Published online Jun 18, 2021. doi: 10.5500/wjt.v11.i6.231
Peer-review started: February 9, 2021
First decision: March 17, 2021
Revised: March 18, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: June 18, 2021
Processing time: 122 Days and 23.7 Hours
Variations in the anatomy of hepatic veins are of interest to transplant surgeons, interventional radiologists, and other medical practitioners who treat liver diseases. The drainage patterns of the right hepatic veins (RHVs) are particularly relevant to transplantation services.
The aim was to identify variations of the patterns of venous drainage from the right side of the liver. To the best of our knowledge, there have been no reports on RHV variations in in a Caribbean population.
Two radiologists independently reviewed 230 contrast-enhanced computed tomography scans performed in 1 year at a hepatobiliary referral center. Venous outflow patterns were observed and RHV variants were described as: (1) Tributaries of the RHV; (2) Variations at the hepatocaval junction (HCJ); and (3) Accessory RHVs.
A total of 118 scans met the inclusion criteria. Only 39% of the scans found conventional anatomy of the main hepatic veins. Accessory RHVs were present 49.2% and included a well-defined inferior RHV draining segment VI (45%) and a middle RHV (4%). At the HCJ, 83 of the 118 (70.3%) had a superior RHV that received no tributaries within 1 cm of the junction (Nakamura and Tsuzuki type I). In 35 individuals (29.7%) there was a short superior RHV with at least one variant tributary. According to the Nakamura and Tsuzuki classification, there were 24 type II variants (20.3%), six type III variants (5.1%) and, five type IV variants (4.2%).
There was significant variation in RHV patterns in this population, each with important relevance to liver surgery. Interventional radiologists and hepatobiliary surgeons practicing in the Caribbean must be cognizant of these differences in order to minimize morbidity during invasive procedures.
Core Tip: There were variations in right hepatic vein (RHV) anatomy in 61% of unselected persons in the eastern Caribbean. They included proximal confluence (61%), accessory RHVs (49.2%), hepatocaval junction variants (29.7%), both dorsal and ventral segment VIII veins entering middle hepatic vein (28%), and absent segment VII tributaries (4.2%). The Nakamura and Tsuzuki classification included type I hepatocaval junction variants in 83 individuals (70.3%), type II in 24 (20.3%), type III in 6 (5.1%), and type IV variants in five (4.2%). Knowledge of the anatomic variations of the RHV are particularly important to optimize transplantation services.
- Citation: Cawich SO, Naraynsingh V, Pearce NW, Deshpande RR, Rampersad R, Gardner MT, Mohammed F, Dindial R, Barrow TA. Surgical relevance of anatomic variations of the right hepatic vein. World J Transplant 2021; 11(6): 231-243
- URL: https://www.wjgnet.com/2220-3230/full/v11/i6/231.htm
- DOI: https://dx.doi.org/10.5500/wjt.v11.i6.231
There are many variations in the venous drainage of the human liver. This information is important to transplant surgeons, interventional radiologists, and other medical practitioners with an interest in treating liver diseases. Anatomic variations of the right hepatic veins (RHVs) are particularly important to optimize transplantation services. We carried out this study to document the venous outflow patterns from the right hemi-liver in a Caribbean population. The information is important to clinicians who treat Caribbean diaspora patients with liver disorders.
This study was carried out at an 850-bed tertiary hepatobiliary referral center in the eastern Caribbean. A multidisciplinary team approach is used at this facility to plan the management of all patients after a detailed review of electronic imaging. Approval was obtained from the local institutional review board to review these images, and the study was carried out in keeping with the agreements reached in the Declaration of Helsinki, revised in October 2000 as drafted by the World Medical Association. All patients had multiphase computed tomography (CT) scans using a 64 slice multi-row detector CT scanner. A nonionic contrast medium, iopromide (Ultravist 300®) 100 mL was administered in all contrast CT abdomen studies using a pressure injector with bolus tracking.
A retrospective evaluation of images from all CT scan series of the abdomen and pelvis was performed over a period of 1 year from August 1, 2017 to July 30, 2018. Two investigators independently evaluated the hepatic veins on each CT scan using HorosTM imaging software tools (Nimble CO LLC, Annapolis, MD, United States). Measurements were taken independently by two radiologists, and the average measurement was used as the final dimension. We included all contrast-enhanced CT scans that adequately covered the entire liver in the venous phase. We excluded duplicate scans, scans with incomplete demographic data, and scans without adequate venous phases from the analysis.
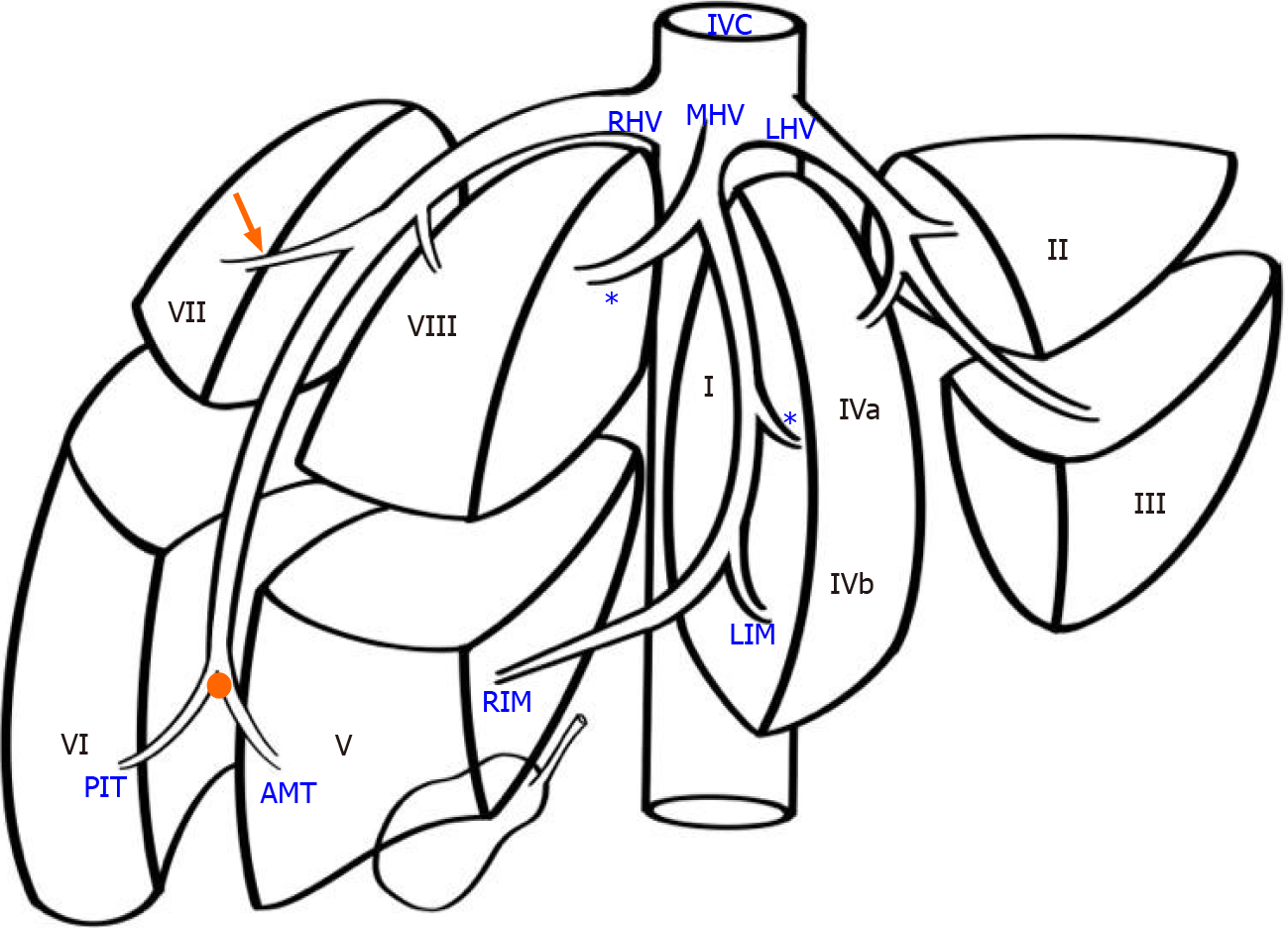
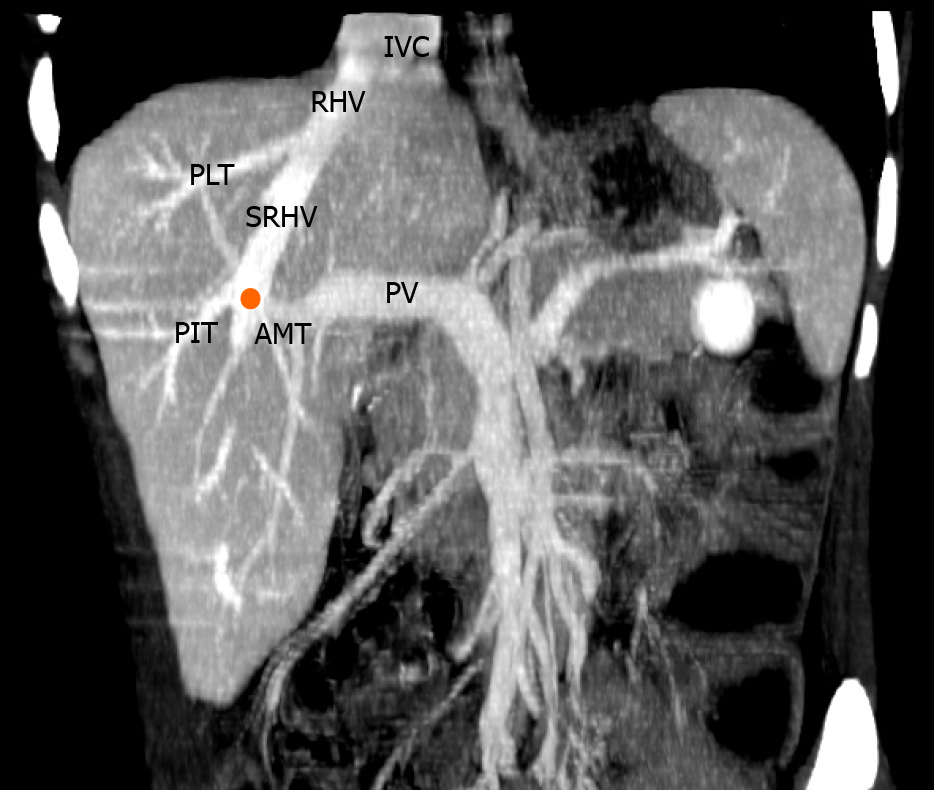
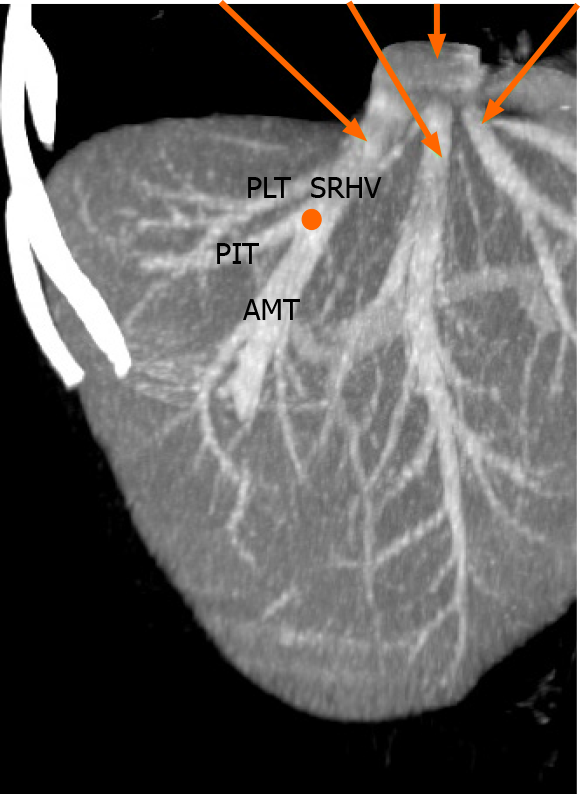
The anatomic pattern of the RHV is multifarious, with numerous proposed descriptions and classifications[1-6]. In most descriptions, a main trunk forms at the junction of two tributaries in the plane between the right anterior and posterior liver sections[5,6]. Liver segments V and VIII are drained by the anteromedial tributary (AMT) and the posterioinferior tributary (PIT) drains segment VI. Those tributaries join to form the main RHV trunk, also known as the superior RHV (SRHV)[2,7,8], that then courses up toward the inferior vena cava (IVC). A constant tributary draining segment VII, sometimes called the right superficial vein[2], consistently joins the SRHV on its posterolateral aspect[5,6]. A single SRHV then continues on to empty into the IVC at the hepatocaval junction (HCJ) approximately 1 cm below the diaphrag
Many previous classifications have attempted to describe variations of the RHV tributaries, but few are of clinical relevance. We used parts of the classification described by De Cecchis et al[6] because we thought that the hepatic venous confluence had clinical significance. De Cecchis et al[6] defined the hepatic venous confluence as the point at which the tributaries joined to form the main RHV trunk. They defined two types of hepatic venous confluences, a distal confluence in which the distance between the HCJ and venous confluence was > 3.5 cm, and a proximal confluence where the distance was ≤ 3.5 cm[6]. In our study, we evaluated the hepatic venous confluence by measuring the distance between the HCJ and the point at which the PIT draining segment VI and the AMT draining segments V/VIII met. We also described the presence of the right superficial vein (constant tributary draining the cranial part of segment VII) and the dorsal vein for segment VIII.
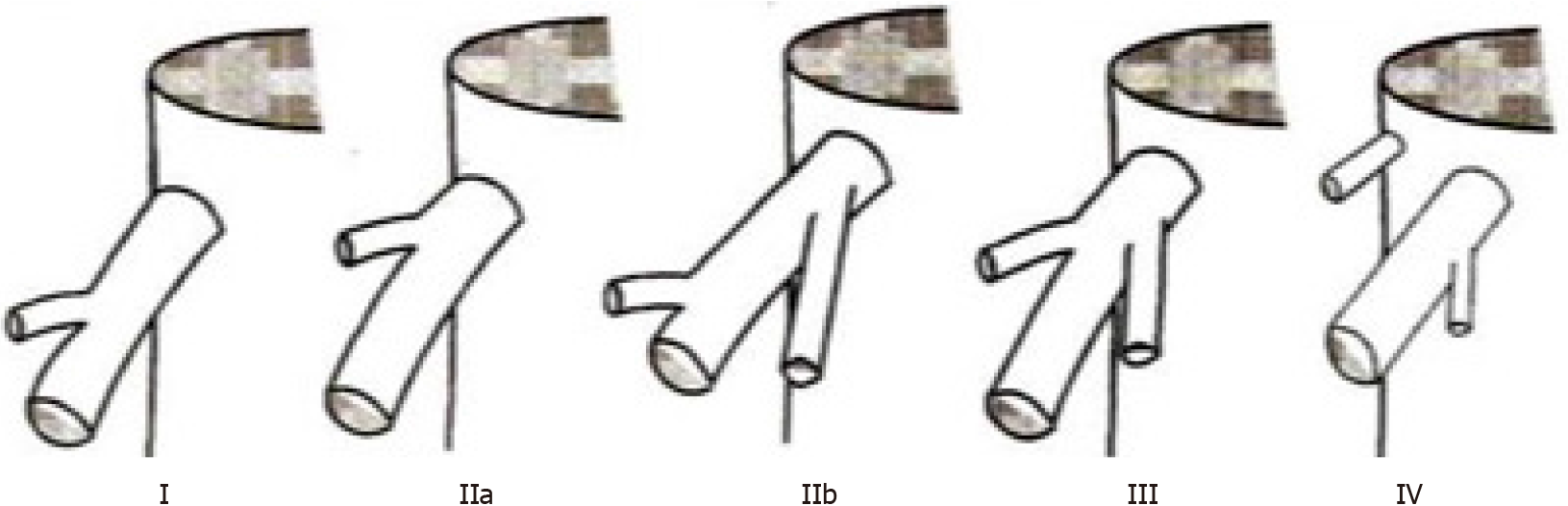
We described variations at the HCJ by the classification proposed by Nakamura and Tsuzuki[4], which includes five variants based on the number of tributaries emptying into the IVC (Figure 2). To describe those variations, we measured the tributary-free SRHV length, which we defined as the distance from the HCJ to the first SRHV tributary.
Classical anatomic descriptions include a single SRHV that empties into the IVC, and any additional vessels from the right liver that empty into the IVC are considered accessory RHVs[2,7,8]. Three variants are recognized. An inferior RHV (IRHV) drains segment VI and empties directly into the IVC just above the inferior border of the liver more than 2 cm from the HCJ[8]. The middle RHV (MRHV) drains segment VII[8] and empties directly into the IVC within 1-2 cm of the HCJ. In this study, we evaluated the accessory veins using the above terminology, the diameter just before entry into the IVC, the distance from the HCJ, and the drainage territories.
The reported prevalence of accessory veins varies widely. Therefore, we performed a systematic literature search of medical archiving platforms, including PubMed, Medline, Google Scholar, and the Cochrane Database of Systematic Reviews. We searched for studies evaluating RHVs using the search terms “right hepatic vein, accessory hepatic vein, superior right hepatic vein, middle right hepatic vein, inferior right hepatic vein, posterior hepatic vein, posteroinferior hepatic vein, segment VI vein, segment VII vein, liver veins, venous drainage, IVC tributaries, supernumerary liver veins and supernumerary hepatic veins.”
We extracted raw data from the published studies retrieved by the systematic literature search and collated the data documenting the prevalence of each accessory vein. The global prevalence of each accessory hepatic vein was calculated as a percentage of the raw data using the number of individuals with accessory veins relative to the total number of individuals included in retrieved studies. We used Z-scores to compare the prevalence of each accessory hepatic vein in our population with the global prevalence of accessory veins. P values < 0.05 were considered significant.
Over the study period, 230 CT scans were reviewed, but only 118 met the inclusion criteria. Of the 112 scans that were excluded, 59 were repeat/duplicated scans, 12 contained incomplete demographic data, and 41 had inadequate venous phases (41).
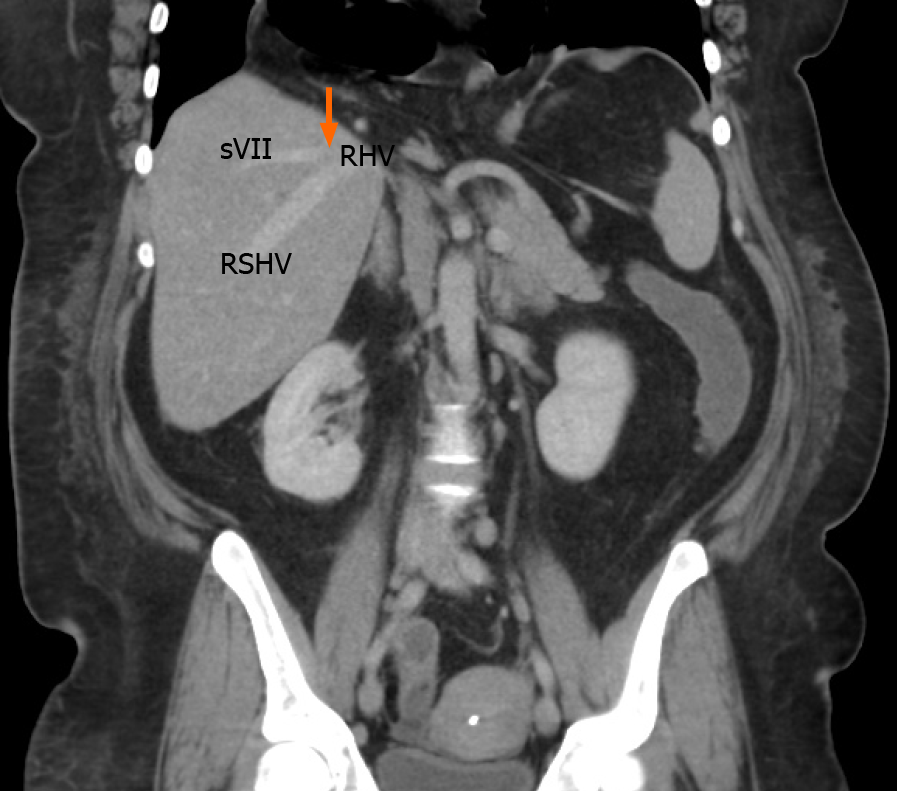
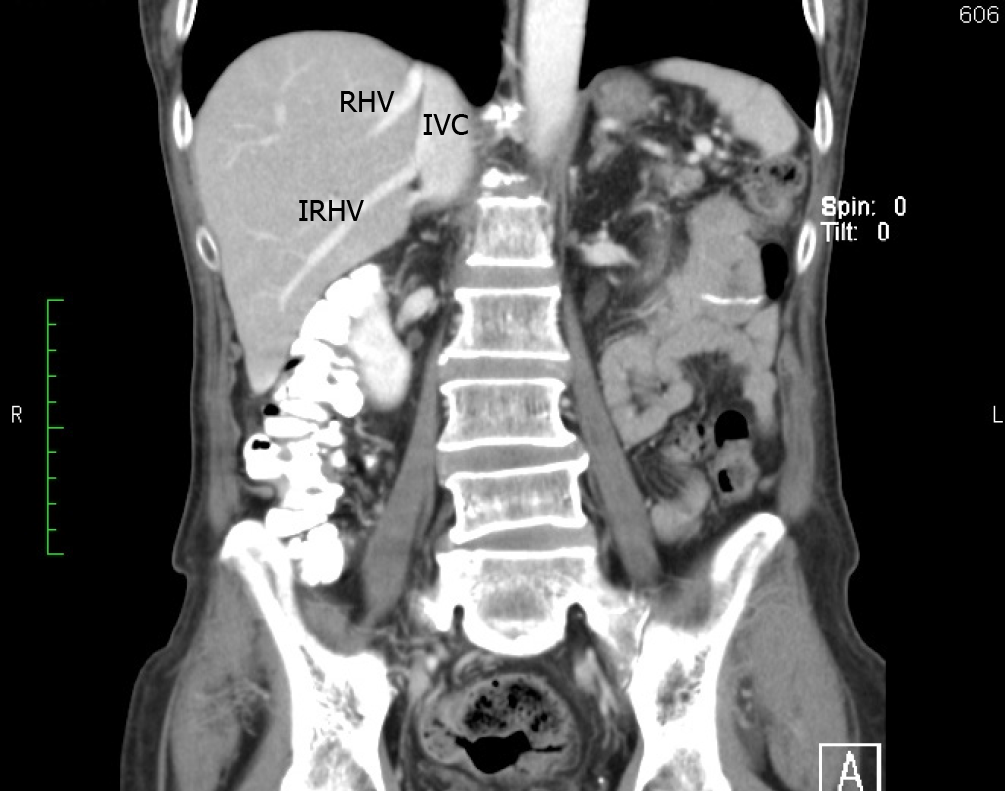
We identified the hepatic venous confluence in all the individuals in our series. Forty-six individuals (39%) had a distal confluence (Figure 3) and 72 (61%) had a proximal confluence (Figure 4). A well-defined segment VII tributary joined the SRHV in 113 (96%) individuals before it emptied into the IVC (Figure 5). An MRHV was present in the five individuals (4%) in whom a segment VII tributary was rudimentary or absent. A dorsal vein for segment VIII emptied into the SRHV in 85 individuals (72%). In the remaining 33 (28%), both dorsal and ventral segment VIII veins emptied into the middle hepatic vein.
Table 1 outlines the variations encountered at the HCJ. In 83 individuals (70.3%), the SRHV accepted no tributaries within 1 cm of the HCJ (Nakamura and Tsuzuki type I). In these type I variants, the tributary-free SRHV had a mean length of 11.9 ± 4.09 mm (range 1.9-21.0 mm; median 12.05 mm) and a mean diameter of 20.4 ± 3.76 mm (range 11.5-31.0 mm; median 19.5 mm). Thirty-five individuals (29.7%) had a short SRHV with at least one tributary within 1 cm of the HCJ. Twenty-four individuals (20.3%) had one tributary (type 2 variant), six (5.1%) had two tributaries (type 3 variant) and five (4.2%) had a vein draining irregular portions of segment VII that emptied flush at the SRHV ostium (type 4 variant).
| Type | Description | Trinidad and Tobago (%) | India[16] (%) | Japan[4] (%) | China[17]1 (%) | Turkey[18]1 (%) | Slovenia[6] (%) | France[19] (%) | Vietnam[39] (%) |
| CT | Cadaver | Cadaver | CT | CT | Cadaver | Cadaver | Cadaver | ||
| I | No tributaries within 1cm of the IVC | 83/118 (70.3) | 82/100 (82) | 51/83 (61.4) | 162/200 (85) | 40/100 (40) | 85/110 (77.3) | 32/64 (50) | 18/20 (90) |
| II | One tributary within 1 cm of IVC; IIa: Right superior vein; IIb: Right antero-superior vein | 24/118 (20.3) | 10/100 (10) | 19/83 (22.8) | 17/200 (8.5) | 57/100 (57) | NS | 20/64 (31.3) | 0 |
| III | Both right superior and right antero-superior veins join within 1cm of IVC | 6/118 (5.1) | 4/100 (4) | 8/83 (9.6) | 6/200 (3) | 3/100 (3) | NS | 10/64 (15.6) | 0 |
| IV | Two veins empty directly into IVC: (1) Right hepatic with a branch of right antero-superior vein; and (2) Independent right superior vein | 5/118 (4.2) | 4/100 (4) | 5/83 (6) | 15/200 (7.5) | 0 | NS | 2/64 (3.1) | 2/20 (10) |
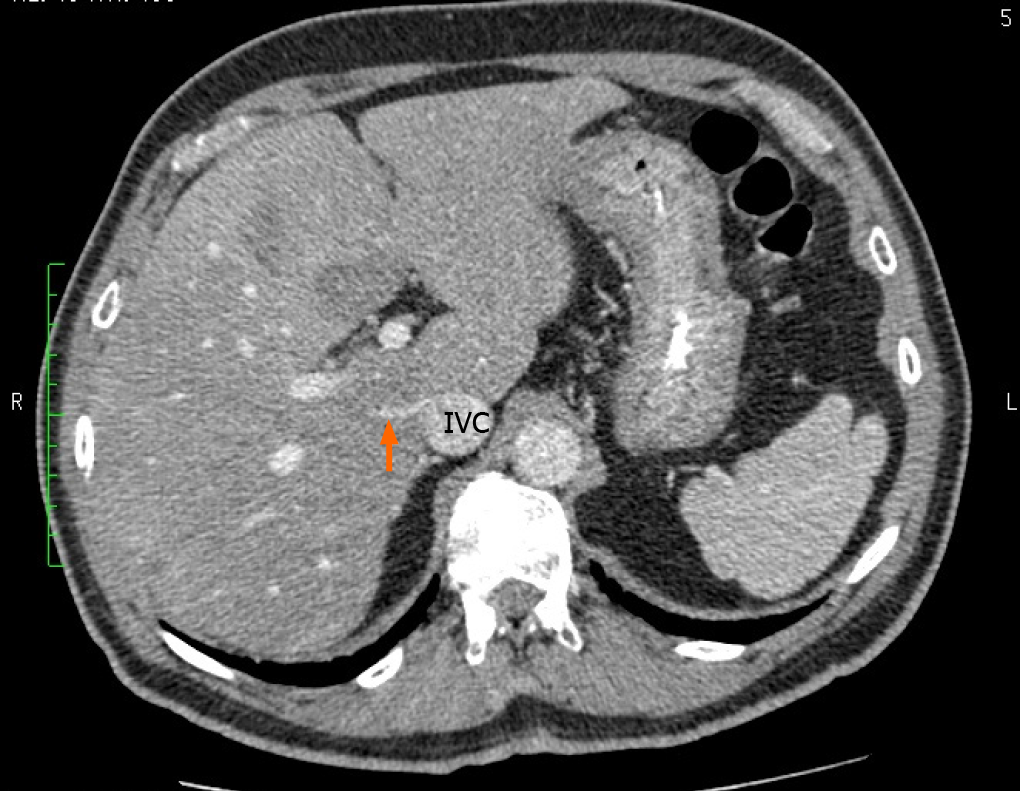
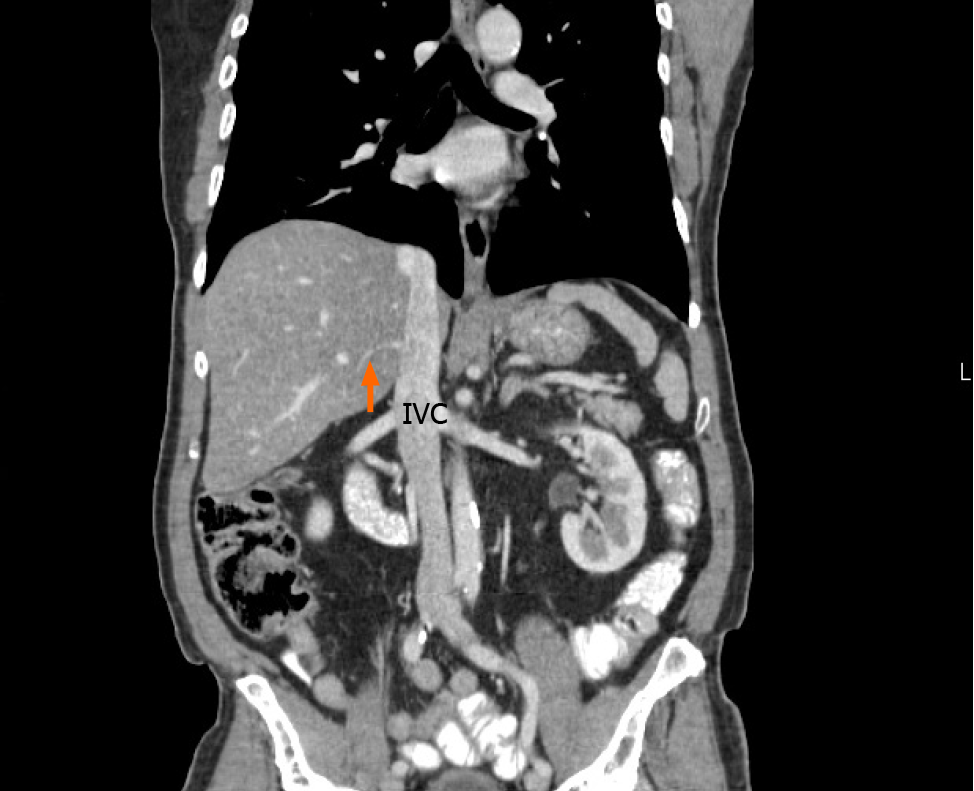
Our literature search returned 26 published studies that evaluated the presence of accessory hepatic veins[2,4,6,8-37]. A variety of study techniques were used. Eleven used cadaveric dissections and 15 that used imaging-based studies. Most of the recent studies included imaging in their research methodology. These studies evaluated the accessory veins in a total of 4657 individuals, and the global prevalence of each hepatic vein is shown in Table 2. In our study, 60 individuals (51%) had a single SRHV draining into the IVC, and accessory RHVs emptied into the IVC at various levels in the remaining 58 (49%). That was significantly lower than the global incidence of solitary SRHVs (51% vs 71.8%; P < 0.0001). A well-defined IRHV draining segment VI was present in 53 (45%) individuals as shown in Figure 6. That was significantly greater than the prevalence of IRHVs in the global population (45% vs 29.2%; P = 0.0002). In all cases, there was a single IRHVs that emptied directly into the IVC just above the lower border of the liver (Figure 7). When present, the mean IRHV diameter was 8.13 ± 1.93 mm (range 4.3-13.0 mm; median 8.2 mm). An MRHV was present in five individuals (4%, Figures 8 and 9), and had a mean diameter of 4.9 ± 0.25 mm (range 4.6-5.2 mm; median 4.9 mm). There was no significant difference between the prevalence of MRHVs in our population and that in the global population (4% vs 5.5%).
| Country | Methodology | None (SRHV only) (%) | MRHV (%) | IRHV (%) |
| Trinidad and Tobago | Imaging (CT) | 60/118 (51) | 5/118 (4) | 53/118 (45) |
| Global prevalence | 3343/4657 (71.8) | 145/2652 (5.5) | 1361/4657 (29.2) | |
| P value | < 0.0001 | 0.562 | 0.0002 | |
| Poland[21]: 1975 | Cadaveric | 79/93 (84.9) | NS | 14/93 (15.1) |
| France[22]: 1978 | Cadaveric | 62/80 (77.5) | NA | 18/80 (22.5) |
| Japan[4]: 19811 | Cadaveric | 32/83 (38.6) | 20/83 (24.1) | 31/83 (37.3) |
| France[23]: 1981 | Cadaveric | 79/100 (79) | NS | 21/100 (21) |
| Japan[24]: 1983 | Ultrasound (operative) | 242/269 (90) | 2/269 (0.7) | 27/269 (10) |
| Korea[25]: 1987 | Ultrasound | 67/124 (54) | NA | 57/124 (46) |
| France[19]: 19881 | Cadaveric | 32/64 (50) | 11/64 (17.2) | 28/64 (43.8) |
| England[26]: 1990 | Imaging (MRI) | 63/82 (76.8) | 0 | 19/82 (23.2) |
| France[27]: 1993 | Cadaveric | 111/125 (88.8) | NA | 14/125 (11.2) |
| United States[28]: 1995 | Imaging (CT) | 62/69 (89.9) | 1/69 (1.5) | 6/69 (8.7) |
| Taiwan[29]: 1997 | Imaging (US) | 306/400 (76.5) | 22/400 (5.5) | 72/400 (18) |
| Slovenia[6]: 2000 | Cadaveric | 79/110 (71.8) | 8/110 (7.3) | 31/110 (28.2) |
| Central India[16]: 2001 | cadaveric | 96/100 (96) | 0 | 4/100 (4) |
| Turkey[18]: 20041 | Imaging (CT) | 53/100 (53) | 19/100 (19) | 28/100 (28) |
| Turkey[30]: 2004 | Imaging (CT) | 243/308 (78.9) | NA | 65/308 (21.1) |
| United States[31]: 2004 | Imaging (CT) | 40/65 (61.5) | 0 | 25/65 (38.5) |
| Turkey[32]: 2005 | Imaging (CT) | 27/52 (51.9) | 0 | 25/52 (48.1) |
| Germany[33]: 2005 | Imaging (CT) | 30/55 (54.5) | NA | 25/55 (45.5) |
| Turkey[8]: 2007 | Imaging (CT) | 764/1120 (68.2) | NA | 356/1120 (31.8) |
| Japan[34], 20081 | Cadaveric | 0/60 | 18/60 (30) | 52/60 (86.7) |
| Scotland[35]: 20101 | Cadaveric | 0/18 | 2/18 (11.1) | 16/18 (88.9) |
| South China[17]: 2012 | Imaging (CT) | 158/200 (79) | 0 | 42/200 (21) |
| Japan[2]: 2016 | Imaging (CT) | 46/100 (46) | 20/100 (20) | 34/100 (34) |
| South Korea[36]: 2017 | Imaging (CT) | 196/360 (54.4) | 22/360 (6.1) | 164/360 (45.6) |
| Northern India[37]: 2019 | Imaging (CT) | 458/500 (91.6) | 0 | 185/500 (37) |
| Vietnam[39]: 2020 | Cadaveric | 18/20 (90) | 0 | 2/20 (10) |
Of the three main hepatic veins, the RHV has the largest drainage territory[2,3] and the greatest number of anatomic variations[5,6,8]. We found RHV variations in 61% of the individuals in this eastern Caribbean series. It is important to identify the variants preoperatively in patients undergoing liver transplantation because their presence can affect the therapeutic interventions[5,6,8-10]. For this reason, we routinely perform contrast-enhanced CT scans with properly-timed venous phases and report the variations in three categories.
Considering the multifarious descriptions in the medical literature, we found RHV tributaries particularly challenging to analyze. We determined the hepatic venous confluence as proposed by De Cecchis et al[6]. We thought it was clinically important because a distal confluence may facilitate parenchymal-sparing liver resection because venous outflow can be preserved in the main trunk plus one of the two tributaries. There was less opportunity for parenchymal-sparing resections in our population because a distal confluence was less prevalent (39% vs 60%) than it was in the European population studied by De Cecchis et al[6].
A well-defined segment VII tributary was fairly consistent (96%) in our population. Thas venous outflow pattern, especially when combined with a distal confluence, may facilitate anterior right sectionectomy. The rationale is that it preserves venous outflow from the future liver remnant (FLR). Venous congestion has been shown to have a deleterious effect on the FLR[10-13]. Kawaguchi et al[11] demonstrated that when they used intraoperative fluorescence during liver resections to demonstrate significant reduction in indocyanine green uptake in veno-occluded FLRs. Reduced perfusion leads to attenuated hepatocyte function and impaired FLR regeneration. For that reason, most authorities recommend venous reconstruction when congested FLRs develop intraoperatively[11-14].
In the model described by Hjortsjo[15], segment VIII is divided into dorsal and ventral regions draining separately into the RHV and middle hepatic veins, respectively. They drain large parenchymal territories, with 21% of the entire liver volume drained by the dorsal vein of segment VIII and 16% drained by the ventral vein[3]. That was the dominant pattern in our population, with the dorsal vein of segment VIII draining into the SRHV in 72% of cases. In 28% of cases there was anomalous drainage from segment VIII, where both dorsal and ventral segment VIII veins emptied into the middle hepatic vein. Considering the fact that those two veins combined drain an estimated 37%[3] to 56%[2] of right liver volume, it is easy to appreciate the relevance of this anomaly. Patients with this unsuspected variant who undergo an anatomic left hepatectomy would have venous congestion in segment VIII, compromising an additional 37% FLR. In these patients, the impaired FLR function could increase operative morbidity and mortality. This variant is also important in transplantation because graft dysfunction can result from venous occlusion if the segment VIII veins are not reconstructed[3,12,13]. Therefore, we routinely reconstruct any outflow tract from segments V or VIII that is larger than 5 mm during right lobe living donation. That also lends support to the recommendation by Kawaguchi et al[11] for routine intraoperative ICG fluorescence to achieve real time evaluation and accurate FLR estimation during liver transplantation. Kawaguchi et al[11] demonstrated that the portal uptake function in veno-occluded regions was only 40% of that in nonoccluded regions.
The most common pattern was a single SRHV with no tributaries within 1 cm of the IVC (Nakamura and Tsuzuki type I). We found that the prevalence in our population was comparable to that in other countries across the globe[4,6,16-19], as shown in Table 1. Although relatively wide in diameter (20.4 mm), a type I SRHV is easier to expose and control outside the liver intraoperatively. It also facilitates the hanging maneuver, where an instrument is passed along the avascular space over the retro
Only 4% of individuals had the dangerous Nakamura and Tsuzuki type IV anomaly in which there are two SRHVs emptying independently into IVC. It is technically difficult to control those veins during hepatectomy, and there is increased risk of inadvertently tearing the large SRHVs if their presence is not anticipated. In such cases, there is the potential for excessive hemorrhage during liver resection.
In our population, 60 individuals (51%) had a solitary SRHV without accessory hepatic veins, which was significantly lower than the global incidence of a solitary SRHV (71.8%). Accessory RHVs were present in 49% of unselected individuals in our population. Globally, the prevalence of accessory RHVs ranges from 4% in India[16] to 100% in Scotland[35]. Comparisons were difficult because authors have applied many different names to these vessels, such as retrohepatic veins[22], accessory RHVs[6], paracaval veins[38], inferior accessory veins[36], segment VI accessory veins[34], posterior or posteroinferior veins[4,19], middle or lower accessory[34] and supernumerary RHVs[2]. In an attempt to analyze the existing variations, we retrospectively examined data, descriptions, and images from existing publications and attempted to conform to the defined nomenclature of IRHV and MRHV[2,7,8]. The results are shown in Table 2.
Consistently, an IRHV is the most common variant reported in the medical literature (Table 2) and it was significantly more common in our population than the global IRHV prevalence (45% vs 29.2%). In our population, the IRHVs were short and wide (8.1 mm mean diameter). Although CT volumetric analysis was not performed in our study, some authors have reported the IRHV is responsible for up to 20% of the venous drainage from the right liver[2,39]. When they are not anticipated and properly controlled, inadvertent avulsion or lacerations may cause significant bleeding during right hepatectomies.
The presence of an IRHV is important in pretransplantation evaluations, especially for adult recipients, often receive right lobe grafts in order to ensure sufficient FLR[18]. Those with large venous outflow territories would need to be re-implanted to prevent venous outflow obstruction and subsequent parenchymal congestion that would threaten the graft[18]. The IRHV anatomic pattern is also important because re-implantation into the IVC becomes more difficult technically as the distance from the HCJ increases[2,8].
The presence of an IRHV may sometimes be beneficial, especially when they are the dominant drainage of segment VI. Tani et al[2] documented that the IRHV drains an average of 70.8% of the venous blood from segment VI (100% in 32% of their cases). That is clinically important because it may allow tailored liver resections in which the FLR drainage is based on the IRHV preserving segment VI venous outflow. Makuuchi et al[40] were the first to report a series of tailored resections of segments VII/VIII while sparing segments V/VI when they identified IRHV preoperatively.
In our population, all the IRHVs encountered were single vessels. Multiple IRHVs have been reported in the medical literature, ranging in incidence from 1%[28] to 7.3%[8]. Other complex patterns include multiple IRHV tributaries forming a short common trunk prior to joining the IVC[34,37] or IRHVs with anomalous drainage patterns[8]. For example, Koc et al[8] reported an IRHV accepting the adrenal veins to form a common trunk emptying into IVC in two of 1120 individuals.
Although an MRHV was less common in our population than in the global population (4% vs 5.5%), the differences did not achieve statistical significance (Table 2). When present, the MRHV was a short and wide channel (4.9 mm) draining directly into the retrohepatic IVC. Unlike the IRHV, which is relatively easy to control intraoperatively at the inferior border of the liver, we have found the MRHV to be more problematic because it is completely concealed behind the liver. When present, we consider MRHV proxies for increased technical difficulty during laparoscopic hepatectomies because they are difficult to reach and control with laparoscopic instruments. In cases where we proceeded with laparoscopic liver resections in the face of a MRHV, the informed consent procedure was adjusted to reflect the technical difficulty and increased risk profile. Fortuitously, MRHVs were only present in 4% of our patients.
Tani et al[2] reported that the MRHV drained 8% of the venous blood from the right liver. Therefore, they may also need to be re-implanted at the time of liver transplan
Only 39% of the individuals in this eastern Caribbean population had conventional venous drainage patterns from the right hemi-liver. The RHV variants in this population included proximal venous confluence (61%), accessory RHVs (49.2%), IRHVs (45%) HCJ variants (29.7%), complete segment VIII drainage to middle hepatic vein (28%), absent segment VII tributaries (4.2%) and MRHVs (4%). Interventional radiologists and hepatobiliary surgeons treating patients from the Caribbean diaspora must be cognizant of these differences in order to minimize morbidity during invasive procedures.
Venous drainage from the liver is known to have many variations. The variations have a direct bearing on invasive procedures performed by transplant surgeons, interventional radiologists, and medical practitioners with an interest in treating liver diseases.
The right hepatic vein (RHV) drainage patterns are particularly important in transplantation procedures. Therefore, the RHV variations were evaluated in this study.
The objective of this study was to document RHV drainage patterns. The information would provide important information that would help to optimize outcomes during invasive liver procedures.
In this study, 230 contrast-enhanced computed tomography scans were independently examined by two radiologists. The venous drainage patterns were described in three categories: (1) tributaries to the RHV; (2) variations at the hepatocaval junction; and (3) accessory RHVs.
Conventional anatomic arrangements of the hepatic veins were present in only 39% of the individuals. The anatomic variations we encountered included accessory RHVs (49%), inferior RHV draining segment VI (45%), middle RHV (4%), Nakamura and Tsuzuki type I variations (70.3%), type II variants (20.3%) type III (5.1%) and type IV variants (4.2%).
There was significant variation in RHV patterns in this population. The variations have great relevance to operative procedures on the liver. Any surgeons performing operations in patients from the Caribbean diaspora must be cognizant of these variations.
The direction of the future investigations stimulated by this research would be to evaluate other associated anatomic anomalies and to compare the results to global statistics.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country/Territory of origin: Trinidad and Tobago
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Thandassery RB S-Editor: Gao CC L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Skandalakis JE, Skandalakis LJ, Skandalakis PN, Mirilas P. Hepatic surgical anatomy. Surg Clin North Am. 2004;84:413-435, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 2. | Tani K, Shindoh J, Akamatsu N, Arita J, Kaneko J, Sakamoto Y, Hasegawa K, Kokudo N. Venous drainage map of the liver for complex hepatobiliary surgery and liver transplantation. HPB (Oxford). 2016;18:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Shindoh J, Satou S, Aoki T, Beck Y, Hasegawa K, Sugawara Y, Kokudo N. Hidden symmetry in asymmetric morphology: significance of Hjortsjo's anatomical model in liver surgery. Hepatogastroenterology. 2012;59:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Nakamura S, Tsuzuki T. Surgical anatomy of the hepatic veins and the inferior vena cava. Surg Gynecol Obstet. 1981;152:43-50. [PubMed] |
| 5. | Barbaro B, Soglia G, Alvaro G, Vellone M, Giuliante F, Nuzzo G, Bonomo L. Hepatic veins in presurgical planning of hepatic resection: what a radiologist should know. Abdom Imaging. 2013;38:442-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | De Cecchis L, Hribernik M, Ravnik D, Gadzijev EM. Anatomical variations in the pattern of the right hepatic veins: possibilities for type classification. J Anat. 2000;197 Pt 3:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Couinaud C, Noguiera C. LesVeines. Sus-Hepatiques Chez l’Homme. Acta Anat. 1958;34:84-110. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Koc Z, Ulusan S, Oguzkurt L, Tokmak N. Venous variants and anomalies on routine abdominal multi-detector row CT. Eur J Radiol. 2007;61:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Kamel IR, Kruskal JB, Pomfret EA, Keogan MT, Warmbrand G, Raptopoulos V. Impact of multidetector CT on donor selection and surgical planning before living adult right lobe liver transplantation. AJR Am J Roentgenol. 2001;176:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Ghobrial RM, Hsieh CB, Lerner S, Winters S, Nissen N, Dawson S, Amersi F, Chen P, Farmer D, Yersiz H, Busuttil RW. Technical challenges of hepatic venous outflow reconstruction in right lobe adult living donor liver transplantation. Liver Transpl. 2001;7:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Kawaguchi Y, Ishizawa T, Miyata Y, Yamashita S, Masuda K, Satou S, Tamura S, Kaneko J, Sakamoto Y, Aoki T, Hasegawa K, Sugawara Y, Kokudo N. Portal uptake function in veno-occlusive regions evaluated by real-time fluorescent imaging using indocyanine green. J Hepatol. 2013;58:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Maema A, Imamura H, Takayama T, Sano K, Hui AM, Sugawara Y, Makuuchi M. Impaired volume regeneration of split livers with partial venous disruption: a latent problem in partial liver transplantation. Transplantation. 2002;73:765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Lee S, Park K, Hwang S, Lee Y, Choi D, Kim K, Koh K, Han S, Choi K, Hwang K, Makuuchi M, Sugawara Y, Min P. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001;71:812-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Mise Y, Hasegawa K, Satou S, Aoki T, Beck Y, Sugawara Y, Makuuchi M, Kokudo N. Venous reconstruction based on virtual liver resection to avoid congestion in the liver remnant. Br J Surg. 2011;98:1742-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Hjortsjo CH. The topography of the intrahepatic duct systems. Acta Anat (Basel). 1951;11:599-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Sharma D, Deshmukh A, Raina VK. Surgical anatomy of retrohepatic inferior vena cava and hepatic veins: a quantitative assessment. Indian J Gastroenterol. 2001;20:136-139. [PubMed] |
| 17. | Fang CH, You JH, Lau WY, Lai EC, Fan YF, Zhong SZ, Li KX, Chen ZX, Su ZH, Bao SS. Anatomical variations of hepatic veins: three-dimensional computed tomography scans of 200 subjects. World J Surg. 2012;36:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Orguc S, Tercan M, Bozoklar A, Akyildiz M, Gurgan U, Celebi A, Nart D, Karasu Z, Icoz G, Zeytunlu M, Yuzer Y, Tokat Y, Kilic M. Variations of hepatic veins: helical computerized tomography experience in 100 consecutive living liver donors with emphasis on right lobe. Transplant Proc. 2004;36:2727-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Chevallier JM. Anatomic basis of vascular exclusion of the liver. Surg Radiol Anat. 1988;10:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Cawich SO, Thomas DAW, Ragoonanan V, Ramjit C, Narinesingh D, Naraynsingh V, Pearce N. Modified hanging manoeuvre facilitates inferior vena cava resection and reconstruction during extended right hepatectomy: A technical case report. Mol Clin Oncol. 2017;7:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Sledziński Z, Tyszkiewicz T. Hepatic veins of the right part of the liver in man. Folia Morphol (Warsz). 1975;34:315-322. [PubMed] |
| 22. | Masselot R, Leborgne J. Anatomical study of the hepatic veins. Anat Clin. 1978;1:109-25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 23. | Couinaud C. Controlled hepatectomies and exposure of the intrahepatic bile ducts. 1st ed. Paris: Couinaud C Pub, 1981: 10-22. |
| 24. | Makuuchi M, Hasegawa H, Yamazaki S, Bandai Y, Watanabe G, Ito T. The inferior right hepatic vein: ultrasonic demonstration. Radiology. 1983;148:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Kim HJ, Chae YS, Park HY, Park BH, Kim YS. Normal Hepatic Vein Patterns on Ultrasound. J Kor Radiol Soc. 1987;23:79-84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 26. | Ng YY, Finn JP, Hall-Craggs MA. Inferior right hepatic veins: MR assessment of prevalence and potential clinical significance in children. Pediatr Radiol. 1990;20:605-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Champetier J, Haouari H, Le Bas JF, Létoublon C, Alnaasan I, Farah I. Large inferior right hepatic vein. Clinical implications. Surg Radiol Anat. 1993;15:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Soyer P, Bluemke DA, Choti MA, Fishman EK. Variations in the intrahepatic portions of the hepatic and portal veins: findings on helical CT scans during arterial portography. AJR Am J Roentgenol. 1995;164:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 29. | Cheng YF, Huang TL, Chen CL, Chen TY, Huang CC, Ko SF, Yang BY, Lee TY. Variations of the middle and inferior right hepatic vein: application in hepatectomy. J Clin Ultrasound. 1997;25:175-182. [PubMed] [DOI] [Full Text] |
| 30. | Akgul E, Inal M, Binokay F, Celiktas M, Aikimbaev K, Soyupak S. The prevalence and variations of inferior right hepatic veins on contrast-enhanced helical CT scanning. Eur J Radiol. 2004;52:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Varotti G, Gondolesi GE, Goldman J, Wayne M, Florman SS, Schwartz ME, Miller CM, Sukru E. Anatomic variations in right liver living donors. J Am Coll Surg. 2004;198:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Saylisoy S, Atasoy C, Ersöz S, Karayalçin K, Akyar S. Multislice CT angiography in the evaluation of hepatic vascular anatomy in potential right lobe donors. Diagn Interv Radiol. 2005;11:51-59. [PubMed] |
| 33. | Radtke A, Schroeder T, Sotiropoulos GC, Molmenti E, Schenk A, Paul A, Nadalin S, Lang H, Saner F, Peitgen HO, Broelsch CE, Malagò M. Anatomical and physiological classification of hepatic vein dominance applied to liver transplantation. Eur J Med Res. 2005;10:187-194. [PubMed] |
| 34. | Buhe S, Miyaki T, Saito T, Sawuti A, Terayama H, Naito M, Yi SQ, Itoh M. A study of the accessory hepatic vein to segments VI and VII with a morphological reconsideration of the human liver. Surg Radiol Anat. 2008;30:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Fersia O, Dawson D. Evaluation of the Variations of the Hepatic Veins. Int J Human Anat. 2010;2:1-7. |
| 36. | Kim HS, Lee CH, Kim SH, Kim JW, Park CM, Yeom SK. Predicting the Presence of an Accessory Hepatic Vein Using Abdominal Computed Tomography. Int J Morphol. 2017;35:21-25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Sureka B, Sharma N, Khera PS, Garg PK, Yadav T. Hepatic vein variations in 500 patients: surgical and radiological significance. Br J Radiol. 2019;92:20190487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Neumann JO, Thorn M, Fischer L, Schöbinger M, Heimann T, Radeleff B, Schmidt J, Meinzer HP, Büchler MW, Schemmer P. Branching patterns and drainage territories of the middle hepatic vein in computer-simulated right living-donor hepatectomies. Am J Transplant. 2006;6:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Tran HV, Vo NT, Duong HV, Talarico EF. Anatomical variations of hepatic veins in Vietnamese adults. Eur J Anat. 2020;24:111-120. |
| 40. | Makuuchi M, Hasegawa H, Yamazaki S, Takayasu K. Four new hepatectomy procedures for resection of the right hepatic vein and preservation of the inferior right hepatic vein. Surg Gynecol Obstet. 1987;164:68-72. [PubMed] |