Published online Oct 18, 2021. doi: 10.5500/wjt.v11.i10.432
Peer-review started: June 20, 2021
First decision: July 28, 2021
Revised: August 27, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: October 18, 2021
Processing time: 115 Days and 13.3 Hours
Solid organ transplantation is a life-saving intervention for end-stage organ disease. Post-transplant diabetes mellitus (PTDM) is a common complication in solid organ transplant recipients, and significantly compromises long-term survival beyond a year.
To perform a systematic review and meta-analysis to estimate incidence of PTDM and compare the effects of the 3 major immunosuppressants on incidence of PTDM.
Two hundred and six eligible studies identified 75595 patients on Tacrolimus, 51242 on Cyclosporine and 3020 on Sirolimus. Random effects meta-analyses was used to calculate incidence.
Network meta-analysis estimated the overall risk of developing PTDM was higher with tacrolimus (OR = 1.4 95%CI: 1.0–2.0) and sirolimus (OR = 1.8; 95%CI: 1.5–2.2) than with Cyclosporine. The overall incidence of PTDM at years 2-3 was 17% for kidney, 19% for liver and 22% for heart. The risk factors for PTDM most frequently identified in the primary studies were age, body mass index, hepatitis C, and African American descent.
Tacrolimus tends to exhibit higher diabetogenicity in the short-term (2-3 years post-transplant), whereas sirolimus exhibits higher diabetogenicity in the long-term (5-10 years post-transplant). This study will aid clinicians in recognition of risk factors for PTDM and encourage careful evaluation of the risk/benefit of different immunosuppressant regimens in transplant recipients.
Core Tip: The aim of this study is to perform a systematic review and meta-analysis to estimate incidence of post-transplant diabetes mellitus (PTDM) and the relative effects of the 3 major immunosuppressants on incidence of PTDM. This study will aid clinicians in recognizing the risk factors for PTDM and careful evaluation of the risk/benefit of different immunosuppressant regimens in transplant recipients.
- Citation: Kotha S, Lawendy B, Asim S, Gomes C, Yu J, Orchanian-Cheff A, Tomlinson G, Bhat M. Impact of immunosuppression on incidence of post-transplant diabetes mellitus in solid organ transplant recipients: Systematic review and meta-analysis. World J Transplant 2021; 11(10): 432-442
- URL: https://www.wjgnet.com/2220-3230/full/v11/i10/432.htm
- DOI: https://dx.doi.org/10.5500/wjt.v11.i10.432
Solid organ transplantation (SOT) has achieved an excellent long-term survival in various end-stage organ diseases. However, it is still associated with several complications including post-transplant diabetes mellitus (PTDM) in the transplant recipients. PTDM may result from both transplant-related factors like immunosuppression as well as traditional risk factors like obesity, ethnicity, lifestyle and genetics. It is associated with significant morbidity and mortality, with increased cardiovascular risk, infection and graft failure [1,2]. The reported incidence of PTDM has been variable due to varying definitions over time, diverse transplant patient populations and variation in immunosuppression regimens. The reported incidence ranges from 4%-25% in renal transplant recipients, 2.5%-25% in liver transplant recipients, 4%-40% in heart transplant recipients, and 30%-35% in lung transplant recipients[3,4]. In some instances, hyperglycemia develops in response to steroid doses and pulsed steroids required during episodes of acute rejection rather than baseline immunosuppression and care must be taken in making the diagnosis.
Based on the 2013 International Consensus meeting[3], the older term new onset diabetes after transplant has been replaced by PTDM, which is defined as newly diagnosed diabetes post-transplant irrespective of timing or whether it was present but undetected prior to transplant. The criteria for diagnosis of PTDM are symptoms of diabetes plus random plasma glucose (PG) concentrations ≥ 200 mg/dL (11.1 mmol/L) or fasting blood glucose ≥ 126 mg/dL (7.0 mmol/L) (on two occasions) or 2-hour PG ≥ 200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test or HbA1C ≥ 6.5%[3]. However, these guidelines mainly focus on kidney transplant patients as most studies were conducted in this cohort.
Historically, PTDM has been attributed to insulin resistance like type 2 diabetes mellitus (DM2). The pathophysiology is incompletely understood, both insulin resistance and impaired insulin secretion due to destruction of pancreatic B-cells have been implicated[5,6]. PTDM is associated with traditional DM2 risk factors such as older age, ethnicity, obesity, family history of DM2 and unique post-transplant risk factors such as immunosuppressant use, CMV positivity, hepatitis C and weight gain[7,8].
PTDM has a significant impact on post-transplant outcomes. Various studies have reported decreased graft survival and an increase in cardiovascular, renal and infection complications[1,2]. Identification of risk factors is helpful in guiding the implementation of measures to prevent PTDM and its associated morbidity and mortality in solid organ transplant recipients. A network meta-analysis (NMA) allows for the comparison of multiple treatments in a single analytical model, allowing direct and indirect comparisons between several treatments.
The objective of this NMA is to determine the relative impact of the 3 main immunosuppressants ie tacrolimus, sirolimus and cyclosporine used in transplant medicine on the incidence of PTDM, thus providing information on clinical risk stratification and prompting physicians to carefully evaluate risk-benefit ratios in transplant recipients.
We performed a systematic review and meta-analysis as per the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement standards. An experienced librarian at the University of Toronto performed the literature search on February 16, 2017.
A systematic literature search was performed in peer reviewed databases of Medline, Medline epub/in-process, EMBASE, CDSR and CCRCCT (search last conducted on February 16, 2017 including studies from 1995 onwards). The search strategy was developed using a combination of database-specific subject headings and text words. Additional key words were mined from sample articles and generated through input from subject specialists on the team. The search strategy was then customized for each database. Appendix 1 outlines the detailed search strategy.
Two independent reviewers screened the titles and abstracts using the inclusion criteria. Disagreement was resolved after discussion.
The inclusion criteria were: (1) English-language studies; (2) Studies with an adult (> 18 years old) human patient population; (3) Studies published between 1995 and the date of the search; (4) Studies using an intervention of maintenance immunosuppression with standard-dose tacrolimus, cyclosporine or sirolimus; (5) Studies about solid-organ transplant recipients (i.e., not hand, pancreatic islet, stem cell transplant); and (6) Studies with a follow-up period of 1 year or longer.
Estimation of incidence: Random effects meta-analyses of the treatment-specific incidence of PTDM overall (using the earliest reported time in each study) as well as at various time points post-transplant (1 year, 2-3 years, and 5 or more years) were conducted using generalized linear mixed models; the input data were the number of patients with follow-up and the number with PTDM at the time point. In this analysis, we only pooled data from cohort and randomized studies where patients with pre-existing DM were excluded. Heterogeneity was assessed using Cochran’s Q, and I2. Meta-regression was used to quantify the variation in incidence attributable to the following study characteristics: mean patient age, mean body mass index (BMI), percentage male, concomitant use of steroids and study design.
Comparisons of relative incidence: To compare incidence between the three immunosuppressants, NMA was used. This allows comparison of multiple treatments in a single analytical model, allowing for direct and indirect comparisons between numerous treatments, so all studies could be included simultaneously. The direct and indirect estimates were compared using the node-splitting procedure. For example, the directly estimated odds ratio from studies comparing sirolimus and tacrolimus can be compared to the indirect estimate that would be expected based on the comparisons of each of them to cyclosporine. When these direct and indirect estimates are consistent, the pooled network estimate can be more precise than the direct estimate. Odds ratios (OR) between pairs of treatments were calculated as well as the 95% credible intervals. All analyses were done in R 4.02 using the Meta package for meta-analysis of incidence and the gemtc packages for NMA[9-11].
Quality grading of studies: The quality of each study in the analysis was assessed based on Newcastle-Ottawa scale (NOS). The scale includes three categories, using scores of 1-9 for assessment. The total score is 9, comprising of 4 for selection, 2 for comparability, and 3 for outcome. Total score ≥ 7 represents a high-quality study.
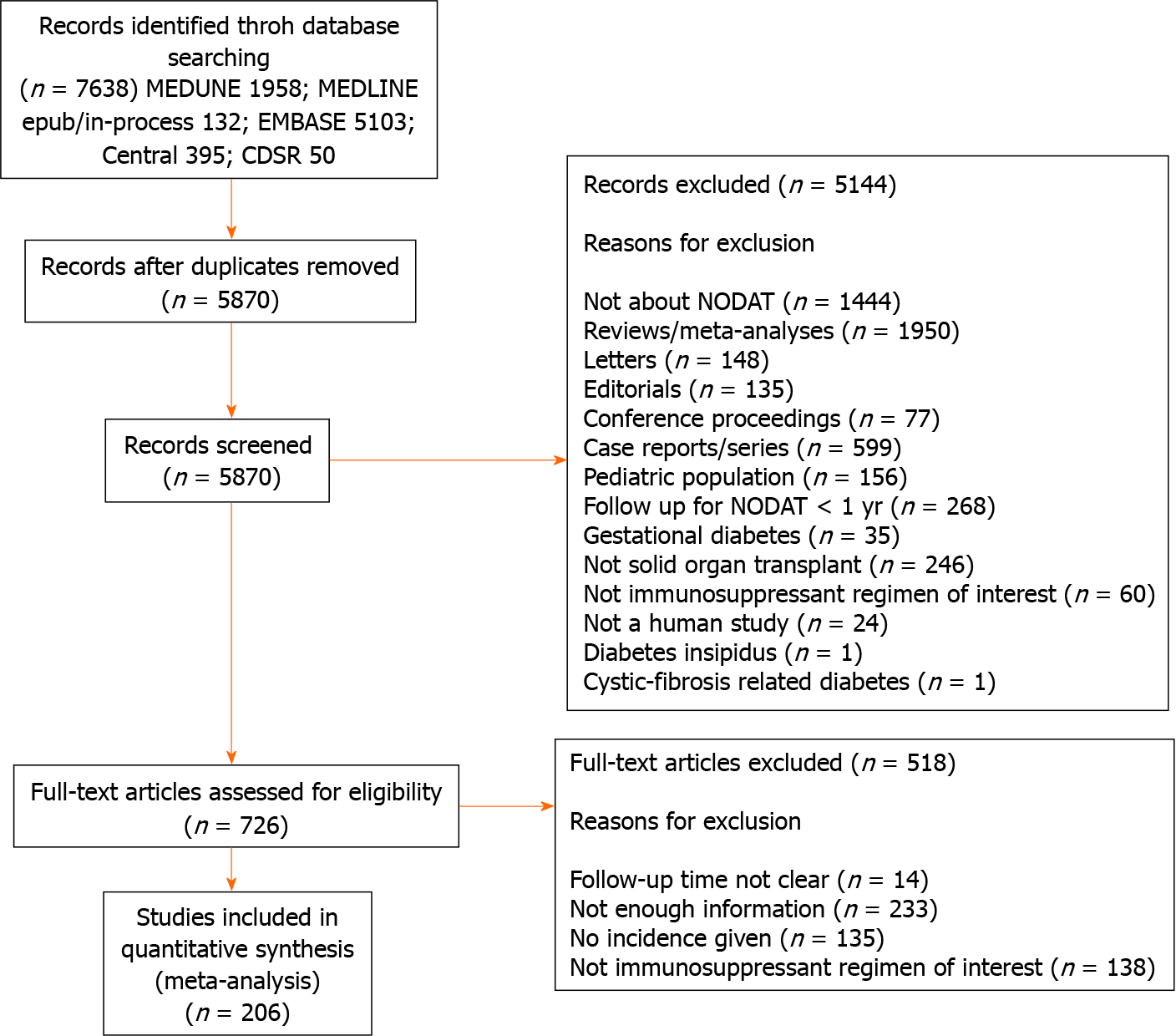
The literature search yielded 7638 records. Following elimination of 1768 duplicates, 5870 articles were identified for screening, of which 5144 records were excluded by title and abstract, leaving 726 eligible for full-text assessment. Case reports/series, conference proceedings, and editorials, reviews, articles in which PTDM was not the primary or secondary outcome of interest, or there was unclear duration of follow-up, or the intervention was not an immunosuppressant regimen of interest and articles with inadequate information were excluded after a full text review. Ultimately, 206 studies were included in our study as shown in the PRISMA diagram (Figure 1). Most of the studies had a NOS score of 7, with a mean of 7.2 indicating that the overall study quality was high. The median year of publication was 2009, and most of the papers were published between 2006 and 2013. The breakdown of the studies by design was as follows: 151 studies were cohort studies, 6 were case-control studies, 10 were cross-sectional studies and 39 studies were randomized controlled trials. The studies included various solid organ transplant patients, with the majority being kidney transplant (163 studies) and liver transplant (26 studies).
A total of 206 eligible studies identified 75595 patients on Tacrolimus, 51242 on Cyclosporine and 3020 on Sirolimus. All patients underwent SOT and received immunosuppression. The mean age of the patients was 45 years old, 62.4% were male and the mean BMI was 24.7.
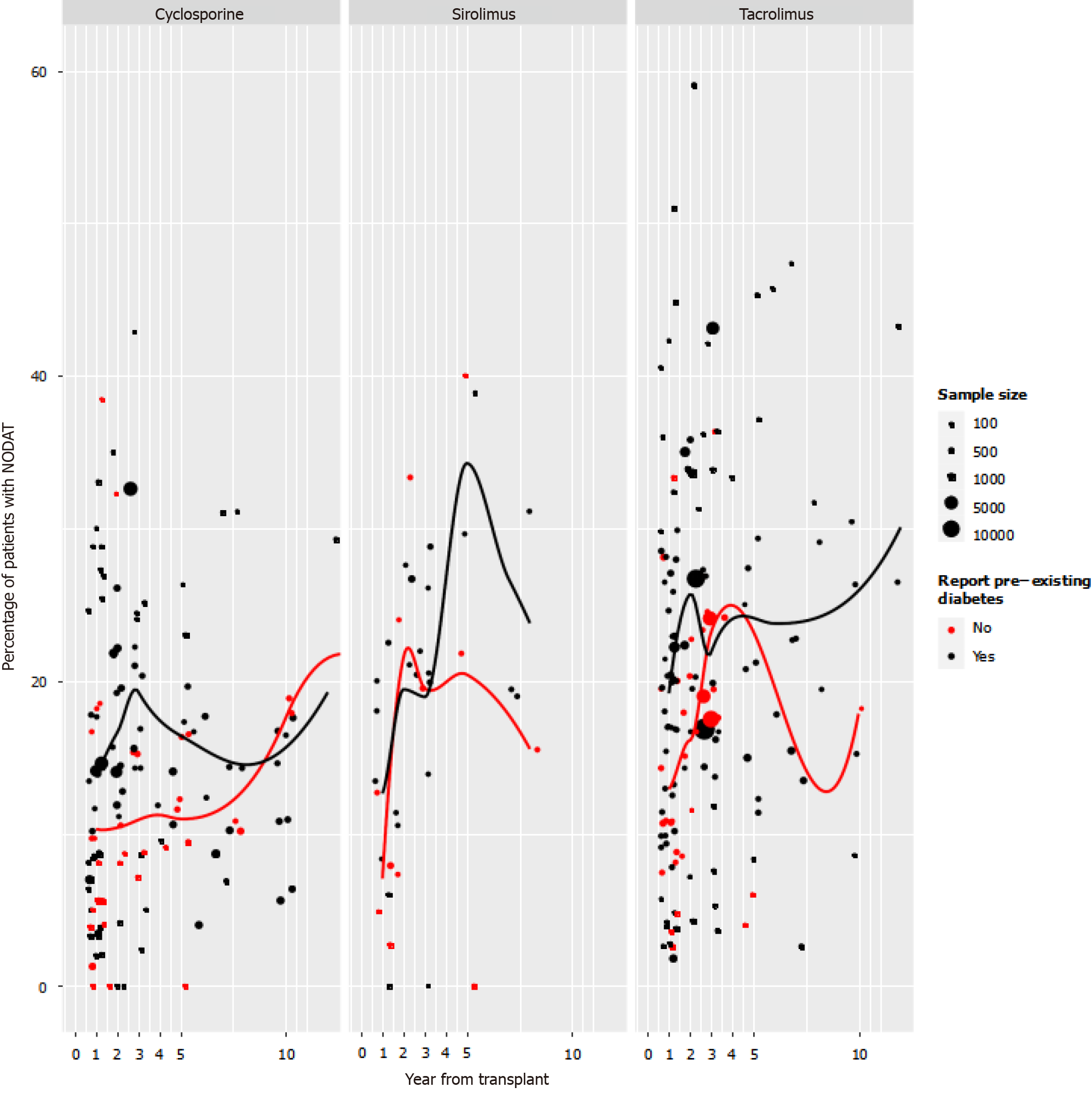
Incidence of PTDM: Figure 2 illustrates the first reported time point per study for incidence of PTDM presented by immunosuppressant and stratified by studies reporting the number of patients with pre-transplant diabetes. It is important to note the wide heterogeneity of incidence rates within every time point. Most studies reported the first time point, with PTDM incidence within the first-year post-transplant.
One hundred and twenty-one studies were used in the meta-analysis of the incidence of PTDM at one-year post-transplant. Forty-five, 65, and 11 studies were used in the analysis of the one-year incidence in studies, which used cyclosporine, tacrolimus, and sirolimus respectively as the main immunosuppressant. The overall proportion of patients developing PTDM at 1 year was 12.3% (95%CI: 10.6%-14.3%, I2 = 95.4 %). Among patients on cyclosporine as the main immunosuppressant, the proportion of patients developing PTDM was 9.9% (95%CI: 7.6%-12.7%, I2 = 96.1%). Whereas the proportions of patients developing PTDM at one year were 14.9% (95%CI: 12.4%-17.8%, I2 = 94.3 %) with tacrolimus and 9.5% (95%CI: 6.1%–14.5%, I2 = 77.0%) with sirolimus.
The analysis of incidence of PTDM at 2-3 years post-transplant included 103 study arms. Forty-one, 46 and 16 studies used cyclosporine, tacrolimus and sirolimus respectively. The overall proportion of patients developing PTDM at 2-3 years was 18.1% (95%CI: 16.2%-20.3%, I2 = 98.2%). The percentages of patients who developed PTDM with each immunosuppression therapy were 15.3% for cyclosporine (95%CI 12.9%-18.1%, I2 = 94.2 %), 20.7% for tacrolimus (95%CI: 17.4%-24.4%, I2 = 99.1%), and 20.8% for sirolimus (95%CI: 17.3%-24.9%, I2 = 58.6%).
The analysis of incidence of PTDM at 5-10 years post-transplant included 78 study arms, 8 with cyclosporine, 31 with tacrolimus, and 9 with sirolimus as the main immunosuppressant. The overall proportion of patients developing PTDM at 5-10 years was (95%CI: 0.1362-0.1840, I2 = 93.2%). The percentages of patients who de
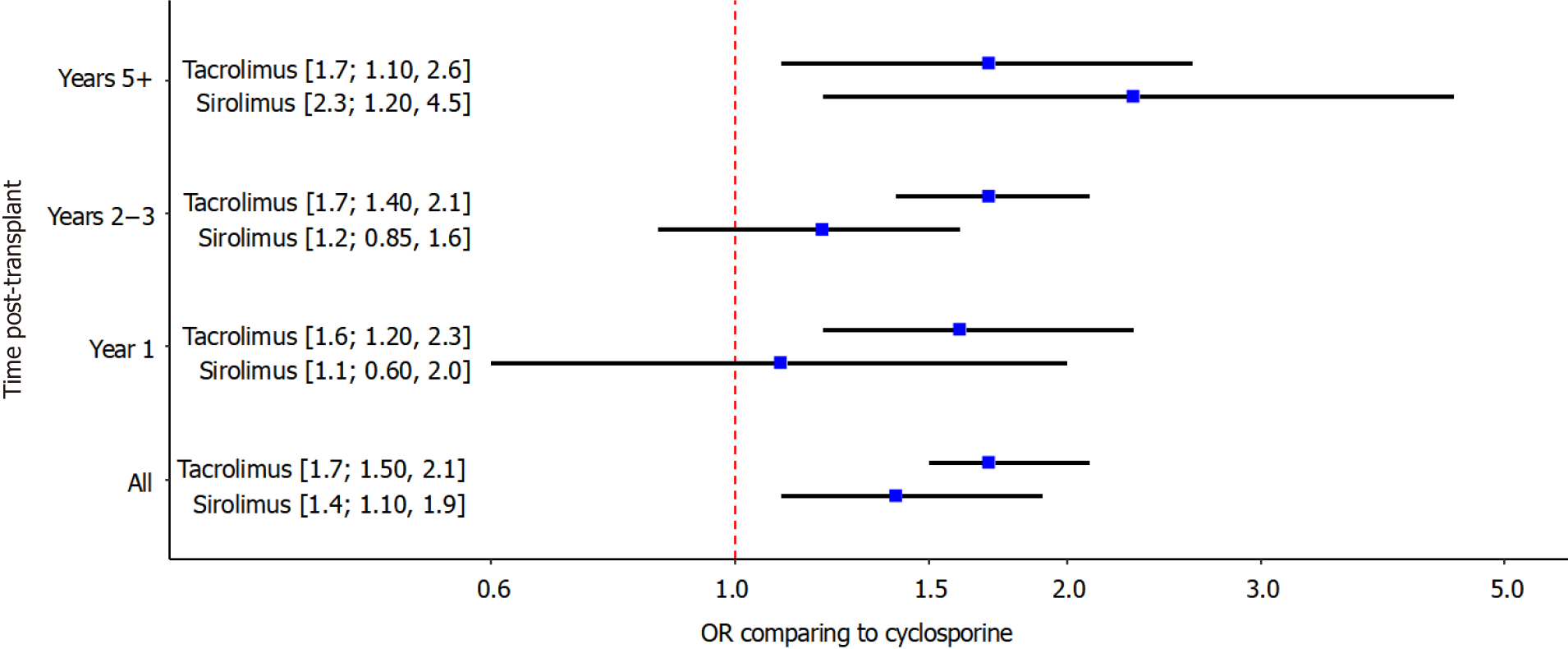
NMA comparing tacrolimus, sirolimus and cyclosporine: The results of the NMA are presented in Figure 3. The direct and indirect estimates were inconsistent, with the exception of one comparison at 5 year that involved a study with zero events in one arm. The overall odds of developing PTDM were higher when tacrolimus or sirolimus were used as main immunosuppressant than with cyclosporine. Across all time points, compared to cyclosporine, the odds of developing PTDM were 1.4 times higher (95%CI: 1.1–1.9) with sirolimus and 1.7 times higher (95%CI: 1.5–2.1) with tacrolimus. The odds ratio between tacrolimus and sirolimus was 1.2 (95%CI: 0.9–1.6). The increased risk with tacrolimus compared to cyclosporine was seen at all time points post-transplant (1-year: 1.6, 95%CI: 1.2–2.3; 2-3 years: 1.7; 95%CI: 1.4-2.1; 5 or more years: 1.7; 95%CI: 1.1-2.6). The increased risk with tacrolimus compared to cyclos
Subgroup analysis by type of solid organ transplanted: Table 1 shows the incidence of PTDM, number of studies in the analysis, incidence, 95%CI and I2 by organ transplanted (liver, kidney, heart and lung) at various time points. This analysis excluded studies where pre-existing DM was unknown. At 2-3 years post-transplant, incidence of PTDM was 18.9% (95%CI: 14.2–24.7) in liver transplant patients, 17.2% (95%CI: 14.9-19.8) in kidney transplant patients, 22.4% (95%CI: 17.1-28.8) in heart transplant patients and 18.8% (95%CI: 8.6-36.3) in lung transplant patients. Heterogeneity in the incidence of PTDM was related to X, Y and Z in meta-regression, but the I2 for each organ-time combination remained high even after accounting for differences between studies in these characteristics.
| Organ | Year | Number of studies | Incidence (%) | 95%CI | I2 (%) |
| Liver | Year 1 | 7 | 12.3 | 5.6-24.8 | 89.3 |
| Liver | Years 2-3 | 16 | 18.9 | 14.2-24.7 | 94.4 |
| Liver | Years 5+ | 5 | 9.0 | 2.9-24.5 | 95.0 |
| Kidney | Year 1 | 108 | 12.2 | 10.5-14.1 | 95.3 |
| Kidney | Years 2-3 | 73 | 17.3 | 15.1-19.7 | 98.2 |
| Kidney | Years 5+ | 71 | 16.3 | 13.9-19 | 93.2 |
| Heart | Year 1 | 3 | 29.3 | 9.5-62 | 84.9 |
| Heart | Years 2-3 | 10 | 22.4 | 17.1-28.8 | 93.6 |
| Heart | Years 5+ | 2 | 17.7 | 14.1-22 | 0.0 |
| Lung | Year 1 | 3 | 6.4 | 0.9-34 | 92.8 |
| Lung | Years 2-3 | 5 | 18.8 | 8.6-36.3 | 96.8 |
| Lung | Years 5+ | 0 | N/A | N/A |
Risk factors for developing PTDM: We did not conduct a meta-analysis of predictors of developing PTDM. However, for each variable assessed as a predictor any paper in the review, we present the number of studies assessing it, and the number of studies that found it to be statistically significant in multivariable analyses (Table 2). Some of the noteworthy variables and the proportions, where they were significant include age (44/50), BMI (36/39), HCV (14/18), and African American ethnicity (22/52).
| Predictor | n | Fraction |
| African-American | 59 | 27/59 |
| Age | 56 | 51/56 |
| BMI | 43 | 39/43 |
| Tac use | 31 | 24/31 |
| HCV | 20 | 15/20 |
| BPAR | 13 | 10/13 |
| Male | 11 | 4/11 |
| Family history of diabetes | 7 | 6/7 |
| Pre transplant triglycerides | 7 | 7/7 |
| Pre transplant impaired fasting glucose | 6 | 6/6 |
| CMV infection | 5 | 2/5 |
| Proteinuria at post-operative day 5 | 3 | 3/3 |
| Number of rejections | 3 | 2/3 |
| Triglyceride lipid increase | 2 | 2/2 |
| HLA mismatch | 2 | 0/2 |
| HBV | 1 | 1/1 |
| Non-Caucasian | 1 | 1/1 |
| Cystic fibrosis | 1 | 1/1 |
| Cadaveric donor | 1 | 1/1 |
PTDM is a recognized complication of SOT, with reported incidence varying widely between 10 and 40%, depending on the transplanted organ[3,4]. This variability is mainly due to lack of definitive diagnostic criteria. PTDM is associated with substantially increased risk of cardiovascular disease, graft failure and premature death across all organ transplant groups[1,2]. Various factors have been noted to influence the development of PTDM.
In this systematic review and meta-analysis, we estimated incidence of PTDM for each of the 3 major immunosuppressants used in SOT (tacrolimus, sirolimus and cyclosporine), both overall and at key time points post-transplant, and used NMA to compare incidence between agents. A total of 206 eligible studies involving 129857 post-transplant patients fulfilling the inclusion criteria were included in one or more parts of the meta-analysis. Renal transplant recipients constituted the largest number of participants.
The overall pooled incidence of PTDM was higher in arms using tacrolimus and sirolimus than in those using cyclosporine across all SOT. The pattern across agents was similar at one, two and 5-10 years following SOT. In NMA combining studies that examined two or more immunosuppressants, tacrolimus had a consistently higher odds of PTDM at each time point than cyclosporine, with increased risk from sirolimus compared to cyclosporine being mainly restricted to the period 5-10 years post-transplant.
There is biological plausibility to sirolimus inducing insulin resistance in the longer term, with in vivo evidence that chronic usage of Sirolimus leads to insulin resistance and diabetes. It has been demonstrated that Sirolimus induces gluconeogenesis in liver and well as downregulation of GLUT-4 Leading to the development of severe glucose intolerance[12,13]. These effects are mediated through the blockade of the mTOR/ S6K1 pathway. Moreover, there is evidence of increased β-cell toxicity induced by the chronic mTOR inhibitor treatment also possibly leading to insulin resistance and diabetes[14]. Conversely, tacrolimus affects the pancreatic B-cells, thereby decreasing insulin secretion resulting in hyperglycemia.
This review found that the variables most frequently associated with PTDM were age, BMI, tacrolimus use and hepatitis C virus. In the literature, numerous risk factors such as age, race, ethnicity, family history, hepatitis C infection, BMI, acute rejection and immunosuppressive agents have been implicated in the development of PTDM (15-19). Increased age as a risk factor for PTDM has been investigated in numerous studies within the transplant population. Khalili et al[20] reported increased age as a predictor of PTDM in 555 Liver transplant recipients whereas Mirabella et al[21] reported an increased age at transplant (> 45 years) as a risk factor in a cohort of 899 recipients. In contrast, studies by Saliba et al[22] and Driscoll et al[23] reported no association with age. Biopsy proven acute rejection is a risk factor for PTDM as bolus doses of steroids along with increase in maintenance immunosuppression with tacrolimus, cyclosporine or sirolimus is used as standard of treatment which lead to increased risk of PTDM.
Evaluating the incidence and predictors of PTDM is important, aspatient survival is significantly compromised by renal disease, cardiovascular disease and infection[24]. However, there is variable evidence regarding the relationship of PTDM with mortality in the post-transplant cohort. In a study by Kasiske et al[7], PTDM was associated with mortality (P < 0.0001), graft failure (P < 0.0001), and death-censored graft failure (P < 0.0001). In contrast, a retrospective analysis of the UNOS/OPTN database (n > 37000) by Kuo et al[25] did not demonstrate the negative impact of PTDM on transplant survival or cardiovascular mortality. Similarly, a retrospective analysis of the UNOS/OPTN database by Kuo et al[26] consisting of over 13000 Liver transplant recipients demonstrated that the presence of both PTDM and acute rejection at 1-year post-transplant but not PTDM alone was associated with higher overall graft failure and mortality risk. This suggests that more robust studies are required to investigate this association further.
Strengths of this study include: (1) Use of a comprehensive and exhaustive search strategy, in order to identify all potentially relevant studies; (2) Evaluation of eligible studies and data extraction by two investigators independently, with discrepancies resolved by consensus; (3) The large total number of studies and participants; (4) Use of rigorous analytic methods to summarize and compare estimates and investigate heterogeneity; (5) Tabulation of all identified risk factors for PTDM across solid organ transplant groups; and (6) Adherence to the PRISMA guidelines and use of standar
An important limitation to the interpretation of results is the significant clinical heterogeneity across studies in immunosuppression protocols, patient populations, and criteria used to define PTDM. In meta-regression, such study-level variables explained only a small amount of heterogeneity in incidence estimates. I2, with or without meta-regression, mainly fell in the range of ‘substantial heterogeneity, meaning that most variability in incidence remains unexplained. Where a study reported incidence at multiple times, we used the earliest time to minimize issues related to loss-to-follow-up in the individual study. This has consequences for the comparisons of incidence of PTDM at different time points; these comparisons should be made with the proviso that the estimates may be based on somewhat different types of patients, as a result of differential drop-out and death. In the NMA, where we compare risk of PTDM between immunosuppressant regimens, RCTs and observational studies were com
Nonetheless, our study represents the most comprehensive study review and meta-analysis to date on the relative impact of the principal maintenance immunosuppressants.
This NMA compares the relative impact of the 3 major immunosuppressants on the development of PTDM, revealing sirolimus and tacrolimus to be significantly more diabetogenic than cyclosporine. Tacrolimus has higher diabetogenicity in the short-term (2-3 years post-transplant), whereas sirolimus tends to exhibit higher diabetogenicity in the long-term (5-10 years post-transplant). This research will aid clinicians in understanding the important risk factors for PTDM, and encourages careful evaluation of the risk-benefit ratio of different immunosuppressant regimens in the transplant recipients.
Post-transplant diabetes mellitus (PTDM) is associated with significant morbidity and mortality, with increased cardiovascular risk, infection and graft failure. The reported incidence of PTDM ranges from 4%-25% in renal transplant recipients, 2.5%-25% in liver transplant recipients, 4%-40% in heart transplant recipients, and 30%-35% in lung transplant recipients.
This research will help clinicians recognise the risk-benefit of various immunosuppressants for PTDM.
The aim of this study is to perform a systematic review and meta-analysis to estimate incidence of PTDM and compare the effects of the 3 major immunosuppressants on incidence of PTDM
The authors performed a systematic review and meta-analysis as per the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement standards.
This network meta-analysis (NMA) reveals sirolimus and tacrolimus to be signi
This NMA reveals sirolimus and tacrolimus to be significantly more diabetogenic than cyclosporine. Tacrolimus is more diabetogenic in the short-term (2-3 years post-transplant), whereas sirolimus tends to exhibit higher diabetogenicity in the long-term (5-10 years post-transplant). This research will aid clinicians in understanding the important risk factors for PTDM, and encourages careful evaluation of the benefit–risk ratio of different immunosuppressant regimens in the transplant patients.
Focused studies on patients on sirolimus to get more information on pathophysiology of PTDM development required.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gonzalez FM S-Editor: Ma YJ L-Editor: A P-Editor: Yuan YY
| 1. | Matas AJ, Gillingham KJ, Humar A, Ibrahim HN, Payne WD, Gruessner RW, Dunn TB, Sutherland DE, Najarian JS, Kandaswamy R. Posttransplant diabetes mellitus and acute rejection: impact on kidney transplant outcome. Transplantation. 2008;85:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Siraj ES, Abacan C, Chinnappa P, Wojtowicz J, Braun W. Risk factors and outcomes associated with posttransplant diabetes mellitus in kidney transplant recipients. Transplant Proc. 2010;42:1685-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Sharif A, Hecking M, de Vries AP, Porrini E, Hornum M, Rasoul-Rockenschaub S, Berlakovich G, Krebs M, Kautzky-Willer A, Schernthaner G, Marchetti P, Pacini G, Ojo A, Takahara S, Larsen JL, Budde K, Eller K, Pascual J, Jardine A, Bakker SJ, Valderhaug TG, Jenssen TG, Cohney S, Säemann MD. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14:1992-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 386] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 4. | Ye X, Kuo HT, Sampaio MS, Jiang Y, Bunnapradist S. Risk factors for development of new-onset diabetes mellitus after transplant in adult lung transplant recipients. Clin Transplant. 2011;25:885-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Chakkera HA, Mandarino LJ. Calcineurin inhibition and new-onset diabetes mellitus after transplantation. Transplantation. 2013;95:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Soleimanpour SA, Crutchlow MF, Ferrari AM, Raum JC, Groff DN, Rankin MM, Liu C, De León DD, Naji A, Kushner JA, Stoffers DA. Calcineurin signaling regulates human islet {beta}-cell survival. J Biol Chem. 2010;285:40050-40059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 998] [Cited by in RCA: 955] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 8. | Cruzado JM, Bestard O, Grinyó JM. Impact of extrahepatic complications (diabetes and glomerulonephritis) associated with hepatitis C virus infection after renal transplantation. Contrib Nephrol. 2012;176:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | R: A language and environment for statistical computing. 2013. Available from: http://www.R-project.org/. |
| 10. | Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1954] [Cited by in RCA: 3020] [Article Influence: 503.3] [Reference Citation Analysis (0)] |
| 11. | van Valkenhoef G, Kuiper J, van Valkenhoef MG. Package ‘gemtc’. 2016. Available from: https://github.com/gertvv/gemtc. |
| 12. | Houde VP, Brûlé S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y, Marette A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 353] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 13. | Veilleux A, Houde VP, Bellmann K, Marette A. Chronic inhibition of the mTORC1/S6K1 pathway increases insulin-induced PI3K activity but inhibits Akt2 and glucose transport stimulation in 3T3-L1 adipocytes. Mol Endocrinol. 2010;24:766-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Kezic A, Popovic L, Lalic K. mTOR Inhibitor Therapy and Metabolic Consequences: Where Do We Stand? Oxid Med Cell Longev. 2018;2018:2640342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Bergrem HA, Valderhaug TG, Hartmann A, Hjelmesaeth J, Leivestad T, Bergrem H, Jenssen T. Undiagnosed diabetes in kidney transplant candidates: a case-finding strategy. Clin J Am Soc Nephrol. 2010;5:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Bhat V, Tazari M, Watt KD, Bhat M. New-Onset Diabetes and Preexisting Diabetes Are Associated With Comparable Reduction in Long-Term Survival After Liver Transplant: A Machine Learning Approach. Mayo Clin Proc. 2018;93:1794-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Aravinthan AD, Fateen W, Doyle AC, Venkatachalapathy SV, Issachar A, Galvin Z, Sapisochin G, Cattral MS, Ghanekar A, McGilvray ID, Selzner M, Grant DR, Selzner N, Lilly LB, Renner EL, Bhat M. The Impact of Preexisting and Post-transplant Diabetes Mellitus on Outcomes Following Liver Transplantation. Transplantation. 2019;103:2523-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Kuo HT, Sampaio MS, Ye X, Reddy P, Martin P, Bunnapradist S. Risk factors for new-onset diabetes mellitus in adult liver transplant recipients, an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing database. Transplantation. 2010;89:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Oufroukhi L, Kamar N, Muscari F, Lavayssière L, Guitard J, Ribes D, Esposito L, Alric L, Hanaire H, Rostaing L. Predictive factors for posttransplant diabetes mellitus within one-year of liver transplantation. Transplantation. 2008;85:1436-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Khalili M, Lim JW, Bass N, Ascher NL, Roberts JP, Terrault NA. New onset diabetes mellitus after liver transplantation: the critical role of hepatitis C infection. Liver Transpl. 2004;10:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Mirabella S, Brunati A, Ricchiuti A, Pierini A, Franchello A, Salizzoni M. New-onset diabetes after liver transplantation. Transplant Proc. 2005;37:2636-2637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Saliba F, Lakehal M, Pageaux GP, Roche B, Vanlemmens C, Duvoux C, Dumortier J, Salamé E, Calmus Y, Maugendre D; Diapason Study Group. Risk factors for new-onset diabetes mellitus following liver transplantation and impact of hepatitis C infection : an observational multicenter study. Liver Transpl. 2007;13:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Driscoll CJ, Cashion AK, Hathaway DK, Thompson C, Conley Y, Gaber O, Vera S, Shokouh-Amiri H. Posttransplant diabetes mellitus in liver transplant recipients. Prog Transplant. 2006;16:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Boudreaux JP, McHugh L, Canafax DM, Ascher N, Sutherland DE, Payne W, Simmons RL, Najarian JS, Fryd DS. The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation. 1987;44:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Kuo HT, Sampaio MS, Vincenti F, Bunnapradist S. Associations of pretransplant diabetes mellitus, new-onset diabetes after transplant, and acute rejection with transplant outcomes: an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database. Am J Kidney Dis. 2010;56:1127-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Kuo HT, Lum E, Martin P, Bunnapradist S. Effect of diabetes and acute rejection on liver transplant outcomes: An analysis of the organ procurement and transplantation network/united network for organ sharing database. Liver Transpl. 2016;22:796-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |