Published online Nov 18, 2022. doi: 10.5500/wjt.v12.i11.365
Peer-review started: June 15, 2022
First decision: August 4, 2022
Revised: August 26, 2022
Accepted: October 18, 2022
Article in press: October 18, 2022
Published online: November 18, 2022
Processing time: 154 Days and 12.9 Hours
Liver transplantation is the most important therapeutic intervention for end-stage liver disease (ELD). The prioritization of these patients is based on the model for end-stage liver disease (MELD), which can successfully predict short-term mortality. However, despite its great validity and value, it cannot fully incor
To evaluate the effects of an active lifestyle on physical frailty on liver transplant candidates.
An observational study was performed within the facilities of the Department of Transplant Surgery of Aristotle University of Thessaloniki. Twenty liver tran
According to their responses in the IPAQ, patients were divided into the following two groups based on their activity level: Active group (A, 10 patients); and sedentary group (S, 10 patients). Comparing mean values of the recorded variables showed the following results: MELD (A: 12.05 ± 5.63 vs S: 13.99 ± 3.60; P > 0.05); peak oxygen uptake (A: 29.78 ± 6.07 mL/kg/min vs S: 18.11 ± 3.39 mL/kg/min; P < 0.001); anaerobic threshold (A: 16.71 ± 2.17 mL/kg/min vs S: 13.96 ± 1.45 mL/kg/min; P < 0.01); 6MWT (A: 458.2 ± 57.5 m vs S: 324.7 ± 55.8 m; P < 0.001); and LFI (A: 3.75 ± 0.31 vs S: 4.42 ± 0.32; P < 0.001).
An active lifestyle can be associated with better musculoskeletal and functional capacity, while simultaneously preventing the evolution of physical frailty in liver transplant candidates. This effect appears to be independent of the liver disease severity.
Core Tip: This study highlights the importance of regular physical activity and exercise of low and medium intensities in the routine of liver transplant candidates. As liver transplantation is a highly demanding procedure, imposing a significant amount of stress across every system, physical frailty is steadily proving to be a factor of great importance, not only due to its role in mortality prediction but also due to its potential improvement via preoperative interventions.
- Citation: Oikonomou IM, Sinakos E, Antoniadis N, Goulis I, Giouleme O, Anifanti M, Katsanos G, Karakasi KE, Tsoulfas G, Kouidi E. Effects of an active lifestyle on the physical frailty of liver transplant candidates. World J Transplant 2022; 12(11): 365-377
- URL: https://www.wjgnet.com/2220-3230/full/v12/i11/365.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i11.365
Liver transplantation is the greatest tool for the management and treatment of end-stage liver disease (ELD)[1]. Nevertheless, there is a worldwide gap between the demand for liver transplants and the availability of organ donations[2], increasing the need for optimization of candidate prioritization and organ distribution[3]. It is well established in the literature that the model for end-stage liver disease (MELD) score is a unique tool in this direction[4]. Nevertheless, there are further clinical parameters that may play a substantial role in the waiting list mortality, especially in patients with lower MELD scores[5].
Sarcopenia is related to waiting list mortality and survival after liver transplantation[6-9]. Fur
Furthermore, physical frailty has been gaining growing attention due to its correlation with mortality prediction in liver transplantation. Physical frailty is a clinical syndrome that is correlated with both sarcopenia and functional capacity and is characterized by reduced strength and stamina, as well as increased mortality risk and postoperative dependence[19-21]. The Liver Frailty Index™ (LFITM) is an innovative tool, developed by Lai et al[22], which appears to significantly improve mortality prediction when combined with MELD, especially in patients with low MELD scores[22,23].
The course of liver disease is well correlated with a gradual diminishment of both functional capacity and musculoskeletal robustness. Taking the importance of the above clinical tools into consideration, not only on mortality prediction but also on patient prioritization, this observational study evaluated the effects of an active lifestyle on indices of physical functioning, in order to identify the effects of physical activity on physical frailty and cardiovascular capacity on liver transplant candidates.
Liver transplant candidates from the Department of Transplant Surgery of the Aristotle University of Thessaloniki in the Hippokration General Hospital of Thessaloniki were recruited for the study. Patients enlisted in the liver transplantation waiting list registry, according to criteria of the Hellenic Transplantation Organization, were deemed eligible for enrollment. The observational study design excluded patients with other comorbidities hindering their activity level or the ones having received instructions from their physicians to limit it, due to a recent acute deterioration of their condition.
Therefore, patients were deemed ineligible if one of the following was true: Recent cardiovascular incident in the preceding 12 mo; grade 2 or higher hepatic encephalopathy; bedridden patients with complete dependence; and recent hospital admission requiring longer than 72 h of inpatient care due to condition deterioration.
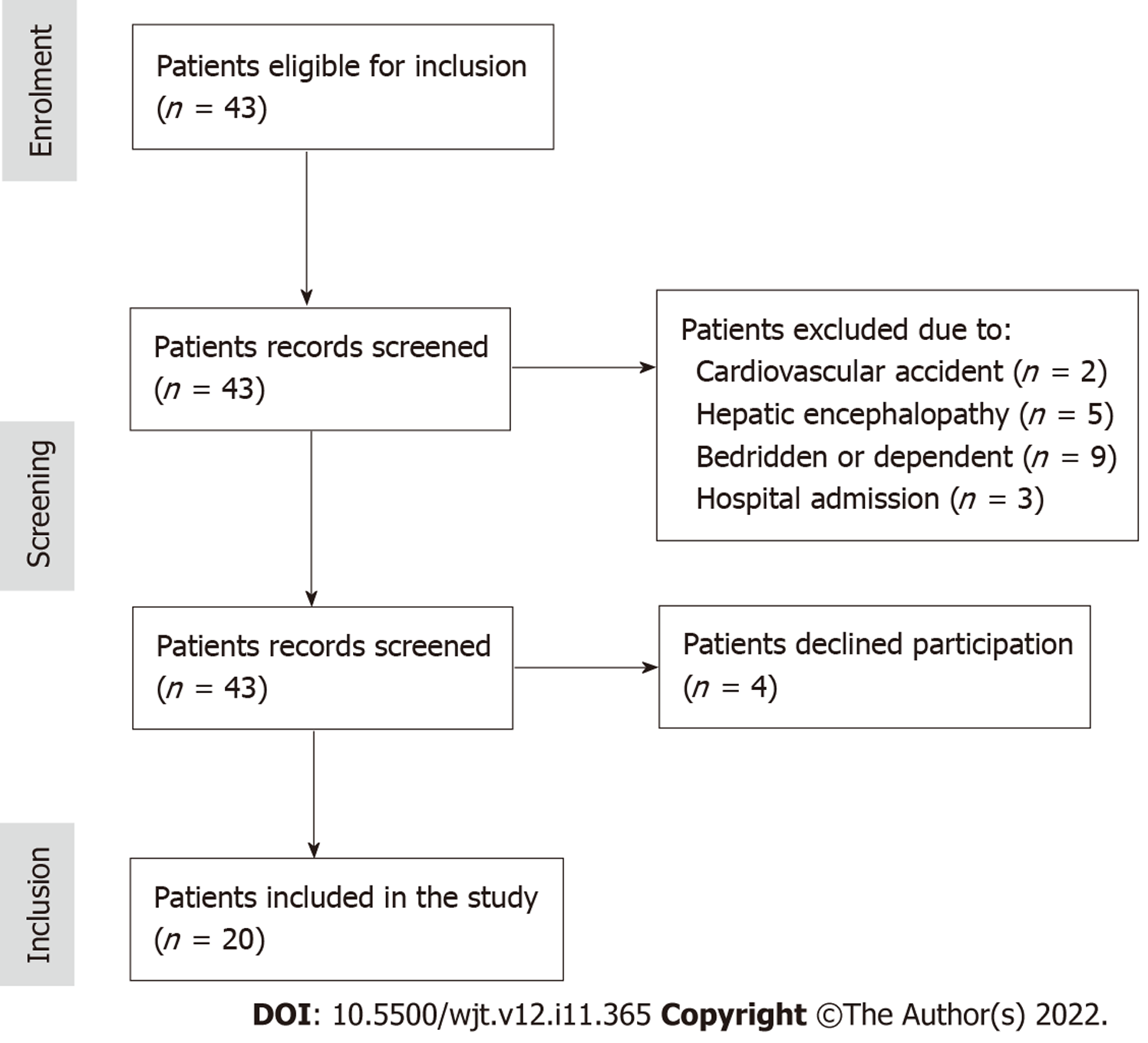
A total of 43 patients had their records screened to be included in the observational study. Following the exclusion criteria described above, 19 patients were excluded. In particular, 2 patients were recovering from a recent cardiovascular incident, 5 were classified with hepatic encephalopathy of grade 2 or higher, 9 were completely bedridden and unable to self-accommodate everyday needs, and finally 3 required long inpatient care within the past 3 mo. The remaining 24 patients were contacted and informed about the study; four declined participation. The recruitment process diagram is presented in Figure 1. All patients participating in the study were informed about the purpose and methodology of the study and provided written informed consent. The study protocol was approved by the Department’s Ethics Committee of Aristotle University of Thessaloniki (Protocol No. 65/2021). The study was performed from February 16 to June 21, 2021.
The self-administered, short form of the International Physical Activity Questionnaire (IPAQ) was used to evaluate the activity level of the participants. The IPAQ questionnaire was completed by the participants independently, without any guidance from the study investigators. It includes seven questions, collecting self-reported information for the number of days and time spent doing vigorous activity, moderate physical activity, walking, and sitting each day during the course of 1 wk[24,25]. The participants completed the Greek version of the questionnaire[26]. Questions 1 and 2 were about the days and time spent on vigorous activities, questions 3 and 4 referred to activities of moderate intensity, questions 5 and 6 referred to walking, and question 7 asked about the time spent sitting. This tool classifies respondents into three categories of physical activity, namely low, moderate, and high, according to the following criteria[27]: (1) Category 1 - low, consisting of individuals failing to meet any of the criteria detailed below; (2) Category 2 - moderate, consisting of individuals that fulfill any of the following three criteria: At least 3 d of vigorous activity, lasting more than 20 min daily; at least 5 d of moderate activity or walking, lasting more than 30 min daily; and at least 5 d of exercise comprising of a combination of walking, moderate, and vigorous activities, equal to 600 metabolic equivalent of task (MET) minutes or more; and (3) Category 3 - high, consisting of individuals that fulfill either of the following: At least 3 d of vigorous activity, reaching at least 1500 MET minutes weekly; and daily exercise comprising of a combination of walking, moderate, and vigorous activities, reaching at least 3000 MET minutes weekly.
Two different methods were used to evaluate the functional capacity of participants, namely CPET and the 6MWT. CPET was performed on the Trackmaster Treadmill (Full Vision Inc., Newton, KS, United States), using the Bruce protocol, whereas gas exchange was measured by the MedGraphics Breeze Suite CPX Ultima (Medical Graphics Corp., St. Paul, MN, United States). The test was performed under the supervision of trained personnel and a cardiologist, within the facilities of the Laboratory of Sports Medicine of the Aristotle University of Thessaloniki. Maximal effort was achieved by all participants, upon reaching a respiratory exchange ratio larger than 1.10. Peak oxygen uptake (VO2peak) and anaerobic threshold (AT) were assessed to evaluate the functional capacity of the participants.
Furthermore, a 6MWT was performed indoors by all participants. The testing design included a 30-m long, flat, and circular track, which was clearly marked for every meter. Patients performed the test twice and the longest distance achieved was recorded as their result. They were also instructed to immediately abandon their attempt if they felt unwell or had uncontrollable fatigue. During the 6MWT, patients received verbal encouragement on the 2nd and 4th min of every attempt and a notification when 60 s were left. Pulse oximetry was used to measure the oxygen saturation and heart rate during the test, whereas the Borg scale Rating of Perceived Exertion was used to monitor exercise intensity.
The LFI was used to evaluate the physical frailty of the study participants[28]. This clinical tool, developed by Lai et al[29], includes three tests that assess balance, neuromuscular coordination, and sarcopenia. The three tests are as follows: (1) Hand grip strength (using a dynamometer in the standard position, the participant squeezes the grip three times while the dynamometer rests on no surface); (2) Sit-to-stand test (from sitting position and keeping both arms folded in front of their chest, the participant is timed while standing up and sitting down five consecutive times); and (3) Balance test (the participant is timed standing up in three different balance positions, with feet side-by-side, semi tandem and tandem, while receiving no further support, for a maximum of 10 s).
IBM SPSS Statistics, version 25.0 (IBM Corp., Armonk, NY, United States) was used for the statistical analyses. Continuous parameters were compared using the independent samples t-test. The values of the parameters of the sample were tested for normal distribution with the Shapiro-Wilk test. Point biserial correlation analysis was used to determine the relationship between activity level and the frailty and functional capacity variables. Difference between values was considered to be of statistical significance for P values less than 0.01. All data are presented as the mean ± standard deviation.
Twenty patients were included in the study, all of whom are listed in the waiting list of the Department of Transplant Surgery in the Hippokration General Hospital of Thessaloniki. The majority of patients came from the city of Thessaloniki (n = 9, 45%), whereas the rest were distributed across the Greek mainland and islands. There were 10 male and 10 female patients included in the study, with a median age of 50.1 years. The primary causes of ELD of the participants were hepatitis B (n = 5, 25%), non-alcoholic fatty liver disease (n = 3, 15%), primary biliary cholangitis (n = 3, 15%), alcohol-related liver disease (n = 2, 10%), liver hemangioma (n = 2, 10%), hepatocellular carcinoma (n = 2, 10%), hepatitis C (n = 1, 5%), autoimmune hepatitis (n = 1, 5%), and hepatic cystadenomas (n = 1, 5%). The mean MELD score for the patients in the study was 13.02 ± 4.71. Demographic details for each patient are listed in Table 1, including the primary cause of ELD per participant.
| No. | Age | Sex | Primary cause |
| 1 | 32 | Female | Primary biliary cholangitis |
| 2 | 53 | Female | Liver hemangioma |
| 3 | 38 | Female | Liver hemangioma |
| 4 | 53 | Male | Hepatitis B virus |
| 5 | 38 | Male | Autoimmune hepatitis |
| 6 | 51 | Female | Hepatocellular carcinoma |
| 7 | 32 | Male | Hepatocellular carcinoma |
| 8 | 61 | Female | Hepatitis B virus |
| 9 | 63 | Male | Non-alcoholic fatty liver disease |
| 10 | 47 | Female | Hepatic cystadenomas |
| 11 | 62 | Female | Primary biliary cholangitis |
| 12 | 54 | Male | Hepatitis C virus |
| 13 | 52 | Male | Alcohol-related liver disease |
| 14 | 63 | Male | Alcohol-related liver disease |
| 15 | 49 | Female | Hepatitis B virus |
| 16 | 52 | Male | Hepatitis B virus |
| 17 | 50 | Male | Hepatitis B virus |
| 18 | 52 | Female | Non-alcoholic fatty liver disease |
| 19 | 50 | Male | Non-alcoholic fatty liver disease |
| 20 | 50 | Female | Primary biliary cholangitis |
All responses collected via the IPAQ can be seen in Table 2. Ten patients were classified as having a moderate physical activity level (category 2), whereas ten patients were found to be in the low physical activity level category (category 1). Using these responses, the sample was divided into two groups; patients with a moderate activity level were characterized as active (A), and patients with low activity level were allocated in the sedentary group (S). The active and sedentary groups were found to be similar regarding their MELD scores (A: 12.05 ± 5.63 vs S: 13.99 ± 3.60, respectively; P > 0.05).
| No. | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Result |
| 1 | 0 d | - | 2 d | 0 h 15 min | 5 d | 1 h 0 min | 8 h 0 min | Moderate |
| 2 | 2 d | 0 h 15 min | 4 d | 30 min | 5 d | 1 h 0 min | 4 h 30 min | Moderate |
| 3 | 0 d | - | 2 d | 0 h 20 min | 7 d | 1 h 30 min | 6 h 0 min | Moderate |
| 4 | 0 d | - | 0 d | - | 3 d | 0 h 30 min | 8 h 0 min | Low |
| 5 | 0 d | - | 3 d | 0 h 30 min | 3 d | 1 h 0 min | 6 h 0 min | Moderate |
| 6 | 0 d | - | 2 d | 0 h 20 min | 4 d | 0 h 45 min | 6 h 30 min | Moderate |
| 7 | 0 d | - | 3 d | 0 h 45 min | 4 d | 1 h 15 min | 4 h 30 min | Moderate |
| 8 | 0 d | - | 2 d | 0 h 15 min | 2 d | 0 h 30 min | 7 h 30 min | Low |
| 9 | 0 d | - | 0 d | - | 3 d | 0 h 15 min | 9 h 30 min | Low |
| 10 | 0 d | - | 3 d | 0 h 30 min | 3 d | 0 h 45 min | 6 h 15 min | Moderate |
| 11 | 0 d | - | 0 d | - | 3 d | 0 h 15 min | 9 h 15 min | Low |
| 12 | 0 d | - | 2 d | 0 h 20 min | 3 d | 0 h 30 min | 6 h 45 min | Low |
| 13 | 0 d | - | 2 d | 0 h 15 min | 4 d | 0 h 20 min | 7 h 0 min | Low |
| 14 | 0 d | - | 0 d | - | 5 d | 0 h 15 min | 8 h 0 min | Low |
| 15 | 0 d | - | 0 d | - | 3 d | 0 h 40 min | 7 h 30 min | Low |
| 16 | 0 d | - | 2 d | 0 h 20 min | 3 d | 0 h 30 min | 6 h 0 min | Low |
| 17 | 0 d | - | 3 d | 0 h 30 min | 4 d | 1 h 30 min | 4 h 0 min | Moderate |
| 18 | 0 d | - | 3 d | 0 h 20 min | 4 d | 1 h 0 min | 6 h 0 min | Moderate |
| 19 | 0 d | - | 0 d | - | 7 d | 1 h 15 min | 5 h 30 min | Moderate |
| 20 | 0 d | - | 0 d | - | 3 d | 0 h 30 min | 8 h 0 min | Low |
All participants successfully completed their CPET, successfully reaching a respiratory exchange ratio equal to 1.10 or higher. No patient had to abandon their examination due to excess fatigue or the presentation of adverse effects. No patient was instructed to terminate the exercise stress test due to changes to their electrocardiogram.
The mean VO2peak achieved by active participants was higher compared to the mean value recorded by the sedentary group (A: 29.78 ± 6.07 mL/kg/min vs S: 18.11 ± 3.39 mL/kg/min, respectively; P < 0.001). Similarly, the AT in active subjects was higher than that in their sedentary counterparts (A: 16.71 ± 2.17 mL/kg/min vs S: 13.96 ± 1.45 mL/kg/min, respectively; P < 0.01). All results for VO2peak and AT are presented in Table 3.
| No. | Group | VO2peak in mL/kg/min | AT in mL/kg/min |
| 1 | Active | 29.9 | 15.8 |
| 2 | Active | 40.8 | 21.1 |
| 3 | Active | 27.1 | 18.0 |
| 4 | Sedentary | 18.9 | 14.8 |
| 5 | Active | 25.7 | 14.1 |
| 6 | Active | 24.2 | 15.0 |
| 7 | Active | 39.6 | 18.8 |
| 8 | Sedentary | 18.4 | 14.2 |
| 9 | Sedentary | 13.8 | 12.8 |
| 10 | Active | 22.2 | 14.2 |
| 11 | Sedentary | 13.2 | 11.6 |
| 12 | Sedentary | 25.3 | 17.0 |
| 13 | Sedentary | 20.0 | 14.7 |
| 14 | Sedentary | 16.9 | 12.8 |
| 15 | Sedentary | 17.0 | 13.8 |
| 16 | Sedentary | 19.5 | 14.0 |
| 17 | Active | 30.0 | 16.9 |
| 18 | Active | 28.5 | 16.5 |
| 19 | Active | 29.8 | 16.7 |
| 20 | Sedentary | 18.1 | 13.9 |
Regarding the 6MWT, all participants successfully completed two attempts, with the longest distance considered the test result. No complication was recorded, and no effort was abandoned due to fatigue or exhaustion. Detailed results per participant are presented in Table 4. The active group covered a larger mean distance on the test compared to the sedentary group (A: 324.7 ± 55.8 m vs S: 458.2 ± 57.5 m, respectively; P < 0.001).
| No. | Group | 6-min walking test in m |
| 1 | Active | 396 |
| 2 | Active | 456 |
| 3 | Active | 595 |
| 4 | Sedentary | 250 |
| 5 | Active | 433 |
| 6 | Active | 397 |
| 7 | Active | 429 |
| 8 | Sedentary | 347 |
| 9 | Sedentary | 264 |
| 10 | Active | 502 |
| 11 | Sedentary | 259 |
| 12 | Sedentary | 360 |
| 13 | Sedentary | 431 |
| 14 | Sedentary | 362 |
| 15 | Sedentary | 320 |
| 16 | Sedentary | 330 |
| 17 | Active | 460 |
| 18 | Active | 456 |
| 19 | Active | 458 |
| 20 | Sedentary | 324 |
The LFI was used to assess the robustness or frailty of the study participants. Patients successfully completed all exercises after first witnessing a demonstration. The sedentary group was more likely to score a greater LFI score and to be frail, whereas its mean value was above the limit for patient classification as frail compared to the active group, which was more likely to score smaller values (S: 4.42 ± 0.32 vs A: 3.75 ± 0.31, respectively; P < 0.001). The detailed performance per test is described in Table 5. Patients with a LFI greater than 4.4 were classified as frail[23,29]. No patient from the active group was classified as frail (LFI < 4.4, n = 10), whereas 6 patients were found to be frail according to the LFI in the sedentary group (LFI > 4.4, n = 6). Mean value comparisons are presented for all variables in Table 6.
| No. | Hand grip strength in kg | Sit-to-stand in s | Balance test in s | LFI | ||||
| Att. 1 | Att. 2 | Att. 3 | Side-by-side | Semi-tandem | Tandem | |||
| 1 | 18 | 19 | 19 | 12.4 | 10.0 | 10.0 | 10.0 | 3.95 |
| 2 | 26 | 26 | 25 | 8.5 | 10.0 | 10.0 | 10.0 | 3.11 |
| 3 | 25 | 24 | 24 | 10.1 | 10.0 | 10.0 | 10.0 | 3.42 |
| 4 | 19 | 18 | 18 | 16.8 | 7.9 | 9.1 | 8.2 | 4.76 |
| 5 | 26 | 27 | 27 | 11.0 | 10.0 | 10.0 | 10.0 | 3.9 |
| 6 | 19 | 18 | 19 | 13.1 | 9.1 | 10.0 | 8.9 | 4.08 |
| 7 | 30 | 28 | 29 | 10.0 | 10.0 | 10.0 | 10.0 | 3.71 |
| 8 | 14 | 14 | 13 | 17.2 | 8.5 | 9.2 | 8.1 | 4.66 |
| 9 | 13 | 14 | 14 | 17.6 | 8.5 | 9.4 | 8.0 | 4.92 |
| 10 | 18 | 17 | 18 | 13.3 | 9.0 | 10.0 | 9.0 | 4.15 |
| 11 | 12 | 11 | 12 | 16.1 | 9.3 | 10.0 | 9.0 | 4.62 |
| 12 | 20 | 19 | 19 | 11.9 | 10.0 | 10.0 | 10.0 | 4.23 |
| 13 | 26 | 27 | 28 | 12.2 | 10.0 | 10.0 | 10.0 | 4.00 |
| 14 | 22 | 21 | 21 | 11.8 | 10.0 | 10.0 | 10.0 | 4.15 |
| 15 | 18 | 18 | 17 | 12.8 | 10.0 | 10.0 | 10.0 | 4.03 |
| 16 | 18 | 19 | 18 | 13.0 | 9.5 | 9.8 | 8.9 | 4.42 |
| 17 | 27 | 27 | 26 | 9.4 | 10.0 | 10.0 | 10.0 | 3.70 |
| 18 | 19 | 20 | 20 | 11.3 | 10.0 | 10.0 | 10.0 | 3.80 |
| 19 | 27 | 28 | 27 | 9.8 | 10.0 | 10.0 | 10.0 | 3.74 |
| 20 | 15 | 14 | 14 | 14.2 | 9.0 | 9.4 | 8.4 | 4.43 |
Pearson correlation analysis was used to determine if disease severity was associated with worse functional capacity or higher frailty scores. Correlation was tested between MELD scores and LFI, VO2max, AT, and 6MWT. No significant correlation was found between MELD and LFI (rp = 0.29, P > 0.05), VO2max (rp = -0.10, P > 0.05), AT (rp = -0.25, P > 0.05) or 6MWT (rp = -0.36, P > 0.05).
Point-biserial correlation was run to determine the relationship between the activity level and functional capacity and physical frailty markers. MELD and activity level was not significantly correlated (rpb = -0.212, P > 0.05), whereas there was significant correlation between activity level and LFI (rpb = -0.747, P < 0.001), VO2peak (rpb = 0.781, P < 0.001), AT (rpb = 0.618, P < 0.01), and 6MWT (rpb = 0.779, P < 0.001). This relationship is presented in Table 7.
| Value | rpb | P value |
| MELD | -0.212 | > 0.05 |
| VO2peak in mL/kg/min | 0.781 | < 0.001 |
| AT in mL/kg/min | 0.618 | < 0.01 |
| 6MWT in m | 0.779 | < 0.001 |
| LFI | -0.747 | < 0.001 |
According to the results of this observational study, physical activity appears to prevent physical frailty and retain cardiovascular capacity in liver transplant candidates, independent of their MELD score. This can be potentially used as a tool for prehabilitation in listed patients for a liver transplant. Availability of liver transplants has always been well below demand, especially in Greece, with the coronavirus disease 2019 pandemic posing an even greater challenge. This study was driven by the need to identify possible important and potentially modifiable clinical parameters, which, when used in concordance with the MELD score, would be able to optimize the capacity of a medium-size transplant center[3,6].
According to the LFI, 30% (n = 6) of the study participants are classified as frail (LFI > 4.4)[23,29], a percentage that is concordant with the results of a previous review study[30]. Physical frailty has been associated with increased waiting list mortality, independently of the MELD score, presence of ascites or hepatic encephalopathy[31]. Furthermore, in the postoperative spectrum, frailty has been associated with increased 30-d mortality, extended inpatient and intensive unit care[32], increased rates of acute cellular rejection[33], increased dependency[34,35], and vertebrae fractures[36]. Constructed, the home-based exercise program appears to positively influence frailty indexes and partially restore musculoskeletal robustness[37-40]. Our study compared each patient’s physical activity level with their physical frailty. Although patients were not under professional trainer guidance, frequent activity such as walking and gardening, appeared to have a preventive effect on the evolvement of physical frailty. This could potentially provide clinicians with an important tool in the preoperative treatment of candidates, while on the waiting list for a transplant, being a tool that could potentially improve transplantation outcomes.
Functional capacity has also been associated with postoperative dependency and mortality. Epstein et al[12] described an increased 100-d mortality in patients with lower peak oxygen uptake, whereas other studies have associated a smaller VO2peak with extended intensive care unit stay and mechanical ventilation dependency[41]. Similarly, smaller distances in the preoperative 6MWT have been associated with increased mortality after liver transplantation[42,43]. In 2021, Henrique et al[18] identified a statistically significant increased risk of cirrhosis decompensation in patients with values smaller than 401.8 m in the 6MWT, whereas Bhanji et al[44] described a double risk of waiting list mortality in patients with values smaller than 250 m and its statistically significant reduction for every 100 m improvement. In our study, active participants were much more likely to record values above 401.8 m (80% vs 10%; P < 0.01), consistent with the findings of the effects of exercise in liver patients in other studies[45,46].
The inclusion of indexes of frailty and functional capacity in the clinical practice of liver tran
The active participants of our study, although not following an organized and formal exercise protocol, had substantially better musculoskeletal and functional status, appeared to be more robust, and could potentially have great tolerance to stressors. This suggests evidence that exercise interventions could have a positive impact on liver transplant candidates, without the need for formal and difficult exercise regimes that bear a higher risk of lower compliance. However, this study had limitations, namely the small sample size and no prospective results. Further data collection and follow-up could confirm the effects of this lifestyle on pretransplantation and posttransplantation survival, dependency, and complications.
In conclusion, an active lifestyle can potentially be a tool of preoperative preparation of liver transplant candidates to reduce mortality, hospitalization, and dependencies. Physical frailty and functional capacity can be improved with exercise training interventions. Clinical tools such as the 6MWT and the LFI could be used for better mortality prediction and patient prioritization, which is of significant importance in smaller and medium-sized transplant centers, where organ donation is unable to meet the existing high demand.
Liver transplantation forces a substantial stress on the human physiology, which is even more significant considered the deconditioning that accompanies end-stage liver disease (ELD). Physical frailty has emerged as an important factor both pre- and postoperatively, aiming to improve results and outcomes.
The limited amount of available organ donations in addition to the high demand in liver transplants, highlight the need for proper planning and prioritization, while at the same time working towards further outcome improvement.
The main objective was to identify if an active lifestyle can significantly improve physical frailty and functional capacity in patients with ELD.
An International Physical Activity Questionnaire, a functional capacity assessment, and a physical frailty evaluation were utilized.
There was a statistically significant difference and statistically significant correlation between the activity level and the Liver Frailty Index, the peak oxygen uptake, the anaerobic threshold, and the 6-min walking distance.
Physical activity can potentially improve functional capacity and frailty in liver transplant candidates.
Future research should focus on the regimen of the exercise that would be more suitable, or better quantify the amount of physical exercise needed for these patients. Furthermore, the potential use of these markers in survival and outcomes should be evaluated.
The authors would like to acknowledge Mr. Lambros Vagiotas, Ms. Aikaterini Tsakni and Ms. Sofia Tsakalidou, Transplant Coordinators of the Department of Transplant Surgery of Hippokration General Hospital of Thessaloniki for their support and contribution.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Balaban HY, Turkey; Bredt LC, Brazil; Sintusek P, Thailand S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Meeks AC, Madill J. Sarcopenia in liver transplantation: A review. Clin Nutr ESPEN. 2017;22:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Kaido T. Selection Criteria and Current Issues in Liver Transplantation for Hepatocellular Carcinoma. Liver Cancer. 2016;5:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Lai JC. Defining the threshold for too sick for transplant. Curr Opin Organ Transplant. 2016;21:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, Wang SC, Segev DL, Sonnenday CJ. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 650] [Cited by in RCA: 621] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 5. | Durand F, Buyse S, Francoz C, Laouénan C, Bruno O, Belghiti J, Moreau R, Vilgrain V, Valla D. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 6. | Sacleux SC, Samuel D. A Critical Review of MELD as a Reliable Tool for Transplant Prioritization. Semin Liver Dis. 2019;39:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Darwish Murad S, Feshtali S, van Ooijen PMA, Polak WG, Porte RJ, van Hoek B, van den Berg AP, Metselaar HJ, IJzermans JNM. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: A competing risk analysis in a national cohort. J Hepatol. 2018;68:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 8. | Masuda T, Shirabe K, Ikegami T, Harimoto N, Yoshizumi T, Soejima Y, Uchiyama H, Ikeda T, Baba H, Maehara Y. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 2014;20:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 9. | van Vugt JLA, Buettner S, Alferink LJM, Bossche N, de Bruin RWF, Darwish Murad S, Polak WG, Metselaar HJ, IJzermans JNM. Low skeletal muscle mass is associated with increased hospital costs in patients with cirrhosis listed for liver transplantation-a retrospective study. Transpl Int. 2018;31:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, Beaumont C, Tandon P, Esfandiari N, Sawyer MB, Kneteman N. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 11. | Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, Beaumont C, Esfandiari N, Myers RP. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol. 2015;6:e102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 12. | Epstein SK, Freeman RB, Khayat A, Unterborn JN, Pratt DS, Kaplan MM. Aerobic capacity is associated with 100-day outcome after hepatic transplantation. Liver Transpl. 2004;10:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Mancuzo EV, Pereira RM, Sanches MD, Mancuzo AV. Pre-Transplant Aerobic Capacity and Prolonged Hospitalization After Liver Transplantation. GE Port J Gastroenterol. 2015;22:87-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Dharancy S, Lemyze M, Boleslawski E, Neviere R, Declerck N, Canva V, Wallaert B, Mathurin P, Pruvot FR. Impact of impaired aerobic capacity on liver transplant candidates. Transplantation. 2008;86:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Forman DE, Myers J, Lavie CJ, Guazzi M, Celli B, Arena R. Cardiopulmonary exercise testing: relevant but underused. Postgrad Med. 2010;122:68-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Carey EJ, Steidley DE, Aqel BA, Byrne TJ, Mekeel KL, Rakela J, Vargas HE, Douglas DD. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 17. | Alameri HF, Sanai FM, Al Dukhayil M, Azzam NA, Al-Swat KA, Hersi AS, Abdo AA. Six Minute Walk Test to assess functional capacity in chronic liver disease patients. World J Gastroenterol. 2007;13:3996-4001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Henrique DMN, Malaguti C, Limonge TM, Siqueira MR, Paticcie TMF, Mira PAC, Laterza MC, Mourão-Junior CA, Pacce FHL, Chebli JMF. Six-Minute Walking Test as a Predictor of Clinical Decompensation in Patients with Cirrhosis. J Gastrointestin Liver Dis. 2021;30:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Gordon AL, Masud T, Gladman JR. Now that we have a definition for physical frailty, what shape should frailty medicine take? Age Ageing. 2014;43:8-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, Gonzalez-Colaço Harmand M, Bergman H, Carcaillon L, Nicholson C, Scuteri A, Sinclair A, Pelaez M, Van der Cammen T, Beland F, Bickenbach J, Delamarche P, Ferrucci L, Fried LP, Gutiérrez-Robledo LM, Rockwood K, Rodríguez Artalejo F, Serviddio G, Vega E; FOD-CC group (Appendix 1). Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 836] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 21. | Williams FR, Milliken D, Lai JC, Armstrong MJ. Assessment of the Frail Patient With End-Stage Liver Disease: A Practical Overview of Sarcopenia, Physical Function, and Disability. Hepatol Commun. 2021;5:923-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Lai JC, Covinsky KE, McCulloch CE, Feng S. The Liver Frailty Index Improves Mortality Prediction of the Subjective Clinician Assessment in Patients With Cirrhosis. Am J Gastroenterol. 2018;113:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 23. | Kardashian A, Ge J, McCulloch CE, Kappus MR, Dunn MA, Duarte-Rojo A, Volk ML, Rahimi RS, Verna EC, Ganger DR, Ladner D, Dodge JL, Boyarsky B, McAdams-DeMarco M, Segev DL, Lai JC. Identifying an Optimal Liver Frailty Index Cutoff to Predict Waitlist Mortality in Liver Transplant Candidates. Hepatology. 2021;73:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 24. | Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11293] [Cited by in RCA: 13617] [Article Influence: 619.0] [Reference Citation Analysis (0)] |
| 25. | Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1231] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 26. | International Physical Activity Questionnaire Group. International Physical Activity Questionnaire. [cited 15 May 2022]. Available from: https://sites.google.com/site/theipaq/questionnaire_links. |
| 27. | International Physical Activity Questionnaire. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ). [cited 15 May 2022]. Available from: https://www.researchgate.net/file.PostFileLoader.html?id=5641f4c36143250eac8b45b7&assetKey=AS%3A294237418606593%401447163075131. |
| 28. | Functional Assessment in Liver Transplantation. Liver Frailty IndexTM. [cited 15 May 2022]. Available from: https://liverfrailtyindex.ucsf.edu/. |
| 29. | Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, Feng S. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 371] [Article Influence: 46.4] [Reference Citation Analysis (1)] |
| 30. | Van Jacobs AC. Frailty Assessment in Patients with Liver Cirrhosis. Clin Liver Dis (Hoboken). 2019;14:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Lai JC, Rahimi RS, Verna EC, Kappus MR, Dunn MA, McAdams-DeMarco M, Haugen CE, Volk ML, Duarte-Rojo A, Ganger DR, O'Leary JG, Dodge JL, Ladner D, Segev DL. Frailty Associated With Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology. 2019;156:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 32. | DeMaria S Jr, Khromava M, Schiano TD, Lin HM, Kim S. Standardized measures of frailty predict hospital length of stay following orthotopic liver transplantation for hepatocellular carcinoma. Clin Transplant. 2019;33:e13746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Fozouni L, Mohamad Y, Lebsack A, Freise C, Stock P, Lai JC. Frailty Is Associated With Increased Rates of Acute Cellular Rejection Within 3 Months After Liver Transplantation. Liver Transpl. 2020;26:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Lai JC, Dodge JL, McCulloch CE, Covinsky KE, Singer JP. Frailty and the Burden of Concurrent and Incident Disability in Patients With Cirrhosis: A Prospective Cohort Study. Hepatol Commun. 2020;4:126-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Owodunni OP, Mostales JC, Qin CX, Gabre-Kidan A, Magnuson T, Gearhart SL. Preoperative Frailty Assessment, Operative Severity Score, and Early Postoperative Loss of Independence in Surgical Patients Age 65 Years or Older. J Am Coll Surg. 2021;232:387-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Saeki C, Kanai T, Nakano M, Oikawa T, Torisu Y, Abo M, Saruta M, Tsubota A. Relationship between Osteosarcopenia and Frailty in Patients with Chronic Liver Disease. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Debette-Gratien M, Tabouret T, Antonini MT, Dalmay F, Carrier P, Legros R, Jacques J, Vincent F, Sautereau D, Samuel D, Loustaud-Ratti V. Personalized adapted physical activity before liver transplantation: acceptability and results. Transplantation. 2015;99:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Chen HW, Ferrando A, White MG, Dennis RA, Xie J, Pauly M, Park S, Bartter T, Dunn MA, Ruiz-Margain A, Kim WR, Duarte-Rojo A. Home-Based Physical Activity and Diet Intervention to Improve Physical Function in Advanced Liver Disease: A Randomized Pilot Trial. Dig Dis Sci. 2020;65:3350-3359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 39. | Trivedi HD, Tapper EB. Interventions to improve physical function and prevent adverse events in cirrhosis. Gastroenterol Rep (Oxf). 2018;6:13-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | West J, Gow PJ, Testro A, Chapman B, Sinclair M. Exercise physiology in cirrhosis and the potential benefits of exercise interventions: A review. J Gastroenterol Hepatol. 2021;36:2687-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Bernal W, Martin-Mateos R, Lipcsey M, Tallis C, Woodsford K, McPhail MJ, Willars C, Auzinger G, Sizer E, Heneghan M, Cottam S, Heaton N, Wendon J. Aerobic capacity during cardiopulmonary exercise testing and survival with and without liver transplantation for patients with chronic liver disease. Liver Transpl. 2014;20:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Mizuno Y, Ito S, Hattori K, Nagaya M, Inoue T, Nishida Y, Onishi Y, Kamei H, Kurata N, Hasegawa Y, Ogura Y. Changes in Muscle Strength and Six-Minute Walk Distance Before and After Living Donor Liver Transplantation. Transplant Proc. 2016;48:3348-3355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Locklear CT, Golabi P, Gerber L, Younossi ZM. Exercise as an intervention for patients with end-stage liver disease: Systematic review. Medicine (Baltimore). 2018;97:e12774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Bhanji RA, Watt KD. Physiologic Reserve Assessment and Application in Clinical and Research Settings in Liver Transplantation. Liver Transpl. 2021;27:1041-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Aamann L, Dam G, Rinnov AR, Vilstrup H, Gluud LL. Physical exercise for people with cirrhosis. Cochrane Database Syst Rev. 2018;12:CD012678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Lima YB, Magalhães CBA, Garcia JHP, Viana CFG, Prudente GFG, Pereira EDB. Association between fatigue and exercise capacity in patients with chronic liver disease awaiting liver transplantation. Arq Gastroenterol. 2019;56:252-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Klein CG, Malamutmann E, Latuske J, Tagay S, Dörri N, Teufel M, Paul A, Oezcelik A. Frailty as a predictive factor for survival after liver transplantation, especially for patients with MELD≤15-a prospective study. Langenbecks Arch Surg. 2021;406:1963-1969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Chen HW, Ferrando AA, Dunn MA, Kim WR, Duarte-Rojo A. Cadence From Physical Activity Trackers for Monitoring of Home-Based Exercise Intensity in Advanced Liver Disease. Liver Transpl. 2020;26:718-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Williams FR, Berzigotti A, Lord JM, Lai JC, Armstrong MJ. Review article: impact of exercise on physical frailty in patients with chronic liver disease. Aliment Pharmacol Ther. 2019;50:988-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 50. | Limongi V, Dos Santos DC, de Oliveira da Silva AM, Boin Ide F, Stucchi RS. Exercise manual for liver disease patients. World J Transplant. 2016;6:429-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Al-Judaibi B, Alqalami I, Sey M, Qumosani K, Howes N, Sinclair L, Chandok N, Eddin AH, Hernandez-Alejandro R, Marotta P, Teriaky A. Exercise Training for Liver Transplant Candidates. Transplant Proc. 2019;51:3330-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Macías-Rodríguez RU, Ruiz-Margáin A, Román-Calleja BM, Moreno-Tavarez E, Weber-Sangri L, González-Arellano MF, Fernández-del-Riveroa G, Ramírez-Sotoa K. Prescripción de ejercicio en pacientes con cirrosis: recomendaciones para la atención clínica. Rev Gastroenterol México. 2019;84:326-343. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |