Published online Oct 18, 2022. doi: 10.5500/wjt.v12.i10.313
Peer-review started: July 3, 2022
First decision: August 4, 2022
Revised: August 5, 2022
Accepted: September 9, 2022
Article in press: September 9, 2022
Published online: October 18, 2022
Processing time: 105 Days and 16.5 Hours
Chronic kidney disease is associated with immunological disorders, presented as phenotypic alterations of T lymphocytes. These changes are expected to be restored after a successful renal transplantation; however, additional parameters may contribute to this process.
To evaluate the impact of positive panel reactive antibodies (PRAs) on the restoration of T cell phenotype, after renal transplantation.
CD4CD28null, CD8CD28null, natural killer cells (NKs), and regulatory T cells (Tregs) were estimated by flow cytometry at T0, T3, and T6 which were the time of transplantation, and 3- and 6-mo follow-up, respectively. Changes were esti
Patients were classified in two groups: PRA(-) (n = 43) and PRA(+) (n = 28) groups. Lymphocyte and their subtypes were similar between the two groups at T0, whereas their percentage was increased at T3 in PRA(-) compared to PRA(+) [23 (10.9-47.9) vs 16.4 (7.5-36.8 μ/L, respectively; P = 0.03]. Lymphocyte changes in PRA(-) patients included a significant increase in CD4 cells (P < 0.0001), CD8 cells (P < 0.0001), and Tregs (P < 0.0001), and a reduction of NKs (P < 0.0001). PRA(+) patients showed an increase in CD4 (P = 0.008) and CD8 (P = 0.0001), and a reduction in NKs (P = 0.07). CD4CD28null and CD8CD28null cells, although initially reduced in both groups, were stabilized thereafter.
Our study described important differences in the immune response between PRA(+) and PRA(-) patients with changes in lymphocytes and lymphocyte subpopulations. PRA(+) patients seemed to have a worse immune profile after 6 mo follow-up, regardless of renal function.
Core Tip: Chronic kidney disease is associated with phenotypic and functional changes in the immune system. This study evaluated the impact of positive panel reactive antibodies (PRAs) on restoration of the T cell phenotype after renal transplantation. Our study described important differences in the immune response between PRA(+) and PRA(-) patients with changes in lymphocytes and lymphocyte subpopulations. PRA(+) patients seemed to have a worse immune profile after 6 mo follow-up, regardless of renal function.
- Citation: Vagiotas L, Stangou M, Kasimatis E, Xochelli A, Myserlis G, Lioulios G, Nikolaidou V, Panteli M, Ouranos K, Antoniadis N, Maria D, Papagianni A, Tsoulfas G, Fylaktou A. Effect of panel reactive antibodies on T cell immunity reinstatement following renal transplantation. World J Transplant 2022; 12(10): 313-324
- URL: https://www.wjgnet.com/2220-3230/full/v12/i10/313.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i10.313
Chronic kidney disease (CKD) is associated with phenotypic and functional changes in the immune system, including both innate and adaptive immunity, causing detrimental clinical consequences. Total lymphopenia is one of the major concerns in CKD, whereas changes in T lymphocytes include both elimination of their population and alterations of their subtypes. Some of these phenotypic and functional changes have been described by investigators[1,2]. We previously showed that CKD, even at the pre-dialysis stage, results in reduced levels of CD4, CD8, and regulatory T cells (Tregs). Furth
The CD28 molecule constitutes a primary co-stimulatory receptor, which is essential for successful T cell activation, proliferation, and survival. It is mainly expressed on naive T cells in humans, but its expression on memory T cells depends on their differentiation status. Expansion of circulating T lymphocytes lacking the CD28 molecule represents an adaptive mechanism following repeated antigenic stimulation, and has been considered an age-associated immunological alteration[3-7].
Initiation of hemodialysis (HD) cannot restore these structural changes of lymphocytes. Even more, the HD itself, as an extracorporeal circulation, use of dialyzers, may have an additive deleterious effect [1]. Conversely, successful renal transplantation allows patients to stop dialysis and reinstates kidney function. Accordingly, as part of returning to normality, it is also expected to restore patients’ immune profile[8,9].
However, despite the indisputable beneficial effect of renal transplantation on immune status, there may be parameters that affect the outcome of graft function and potentially influence the reestablishment of immunological disorders. Most of these parameters are closely associated with the patient’s immune status at the time of transplantation. Immune status of the CKD patient is determined by phenotypic and functional alterations of lymphocytes due to CKD, and even more interesting for those patients undergoing renal transplantation, by the presence of human leukocyte antigen (HLA) sensitization. HLA sensitization refers to the presence of antibodies in the potential recipient against HLA molecules of the selected donor. While on the waiting list, CKD patients may develop antibodies against HLA antigens as a result of blood transfusions, previous transplantations, or pregnancies[10,11], generally described as panel reactive antibodies (PRAs)[12]. The risk of sensitization increases as there is exposure to more than one sensitizing factor[9,13]. PRA screening is routinely performed in CKD patients before renal transplantation to assess recipients’ exposure and sensitization. PRA titers before kidney transplantation may be used to predict acute rejection and guide the immunosuppressive treatment, including induction treatment. The presence of PRAs is not uncommon, as patients have to wait long for a kidney transplant, and meanwhile, are exposed to blood transfusions or get pregnant[12]. The purpose of this study was to evaluate the effect of positive PRA on restoration of the immunological T cell phenotype following successful renal transplantation.
The study was conducted between January 2020 and October 2021 at the Department of Renal Tran
Inclusion criteria: Patients eligible for the study were 13-70-years-old, and had undergone a living or deceased donor kidney transplantation. Regarding the deceased donors, we included only Donation after Brain Death and not Donation after Cardiac Death transplants. All transplantations were ABO-compatible with a negative complement-dependent crossmatch. The patients were followed for 6 mo in the outpatient clinic, and all were treated with the same treatment protocol.
Exclusion criteria: Patients were excluded from the study in case of recent (less than 3 mo) cytomegalovirus (CMV) or bacterial infection; recent malignancy (less than 5 years); or active autoimmune, inflammatory disease, or hematological disorder. Also, patients who had been on immunosuppressive treatment during the last 12 mo prior to kidney transplantation were excluded, as were patients not compliant with the treatment instructions.
Each patient receiving a kidney transplantation was assessed for eligibility to be included in the study. For patients who fulfilled the inclusion criteria, as described above, the day of enrollment in the study was the day of transplantation. Blood samples were taken in the morning, before the administration of any immunosuppressive treatment, and used for laboratory and immunological assessments. During the posttransplant period, renal function, medication, and possible side effects were recorded. Following discharge from the hospital, after renal transplantation, all patients were regularly followed up at the outpatient clinic on a monthly basis. Their immune profile was recorded on the day of transplantation (T0), and at the 3- and 6-mo follow-up (T3 and T6, respectively). At the same time intervals, the function of the renal graft was evaluated and the results were correlated with the immunophenotype.
Demographic, clinical data from donors and recipients, HLA mismatches, and cold ischemia time were recorded at T0, and delayed graft function (DGF), acute rejection episodes, infections, and hospitalization time were recorded and analyzed at T3 and T6, 3 and 6 mo after transplantation. All patients received the same immunosuppressive regimen, according to the Immunosuppressive Protocol, including basiliximab or antithymocyte globulin (ATG), steroids, tacrolimus, and multimode fiber. Eleven patients (15.5%) received ATG, reasons to receive ATG were as follows: 4/11 because of retr
Flow cytometry: T cell subsets were identified using multicolor flow cytometry with standard techniques on the Navios EX flow cytometer (Beckman Coulter, Sykesville, MD, United States). Whole blood samples were drawn from patients at the scheduled time points (T0, T3, and T6), collected in EDTA tubes, and processed for the evaluation of lymphocyte count and their subpopulations. T lymphocyte subsets determined were CD3+CD4+, CD3+CD8+, CD3-CD16+CD56+, CD3+CD4+CD28-, and CD3+CD8+CD28-, using the following monoclonal antibodies: CD3-FITC (clone: UCHT1; Beckman Coulter), CD16 (clone: 3G8; Beckman Coulter), CD56 clone: N901(NKH-1)-PE; Beckman Coulter), CD4-APC (clone: 13B8.2; Beckman Coulter), CD8 PC5.5 (clone: B9.11l Beckman Coulter), CD28-ECD (clone: CD28.2; Beckman Coulter), and CD45-PC7 (clone: J33; Beckman Coulter). Peripheral blood mononuclear cells were obtained by Ficoll density gradient centrifugation. Immunophenotyping of Tregs was performed with the combination of the following monoclonal antibodies: CD45-PC7 (clone: J33; Beckman Coulter), CD4-FITC (clone: 13B8.2; Beckman Coulter), CD25-PC5 (clone: B1.49.9; Beckman Coulter), and FOXP3-PE (clone: 259D; Beckman Coulter).
Statistical analyses were performed using the Statistical Package for Social Sciences for Windows, version 27.0 (SPSS Inc., Chicago, IL, United States). The Shapiro-Wilk or Kolmogorov-Smirnov test was applied to examine the normality of distribution for continuous variables. For all comparisons, P < 0.05 was considered statistically significant. Mean ± SD and medians and interquartile range were used to describe data from normally distributed and non-parametric variables, respectively. Similarly, the student’s t-test for non-paired and paired variables, and Mann-Whitney U test and Wilcoxon signed-rank test were respectively performed to compare differences between groups. To investigate the change in subpopulations among T0, T3, and T6, repeated measures analysis of variance (ANOVA) for parametric variables or Friedman’s ANOVA for non-parametric variables was used.
Seventy-one recipients of a kidney transplant were included in the study. Characteristics of patients are depicted in Table 1.
| Characteristics | |
| Age, yr, median (range) | 46 (13-70) |
| Male/female | 49/22 |
| Living kidney donor | 22.5% |
| Deceased kidney donor | 77.5% |
| Previous kidney transplant | 7.0% |
| Preemptive transplantation | 4.2% |
| PRA(-) | 60.5% |
| Early rejection, within first 6 mo after KT | 4.2% |
| Induction therapy | |
| Basiliximab | 84.5% |
| ATG | 15.5% |
| Maintenance immune suppression | |
| Tacrolimus/mycophenolate/prednisone | 100.0% |
| Other | 0.0% |
| Distribution of underlying kidney disease | |
| Polycystic kidney disease | 22.5% |
| Primary glomerulopathies | 21.1% |
| Reflux nephropathy | 12.6% |
| Diabetes mellitus | 4.2% |
| Nephrosclerosis/hypertension | 4.2% |
| Urinary tract infections/ stones | 3.7% |
| Other | 16.2% |
| Unknown | 15.5% |
Differences between PRA(-) and PRA(+) patients
Differences in clinical and laboratory findings: Of the study population, 43 patients had negative PRA, and were classified as PRA(-), whereas 28 had positive PRA, and were classified as PRA(+). There were no differences between the two groups in terms of age, sex, and time on HD, [defined as HD vintage (HDV)]. Also, no differences were found between the two groups in the proportion of patients who underwent preemptive transplantation, had an episode of acute rejection or were administered ATG, as well as in those who had DGF (Table 2).
| Parameter | T0 (at renal transplantation) | ||
| PRA(-) | PRA(+) | P value | |
| Age, yr | 45 (13-65) | 47 (14-70) | NS |
| HDV, mo | 82.5 (0-251) | 112 (0-165) | NS |
| Time of cold ischemia, h | 18 (0-30) | 16.5 (0-30.5) | NS |
| Pre-emptive RT, % | 6 (13.6) | 1 (3.6) | NS |
| Acute rejection episode, % | 2 (4.5) | 1 (3.6) | NS |
| ATG administration, % | 5 (11.4) | 7 (25) | NS |
| DGF, % | 7 (15.9) | 4 (14.3) | NS |
No significant differences in lymphocyte numbers and T lymphocyte subpopulations were noticed between PRA(-) and PRA(+) patients at the time of transplantation. An increase in percentage of CD4CD28null and CD8CD28null cell within PRA(+) patients did not reach statistical difference (Table 3).
| Parameter | T0, at renal transplantation | ||
| All patients | PRA(-) | PRA(+) | |
| n | 71 | 43 | 28 |
| Lymphocyte, % | 18.1 (6.4-40) | 18.8 (6.4-38.4) | 17.8 (11.2-40) |
| Lymphocyte, cells/μL | 1200 (700-2800) | 1200 (700-2800) | 1100 (700-2600) |
| CD4+, % | 42.0 (20.6-68.6) | 44.4 (20.6-68.6) | 41.5 (25.3-59.5) |
| CD4+, cells/μL | 515 (206-1453.2) | 557 (206-1453.2) | 435 (253-1362.4) |
| CD8+, % | 24.55 (10.5-53.1) | 25.1 (12,2-37.7) | 23.4 (10.5-53.1) |
| CD8+, cells/μL | 301.5 (91.7-665.6) | 301.5 (102.9-641.7) | 294.9 (91.7-665.6) |
| CD4+/CD8+ | 1.7 (0.6-5.6) | 1.5 (0.9-5.6) | 2 (0.6-5) |
| CD4+CD28-, % | 5.4 (0.0-33.7) | 4.8 (0.2-33.7) | 7.2 (0-32.1) |
| CD4+CD28-, cells/μL | 26.9 (0.0-206) | 26.7 (0-160) | 27.3 (0-206) |
| CD8+CD28-, % | 38.6 (6.1-91.5) | 38.3 (6.1-68.2) | 48.4 (15.1-91.5) |
| CD8+CD28-, cells/μL | 121.5 (13-583) | 113.6 (17-315) | 122 (13-583) |
| CD16/56, % | 18 (3.6-50.6) | 17.7 (3.6-50.6) | 18.4 (4.4-34.2) |
| CD16/56, cells/μL | 198.1 (50.4-750.5) | 210 (50.4-750.5) | 190.4 (94.8-393.6) |
| Tregs, %, on CD4 | 4 (0.1-11.5) | 3.9 (0.1-11.5) | 4.2 (1.5-7.3) |
| Tregs, cells/μL | 20 (0.52-74.38) | 20.2 (0.5-74.3) | 18.9 (5.8-73.5) |
In the whole cohort of patients, age was significantly correlated with the percentage of CD4CD28null (r = 0.3, P = 0.03), percentage and number of CD8CD28null (r = 0.4, P < 0.001 and r = 0.3, P = 0.03, respectively) and percentage of ΝΚ cells (r = 0.3, P = 0.02). HDV had a negative correlation with total lymphocyte number (r = -0.3, P = 0.04), CD4+ lymphocytes (r = -0.3, P = 0.01), and Tregs (r = -0.4, P = 0.006). Patients who underwent preemptive kidney transplantation had a better immune profile than patients already enrolled in HD or continuous ambulatory peritoneal dialysis. In these patients, a significantly increased percentage and number of lymphocytes was observed, 27.9 (14%-37.7%) vs 18 (6.4%-40%) P = 0.03, and 1705 (100-2800) vs 1200 (700-2700) cells/μL, P = 0.03, respectively. Reduction in the percentage of CD4CD28null, 1.7 (0.4%-2.9%) vs 6.7 (0%-33.7%), P = 0.04 and CD8CD28null, [14.9 (6.1%-22.1%) vs 39.7 (114%-91%), P = 0.002, 207 (85-266) vs 477 (105-1131), P = 0.002] were also noticed as well as a significant increase in Tregs, affecting both percentage, 5.6 (1.7%-8.3%) vs 3.9 (0.1%-11.5%) P = 0.05, and total number of Tregs, 32.1 (24-47) cells/μL vs 18.9 (0.5-74) cells/μL, P = 0.006.
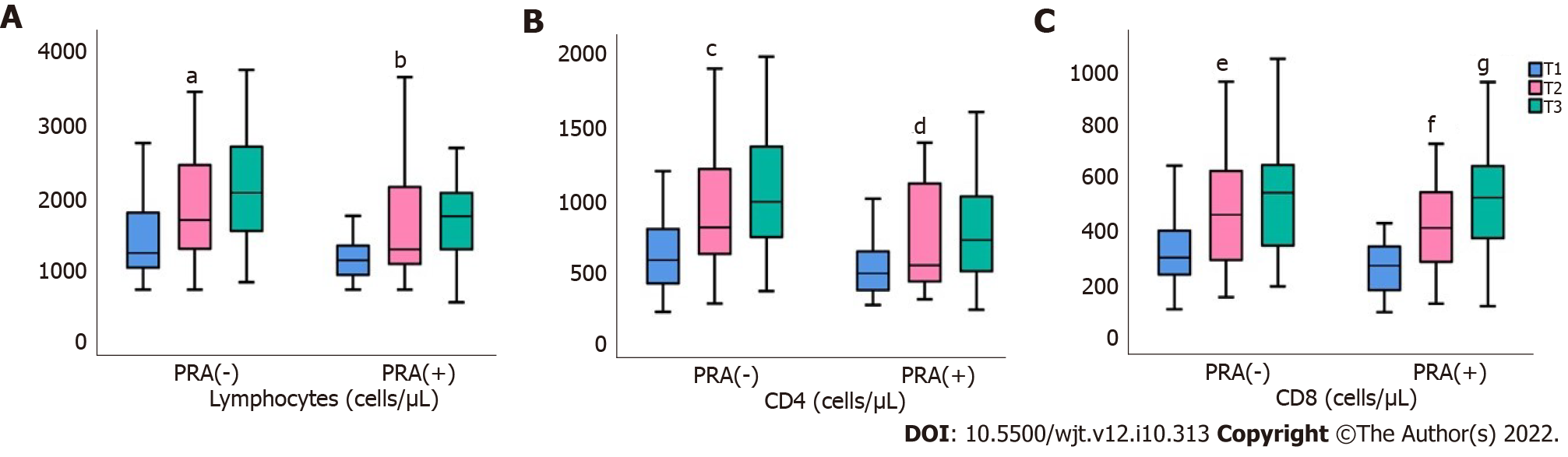
Changes in lymphocytes and their subpopulations following renal transplantation are depicted in Tables 4 and 5, for PRA(-) and PRA(+) patients, respectively. In both groups, PRA(-) and PRA(+), the percentage and total number of lymphocytes were increased. However, the response of lymphocyte changes was earlier and stronger in PRA(-) patients, as their percentage raised from T0 to T3, mean rank 15.35 to 20.98, P = 0.002, compared to 10.2 and 13.9, P = NS in PRA(+). This prompt response resulted in a significant increase in the number of total lymphocytes, in PRA(-), during the period T0 to T3, mean rank 10.57 to 20.41, P < 0.0001.
| Parameter | T0 | T3 | T6 | P value |
| Lymphocyte, % | 18.8 (6.4-38.4) | 23 (10.9-47.9) | 25.4 (8.4-52) | 0.001 |
| Lymphocyte, cells/μL | 1200 (700-2800) | 1650 (700-4100) | 1900 (800-3700) | < 0.0001 |
| CD4+, % | 44.4 (20.6-68.6) | 49.8 (22.7-77.1) | 49.1 (16.2-71.4) | 0.004 |
| CD4+, cells/μL | 557 (206-1453.2) | 782 (261.8-1951.6) | 872 (330-2001.6) | < 0.0001 |
| CD8+, % | 25.1 (12.2-37.7) | 26.9 (12.4-50.1) | 27.4 (13.3-49) | NS |
| CD8+, cells/μL | 301.5 (102.9-641.7) | 456.3 (148.6-1402.8) | 514.5 (189.2-1397.8) | < 0.0001 |
| CD4CD28null, % | 4.8 (0.2-33.7) | 2.8 (0-21.1) | 2.7 (0.1-36.4) | NS |
| CD4CD28null, cells/μL | 26.7 (0.9-149) | 27.5 (0-160) | 26.5 (09-241) | NS |
| CD8CD28null, % | 38.3 (6.1-68.2) | 28.4 (8.3-80.5) | 32.8 (6.7-90.7) | NS |
| CD8CD28null, cells/μL | 113.6 (17-315) | 112.6 (28-1129) | 158 (18-1267) | NS |
| CD16/56, % | 17.7 (3.6-50.6) | 6.6 (1.9-24.2) | 9.3 (2.9-28.6) | < 0.0001 |
| CD16/56, cells/μL | 210 (50.4-750.5) | 121.6 (33-622.2) | 151.2 (44-774.4) | < 0.0001 |
| Tregs, %, on CD4 | 3.9 (0.1-11.5) | 3.3 (0.9-6.8) | 4.1 (1.4-8.8) | NS |
| Tregs, cells/μL | 20.2 (0.5-74.3) | 29.4 (7.5-122.9) | 38.4 (8-104) | < 0.0001 |
| Parameter | T0 | T3 | T6 | P value |
| Lymphocyte, % | 17.8 (11.2-40) | 16.4 (7.5-36.8) | 20.9 (12.2-36.4) | 0.07 |
| Lymphocyte, cells/μL | 1100 (700-2600) | 1300 (700-3600) | 1700 (525-3200) | 0.009 |
| CD4+, % | 41.5 (25.3-59.5) | 42.3 (29.2-65.3) | 46.5 (27.4-62) | NS |
| CD4+, cells/μL | 435 (253-1362.4) | 548.9 (292-1371.3) | 744 (220-1888) | 0.008 |
| CD8+, % | 23.4 (10.5-53.1) | 27.4 (10.3-53.6) | 29.9 (11.6-56.2) | 0.005 |
| CD8+, cells/μL | 294.9 (91.7-665.6) | 408 (123.6-1234.8) | 504.9 (114.4-955.4) | < 0.0001 |
| CD4CD28null, % | 7.2 (0-32.1) | 5.3 (0.2-24.8) | 4 (0.1-28.6) | NS |
| CD4CD28null, cells/μL | 27.3 (0-206) | 22.8 (1.5-234) | 24.2 (1.3-244) | NS |
| CD8CD28null, % | 48.4 (15.1-91.5) | 47.1 (10.7-82.1) | 36.5 (7.7-82) | NS |
| CD8CD28null, cells/μL | 122.2 (13-583) | 200 (19-547) | 160 (22-726) | NS |
| CD16/56, % | 18.4 (4.4-34.2) | 11.4 (2.9-26) | 7.9 (3-24.6) | < 0.0001 |
| CD16/56, cells/μL | 190.4 (94.8-393.6) | 157.5 (34.8-450) | 135.7 (23.76-385.7) | 0.07 |
| Tregs, %, on CD4 | 4.2 (1.5-7.3) | 3.3 (1.2-6.8) | 4.4 (1.4-8.6) | NS |
| Tregs, cells/μL | 18.9 (5.8-73.5) | 20.9 (7.4-65.8) | 26.7 (8.5-103.8) | NS |
Although at time point T0, there was no significant difference in the percentage or total number of lymphocytes between the two groups of patients, at T3, PRA(-) had significantly increased percentage of lymphocytes, compared to PRA(+), 23 (10.9-47.9) vs 16.4 (7.5-36.8) μ/L, respectively, P = 0.03. At time T6, although there was still a superiority in PRA(-) patients the difference did not reach statistical significance, P = 0.06.
Both CD4 and CD8 cells were significantly increased in the two groups of patients, from T0 to T3. Figure 1 depicts changes of total lymphocytes, and also, in CD4 and CD8 cells after transplantation in PRA(-) and PRA(+) patients. There was a definite increase and gradual increase of total lymphocytes, together with CD4 and CD8 cells, from T0 towards T6 in both groups of patients, with changes in all three cell types being statistically significant even during the first 3 mo following transplantation.
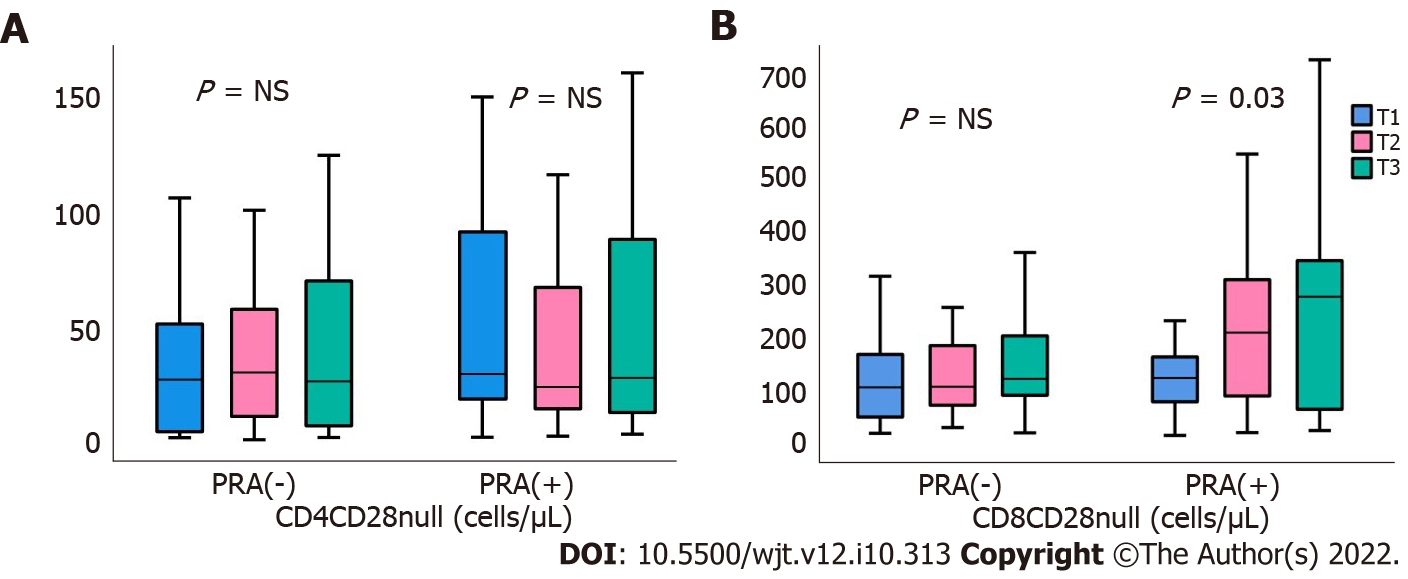
Regarding CD4CD28null cells, although there was a significant reduction in the percentage of CD4CD28null subtypes from T0 to T3, in both PRA(-) and PRA(+) patients, P = 0.04 and 0.01, respectively, population of cells and their percentage were stabilized thereafter, until T6, leading to no significant changes in these cell types during follow up, regardless of the presence of PRA. The results are descried in Tables 3 and 4 and depicted at Figure 2. On the other hand, there was a marked reduction in CD8CD28null cells, both percentage and numbers only in PRA(-) patients, from T0 to T3, P = 0.03, and from T3 to T6, P = 0.02. Such changes were not evident in PRA(+) patients, in contrast there was a significant increase in these cells during the first 3 mo (from T0 to T3).
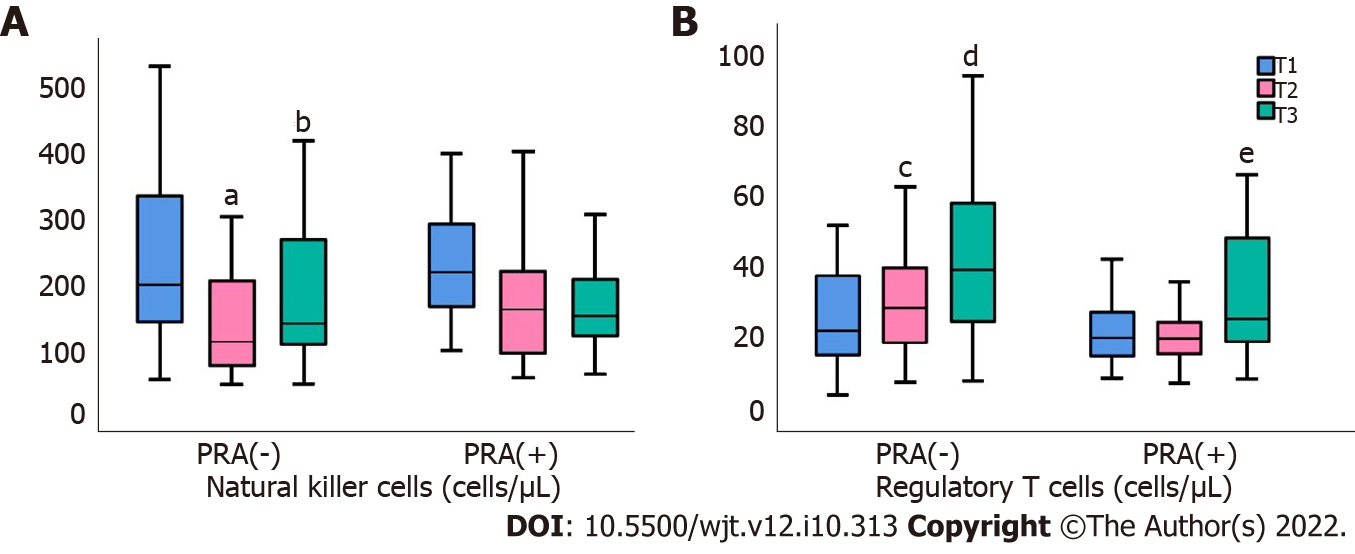
In PRA(-) there was a significant reduction in the percentage of NKs after renal transplantation, from T0 to T3 and from T3 to T6, P < 0.0001 and P = 0.006, respectively, and this was accompanied by significant elimination in the number of NK cells, (P = 0.002 and P = 0.005, respectively) in Figure 2. In contrast, within PRA(+) patients, the only significant changes were reported in the percentage of NK cells, during the time period, from T0 to T3, P = 0.001.
Similar differences were noticed between the two groups of patients regarding Tregs. The percentage of Tregs was increased only in PRA(-) patients, and this alteration was restricted only in the time period 3 to 6 mo, from T3 to T6, P = 0.02. Regulatory T cell population, however, was increased significantly in the same group, from T0 to T3, P = 0.01 and from T3 to T6, P = 0.003, while these cells showed no difference in PRA(+) patients from T0 to T3, and only mild restoration fromT3 to T6 (Figure 3).
The presence of high PRA levels, as a consequence of previous exposure to foreign HLAs[13], represents an increased possibility of preformed DSA occurrence, which is associated with the highest likelihood of graft loss[9,14]. Sensitization leads to the production of antibodies against HLA class I and HLA class II antigens, and activates different cell subpopulations, inducing immune response and possible rejection. The presence of HLA antibodies in the early term of transplantation may be more harmful to allografts, as they are associated with a higher incidence of acute rejection compared to patients who may develop antibodies later[12].
In this study, we evaluated the effect of PRA on the alterations of total lymphocytes and their subpopulations, following successful renal transplantation. For this reason, patients undergoing renal transplantation were divided in two groups, PRA(+) and PRA(-), according to the presence or absence of PRA at time of transplantation. All patients were followed prospectively for 6 mo at the Renal Transplant Outpatient clinic, and their renal function, medication, and clinical and laboratory parameters were assessed every month. Likewise, total lymphocytes, CD4, CD8, their subsets, CD4CD28null and CD 8CD28null, natural killer (NK) cells and Tregs were estimated by flow cytometry at the time of transplantation, and the 3- and 6-mo follow-up.
Although lymphocyte number was significantly and rapidly increased very early during follow-up, there were important differences in the immune response between PRA(-) and PRA(+) patients. The percentage and total number of lymphocytes were significantly improved during the first 3 mo in PRA(-) patients after transplantation. By contrast, the former showed a delayed and weak response in PRA(+) patients. Also, changes in lymphocyte subpopulations showed differences between the two groups. PRA(+) patients were characterized by a shift towards the CD8+ cell population, while in PRA(-) patients, CD4+ cells predominated during follow-up. As the presence of PRA was not associated with sex, age, time on HD, or impaired renal function, we anticipated that differences in T lymphocytes between PRA(-) and PRA(+) patients could not be attributed to other parameters such as HDV or renal function impairment, but rather were directly connected to the effect of PRA.
Interestingly, the expression of CD28 antigen on both CD4 and CD8 cells was not substantially affected by transplantation. CD28 loss is related to normal aging, but is also a consequence of chronic autoimmune and inflammatory diseases[15-19], while recently, CD28 elimination has been described in patients with CKD. The reduction of this receptor in CKD patients has been attributed to uremia, chronic inflammation, oxidative stress, CMV infection, and chronic dialysis[1,17-20].
We found that the percentage of CD4CD28null cells showed a reduction in both groups during the first 3 mo, yet they were subsequently stabilized until the end of follow-up. Regarding CD8CD28null cells, the beneficial effect was proven only in PRA(-) and not in PRA(+) patients, in whom there was a significant increase after the 3rd mo posttransplantation. This is in accordance with previous studies, which showed that CD28 antigen was significantly eliminated in both CD4 and CD8 cells after renal transplantation[21]. In a recent study, lymphocytes from renal transplant patients, who were followed for up to 5 years posttransplant, showed a tendency towards senescent phenotype, including a gradual increase in CD4CD28null and CD8CD28null cells. These findings indicate that despite restoring renal function with a successful renal transplantation, immune phenotype cannot be completely retained. Apparently, immunosuppression and steroid administration have a crucial role in this phenomenon, and this has been proved by the alterations in T cell phenotypes, after the withdrawal of steroids[22].
CD4+CD28null T cells are differentiated from classic T helper cells and share many features of cytotoxic CD8+ T cells and NK cells. They express a cytotoxic profile by producing proinflammatory cytokines, such as interferon gamma (IFN-γ), tumor necrosis factor alpha, and cytotoxic molecules[18,23,24]. CD28null T cells are considered terminally differentiated senescent cells, with shortened telomeres and great ability of cytotoxicity[19]. Thus, any alloreactivity of these cells may be detrimental for the transplant[20]. The gradual disappearance of CD28 following transplantation is controversial, with some investigators showing that loss of CD28 on CD4 T cells promotes immunosuppression resistance and allograft rejection[25,26], while others showing that loss of CD28 on T cells is related to immunosuppressive activity[17], leading to allograft tolerance and stabilization and is also associated with a lower frequency of late rejection and graft loss[27-29]. The role of PRA in CD28 expression seems crucial; however, there is a shortage of related information in the literature. The presence of anti-HLA antibodies may simply reflect the activation of adaptive immunity; however, they can induce endothelial damage, leading to de novo expression of endothelial neoantigens and vascular remodeling, as well as immune activation and chronic inflammation[30]. Therefore, the indirect effects of PRAs on the persistence of lymphocytes with cytotoxic activity may explain the increased levels of CD28null cells, but also their correlation with NK cells and regulatory T cells.
Changes in NK cells after transplant were more prominent. In both groups of patients, the percentage of NK cells was rapidly reduced during the first 3 mo, but only in PRA(-) patients was a reduction in the percentage of cells followed by the elimination of NK cell absolute numbers. NK cells play a crucial role in antibody-mediated rejection as occurs by the presence of HLA-DSAs[31-33]. NK cells are a source of IFN-γ production and they stimulate the T helper type 1 immune response. A direct interaction of NK cells with CD4+ T lymphocytes[34] increases their reactivity, which may motivate the mechanisms of acute rejection[33].
Most investigators support a mutual antagonism between NK and Treg cells[35]. Tregs seem to play major role in the long-term outcome of renal transplantation, as their population in the 6th and 12th mo posttransplantation was found to maintain immune tolerance in transplantation and is associated with better long-term graft survival[28,36-38], and some investigators have proven a time-dependent reduction of Tregs after kidney transplantation as a result of immunosuppressive treatment[28]. In our study, Tregs were almost spontaneously increased in PRA(-) patients during the first 3 mo of follow up, and continued to improve thereafter until the end of follow-up; by contrast, they showed only a delayed increase in PRA(+) patients.
In conclusion, this study demonstrated that T cell reinstatement following renal transplantation was closely affected by the presence of PRAs. Although lymphocyte population increased early after transplant, this beneficial effect did not involve all subpopulations. NK cells were reduced in both groups, Tregs were increased, but only in PRA(-) patients, whereas CD28null cells were not significantly restored regardless of the presence of PRAs.
It is essential to try to both understand and evaluate the effect of panel reactive antibodies (PRAs) on T cell immunity reinstatement, which follows renal transplantation. The potential association between subset changes and posttransplant graft function should be studied further.
This study demonstrated that T cell reinstatement following renal transplantation was closely affected by the presence of PRAs. Although the lymphocyte population increased early after kidney transplantation, this beneficial effect did not involve all subpopulations. Natural killer (NK) cells are reduced in both groups, regulatory T cells (Tregs) were increased, but only in PRA(-) patients, whereas CD28null cells were not significantly restored regardless of the presence of PRAs.
Patients were classified into two groups: PRA(-) (n = 43) and PRA(+) (n = 28). Patients who underwent preemptive kidney transplantation had a better immune profile than those already enrolled in hemodialysis or continuous ambulatory peritoneal dialysis.
Flow cytometry analysis was performed in 71 recipients of kidney transplantation at the time of transplantation, and at 3 and 6 mo after transplantation to estimate CD4CD28null, CD8CD28null, NK, and Treg cells.
The impact of positive PRA on the restoration of T cell phenotype after renal transplantation was evaluated.
Given the fact that PRA screening is a widely used test performed routinely in patients with chronic kidney disease (CKD) before renal transplantation to assess recipients’ exposure and sensitization, we believe it is essential to try to both understand and carefully evaluate the effect of PRA on T cell immunity reinstatement, which follows renal transplantation.
CKD is associated with phenotypic and functional changes in the immune system, including both innate and adaptive immunity, with detrimental clinical consequences. A successful renal transplantation will allow patients to stop dialysis and reinstates kidney function. Accordingly, as part of returning to normality, it is also expected to restore patients’ immune profile.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chan WYK, China; Ghazanfar A, United Kingdom; Sahin TT, Turkey S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Sampani E, Daikidou DV, Lioulios G, Xochelli A, Mitsoglou Z, Nikolaidou V, Dimitriadis C, Fylaktou A, Papagianni A, Stangou M. CD28null and Regulatory T Cells Are Substantially Disrupted in Patients with End-Stage Renal Disease Due to Diabetes Mellitus. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Lioulios G, Fylaktou A, Papagianni A, Stangou M. T cell markers recount the course of immunosenescence in healthy individuals and chronic kidney disease. Clin Immunol. 2021;225:108685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 3. | Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 563] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 4. | Derhovanessian E, Maier AB, Hähnel K, Beck R, de Craen AJM, Slagboom EP, Westendorp RGJ, Pawelec G. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol. 2011;92:2746-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Vallejo AN, Bryl E, Klarskov K, Naylor S, Weyand CM, Goronzy JJ. Molecular basis for the loss of CD28 expression in senescent T cells. J Biol Chem. 2002;277:46940-46949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 349] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 7. | Lee GH, Lee WW. Unusual CD4+CD28- T Cells and Their Pathogenic Role in Chronic Inflammatory Disorders. Immune Netw. 2016;16:322-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | van Walraven C, Austin PC, Knoll G. Predicting potential survival benefit of renal transplantation in patients with chronic kidney disease. CMAJ. 2010;182:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Alelign T, Ahmed MM, Bobosha K, Tadesse Y, Howe R, Petros B. Kidney Transplantation: The Challenge of Human Leukocyte Antigen and Its Therapeutic Strategies. J Immunol Res. 2018;2018:5986740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Jordan SC, Choi J, Kahwaji J, Vo A. Complement Inhibition for Prevention and Treatment of Antibody-Mediated Rejection in Renal Allograft Recipients. Transplant Proc. 2016;48:806-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Zhang R. Donor-Specific Antibodies in Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2018;13:182-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 12. | Akgul SU, Ciftci HS, Temurhan S, Caliskan Y, Bayraktar A, Tefik T, Kaya IA, Canitez IO, Demir E, Yazici H, Bakkaloglu H, Aydin AE, Turkmen A, Nane I, Aydin F, Oguz FS. Association Between HLA Antibodies and Different Sensitization Events in Renal Transplant Candidates. Transplant Proc. 2017;49:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Guichard-Romero A, Marino-Vazquez LA, Castelán N, López M, González-Tableros N, Arvizu A, De Santiago A, Alberú J, Morales-Buenrostro LE. Impact of pretransplant exposure to allosensitization factors generating HLA antibodies in the Luminex era. Transpl Immunol. 2016;38:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Elgueta S, Fuentes C, López M, Hernández J, Arenas A, Jiménez M, Gajardo JG, Rodríguez H, Labraña C. Effect of implementing anti-HLA antibody detection by Luminex in the kidney transplant program in Chile. Transplant Proc. 2011;43:3324-3326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Van Loon E, Lamarthée B, Barba T, Claes S, Coemans M, de Loor H, Emonds MP, Koshy P, Kuypers D, Proost P, Senev A, Sprangers B, Tinel C, Thaunat O, Van Craenenbroeck AH, Schols D, Naesens M. Circulating Donor-Specific Anti-HLA Antibodies Associate With Immune Activation Independent of Kidney Transplant Histopathological Findings. Front Immunol. 2022;13:818569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Żabińska M, Krajewska M, Kościelska-Kasprzak K, Klinger M. CD3(+)CD8(+)CD28(-) T Lymphocytes in Patients with Lupus Nephritis. J Immunol Res. 2016;2016:1058165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 372] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 18. | Mou D, Espinosa J, Lo DJ, Kirk AD. CD28 negative T cells: is their loss our gain? Am J Transplant. 2014;14:2460-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Rodriguez IJ, Lalinde Ruiz N, Llano León M, Martínez Enríquez L, Montilla Velásquez MDP, Ortiz Aguirre JP, Rodríguez Bohórquez OM, Velandia Vargas EA, Hernández ED, Parra López CA. Immunosenescence Study of T Cells: A Systematic Review. Front Immunol. 2020;11:604591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 20. | Dedeoglu B, Litjens NHR, de Weerd AE, Dor FJ, Klepper M, Reijerkerk D, Baan CC, Betjes MGH. T-Cell Composition of the Lymph Node Is Associated with the Risk for Early Rejection after Renal Transplantation. Front Immunol. 2017;8:1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Dedeoglu B, Meijers RW, Klepper M, Hesselink DA, Baan CC, Litjens NH, Betjes MG. Loss of CD28 on Peripheral T Cells Decreases the Risk for Early Acute Rejection after Kidney Transplantation. PLoS One. 2016;11:e0150826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Llinàs-Mallol L, Redondo-Pachón D, Pérez-Sáez MJ, Raïch-Regué D, Mir M, Yélamos J, López-Botet M, Pascual J, Crespo M. Peripheral blood lymphocyte subsets change after steroid withdrawal in renal allograft recipients: a prospective study. Sci Rep. 2019;9:7453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Moro-García MA, Alonso-Arias R, López-Larrea C. When Aging Reaches CD4+ T-Cells: Phenotypic and Functional Changes. Front Immunol. 2013;4:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Betjes MG, Meijers RW, de Wit LE, Litjens NH. A killer on the road: circulating CD4(+)CD28null T cells as cardiovascular risk factor in ESRD patients. J Nephrol. 2012;25:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Trzonkowski P, Zilvetti M, Chapman S, Wieckiewicz J, Sutherland A, Friend P, Wood KJ. Homeostatic repopulation by CD28-CD8+ T cells in alemtuzumab-depleted kidney transplant recipients treated with reduced immunosuppression. Am J Transplant. 2008;8:338-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | de Graav GN, Hesselink DA, Dieterich M, Kraaijeveld R, Douben H, de Klein A, Roelen DL, Weimar W, Roodnat JI, Clahsen-van Groningen MC, Baan CC. An Acute Cellular Rejection With Detrimental Outcome Occurring Under Belatacept-Based Immunosuppressive Therapy: An Immunological Analysis. Transplantation. 2016;100:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Klaus G, Mostert K, Reckzeh B, Mueller TF. Phenotypic changes in lymphocyte subpopulations in pediatric renal-transplant patients after T-cell depletion. Transplantation. 2003;76:1719-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Krajewska M, Kościelska-Kasprzak K, Kamińska D, Żabińska M, Myszka-Kozłowska M, Gomułkiewicz A, Dzięgiel P, Klinger M. Kidney Transplant Outcome Is Associated with Regulatory T Cell Population and Gene Expression Early after Transplantation. J Immunol Res. 2019;2019:7452019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Betjes MG. Clinical consequences of circulating CD28-negative T cells for solid organ transplantation. Transpl Int. 2016;29:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Van Laecke S, Malfait T, Schepers E, Van Biesen W. Cardiovascular disease after transplantation: an emerging role of the immune system. Transpl Int. 2018;31:689-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Resch T, Fabritius C, Ebner S, Ritschl P, Kotsch K. The Role of Natural Killer Cells in Humoral Rejection. Transplantation. 2015;99:1335-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Akiyoshi T, Hirohashi T, Alessandrini A, Chase CM, Farkash EA, Neal Smith R, Madsen JC, Russell PS, Colvin RB. Role of complement and NK cells in antibody mediated rejection. Hum Immunol. 2012;73:1226-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Pontrelli P, Rascio F, Castellano G, Grandaliano G, Gesualdo L, Stallone G. The Role of Natural Killer Cells in the Immune Response in Kidney Transplantation. Front Immunol. 2020;11:1454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Carlin LE, Hemann EA, Zacharias ZR, Heusel JW, Legge KL. Natural Killer Cell Recruitment to the Lung During Influenza A Virus Infection Is Dependent on CXCR3, CCR5, and Virus Exposure Dose. Front Immunol. 2018;9:781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Pontrelli P, Grandaliano G, Van Kooten C. Editorial: Kidney Transplantation and Innate Immunity. Front Immunol. 2020;11:603982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | San Segundo D, Fernández-Fresnedo G, Rodrigo E, Ruiz JC, González M, Gómez-Alamillo C, Arias M, López-Hoyos M. High regulatory T-cell levels at 1 year posttransplantation predict long-term graft survival among kidney transplant recipients. Transplant Proc. 2012;44:2538-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12:417-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 334] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 38. | Safinia N, Vaikunthanathan T, Fraser H, Thirkell S, Lowe K, Blackmore L, Whitehouse G, Martinez-Llordella M, Jassem W, Sanchez-Fueyo A, Lechler RI, Lombardi G. Successful expansion of functional and stable regulatory T cells for immunotherapy in liver transplantation. Oncotarget. 2016;7:7563-7577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |