graft dysfunction, recurrent or de novo autoimmune hepatitis should always be considered in differential diagnosis. Early diagnosis and intervention are vital in de novo and recurrent autoimmune hepatitis because they cause graft loss and subsequent re-transplantation if they are not treated properly.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Fallatah H, Zhou S S-Editor: Wang JJ L-Editor: Kerr C P-Editor: Wang JJ
| 1. | Wang Q, Yang F, Miao Q, Krawitt EL, Gershwin ME, Ma X. The clinical phenotypes of autoimmune hepatitis: A comprehensive review. J Autoimmun. 2016;66:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Gleeson D, Heneghan MA; British Society of Gastroenterology. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2011;60:1611-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1010] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 4. | Mendes F, Couto CA, Levy C. Recurrent and de novo autoimmune liver diseases. Clin Liver Dis. 2011;15:859-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, Pollard S, Lerut J, Paul A, Garcia-Valdecasas JC, Rodríguez FS, Burroughs A; All contributing centers (www. eltr.org); European Liver and Intestine Transplant Association (ELITA). Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 642] [Article Influence: 49.4] [Reference Citation Analysis (2)] |
| 6. | Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 571] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 7. | Futagawa Y, Terasaki PI. An analysis of the OPTN/UNOS Liver Transplant Registry. Clin Transpl. 2004;315-329. [PubMed] |
| 8. | Aravinthan AD, Doyle AC, Issachar A, Dib M, Peretz D, Cattral MS, Ghanekar A, McGilvray ID, Selzner M, Greig PD, Grant DR, Selzner N, Lilly LB, Renner EL. First-Degree Living-Related Donor Liver Transplantation in Autoimmune Liver Diseases. Am J Transplant. 2016;16:3512-3521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Kerkar N, Yanni G. ‘De novo’ and ‘recurrent’ autoimmune hepatitis after liver transplantation: A comprehensive review. J Autoimmun. 2016;66:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Banff Working Group; Demetris AJ, Adeyi O, Bellamy CO, Clouston A, Charlotte F, Czaja A, Daskal I, El-Monayeri MS, Fontes P, Fung J, Gridelli B, Guido M, Haga H, Hart J, Honsova E, Hubscher S, Itoh T, Jhala N, Jungmann P, Khettry U, Lassman C, Ligato S, Lunz JG 3rd, Marcos A, Minervini MI, Mölne J, Nalesnik M, Nasser I, Neil D, Ochoa E, Pappo O, Randhawa P, Reinholt FP, Ruiz P, Sebagh M, Spada M, Sonzogni A, Tsamandas AC, Wernerson A, Wu T, Yilmaz F. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 2006;44:489-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Stirnimann G, Ebadi M, Czaja AJ, Montano-Loza AJ. Recurrent and De Novo Autoimmune Hepatitis. Liver Transpl. 2019;25:152-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Ratziu V, Samuel D, Sebagh M, Farges O, Saliba F, Ichai P, Farahmand H, Gigou M, Féray C, Reynès M, Bismuth H. Long-term follow-up after liver transplantation for autoimmune hepatitis: evidence of recurrence of primary disease. J Hepatol. 1999;30:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Reich DJ, Fiel I, Guarrera JV, Emre S, Guy SR, Schwartz ME, Miller CM, Sheiner PA. Liver transplantation for autoimmune hepatitis. Hepatology. 2000;32:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Demetris AJ, Bellamy C, Hübscher SG, O’Leary J, Randhawa PS, Feng S, Neil D, Colvin RB, McCaughan G, Fung JJ, Del Bello A, Reinholt FP, Haga H, Adeyi O, Czaja AJ, Schiano T, Fiel MI, Smith ML, Sebagh M, Tanigawa RY, Yilmaz F, Alexander G, Baiocchi L, Balasubramanian M, Batal I, Bhan AK, Bucuvalas J, Cerski CTS, Charlotte F, de Vera ME, ElMonayeri M, Fontes P, Furth EE, Gouw ASH, Hafezi-Bakhtiari S, Hart J, Honsova E, Ismail W, Itoh T, Jhala NC, Khettry U, Klintmalm GB, Knechtle S, Koshiba T, Kozlowski T, Lassman CR, Lerut J, Levitsky J, Licini L, Liotta R, Mazariegos G, Minervini MI, Misdraji J, Mohanakumar T, Mölne J, Nasser I, Neuberger J, O’Neil M, Pappo O, Petrovic L, Ruiz P, Sağol Ö, sanchez Fueyo A, Sasatomi E, Sha’ed A, Shiller M, Shimizu T, Sis B, Sonzogni A, Stevenson HL, Thung SN, Tisone G, Tsamandas AC, Wernerson A, Wu T, Zeevi A, Zen Y. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am J Transplant. 2016;16:2816-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 430] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 15. | González IA, Hartley CP, Nalbantoglu I. Recurrent Autoimmune Hepatitis and De Novo Autoimmune Hepatitis in the Liver Allograft. Am J Clin Pathol. 2021;155:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Montano-Loza AJ, Vargas-Vorackova F, Ma M, Bain VG, Burak K, Kumar T, Mason AL. Incidence and risk factors associated with de novo autoimmune hepatitis after liver transplantation. Liver Int. 2012;32:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Kerkar N, Vergani D. De novo autoimmune hepatitis -is this different in adults compared to children? J Autoimmun. 2018;95:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 457] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Krishnamoorthy TL, Miezynska-Kurtycz J, Hodson J, Gunson BK, Neuberger J, Milkiewicz P, Oo YH. Longterm corticosteroid use after liver transplantation for autoimmune hepatitis is safe and associated with a lower incidence of recurrent disease. Liver Transpl. 2016;22:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 847] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 21. | Theocharidou E, Heneghan MA. Con: Steroids Should Not Be Withdrawn in Transplant Recipients With Autoimmune Hepatitis. Liver Transpl. 2018;24:1113-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |