INTRODUCTION
Heart transplantation is the gold standard treatment of end-stage heart failure (HF), although important advances in the field of mechanical circulatory support and technology have been noticed during the last 30 years. Since the first human-to-human heart transplant operation by a cardiac surgeon called Christiaan Neethling Barnard back in 1967, many adult heart transplants have been performed worldwide, especially in patients with end-stage HF. A continuous improvement in morbidity and mortality of transplanted recipients has been noticed despite the fact that they may be older with higher risk[1]. Heart transplantation remains, however, a difficult operation with significant short-term and long-term post-surgery outcomes including graft-related complications such as early graft dysfunction, acute allograft rejection and cardiac allograft vasculopathy, and non-graft-related complications such as infections, acute and chronic renal injury and malignancies[2]. All these complications usually lead to higher morbidity and mortality[3].
Despite the fact that donor and recipient age and comorbidity are being increased over the last years, heart transplantation survival rates seem to have progressively improved. It is estimated that, worldwide, almost 5000 transplants are being performed each year. The median survival for adult heart transplant recipients varies and ranges from 10.7 to 12.2 years approximately, with 82% one-year survival and 69% at five-years[1]. Survival for women is slightly better compared to men[1]. The highest incidence of mortality most often occurs within the first 6 mo after the transplant[1]. After 12 mo, the mortality rate decreases to 3.4% per year[1].
Heart transplantation patients present lower exercise capacity due to cardiac, vascular and skeletal muscle abnormalities leading thus to poor quality of life and reduction in the ability of daily self-service[4]. Impaired vascular function and diastolic dysfunction cause lower cardiac output (CO) while decreased skeletal muscle oxidative fibers, enzymes and capillarity cause arteriovenous oxygen difference, leading thus to decreased peak oxygen uptake (VO2) in heart transplant recipients which is lower at about 40% to 50% than age, sex, and activity matched healthy controls[4-6]. Exercise has been proven to improve exercise capacity and vascular endothelial function in patients with chronic HF and thus in patients with vascular endothelial impairment[7-10]. Exercise also improves aerobic capacity via the suppression of the oxidative stress, the increase of the bioavailability of nitric oxide (NO) and the induction of vasodilation[11,12].
The aim of this narrative review was to demonstrate the existing knowledge on the training protocols and highlight the benefits of exercise training in patients after heart transplantation.
CARDIOVASCULAR AND MUSCULOSKELETAL ALTERATIONS IN HEART TRANSPLANT RECIPIENTS
Cardiovascular alterations
One possible complication after heart transplantation is that the donor heart is surgically denervated and loses efferent and afferent autonomic connections. As a result, the regulation and function of the cardiovascular system is being affected and its reflex reactions are reduced[13,14] (Figure 1). Hypertension and peripheral vasoconstriction are usually the first signs in heart transplant recipients[14]. A possible explanation could be the permanent denervation of low-pressure cardiopulmonary baroreceptors in the heart and the permanent enhancement of sympathetic vasomotion due to lack of afferent impulses[15,16]. Left ventricular (LV) mass and wall thickness is increased, either within the first 30 d after heart transplantation or secondary as a consequence by immunosuppressive agents which trigger chronic tachycardia, hypertension and multiple rejection episodes[17,18].
Figure 1 Cardiovascular and musculoskeletal alterations in recipients after heart transplantation.
Despite the fact that the atrial remnant of the heart transplant recipients is innervated, the suture line causes higher intrinsic rate of the atrium and reduced heart rate variability[19]. Moreover, although some cardiac functions and mechanisms such as Frank-Starling are usually not affected in transplant patients, most heart responses to haemodynamic changes and heart rate variations are impaired[19]. A study by Nygaard et al[20], has shown that patients after heart transplantation present altered cardiovascular responses than healthy controls. Most specifically, their blood pressure and total peripheral resistance is higher at supine rest, attenuated during orthostatic challenge and preserved during isometric exercise. Cardiac denervation mediated chronotropic and allograft diastolic dysfunction after heart transplantation results in lower peak exercise CO[21,22]. Peak exercise CO in heart transplant recipients is 30% to 40% lower than age-matched healthy controls[21-23]. Lower CO leads to a higher resting heart rate and slower increase during exercise[24]. However, physical activity status and cardiac allograft reinnervation are important factors of the variety of heart rate impairment[4]. Moreover, diuretics leading to reduced diastolic filling might contribute to lower CO responses[20].
Heart transplant recipients present 20% lower peak exercise end-diastolic volume and stroke volume than healthy people[21,22]. This is another significant pathophysiological alteration. The impaired LV relaxation and the increased LV stiffness could be a possible explanation of this alteration[4,21]. In addition, previous studies have shown the significantly increased pulmonary capillary wedge pressure (PCWP)/ end-diastolic volume index ratio during maximal exercise in these patients compared to age-matched sedentary normal controls[21]. This elevated peak exercise mean PCWP results in dysfunction in LV strain and peak systolic velocity of the mitral valve, despite the preserved LV ejection fraction[25]. A possible potential pathway that causes LV diastolic dysfunction may be the decreased adrenergic tone associated with complications after the heart transplantation including denervation of the allograft, injury, myocardial ischemia due to vasculopathy or immunosuppression therapy[4,23].
Endothelial dysfunction is a major cause of disability and lower life expectancy in heart transplant recipients which increases exercise systemic vascular resistance, leading thus to less O2 provided to skeletal muscles[1,4] (Figure 1). As a result, peak VO2 is significantly reduced[1,4]. Several studies have shown that their peak exercise systemic vascular resistance is approximately 50% higher than healthy controls because of the impaired endothelial-dependent vasodilation of peripheral conduit arteries and resistance arterioles[21,22,26,27]. A significant observation is that the severity of the impairment of endothelial function appears to be related to the etiology of HF[28,29]. Most specifically, endothelial function is usually improved in similar levels to healthy age-matched controls in heart transplant recipients of non-ischemic cardiomyopathy compared to ischemic cardiomyopathy due to slower pulmonary VO2 kinetics during ischemia[26,29].
Musculoskeletal alterations
The last significant impairment after heart transplantation concerns abnormalities in skeletal muscles leading in impaired peak VO2 (Figure 1). Heart transplant recipients present lower body and leg lean mass, as well as muscle strength, compared to healthy, sedentary age-matched controls[30].
Before heart transplantation, reduced oxidative muscle fibers (Type I), capillary density, mitochondrial volume and oxidative enzyme capacity are usual abnormalities of the skeletal muscles, directly associated with the syndrome of HF and worsen according to its severity[30-32]. More precisely, a reduction in aerobic, Type I and an increase in anaerobic, glycolytic fibers (Type II) is observed in HF patients[33]. Their diaphragm is also metabolically affected with significant atrophy[33-35]. Moreover, reduced levels of enzymes and proteins such as citrate synthase, creatine kinase (CK), MM-CK and lactate dehydrogenase (LDH) prove a major contribution of altered skeletal muscle metabolism to exercise intolerance[36]. As far as muscle metabolism is concerned, electrolyte and phosphocreatine (PCr) disorders, metabolic acidosis and delayed PCr recovery after exercise are common characteristics of patients with HF[33,34]. Finally, muscle atrophy is caused by a decrease of anabolic mechanisms, increased protein degradation or sometimes both of them[33]. Enhanced protein degradation including impaired function of enzymes ubiquitin-ligases MuRF1 and Atrogin-1, impaired growth factor signaling and protein synthesis including decreased levels of circulating total testosterone, dehydroepiandrosterone and insulin like growth factor-1 and skeletal muscle inflammation including inflammatory mediators released into the circulation such as interleukin 1 (IL-1) and IL-6 and tumor necrosis factor α are the major mechanisms that promote muscle atrophy and skeletal muscle alterations[33,37,38].
Size of muscle fiber and mitochondrial volume density increases after heart transplantation reaching almost equal levels to healthy age-matched individuals[30,39]. However, reductions in capillary density persist[30,32,39]. Moreover, endurance in exercise performance seems to be impaired by immunosuppression therapy including cyclosporine and corticosteroids[40,41]. A consequence of all these musculoskeletal abnormalities is a decrease in bone density and oxygen utilization, and possible osteoporotic fractures[4,38,42,43].
THE EFFECT OF EXERCISE TRAINING IN THE CARDIOMYOCYTES, VASCULAR ENDOTHELIAL FUNCTION AND SKELETAL MUSCLES
Exercise training has a beneficial effect in the cardiomyocytes and the function of the vascular endothelial system. As far as the cardiomyocytes are concerned, exercise results in a beneficial form of cardiac remodeling that involves cardiomyocyte growth and proliferation[44,45]. Regular physical exercise has been proven to improve LV contractility, calcium function in the heart and cardiomyocytes size[46,47]. Isometric or static exercises result in mild concentric hypertrophy and usually a normal left atrium while endurance training LV hypertrophy, right ventricular (RV) dilation, and biatrial enlargement[45,48]. In the first case, the increase in cardiac wall thickness is caused by the parallel addition of sarcomeres within cardiomyocytes while in the second case by the addition of cardiomyocyte sarcomeres in series[45,48]. Cardiomyocyte hypertrophy is not the only process in exercise-induced cardiac remodeling. The increased levels of circulating endothelial cells (CECs) and endothelial progenitor cells (EPCs) after acute and long-term exercise seem to play a crucial role in augmentation of vascular density and cardiac repair[49-51].
Exercise training can also contribute to the proliferation of cardiomyocytes, a significant process of cardiomyogenesis[52]. Metabolically, exercise has beneficial effect on LV contractility and increases catabolism of fatty acids and lactate, and therefore of ATP production[53-55]. Circulating metabolites including palmitoleate (C16:1n7), G protein-coupled receptors, Akt, and nuclear receptors are important regulators of exercise-induced cardiac growth[53,56,57].
Regarding the vascular endothelium, exercise has been proven to suppress the generation of free radicals and oxidative stress and increase the bioavailability of NO[11,12]. As far as the potential mechanisms are concerned, shear stress is a procedure that activates eNOs, increases the concentration of NO and induces vasodilation[11,12,58,59]. Exercise increases shear stress, and thus, improves aerobic capacity[11,12,58,59]. Moreover, exercise induces the hypoxic stimuli, as observed by alterations in microcirculation indexes during exercise sessions[59,60]. All these pathophysiological mechanisms may relate to up-regulation of transcriptional factors, including vascular endothelial growth factor (VEGF), matrix metalloproteinases and stromal cell-derived factor 1, and lead to angiogenesis during exercise in healthy controls and patients with comorbidities[60-63]. In healthy subjects, exercise improves peripheral vascular function through the reduction in blood pressure, the endothelin-1 levels and the improvement in vasodilation[64,65].
EPCs and CECs have been shown to restore the dysfunctional or injured endothelium and protect it, regulate vascular homeostasis and promote angiogenesis. Therefore, reflecting the condition of the vascular endothelial function[12,66]. EPCs level is a predicting factor of the occurrence of a cardiovascular event and cardiovascular mortality[67]. Several studies have shown that in both healthy people and population with comorbidities, exercise training increases the number and the function of EPCs[68,69]. We extended previous findings by showing that a single bout of maximal exercise, as well as many bouts organized as an exercise training program, stimulates the mobilization of EPCs and CECs from the bone marrow in patients with chronic HF[8,10]. This beneficial effect of exercise seems to be similar in chronic HF patients of different severity[9].
Physical exercise has the beneficial effect to modify metabolic potential, morphology, and physiology of skeletal muscle[70]. Exercise is a triggering factor for the metabolic and structural skeletal muscle remodeling[70,71]. This remodeling has positive effect in angiogenesis and fatigue[70,71]. Resistance exercise increases muscle mass and strength while endurance training affects mitochondrial function and oxidation[70,72]. Regular exercise mediates molecular and metabolic pathways that are activated by muscle contraction. Intracellular sensors trigger intracellular signaling cascades including several transcription factors[70,72]. These factors are responsible for the remodeling of skeletal muscle via upregulation of mitochondrial metabolism and fiber-type transformation[70,72]. Finally, other potential mechanisms for muscle remodeling such as redox signaling seem to be involved in metabolic adaptation to exercise[70,73,74].
CARDIAC REHABILITATION IN HEART TRANSPLANTATION
Cardiac rehabilitation programs are being implemented all over the world for patients after major cardiovascular disease. A cardiac rehabilitation program is characterized by an interdisciplinary approach and consists of different specialties and health care professionals including cardiologists, physiotherapists, nurses, dieticians, pharmacists, psychologists, physiologists, other specialties such as internists, neurologists, diabetologists and cardiac surgeons, general practitioners and social services experts[75]. One of the most important roles is the role of the program director. A program director could be of any specialty with good organizing and management skills.
Cardiac rehabilitation is a type of secondary prevention in patients with cardiovascular disease. The aim of rehabilitation is to reduce anxiety and depression and instill confidence so that to change lifestyle of patients aimed at preventing further disease[76]. Each patient could benefit from either an in-patient or out-patient cardiac rehabilitation program. The core principles of a cardiac rehabilitation program are patients’ medical evaluation, counselling for exercise training and diet, continuous assessment of weight, blood pressure, lipidemic profile, and psychosocial support[75]. The expected outcomes of a cardiac rehabilitation program are improvement of clinical stability and symptom control of patients, reduce of cardiovascular risk, better compliance to medical therapy, and improved quality of life, social integration and prognosis[75].
Another important parameter of a successful cardiac rehabilitation program is the equipment. A cardiac rehabilitation center should provide the appropriate equipment for the assessment of patient’s clinical status, LV function, arrhythmias, functional capacity, psychosocial status and equipment for conducting an exercise training program. These include stethoscopes and sphygmomanometers, electrocardiogram, echocardiography, echocardiography, graded exercise testing on treadmills or cycles, cardiopulmonary exercise testing (CEPT), six-minute walk test (6-MWT), questionnaires about quality of life and psychological status and exercise equipment such as treadmills, cycle ergometers and weight training equipment[75]. Moreover, emergency equipment for complications during exercise is always mandatory.
Phases of cardiac rehabilitation
Rehabilitation is a complex process, individualized for each patient. Three main phases of rehabilitation can be differentiated according to the updated guidelines about preventive cardiology and rehabilitation of the ESC[75] (Figure 2): (1) Phase 1 is the phase of the in-hospital rehabilitation including early interventions and mobilization immediately after hospital admission[75]; (2) Phase 2 is probably the most critical part in patients with heart transplantation. It is being implemented just after the hospital discharge. It promotes and delivers in-patient and out-patient rehabilitative services for clinical stabilization[75]. In-patient cardiac rehabilitation is being performed to unstable patients in order to stabilize them before the longer-term cardiac rehabilitation program after hospital discharge. Clinically unstable patients after an acute event, with advanced HF under continuous medication or with implantable devices, heart transplant recipients and patients unable to attend a formal outpatient rehabilitation program for any personal reasons are considered as high risk[75]. On the other hand, early out-patient cardiac rehabilitation is being used for independent patients early after hospital discharge, usually within 3 to 6 mo after a cardiovascular event. The mean duration is 8 to 12 wk, most times continuing for one year after the event[75]. Finally, a home-based program is another form of rehabilitation assessed and supported by the rehabilitation group at patient’s home. It may include regular visits to the rehabilitation center and contacts with the team. The activities of a home-based program are similar to those of an early outpatient cardiac rehabilitation program[75]; and (3) Phase 3 is the long-term out-patient type of cardiac rehabilitation. The main aim of phase 3 rehabilitation is to promote long-term exercise and rehabilitation in patients out of hospital and the community. Moreover, it usually results in maintenance of the fitness level and better outcomes in heart transplant recipients[75].
Figure 2 Stages of a cardiac rehabilitation program.
Another important phase of rehabilitation is the “pre-rehabilitation” stage. Heart transplant recipients are doing regular aerobic or combined exercise before transplantation in order to maintain a higher fitness level and reduce complications afterwards like intensive care unit (ICU) acquired weakness or cardiac cachexia.
Significant components of a cardiac rehabilitation program for heart transplant patients
The initial step of the enrollment of a heart transplant recipient in a cardiac rehabilitation program is the risk assessment of the patient by the rehabilitation team. The risk assessment consists of clinical examination including sings such as examination of the wound healing or symptoms of the transplant’s rejection, imaging techniques such as chest X ray for infection, pleural effusion or diaphragm paralysis and echocardiography for RV and LV function or pericardial effusion[75]. Moreover, tests for exercise capacity including CPET 30 d after transplantation or bicycle ergometer and modified Bruce protocols and Naughton protocols on treadmill are recommended[75]. Patient education on the risk of acute rejection is also a significant variable of a rehabilitation program. Patients should be instructed to practice self-monitoring during their rehabilitation process. In the case of transplant rejection, usually presented with significant reduce in blood pressure, unexpected variations of heart rate, fever or fatigue, exercise training should be immediately stopped and appropriate interventions are needed[75]. As far as health care professionals are concerned, they need to be aware of all aspects of this condition. For example, physicians should have full knowledge regarding the possible reasons for patients’ limited exercise tolerance which could possibly be the immune-suppression therapy side effects, chronotropic incompetence or LV diastolic dysfunction[75]. They should also be aware of all necessary actions to prevent complications which could harm patients and avoid infections, and therefore transplant rejection[75].
The second step of a cardiac rehabilitation program is physical activity counselling. Most specifically, heart transplant recipients enrolling a rehabilitation program should perform chronic dynamic and resistance exercises in order to prevent the side-effects of immunosuppressive therapy. In addition, exercise intensity should be increased slowly over time so that patients could reach a score of 12-14 in the Borg scale[75].
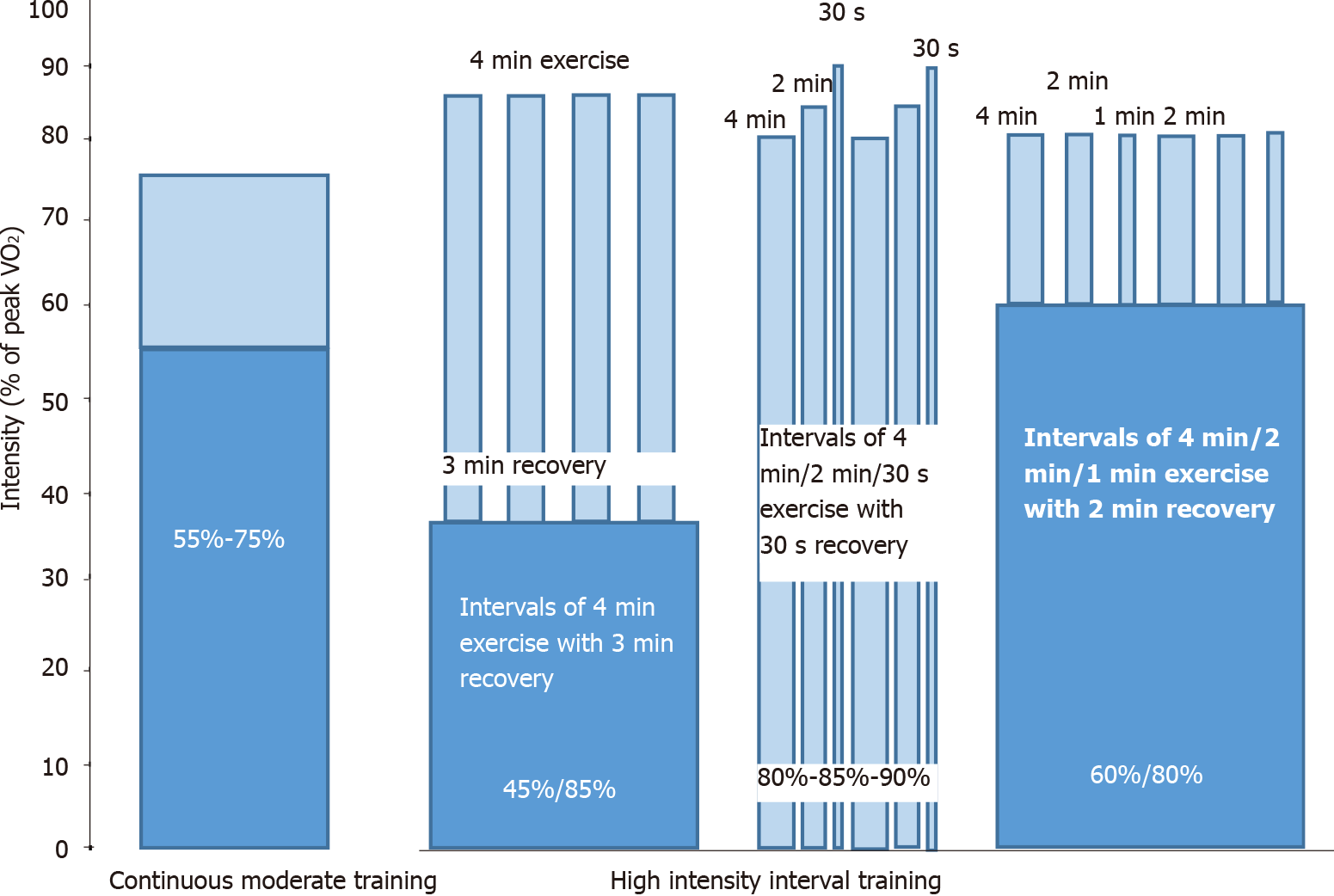
Exercise training is the most important aspect of a cardiac rehabilitation program. Early training program could be beneficial in the early and the long-term post-operative period. Early mobilization of heart transplant recipients could be achieved by implementing kinesiotherapy of the upper and lower limbs and prevention of respiratory infections could be achieved by performing respiratory physiotherapy[75]. Supervised exercise programs during the initial phase may be crucial to verify individual responses, tolerability and adaptability to exercise and clinical stability[75]. Aerobic exercise should be performed immediately after CPET for patients’ prescription. Specifically, regular aerobic exercise may start in the second or third week after transplantation while resistance exercise should be added after 6 to 8 wk. However, exercise should be discontinued during corticosteroid bolus therapy for rejection[75]. A duration of at least 30-40 min/d of combined aerobic and resistance training at moderate level, slowly progressing warm-up, closed-chain resistive activities and cycling in each exercise training session should be achieved[75]. The intensity of aerobic exercise could be calculated according to peak VO2 (< 50% or 10% below Ventilatory Anaerobic Threshold determined by CEPT) or peak work load (< 50%)[77]. Resistance training should consist of 2-3 sets with 10-12 repetitions per set at 40%-70% of the 1-repetition maximum (RM) test with > 1 min recovery between sets in order to achieve 5 sets of 10 repetitions at 70% of the 1-RM test[75]. Aerobic exercise could be either continuous moderate training (COMT) or high intensity interval training (HIIT) (Figure 3). COMT includes sessions consisted of aerobic exercise of 40 min with a continuous intensity of 55%-75% of peak VO2[75,78]. HIIT may varies between different rehabilitation centers. It could either consist of 40-min exercise of high intensity (blocks of 4 min-2 min-30 s according to 80%, 85% and 90% of peak VO2 with 1 or 2 min recovery)[79] or 16-min interval training (intervals of 4, 2 and 1-min duration at > 80% of peak VO2 with a 2-min active rest period of approximately 60% of peak VO2)[78]. Another very common HIIT protocol consists of 4 min × 4 min exercise bouts at 85%-95% of maximum heart rate, with 3 min recovery between them corresponding to 11-13 on the Borg scale[80]. Duration between COMT and HIIT sessions is similar and a 10-min pre-training warm up above 50% of peak VO2, as well as a 10-min post-training stretching and exercises are included in both protocols. HIIT is suggested for hemodynamic stable heart transplant recipients with beneficial effects for them[75,78-80].
Figure 3 Different protocols of continuous moderate training and high intensity interval training.
VO2: Oxygen uptake.
Except for exercise training, there are other important parameters which contribute to the success level of a cardiac rehabilitation program. Patients should be guided by expert nutritionists in order to maintain a balanced diet without sudden weight gain that could increase the risk of cardiac allograft vasculopathy or other classical cardiovascular risk factors[75] and avoid food that could lead to infection such as raw meat or seafood, un-pasteurized milk or cheese and raw eggs. A healthy lifestyle should be adopted by patients in their daily program. Monitoring of blood pressure, lower sodium intake, avoidance of hyperlipidemia and tobacco smoking, and adherence to the suggested medication would increase the beneficial effect of rehabilitation and reduce drug side effects. Appropriate medication with diltiazem, amlodipine and angiotensin-converting enzyme inhibitors, usually completed by diuretics, is mandatory. Also, statins, daily exercise and healthy diet should be applied in patients with hyperlipidemia in order to reduce the possibility of cardiovascular disease and improve survival[75]. Finally, psychosocial management is being considered as an important element in each cardiac rehabilitation program. Patients usually present high levels of depression, apprehensiveness or anxiety, and therefore support coping strategies should be implemented by expert psychologists[75].
Effects of exercise training in heart transplant patients
As far as pre-rehabilitation stage is concerned, 2 clinical trials were recently conducted in patients awaiting heart transplantation. In the first study[81], 7 end-stage HF patients awaiting heart transplantation while on intravenous inotropic support performed exercise training on a cycle ergometer while 11 patients followed the conventional protocol. 6-MWT assessed exercise capacity and manovacuometry assessed inspiratory muscle strength before and after each protocol. The intergroup comparison revealed significant increase in 6-MWT and inspiratory muscle strength in the intervention group compared to the control (P < 0.01)[81]. In the second study[82], 24 HF patients with advanced symptoms awaiting heart transplantation performed HIIT during hospitalization. HIIT was shown to improve skeletal muscle strength, and most specifically knee extensor strength, and decrease brain natriuretic peptide levels in these patients, however, without having any effect on hand grip strength[82].
Exercise training, as early as possible after hospital discharge, is being considered beneficial for the acute and long-term outcomes of heart transplant patients[83-86]. It has been shown to improve endothelial function assessed by brachial artery flow-mediated dilatation (FMD)[79]. In addition, it reduces systolic blood pressure, pro-atrial natriuretic peptide and high sensitive C-reactive protein (CRP)[79]. Two clinical trials investigated the effects of exercise training within the first year of hospital discharge after transplantation. In Braith et al[85] study, 8 wk after transplantation, 10 heart transplant recipients performed COMT on a treadmill 3 d/wk for 12 wk and 10 recipients took standard medical care for the same time period. Patients performed warm-up for 5 min, treadmill walking for 30 min and cooldown for 5 min within the first month. After the first month, treadmill walking increased to 35-40 min with an intensity between 11 and 13 or 12 and 14 of the Borg scale. Brachial artery reactivity was assessed using flow-mediated dilation. This randomized clinical trial proved the benefit of aerobic exercise on peripheral artery function in the early period after heart transplantation, demonstrating increase in brachial artery FMD in contrast with the progressive decline in patients who did not undergo rehabilitation. In addition, resting norepinephrine decreased significantly (P < 0.05) after exercise in the training group compared to controls and peak VO2 increased 26% in the trained patients but remained unchanged in controls[85]. In another study of the same institution[42], 8 heart transplant recipients, 2 mo after transplant, underwent a 6-mo resistance training program (2 d/wk, 10-15 repetitions at 50% of 1-RM in the beginning and then increase by 5%-10% in resistance in each set) for upper and lower body while 7 recipients were used as a control group. The aim of the study was to show the shift of type II fibers to type I fibers through biopsy of the right vastus lateralis, and therefore the beneficial effect of resistance training in the reverse of skeletal muscle myopathy even within a few days after heart transplantation[42].
Several studies have shown the effects of exercise training in heart transplant patients who enrolled a cardiac rehabilitation program in more than one year after hospital discharge. Most of these studies examined the effect of HIIT protocol in different functional capacity and vascular endothelial function indices. Nytrøen et al[80], included 48 clinically stable heart transplant recipients 1-8 (mean time: 4.1 ± 2.2) years after transplantation. Maximal CPET on a treadmill was performed in both 12-mo HIIT patients (intervention group) and patients who received usual care for the same time period. The HIIT group performed warm up for 10 min, followed by four 4 min exercise bouts at 85% to 95% of their maximum heart rate, with 3 min recovery time between them (intensity 11-13 on the Borg scale). Exercise group presented higher mean peak VO2 and predicted peak VO2 compared to controls (P < 0.001). Muscular exercise capacity and general health were also improved. Hermann et al[79], examined the effect of HIIT on peak VO2 and FMD of the brachial artery in 14 patients after heart transplantation who performed an 8-wk HIIT program. Each session included a warm up above 50% of peak VO2 and then 42 min of HIIT divided in 4 min-2 min-30 s intervals at 80%, 85% and 90% of peak VO2 (intensity at 18-19 of the Borg scale) with 1- or 2-min recovery between the intervals. There was also a control group of 13 patients after heart transplantation who did not exercise. Blood pressure and several indices were also evaluated at baseline and 8 wk later. There was a significant increase in peak VO2 and FMD in patients performed HIIT compared to controls, but nitroglycerin-induced vasodilation remained unchanged. Moreover, HIIT reduced systolic blood pressure in heart transplant recipients while it remained unchanged in controls, indicating thus the benefits of HIIT in endothelial after transplantation. Monk-Hansen et al[87], did not observe improvement of LV function in heart transplant recipients after an 8-wk exercise training program, although an increase in peak VO2 was noticed.
A single study recently examined the effects of COMT on ambulatory blood pressure and arterial stiffness of heart transplant recipients. In this study[88], 40 patients either performed 40 min endurance exercise at 70% of peak VO2 (3 times per week) for 12 wk or did not perform any kind of exercise. All patients underwent CPET, 24-h ambulatory blood pressure monitoring, and carotid-femoral pulse wave velocity assessment in 2 time periods; at baseline and after 12 wk. COMT reduced ambulatory blood pressure but pulse wave velocity remained unchanged, suggesting thus that it could be beneficial for the treatment of hypertension in heart transplant recipients.
Comparing HIIT an COMT, Yardley et al[89] showed that heart transplant patients had similar beneficial effect in inflammatory indices such as CRP, blood platelets and angiogenesis, but indices of angiogenesis including VEGF and angiopoietin 2 after HIIT seemed to increase more than COMT.
Finally, combined exercise, including aerobic exercise and muscle strength training, is still under investigation.
Limitations and perspectives
In most studies there are gaps in methodology which could lead to bias. Inclusion criteria, different baseline exercise capacity and fitness level, differentiations in exercise training protocols and small number of samples are some variables that may lead to systemic bias and underpowered conclusions. Taking these factors into consideration, it would be safer to reach a conclusion that HIIT is effective and feasible in heart transplant patients rather than state that it is more beneficial than exercise with moderate intensity. Moreover, patients may drop out of the program either for logistic reasons or for complications of the transplantation caused by transplant rejection, infections and side effects of the immunosuppressive therapy.
Many cases of heart transplant patients could be inspiring examples of the remarkable human exercise capacity. A combination between the conventional post-heart transplantation multi-disciplinary medical therapy with the carefully monitored aerobic or combined endurance exercise training could be a real breakthrough in the field of medicine.