Published online Aug 18, 2021. doi: 10.5500/wjt.v11.i8.335
Peer-review started: February 16, 2021
First decision: May 14, 2021
Revised: May 25, 2021
Accepted: August 10, 2021
Article in press: August 10, 2021
Published online: August 18, 2021
Processing time: 177 Days and 2 Hours
Hematopoietic stem cell transplantation (HSCT) is widely performed as a treatment for malignant blood disorders, such as leukemia. To achieve good clinical outcomes in HSCT, it is necessary to minimize the unfavorable effects of acute graft-vs-host disease (GVHD) and induce the more tolerable, chronic form of the disease. For better management of GVHD, sensitive and specific biomarkers that predict the severity and prognosis of the disease have been intensively investigated using proteomics, transcriptomics, genomics, cytomics, and tandem mass spectrometry methods. Here, I will briefly review the current understanding of GVHD biomarkers and future prospects.
Core Tip: Graft-vs-host disease (GVHD) is the most unfavorable complication of hematopoietic stem cell transplantation (HSCT). Minimizing acute GVHD and inducing its chronic form is necessary to achieve good clinical outcomes in HSCT. GVHD consists of inflammation induced by the conditioning regimen, the alloimmune response of the T lymphocytes, and organ damage due to the graft-vs-host reaction. Biological factors have been comprehensively analyzed to identify novel combinations of biomarkers that predict acute GVHD severity and prognosis more efficiently. Currently, there are no useful biomarkers that can predict the severity and prognosis of chronic GVHD or serve a practical clinical use.
- Citation: Nagasawa M. Biomarkers of graft-vs-host disease: Understanding and applications for the future. World J Transplant 2021; 11(8): 335-343
- URL: https://www.wjgnet.com/2220-3230/full/v11/i8/335.htm
- DOI: https://dx.doi.org/10.5500/wjt.v11.i8.335
Hematopoietic stem cell transplantation (HSCT) is widely performed as a treatment for malignant blood disorders, such as leukemia. Graft-vs-host disease (GVHD) is the most unfavorable complication of HSCT. Although the diagnostic process and management of GVHD are improving, GVHD remains a major clinical problem in transplantation medicine[1,2].
GVHD develops in the background of the immune response of the transplanted immune-competent cells against the alloantigens of the host, which is called the graft-vs-host (GVH) reaction. Such a reaction can cause allogeneic HSCT to induce GVHD. When the GVH reaction induces unfavorable symptoms, usually symptomatic organ damage, it is considered to be GVHD. When HSCT is performed as a treatment for malignant diseases in particular, the GVH reaction is therapeutically necessary, and it has been shown that a chronic GVH reaction (chronic GVHD) is correlated with the prevention of disease relapse and improved disease-free survival[3]. In acute GVHD, the relapse prevention effect is offset by its life-threatening complications, and a significant improvement in disease-free survival is difficult to prove in general[4]. Therefore, it is necessary to minimize the unfavorable effects of acute GVHD and induce tolerable chronic GVHD to achieve good clinical outcomes in HSCT for malignant diseases.
The severity of acute GVHD or the degree of organ damage is classically evaluated based on the clinical symptoms of three organs: the skin, liver, and intestine[5]. Therapeutic intervention for acute GVHD is usually considered in cases of grade 2 or above. It is difficult to predict responsiveness to treatment based on clinical severity and laboratory data before the intervention. It has been reported that the treatment response evaluated through clinical manifestations is rather important to predict prognosis. However, at least 4 wks of observation is necessary to determine the clinical improvement[6] and it is reported that 1 wk of clinical observation is not enough to predict the prognosis[7]. Therefore, a useful biomarker that can predict the responsiveness and prognosis of acute GVHD during a short interval has been intensively investigated. In addition, it must be considered that changes in the conditioning regimen, transplanted stem cells, and therapeutic immunosuppressive drugs may affect the utility of the rating system. Furthermore, we may have to re-consider the validity of conventional GVHD grading for the utility of novel biomarkers.
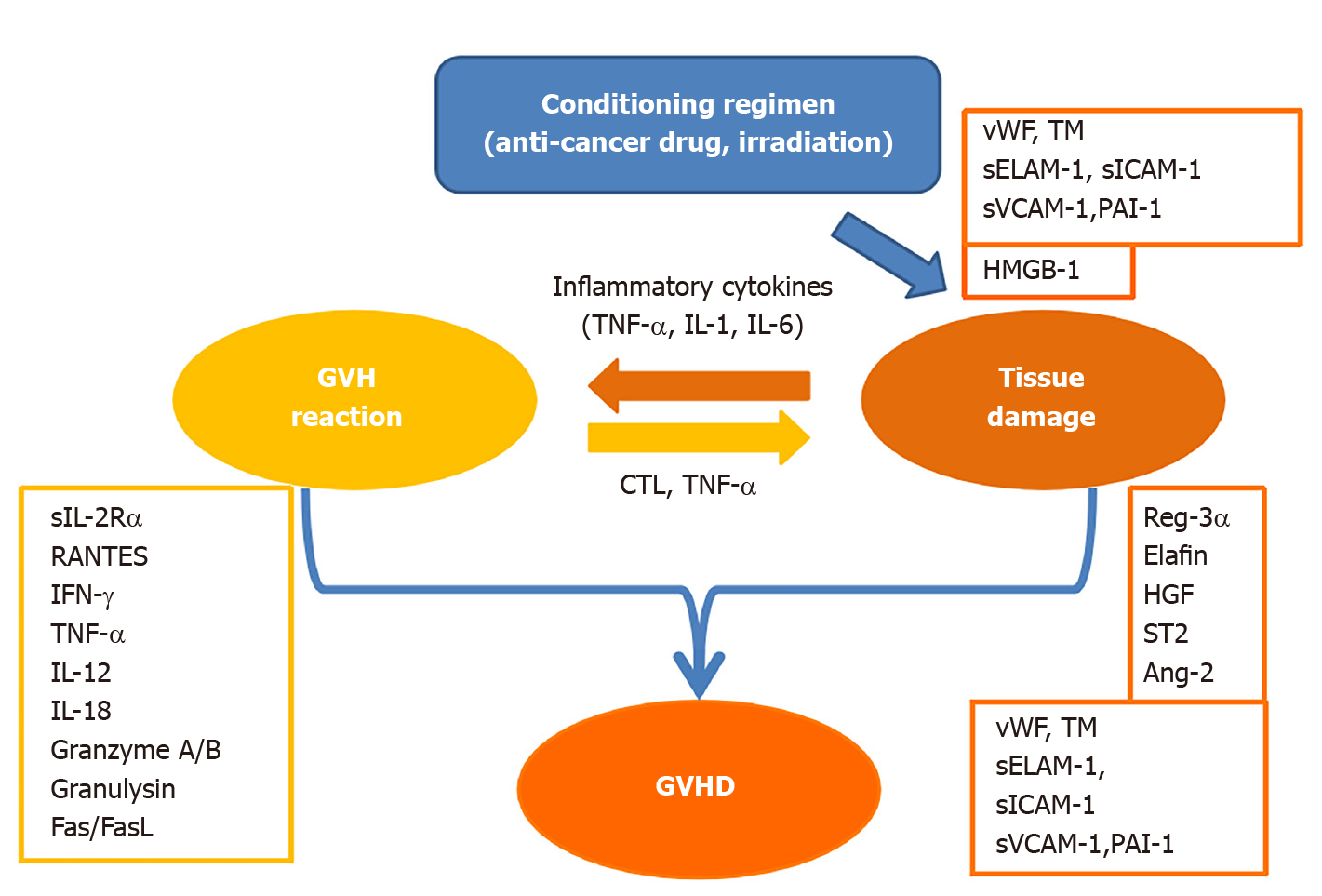
In this short review, acute GVHD-related biomarkers are discussed according to three divided phases: (1) An initiating proinflammatory period; (2) A GVH reaction induced by an immune response to alloantigens; and (3) The induction of organ damage as a result of GVHD (Figure 1). It is reasonable to expect that the biomarker of the GVH reaction is correlated with the intensity of GVHD. However, this is to be answered in terms of GVHD prognosis. Is the absolute value of the biomarker important? Is the duration of elevated biomarkers important? Otherwise, is responsiveness to the treatment important? Furthermore, the prognosis of GVHD is affected by host factors, which makes this problem more complicated.
Inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6 are known to be released early in the transplant from the tissue or vascular endothelium damaged by the anticancer drugs or irradiation used in the conditioning regimens. These tissue-derived cytokines promote vascular endothelial damage and are thought to amplify the GVH reaction of transplanted lymphocytes through the activation of antigen-presenting cells[8]. When these cytokines are excessively produced, they are referred to as cytokine storms and are thought to be involved in hyperacute GVHD.
The GVH reaction appears as an alloimmune response of T lymphocytes, inducing the activation of T lymphocytes, overproduction of cytokines, and development of primarily CD8-positive cytotoxic T lymphocytes (CTLs).
The most frequently used biomarker of T cell activation is a soluble IL-2 receptor (sIL-2R)[9]. regulated upon activation, normal T cell expressed and secreted (RANTES) is also used[10]. Recently, a sensitive assay system has been developed for several T cell derived extracellular vesicles (TDEVs), which are triggered for release by activation[11]. It was found that TDEVs accurately reflect the GVH reaction and acute GVHD specifically[12]. In addition, interferon-g (IFN-g) released by activated T lymphocytes activates macrophages to produce TNF-α, which promotes tissue injury[13] and is reported to be a useful biomarker of GVHD[14,15]. In contrast, ferritin and soluble CD163, which are also produced by activated macrophages in this phase, are reported to be associated with the prognosis of HSCT rather than GVHD[16]. It has been reported that type 1 T helper (Th1) immunity is the main component of acute GVHD, and that Th1 cytokines, IL-12, and IL-18 are biomarkers of GVHD[17].
The activated biomarkers of CTLs include granzyme A/B[18] and granulysin[19]. The former exhibits direct killing activity against all target cells in the presence of perforin, while the latter displays direct killing activity towards all target cells by itself[20]. Additionally, there is the Fas and Fas Ligand (Fas-FasL) system, which exhibits killing activity against only the target cells expressing Fas[21], such as that of hepatocytes and the epidermis[22].
The abovementioned biomarkers are indicators of the GVH reaction but are not directly related to the severity of GVHD, which is based on organ injury, damage, or dysfunction. A component of the biomarkers produced by the GVH reaction is the so-called inflammatory cytokine that induces fever[23], but the fever does not necessarily correlate with the severity of GVHD.
Regenerating islet-derived 3α (Reg-3α) is a C-type lectin that was discovered in regenerating islet cells. Reg-3α has antibacterial activity against gram-positive bacteria and is thought to be produced during the destruction and repair of intestinal tissue. It has been reported that the level of Reg-3α on days 7 and 14 of HSCT predicts acute GVHD and non-relapse mortality (NRM) very well[24,25].
Elafin, also known as skin-derived antileukoprotease or peptidase inhibitor 3, correlates well with skin symptoms due to GVHD[26]. It is thought to be produced as part of the tissue repair process for skin lesions caused by GVHD.
Hepatocyte growth factor (HGF) regenerates hepatocytes, and serum HGF levels are high in acute GVHD[27]. It is considered to reflect the regeneration response of hepatocytes damaged by GVHD. Interestingly, HGF has the ability to relieve acute GVHD in mice[28].
Vascular endothelial injury related to GVHD is a complicated problem associated with HSCT. There are no characteristic clinical manifestations of vascular endothelial injury, such as with GVHD of the skin (rash), liver (jaundice), and intestine (diarrhea), and differentiation from other complications such as thrombotic microangiopathy or veno-occlusive disease that develop in the background of endothelial injury during the process of HSCT is quite difficult. GVHD-related vascular endothelial injury usually spans across various organs.
The presence of vascular endothelial injury has been reported to influence the onset and prognosis of acute GVHD[29]. The following biomarkers of vascular endothelial injury have been reported: von Willebrand factor (vWF)[30], thrombomodulin (TM)[31], soluble adhesion molecules (sELAM-1, sICAM-1, and sVCAM-1)[32], plasmino
At present, it is considered that GVHD is an exacerbating factor of vascular endothelial injury, and intractable vascular endothelial disorder and the elevated vascular injury biomarker are thought to be associated with refractory GVHD[35] and poor prognosis[36,37] rather than a predictor of GVHD.
Suppression of tumorigenicity 2 (ST2) belongs to the IL-1 receptor family, binds to IL-33, and induces a Th2 immune response. Soluble ST2 inhibits IL-33 as a decoy receptor, and it is said to act on a Th1 deviated response[38]. ST2 has been reported as a biomarker of cardiovascular diseases and is thought to reflect myocardial repair and remodeling. When ST2 is high, myocardial damage is considered severe, and the prognosis of cardiovascular diseases is poor[39].
ST2 is reported to reflect refractory GVHD in the examination of acute GVHD[40]. It has been reported that the ST2 value on day 28 post-transplantation is associated with grade 3–4 GVHD with a much better NRM rate than TNF-α, IL-8, Reg-3a, sIL-2Ra, elafin, and HGF in the cord blood transplantation cohort[41]. It is speculated that ST2 is elevated along with tissue damage in refractory GVHD, and then the increased ST2 may induce a Th1 response by inhibiting IL-33, thus further aggravating the acute GVHD.
High-mobility group box 1 (HMGB-1) is a ubiquitous nuclear protein that regulates chromatin function, similar to histones. HMGB-1 is released from activated macrophages or damaged tissues and induces inflammation via interactions with toll-like receptors (TLR2 or TLR4). Therefore, HMGB-1 is considered a damage-associated molecular pattern (DAMP). In a mouse model, HMGB-1 has been reported to be involved in the pathogenesis of GVHD[42,43]. In humans, increased HMGB-1 levels on day 0 are reported to be associated with vascular injury, but not necessarily with GVHD[44].
It is reasonable that the GVH reaction biomarkers are related to GVHD because the latter is fundamentally based on the former. However, it is also true that it is quite difficult for one or a few biomarkers to predict GVHD prognosis precisely, because there are numerous and diverse pathological factors involved in the process of HSCT, in addition to the heterogeneity of the donor and recipient.
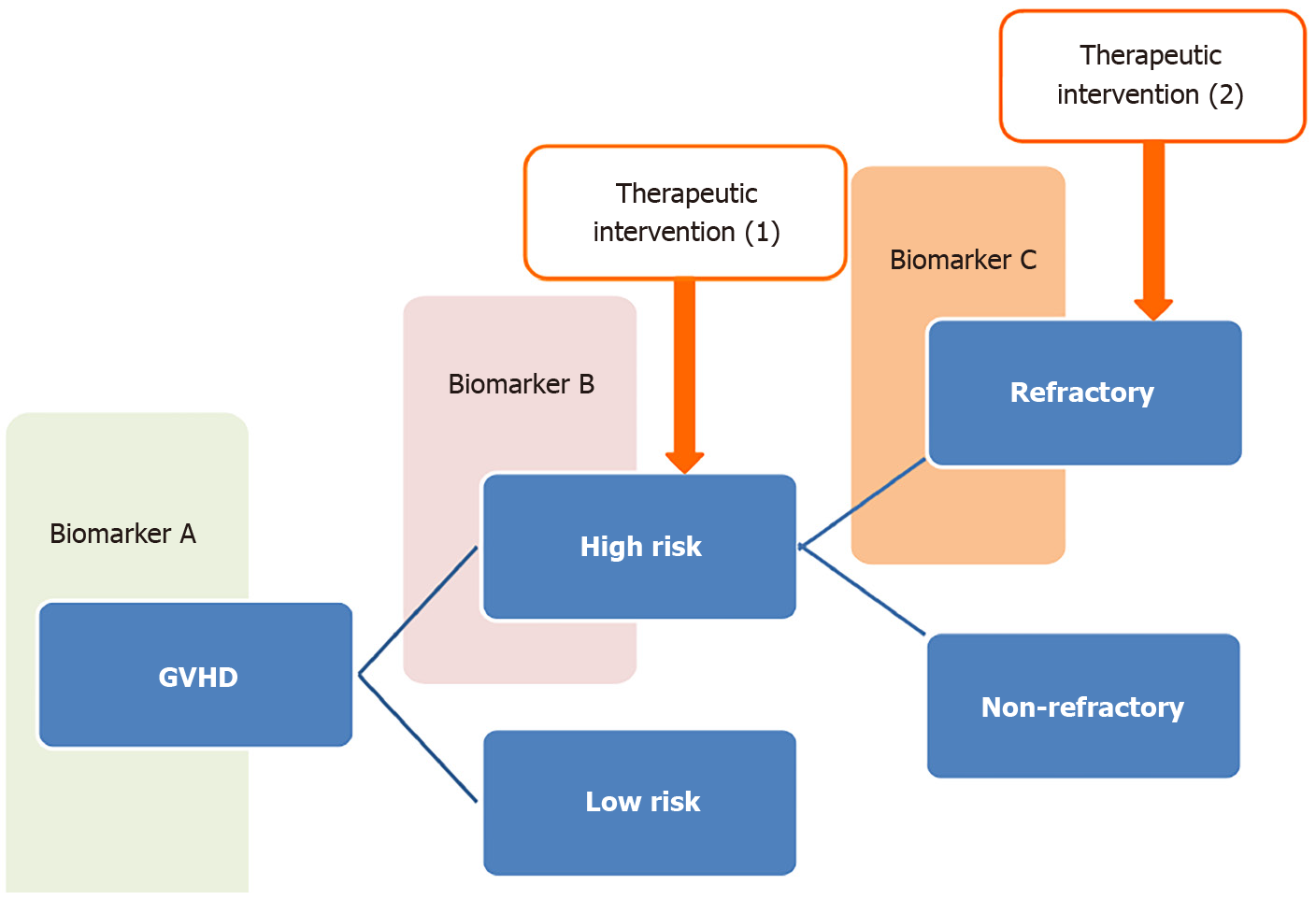
Recently, a comprehensive analysis of biological factors has been performed using proteomics, transcriptomics, genomics, cytomics, and tandem mass spectrometry methods to identify novel combinations of biomarkers that predict the severity and prognosis of GVHD more efficiently rather than searching useful biomarkers by disclosing the basic pathogenesis of GVHD[45].
In the treatment of pediatric acute lymphocytic leukemia, a remarkable improvement in prognosis has been achieved under the two-step stratification of therapy by pre-treatment and steroid-sensitive factors, without the introduction of new anticancer drugs between 1960 and 1995[46].
Based on a similar concept, it will be necessary to stratify therapeutic strategies by considering both the severity of GVHD and responsiveness to treatment according to the proper combination of biomarkers to overcome refractory GVHD (Figure 2). There are several reports of biomarkers that predict responsiveness to GVHD therapy in a short observation period[47,48]. However, it must also be taken into consideration that the clinical outcomes of GVHD are affected by the progress of preventive and targeted therapy for GVHD.
Chronic GVHD is similar to collagen disease in that its pathological essence is based on immune dysregulation[49]. In chronic GVHD, a high level of soluble B cell-activating factor, an abnormality of the B lymphocyte subset[50], and a decrease in regulatory T cells (CD4+CD25+FOXP+/CD4 ratio)[51] have been reported. matrix metalloproteinase-3 (MMP-3) has been reported to be associated with non-infectious obstructive bronchiolitis[52], which is one of the most intractable and lethal complications of chronic GVHD. Currently, there are no useful biomarkers that can predict the severity and prognosis of chronic GVHD or serve a practical clinical use.
Recently, there has been much progress in the understanding of acute and chronic inflammation based on basic immunology[53,54]. In these studies, it was reported that chromatin and DNA modifications in immune cells are significant in chronic inflammation. Based on a new approach towards and an understanding of chronic GVHD, it is expected that its essential pathology will be disclosed and the available biomarkers will be discovered in the near future.
The sensitive and specific biomarkers that predict the severity and prognosis of GVHD have been intensively investigated through using proteomics, transcriptomics, genomics, cytomics, and tandem mass spectrometry methods. Although the utility of available biomarkers have limitations for the clinical decisions, it is expected that its essential pathology will be disclosed and the more useful biomarkers will be discovered in the near future.
I appreciate all of the staff at the Tokyo Medical and Dental University Hospital who were involved in the medical care of patients with SCT.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aguilera I, Lei YC S-Editor: Yan JP L-Editor: A P-Editor: Guo X
| 1. | Nimer SD, Giorgi J, Gajewski JL, Ku N, Schiller GJ, Lee K, Territo M, Ho W, Feig S, Selch M. Selective depletion of CD8+ cells for prevention of graft-versus-host disease after bone marrow transplantation. A randomized controlled trial. Transplantation. 1994;57:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 110] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Champlin RE, Passweg JR, Zhang MJ, Rowlings PA, Pelz CJ, Atkinson KA, Barrett AJ, Cahn JY, Drobyski WR, Gale RP, Goldman JM, Gratwohl A, Gordon-Smith EC, Henslee-Downey PJ, Herzig RH, Klein JP, Marmont AM, O'Reilly RJ, Ringdén O, Slavin S, Sobocinski KA, Speck B, Weiner RS, Horowitz MM. T-cell depletion of bone marrow transplants for leukemia from donors other than HLA-identical siblings: advantage of T-cell antibodies with narrow specificities. Blood. 2000;95:3996-4003. [PubMed] |
| 3. | Ringdén O, Labopin M, Gluckman E, Reiffers J, Vernant JP, Jouet JP, Harousseau JL, Fiere D, Bacigalupo A, Frassoni F, Gorin NC. Strong antileukemic effect of chronic graft-versus-host disease in allogeneic marrow transplant recipients having acute leukemia treated with methotrexate and cyclosporine. The Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Transplant Proc. 1997;29:733-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringdén O, Rozman C, Speck B. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555-562. [PubMed] |
| 5. | Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825-828. [PubMed] |
| 6. | MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115:5412-5417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Saliba RM, Couriel DR, Giralt S, Rondon G, Okoroji GJ, Rashid A, Champlin RE, Alousi AM. Prognostic value of response after upfront therapy for acute GVHD. Bone Marrow Transplant. 2012;47:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1987] [Cited by in RCA: 1843] [Article Influence: 115.2] [Reference Citation Analysis (0)] |
| 9. | Paczesny S, Krijanovski OI, Braun TM, Choi SW, Clouthier SG, Kuick R, Misek DE, Cooke KR, Kitko CL, Weyand A, Bickley D, Jones D, Whitfield J, Reddy P, Levine JE, Hanash SM, Ferrara JL. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 297] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 10. | Nomura S, Ishii K, Kanazawa S, Inami N, Kamitsuji Y, Uoshima N, Ishida H, Yoshihara T, Kitayama H, Hayashi K. Role of platelet-derived chemokines (RANTES and ENA-78) after stem cell transplantation. Transpl Immunol. 2006;15:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Oba R, Isomura M, Igarashi A, Nagata K. Circulating CD3+HLA-DR+ Extracellular Vesicles as a Marker for Th1/Tc1-Type Immune Responses. J Immunol Res. 2019;2019:6720819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Nagasawa M, Mitsuiki N, Yanagimachi M, Yamamoto M, Fukuda T, Miura O, Oba R, Igarashi A, Nagata K, Morio T. Utility of novel T-cell-specific extracellular vesicles in monitoring and evaluation of acute GVHD. Int J Hematol. 2021;113:910-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Teshima T, Ordemann R, Reddy P, Gagin S, Liu C, Cooke KR, Ferrara JL. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 394] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 14. | Remberger M, Ringden O, Markling L. TNF alpha levels are increased during bone marrow transplantation conditioning in patients who develop acute GVHD. Bone Marrow Transplant. 1995;15:99-104. [PubMed] |
| 15. | Levine JE. Implications of TNF-α in the pathogenesis and management of GVHD. Int J Hematol. 2011;93:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Großekatthöfer M, Güclü ED, Lawitschka A, Matthes-Martin S, Mann G, Minkov M, Peters C, Seidel MG. Ferritin concentrations correlate to outcome of hematopoietic stem cell transplantation but do not serve as biomarker of graft-versus-host disease. Ann Hematol 2013; 92: 1121-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Chen YB, Cutler CS. Biomarkers for acute GVHD: can we predict the unpredictable? Bone Marrow Transplant. 2013;48:755-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Kircher B, Schumacher P, Nachbaur D. Granzymes A and B serum levels in allo-SCT. Bone Marrow Transplant. 2009;43:787-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Nagasawa M, Isoda T, Itoh S, Kajiwara M, Morio T, Shimizu N, Ogawa K, Nagata K, Nakamura M, Mizutani S. Analysis of serum granulysin in patients with hematopoietic stem-cell transplantation: its usefulness as a marker of graft-versus-host reaction. Am J Hematol. 2006;81:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Nagasawa M. Granulysin and its clinical sugnificance as a biomarker of immune response and NK-cell related neoplasms. World J Hematol. 2014;3:128-137. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (2)] |
| 21. | Nomura S, Ishii K, Inami N, Uoshima N, Ishida H, Yoshihara T, Kitayama H, Hayashi K. Role of soluble tumor necrosis factor-related apoptosis-inducing ligand concentrations after stem cell transplantation. Transpl Immunol. 2007;18:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Lee S, Chong SY, Lee JW, Kim SC, Min YH, Hahn JS, Ko YW. Difference in the expression of Fas/Fas-ligand and the lymphocyte subset reconstitution according to the occurrence of acute GVHD. Bone Marrow Transplant. 1997;20:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Tanaka J, Imamura M, Kasai M, Masauzi N, Matsuura A, Ohizumi H, Morii K, Kiyama Y, Naohara T, Saitho M. Cytokine gene expression in peripheral blood mononuclear cells during graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol. 1993;85:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Hartwell MJ, Özbek U, Holler E, Renteria AS, Major-Monfried H, Reddy P, Aziz M, Hogan WJ, Ayuk F, Efebera YA, Hexner EO, Bunworasate U, Qayed M, Ordemann R, Wölfl M, Mielke S, Pawarode A, Chen YB, Devine S, Harris AC, Jagasia M, Kitko CL, Litzow MR, Kröger N, Locatelli F, Morales G, Nakamura R, Reshef R, Rösler W, Weber D, Wudhikarn K, Yanik GA, Levine JE, Ferrara JL. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. 2017;2:e89798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 174] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 25. | McDonald GB, Tabellini L, Storer BE, Lawler RL, Martin PJ, Hansen JA. Plasma biomarkers of acute GVHD and nonrelapse mortality: predictive value of measurements before GVHD onset and treatment. Blood. 2015;126:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 26. | Levine JE, Logan BR, Wu J, Alousi AM, Bolaños-Meade J, Ferrara JL, Ho VT, Weisdorf DJ, Paczesny S. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a Blood and Marrow Transplant Clinical Trials Network study. Blood. 2012;119:3854-3860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Okamoto T, Takatsuka H, Fujimori Y, Wada H, Iwasaki T, Kakishita E. Increased hepatocyte growth factor in serum in acute graft-versus-host disease. Bone Marrow Transplant. 2001;28:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Kuroiwa T, Kakishita E, Hamano T, Kataoka Y, Seto Y, Iwata N, Kaneda Y, Matsumoto K, Nakamura T, Ueki T, Fujimoto J, Iwasaki T. Hepatocyte growth factor ameliorates acute graft-versus-host disease and promotes hematopoietic function. J Clin Invest. 2001;107:1365-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Nagasawa M, Ohkawa T, Endo A, Mitsuiki N, Ono T, Aoki Y, Isoda T, Tomizawa D, Takagi M, Kajiwara M, Morio T, Mizutani S. Early coagulation disorder after allogeneic stem cell transplantation is a strong prognostic factor for transplantation-related mortality, and intervention with recombinant human thrombomodulin improves the outcome: a single-center experience. Int J Hematol. 2013;98:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Nomura S, Ishii K, Kanazawa S, Inami N, Uoshima N, Ishida H, Yoshihara T, Kitayama H, Hayashi K. Significance of elevation in cell-derived microparticles after allogeneic stem cell transplantation: transient elevation of platelet-derived microparticles in TMA/TTP. Bone Marrow Transplant. 2005;36:921-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Nomura S, Ozasa R, Nakanishi T, Fujita S, Miyaji M, Mori S, Yokoi T, Ito T, Ishii K. Can recombinant thrombomodulin play a preventive role for veno-occlusive disease after haematopoietic stem cell transplantation? Thromb Haemost. 2011;105:1118-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Tanikawa S, Mori S, Ohhashi K, Akiyama H, Sasaki T, Kaku H, Hiruma K, Matsunaga T, Morita T, Sakamaki H. Predictive markers for hepatic veno-occlusive disease after hematopoietic stem cell transplantation in adults: a prospective single center study. Bone Marrow Transplant. 2000;26:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Woywodt A, Scheer J, Hambach L, Buchholz S, Ganser A, Haller H, Hertenstein B, Haubitz M. Circulating endothelial cells as a marker of endothelial damage in allogeneic hematopoietic stem cell transplantation. Blood. 2004;103:3603-3605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Nomura S, Ishii K, Inami N, Kimura Y, Uoshima N, Ishida H, Yoshihara T, Urase F, Maeda Y, Hayashi K. Evaluation of angiopoietins and cell-derived microparticles after stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Luft T, Dietrich S, Falk C, Conzelmann M, Hess M, Benner A, Neumann F, Isermann B, Hegenbart U, Ho AD, Dreger P. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118:1685-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 36. | Porkholm M, Bono P, Saarinen-Pihkala UM, Kivivuori SM. Higher angiopoietin-2 and VEGF levels predict shorter EFS and increased non-relapse mortality after pediatric hematopoietic SCT. Bone Marrow Transplant. 2013;48:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Mir E, Palomo M, Rovira M, Pereira A, Escolar G, Penack O, Holler E, Carreras E, Diaz-Ricart M. Endothelial damage is aggravated in acute GvHD and could predict its development. Bone Marrow Transplant. 2017;52:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1453] [Article Influence: 132.1] [Reference Citation Analysis (0)] |
| 39. | Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 393] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 40. | Vander Lugt MT, Braun TM, Hanash S, Ritz J, Ho VT, Antin JH, Zhang Q, Wong CH, Wang H, Chin A, Gomez A, Harris AC, Levine JE, Choi SW, Couriel D, Reddy P, Ferrara JL, Paczesny S. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med. 2013;369:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 315] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 41. | Ponce DM, Hilden P, Mumaw C, Devlin SM, Lubin M, Giralt S, Goldberg JD, Hanash A, Hsu K, Jenq R, Perales MA, Sauter C, van den Brink MR, Young JW, Brentjens R, Kernan NA, Prockop SE, O'Reilly RJ, Scaradavou A, Paczesny S, Barker JN. High day 28 ST2 levels predict for acute graft-versus-host disease and transplant-related mortality after cord blood transplantation. Blood. 2015;125:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 42. | Im KI, Kim N, Lim JY, Nam YS, Lee ES, Kim EJ, Kim HJ, Kim SH, Cho SG. The Free Radical Scavenger NecroX-7 Attenuates Acute Graft-versus-Host Disease via Reciprocal Regulation of Th1/Regulatory T Cells and Inhibition of HMGB1 Release. J Immunol. 2015;194:5223-5232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Apostolova P, Zeiser R. The role of danger signals and ectonucleotidases in acute graft-versus-host disease. Hum Immunol. 2016;77:1037-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Nomura S, Maeda Y, Ishii K, Katayama Y, Yagi H, Fujishima N, Ota S, Moriyama M, Ikezoe T, Miyazaki Y, Hayashi K, Fujita S, Satake A, Ito T, Kyo T, Tanimoto M. Relationship between HMGB1 and PAI-1 after allogeneic hematopoietic stem cell transplantation. J Blood Med. 2016;7:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Paczesny S. Biomarkers for posttransplantation outcomes. Blood. 2018;131:2193-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 46. | Pui CH. Childhood leukemias. N Engl J Med. 1995;332:1618-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 336] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 47. | Major-Monfried H, Renteria AS, Pawarode A, Reddy P, Ayuk F, Holler E, Efebera YA, Hogan WJ, Wölfl M, Qayed M, Hexner EO, Wudhikarn K, Ordemann R, Young R, Shah J, Hartwell MJ, Chaudhry MS, Aziz M, Etra A, Yanik GA, Kröger N, Weber D, Chen YB, Nakamura R, Rösler W, Kitko CL, Harris AC, Pulsipher M, Reshef R, Kowalyk S, Morales G, Torres I, Özbek U, Ferrara JLM, Levine JE. MAGIC biomarkers predict long-term outcomes for steroid-resistant acute GVHD. Blood. 2018;131:2846-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 48. | Solán L, Kwon M, Carbonell D, Dorado N, Balsalobre P, Serrano D, Chicano-Lavilla M, Anguita J, Gayoso J, Díez-Martín JL, Martínez-Laperche C, Buño I. ST2 and REG3α as Predictive Biomarkers After Haploidentical Stem Cell Transplantation Using Post-transplantation High-Dose Cyclophosphamide. Front Immunol. 2019;10:2338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Zeiser R, Blazar BR. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N Engl J Med. 2017;377:2565-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 512] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 50. | Sarantopoulos S, Ritz J. Aberrant B-cell homeostasis in chronic GVHD. Blood. 2015;125:1703-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 51. | Alho AC, Kim HT, Chammas MJ, Reynolds CG, Matos TR, Forcade E, Whangbo J, Nikiforow S, Cutler CS, Koreth J, Ho VT, Armand P, Antin JH, Alyea EP, Lacerda JF, Soiffer RJ, Ritz J. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016;127:646-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 52. | Liu X, Yue Z, Yu J, Daguindau E, Kushekhar K, Zhang Q, Ogata Y, Gafken PR, Inamoto Y, Gracon A, Wilkes DS, Hansen JA, Lee SJ, Chen JY, Paczesny S. Proteomic Characterization Reveals That MMP-3 Correlates With Bronchiolitis Obliterans Syndrome Following Allogeneic Hematopoietic Cell and Lung Transplantation. Am J Transplant. 2016;16:2342-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523:612-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 499] [Cited by in RCA: 514] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 54. | Utzschneider DT, Gabriel SS, Chisanga D, Gloury R, Gubser PM, Vasanthakumar A, Shi W, Kallies A. Early precursor T cells establish and propagate T cell exhaustion in chronic infection. Nat Immunol. 2020;21:1256-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |