Published online Jun 29, 2020. doi: 10.5500/wjt.v10.i6.173
Peer-review started: February 24, 2020
First decision: April 18, 2020
Revised: May 19, 2020
Accepted: May 21, 2020
Article in press: May 21, 2020
Published online: June 29, 2020
Processing time: 119 Days and 15.2 Hours
The key question in living kidney donor assessment is how best to determine the contribution of each kidney to overall renal function and guide selection of which kidney to donate, ensuring safety of procedure and good outcome for both recipient and donor. It is thought that a length difference > 2 cm may indicate significant difference in function and therefore need for measurement of differential function.
To determine the effect of using kidney length to decide which kidney to donate in a retrospective cohort of potential donors.
All 333 potential living kidney donors between January 2009 and August 2018 who completed assessment were retrospectively evaluated. Donor assessment was performed as per United Kingdom guidelines. Data included age, sex, kidney length (cranio-caudal) obtained by computed tomography/ultrasono-graphy,51-chromium ethylenediamine tetraacetatic acid measured glomerular filtration rate, mercapto acetyl tri glycine split function and vascular anatomy. There were 48 exclusions due to inadequate data or incomplete investigations. Statistical analysis was performed using Excel pivot tables and GraphPad Prism. Correlation between kidney length and differential function was determined with Pearson’s correlation coefficient.
Of 285 potential donors included in the study, there were 144 males (mean age 49.9 ± 14.75) and 141 females (mean age 51.2 ± 11.23). Overall, the Pearson’s correlation between differences in length and divided function of kidney pairs was 0.1630, P = 0.0058. Of 73 with significant difference (> 10%) in divided function, 18 (24.7%) had no difference in kidney length; 54 (74%) had a difference of < 2 cm and only one of > 2 cm. Using a length difference of > 1 cm would only predict significant difference in divided function in 8/34 (23.5%) of cases. Using a difference of > 2 cm as cut off for performing split function would lead to false reassurance in 72 patients (6 had > 20% difference in divided function whereas 66 had 10%-20% difference).
Length difference between kidney pairs alone is not sufficient to replace measurement of divided function. This issue requires a randomised controlled trial to resolve it.
Core tip: Selection of which kidney to donate is of critical importance in living kidney donation. The decision-making process based on divided function and vascular anatomy was used to validate a retrospective “what if” analysis of prospectively reported kidney length measurements in a cohort of 285 potential donors. This study shows a significant risk for making wrong/harmful decision (removing the significantly better functioning kidney) if kidney length alone is used for decision making -25% if using 2 cm difference as cut off. Difference in length between kidney pairs alone is not sufficient to replace measurement of divided function.
- Citation: Akoh JA, Schumacher KJ. Living kidney donor assessment: Kidney length vs differential function. World J Transplant 2020; 10(6): 173-182
- URL: https://www.wjgnet.com/2220-3230/full/v10/i6/173.htm
- DOI: https://dx.doi.org/10.5500/wjt.v10.i6.173
The continuing scarcity of deceased organs coupled with the evidently better results associated with living donor transplantation has focused attention on living donor wellbeing and long-term outcome. The risk of developing end-stage renal disease, cardiovascular disease and increased all-cause mortality in donors[1] demands that every effort must be made to eliminate risks in living donors. This is in line with the well-known principle of “primum non nocere” (“first do no harm”). The goals of assessment of living kidney donors are to ensure the donor is well enough to go through the donation process with minimal or no morbidity and avoidance of mortality; that the recipient achieves an uncomplicated transplant with a beneficial long-term outcome. Donor welfare and safety is paramount; therefore, potential candidates must have sufficient renal function post donation in order to minimize future risks when living with a single kidney. The aim is to retrieve the kidney that will allow the donor to preserve the better kidney[2]. Sometimes this means that a multi-artery left kidney is preferable to a right kidney[3].
Determining whether both kidneys contribute equally or significantly differently to overall function is fundamental to the decision as to which kidney to donate. In the context of living kidney donation, this involves accurate determination of the donor glomerular filtration rate (GFR) and ensuring that it is within the recommended range as advised by the British Transplantation Society/Renal Association (BTS/RA) Guidelines[2]; and evaluation of renal parenchyma, urinary system and vascular anatomy by means of ultrasonography (US), Computed tomography (CT) or magnetic resonance angiography; and only removing the kidney with the lower contribution when the divided function is significantly different. The key question is how best to determine the contribution of each kidney to the overall function. Isotope differential renal function is not uniformly performed in all transplant centres, with many relying on kidney size measurements. Such an approach is supported by the BTS/RA Living Donor Kidney Transplantation Guidelines (2018)[2] which states that differential kidney function, determined by 99mTechnitium dimercaptosuccinic acid (99mTcDMSA) is recommended where there is > 10% variation in kidney size or significant renal anatomical abnormality. It further states that, “A difference in size of 2 cm or more indicates the possibility of a significant difference in GFR between the two kidneys”. Until the recent BTS/RA Guidelines[2], our centre performed mercapto acetyl tri glycine (MAG3) split function and CT and/or US imaging for all patients. This study aimed to determine the effect of using kidney length to decide which kidney to donate in a retrospective cohort of potential donors. It also studied the correlation between differences in kidney length and split renal function; whether a difference in length of > 2 cm is associated with significant differences in divided function; and whether a < 1 cm difference is sufficient evidence that the split renal function is not significantly different between both kidneys.
This retrospective evaluation of prospectively collected data on potential living kidney donors was approved by the institutional review board and individual informed consent was waived. During the period between January 2009 and August 2018, all potential living kidney donors who completed the assessment process up to CT angiography were studied whether they proceeded to donation or not. Donor assessment was performed as per United Kingdom guidelines.
Pole to pole (cranio-caudal) length as determined by CT and/or ultrasound scan (US) was documented for each kidney in all prospective donors. Where data from both modalities of imaging were available, CT length measurement was used preferentially over US imaging to assess length of kidney. Length measured on US scan was only used where CT was not reported, not completed, or unavailable as completed at different centres, or where CT reports were not specific to true length of kidneys. Other dimensions of the kidney were not reported in this centre.
Each potential donor underwent 51-chromium ethylenediamine tetraacetatic acid (51Cr-EDTA) scans for GFR measurement (Brochner-Mortensen GFR for 1.73 m2 BSA). To determine the split or divided function, a radioisotope renogram with diuretic followed by an indirect micturating cystourethrogram combined with a MAG3 scan was performed with the diuretic administered at the same time as the radiopharmaceutical to ensure good diuresis. Divided function was calculated using geometric mean data. Uptake of tracer in both kidneys as well as normal drainage and excretion bilaterally; evidence of obstruction in either kidney; evidence of reflux on the indirect micturition cystogram component of the study were determined/ reported.
Pre-donation assessment culminated in CT imaging in order to assess length measurement, renal pathology and vascular anatomy. For potential donors with qualifying GFR, the divided function (if ≤ 20%) in conjunction with the vascular anatomy was used to guide suitability decision for donation and appropriate selection of which kidney to donate. The renal length was not utilised in the decision making process during the study period.
The list of potential donors and their key data was maintained prospectively on an Excel spreadsheet. Further information was retrieved from the renal computer system – VitalData Clinical Information System (Vitalpulse Limited 1997-2019). Imaging results were obtained from Insignia Medical Systems [Insight PACS (Picture Archiving and Communication Systems)].
Data collected included donor age, sex, GFR, differential function, US kidney length, CT kidney length and donated GFR. The differences in length and the differential function between left and right kidney pairs were categorised as shown in Table 1 for the purpose of analysis. The donated GFR was calculated as a percentage of the total donor GFR according to the split function of the donated or potentially donated kidney[4].
| Length difference | Difference in divided function | Category |
| > 2 cm | > 20% | 4 |
| 1.01-2.0 cm | 10%-20% | 3 |
| 0.01-1.0 cm | < 10% | 2 |
| 0 | 0% | 1 |
Forty eight potential donors were excluded from the study: 31 due to inadequate reporting of imaging (no length measurement was available, either from CT or US); 14 due to incomplete investigations (late withdrawal from the process, no CT or no MAG3); and three due to inadequate data from an external unit that performed the assessment.
Detailed information on the potential donors were entered in to an Excel database and analysed using Excel pivot tables. Further statistical analysis was conducted using GraphPad Prism (GraphPad Software, San Diego, CA, United States). The difference between means was tested using the unpaired t-test. Correlation between kidney length and divided function; and difference in length of kidney pairs versus differential function were determined using Pearson’s correlation coefficient. A P < 0.05 was regarded as statistically significant.
Of the 285 included in the study, there were 144 males (mean age 49.9 ± 14.75) and 141 females (mean age 51.2 ± 11.23). The difference between the means of 1.3 [95% confidence interval (CI): −1.7615 to 4.3615; t = 0.8358; u = 283] was not statistically significant (P = 0.4040). The average GFR of female potential donors of 86.85 ± 13.51 mL/min was comparable to the 89.63 ± 14.66 mL/min for male potential donors. The difference between the means of -2.78 (95%CI from -6.0707 to 0.5073; t = 1.6648; u = 283; SED = 1.671) was not statistically significant (P = 0.0971).
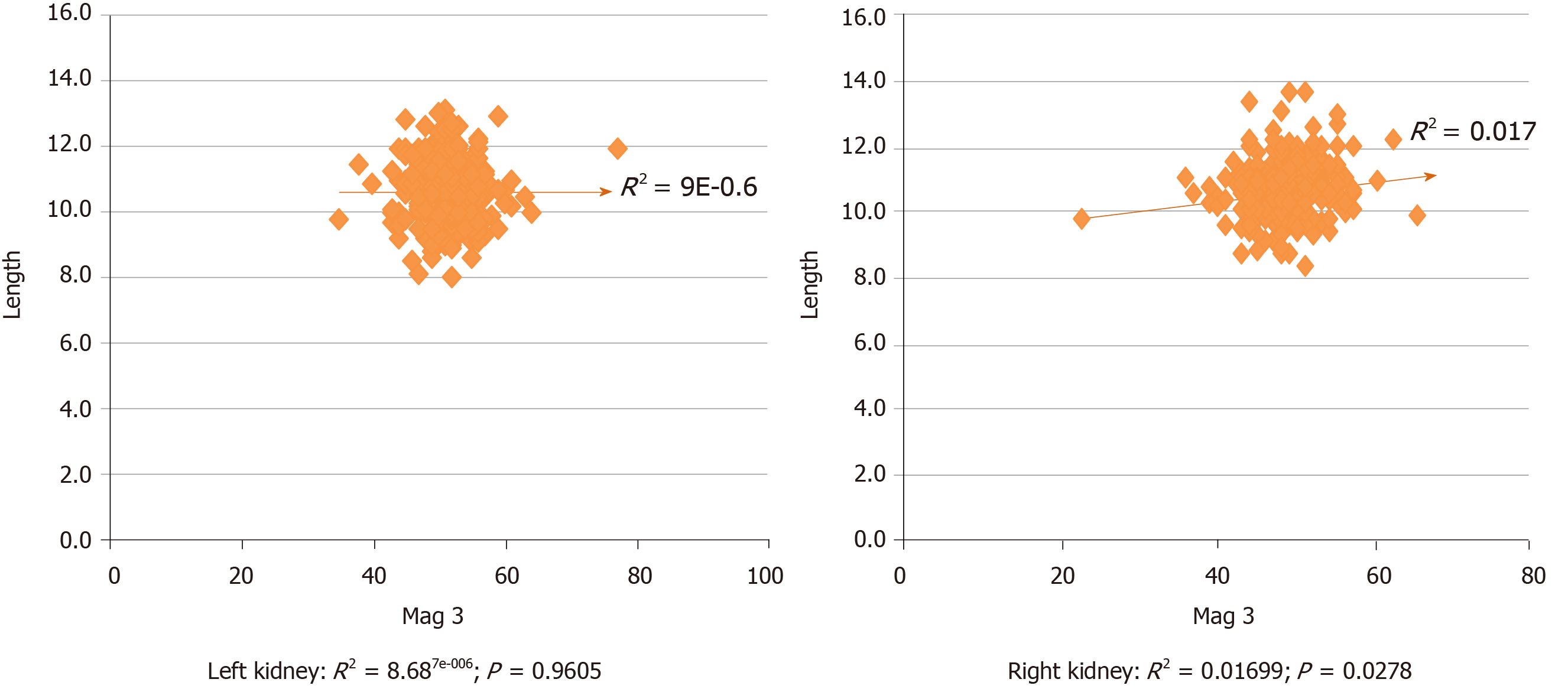
The length of kidneys was determined by CT scan in 237 (83.2%) potential donors and by US alone in 48 (16.8%). Correlation between the cranio-caudal length of kidneys and their contribution to overall function is shown in Figure 1. Whereas there was no significant association with the left kidneys (Pearson r = −0.0029 (95%CI: −0.1191 to 0.1133); R2 = 8.687e-006; P = 0.9605), there was a statistically significant correlation with the right kidneys [Pearson r = 0.1303 (95%CI: 0.01438 to 0.2429); R2 = 0.01699; P = 0.0278].
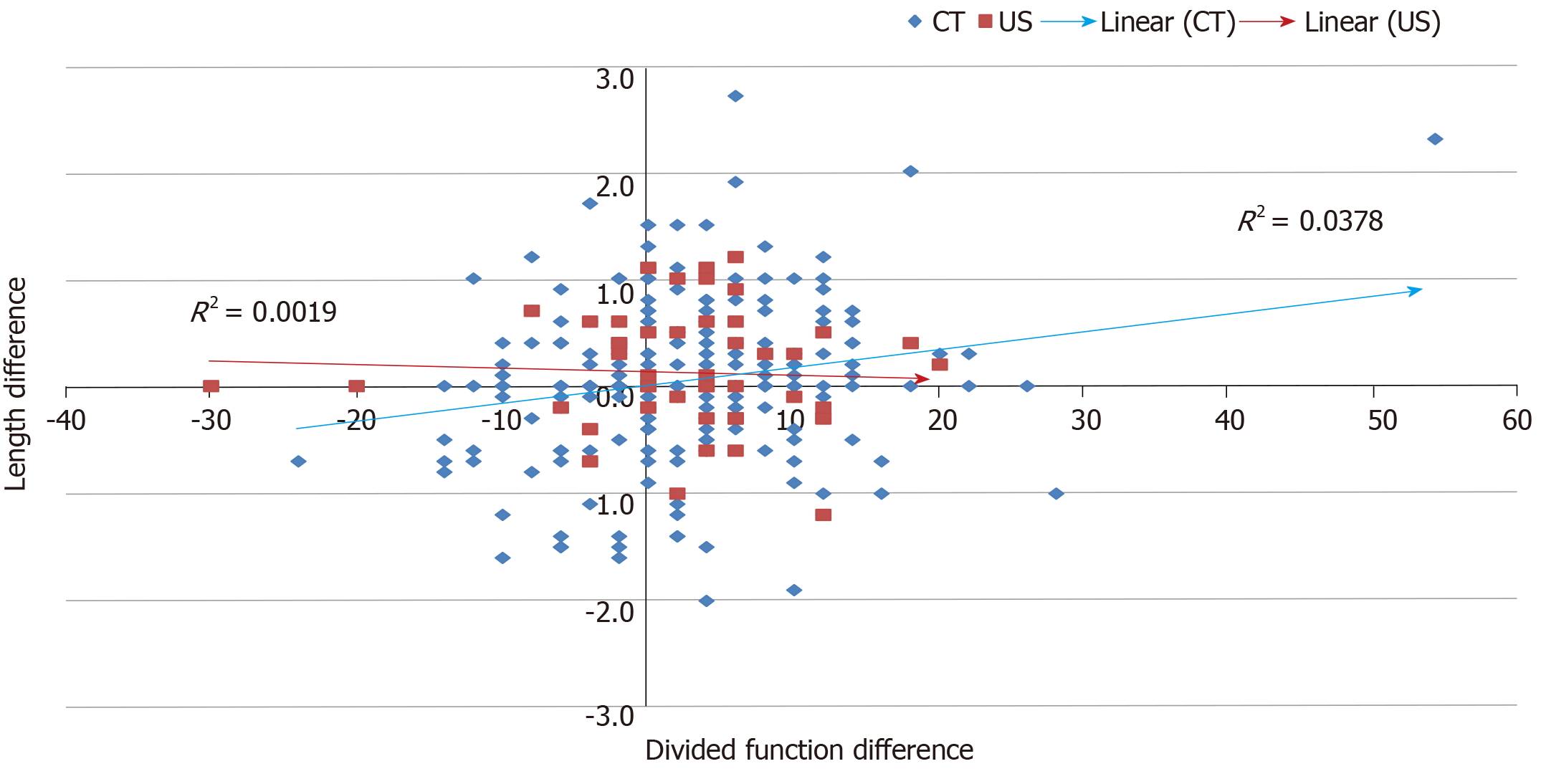
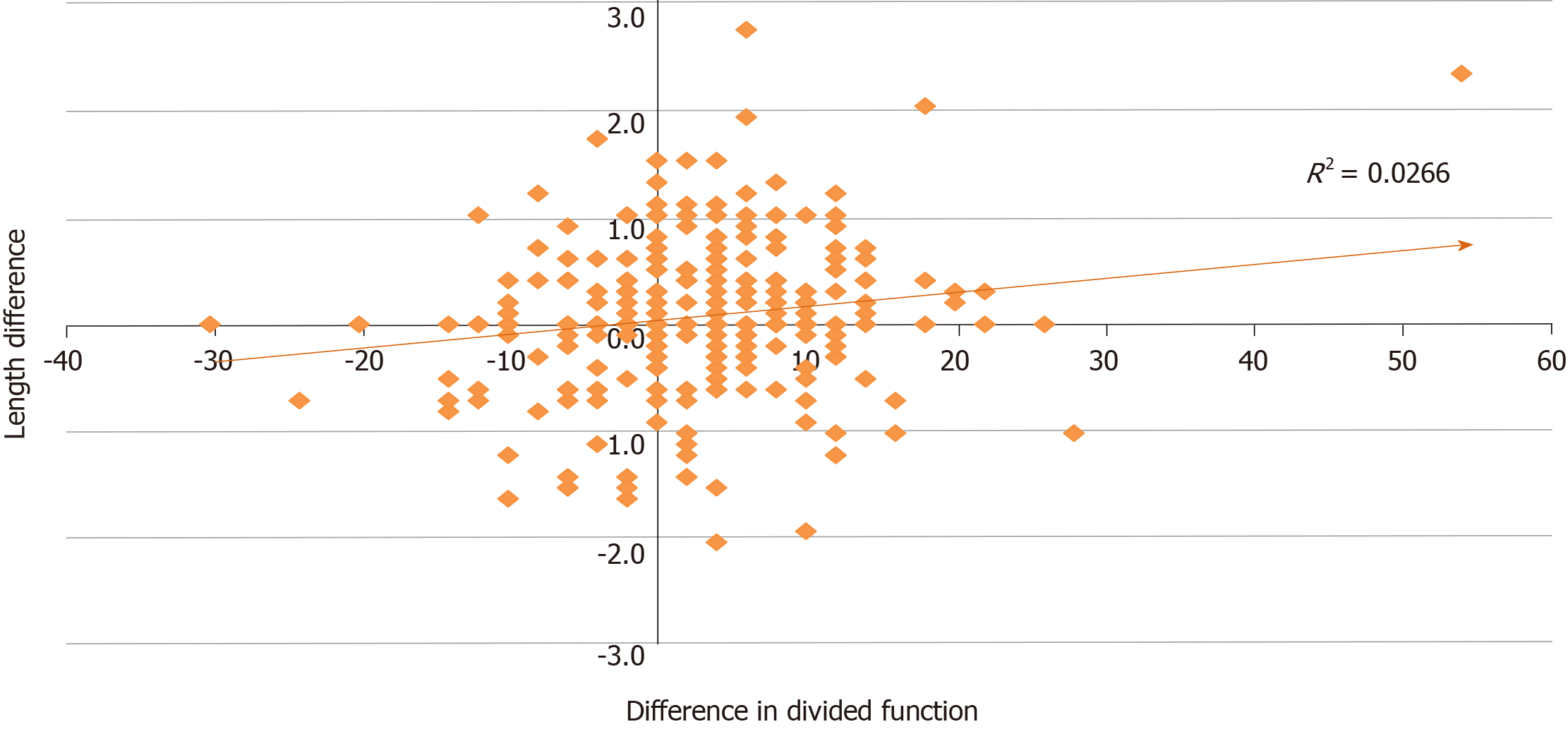
Correlation between difference in length according to imaging modality and divided function of kidney pairs is shown in Figure 2. Though weak, CT-measured kidney length provided a stronger correlation with divided function than US-measured length in this series (R2 = 0.0378 vs 0.0019 respectively). Overall, the correlation between differences in length and differential function of kidney pairs was 0.1630 (Pearson’s) (95%CI from 0.0477 to 0.2740; R2 = 0.0266; P = 0.0058; Figure 3).
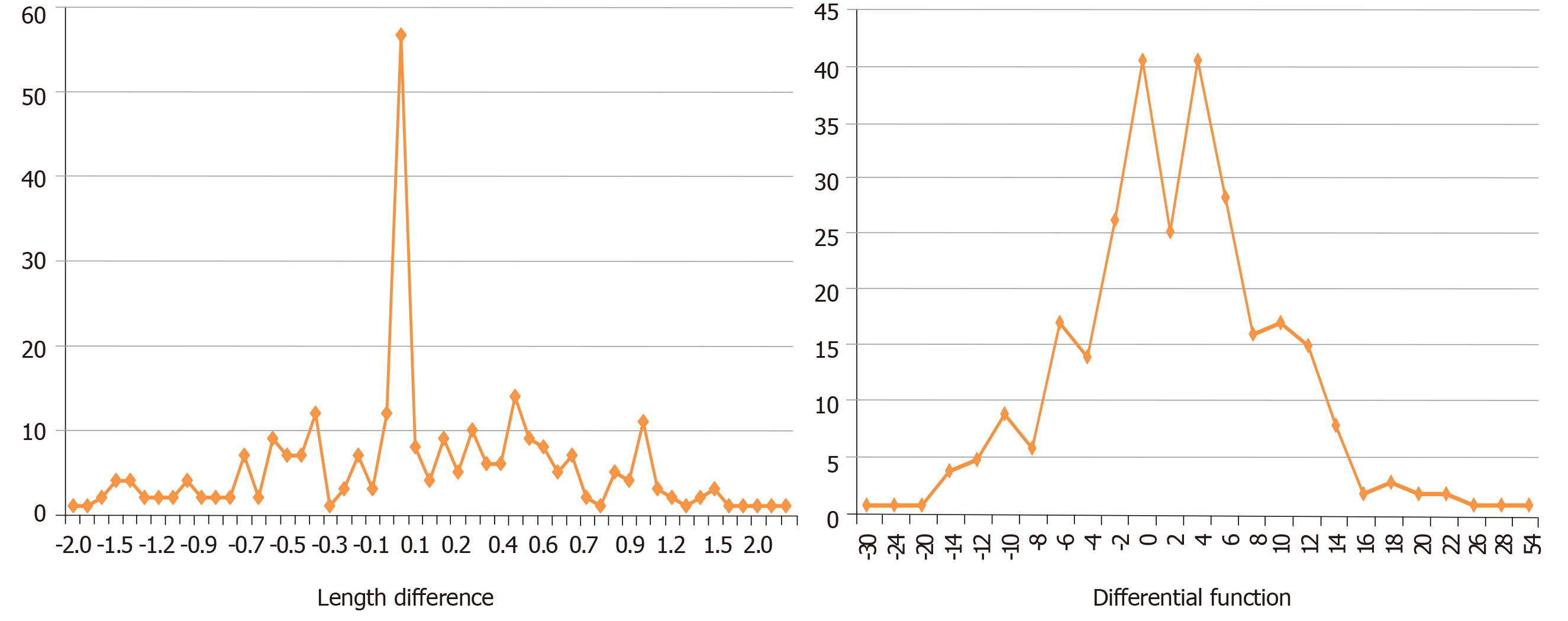
The frequency distribution of the differences in length and divided function between 285 kidney pairs is shown in Figure 4. Fifty seven (20%) donors had no difference in the length of their kidneys compared to 40 who had no difference in the divided function of their kidney pairs – x2 = 3.5904, P = 0.058. However, when the proportion of donors with a difference in length above 1 cm (34/285) was compared with those with a differential function of 10% or higher (73/285), this was found to be highly statistically significant – x2 = 17.5001, P = 0.00003. Of 34 potential donors with a difference in length of at least 1 cm, seven had differential function of 10%-20% with one over 20% (Table 2). Conversely, of seven patients with > 20% difference in function, only one had > 2 cm difference in length (2.3 cm; Table 3). In the remaining six the difference in length ranged from −1 to 0.3. Three of these have donated, three were declined, one donor has moved from the area.
| Differential functioncategory | Category of length difference | Total | |||
| 1 | 2 | 3 | 4 | ||
| 1 | 8 | 29 | 3 | 40 | |
| 2 | 32 | 117 | 22 | 1 | 172 |
| 3 | 15 | 44 | 7 | 66 | |
| 4 | 3 | 3 | 1 | 7 | |
| Total | 58 | 193 | 32 | 2 | 285 |
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Donor GFR | 63 | 69 | 69 | 69 | 87 | 93 | 109 |
| Length difference | 0.0 | 0.0 | 0.0 | 2.3 | 0.3 | −0.7 | −1.0 |
| MAG3 left (%) | 35 | 61 | 63 | 77 | 61 | 38 | 64 |
| MAG3 right (%) | 65 | 39 | 37 | 23 | 39 | 62 | 36 |
| ND | −30 | 22 | 26 | 54 | 22 | −24 | 28 |
| “Donated” GFR of kidney with less function (mL/min) | 22.1 | 26.9 | 25.5 | 15.9 | 33.9 | 35.3 | 39.2 |
| Donated | No | Yes | No | No | Yes | No1 | Yes |
Of 73 with a significant difference in divided function (categories 3 and 4, Table 2), 18 (24.7%) had no difference in kidney length; 54 (74%) had a difference of < 2 cm and only one of > 2 cm. Only these 73 (25.6%) potential donors would present any dilemma regarding which kidney to select for donation, if at all. Of these, using difference in length alone would lead to a false reassurance in 65 as the difference in length would be < 1.0 cm - only eight would raise the need for measurement of differential function. Similarly, using a difference in length of 2 cm as cut off for performing split function would lead to false reassurance in 72 patients (6 had > 20% difference in divided function whereas 66 had 10%-20% difference).
The data on number of vessels was missing in two patients. Of the remaining 283, there were single vessels in 207 left kidneys and 200 right kidneys; and there were two or more vessels in 76 left kidneys and 83 right kidneys. The distribution of multiple vessels between left and right kidneys was not statistically different x2 = 0.4286; P = 0.5126.
The key findings in this study include the following: Equal sex distribution among potential donors whose mean ages and GFRs were comparable; weak correlation between difference in length and divided function of kidney pairs (CT-measured kidney length provided a stronger correlation than US-measured length); the proportion of donors with a difference in length above 1 cm (34/285) was statistically significantly different from those with a differential function of 10% or higher (73/285); and of 73 with a split function greater than 10, 18 (24.7%) had no difference in kidney length; 54 (74%) had a difference of < 2 cm and only one of > 2 cm. Furthermore, using a difference in length of 2 cm as cut off for performing split function would lead to false reassurance in 72 patients (25%).
This study is unique in presenting the results of a retrospective “what if” analysis of prospectively reported kidney length measurements that were not used in the decision-making process as to which organ to donate. The decisions were made on the basis of divided function and vascular anatomy. Analysis of a large number of potential donors this way provides a useful tool in validating the use of kidney size alone in making decisions about which kidney to donate. Our study measured kidney length by CT and US in line with many authors. In a study of 100 living kidney donors, Ninan and co-workers[5] demonstrated that ultrasonographically measured bipolar kidney length was more accurate than measurements using plain X-ray, intravenous urography, and renal angiogram. However, they also found that US tends to underestimate while radiological methods overestimated the size of the kidney. Widjaja et al[6] showed there was significant correlation between ultrasound measured length and CT volume (r = 0.74, P < 0.01). Our decision to prefer CT-measured kidney length is supported by Kang and co-workers[7] who showed that abdominal coronal CT section assessed kidney length more accurately than other radiological methods.
Our study shows low but significant correlation between differences in length between kidney pairs and divided function (Figure 3). The distribution of the differences in length and divided function was not similar (Figure 4). Kidney size can be estimated by measuring renal length, renal volume, cortical volume, or renal weight. Kidney length provides a good indication of kidney size[8] and close correlation with GFR[9,10]. However, Sanusi et al[11] showed a positive correlation between US‐-determined kidney volume and GFR and suggested that kidney volume was a better indicator of kidney size in health or renal disease. Though the donor kidney size (length, weight or volume) is now largely accepted as a predictive factor for recipient allograft function[9,10,12] and as an important predictor of long term donor kidney function[13], volumetric measurement of the donor kidneys provide better correlation with donor kidney function and possibly with outcomes[6,9,12,14]. It is not clear why right kidneys had better (significant) correlation between length and divided function (Figure 1). We speculate that this may have to do with the shapes of the kidneys and that such differences would be eliminated on volume-based analysis.
This study highlights the significant potential for making wrong/harmful decision (removing the significantly better functioning kidney) if kidney length alone is used for decision making. A wrong decision would be made possible in 65/285 (23%) if the trigger for measuring split function were a difference in length of 1 cm; and in 72/285 (25%) if 2 cm were used in the presence of significantly different divided function. If length difference alone was used all could have been allowed to donate except one with a difference of 2.3 cm and a differential function of 54. A length difference greater than 1 cm would only predict a significant difference in divided function in 8/34 (23.5%) of cases. By measuring the split function, it would be possible to consider donation even in potential donors with a greater than 20% differential function. As shown in Table 3, if the concept of donated GFR was considered when using kidneys with the smaller contribution to overall function in potential donors with significant difference in divided function, three or possibly four of these donors would qualify.
Renal volume is thought to be the most precise predictor of kidney size[15,16]. There is sufficient evidence for correlation from CT based volume measurements to split renal function, that CT volumetric measurement of kidney size could replace the need for split function assessment[17,18]. Halleck et al[17] compared CT-measured renal cortex volume with DTPA-clearance combined with MAG3-scintigraphy in 167 consecutive living kidney donors and showed a strong correlation between CT-measured split cortex volume and MAG3-measured split renal function (r = 0.93; P < 0.001). Gardan and co-workers[19] determined pre-donation kidney volume for 105 donors using three methods: Total parenchymal three-dimensional renal volume, total parenchymal renal volume contouring, and renal cortical volume and tested for correlation of each volume with measured GFR. They found that for all methods, total kidney volume was significantly associated with pre-donation GFR (P < 0.001) and concluded that cortical volumetry was the best volumetric technique to use as a surrogate to scintigraphy for estimating pre-donation split renal function. Other workers showed that renal volume calculation using the ellipsoid method (length x antero-posterior diameter x lateral diameter x π/6) compared favourably with volume determined using volumetric software[20]. CT volume can replace nuclear renography for evaluation of relative function, as volume has been shown as a surrogate marker for nephron mass[12,21-24].
This study has important limitations. The retrospective analytical nature of this study resulted in a large number of exclusions due to insufficient, unavailable or non-specific data; and acceptance of kidney length measurements determined by CT or US. It is not clear whether the CT length measurements were all performed in the coronal plane. During the study period, measurement of kidney length was not regarded as a critical component of CT renal angiography and the quality and detail of the reporting varied between radiologists. There is also the possibility of inter-observer variability in reporting kidney lengths. Only measurement for kidney length was available at our centre – other dimensions of the kidney were not reported and it was therefore not possible to calculate kidney volumes using the ellipsoid formula. Furthermore, data on the body habitus of potential donors was scanty and therefore not included in the analysis. Despite the foregoing, the findings of this study provide justification for avoiding the use of kidney length alone in making decisions about which kidney to donate or who needs split function.
In conclusion, length difference between kidney pairs alone is not sufficient to replace measurement of divided function. It may well be that after excluding cases with anatomical abnormalities, volume differences may restrict but not totally eliminate isotopic measurement of divided function in prospective donors. This issue is of vital importance and requires a randomised controlled trial to resolve it.
Potential candidates for kidney donation must have sufficient renal function post donation in order to minimize future risks when living with a single kidney. Currently most transplant units use split function between the kidney pairs in addition to other factors to make a decision on which kidney to donate. However, isotope differential renal function is not uniformly performed in all transplant centres, with many relying on kidney size measurements. Such an approach is supported by the BTS/RA Living Donor Kidney Transplantation Guidelines (2018) which state that differential kidney function, determined by 99mTechnitium dimercaptosuccinic acid (99mTcDMSA) is recommended where there is > 10% variation in kidney size or significant renal anatomical abnormality. It further states that, “A difference in size of 2 cm or more indicates the possibility of a significant difference in GFR between the two kidneys”. Hence this study.
The key question in living kidney donor assessment is how best to determine the contribution of each kidney to overall renal function and guide selection of which kidney to donate, ensuring safety of procedure and good outcome for both recipient and donor. With many units, particularly in the United Kingdom adopting the use of kidney length in the decision making process, there is risk of making wrong or harmful decisions with respect to living kidney donors unless it can be demonstrated that there is strong correlation between kidney length and split function.
This study aimed to determine the effect of using kidney length to decide which kidney to donate in a retrospective cohort of potential donors. Realisation of this objective would confirm the new approach as safe and reliable otherwise alternative approaches would need to be adopted such as use of kidney volume measurements and where indicated isotope differential renal function.
All potential living kidney donors who completed assessment over a ten years period were retrospectively evaluated. Donor assessment was performed as per UK guidelines. This study is unique in presenting the results of a retrospective “what if” analysis of prospectively reported kidney length measurements that were not used in the decision-making process as to which organ to donate. During the study period, decisions were made on the basis of divided function and vascular anatomy. Analysis of a large number of potential donors in this way provides a useful tool in validating the use of kidney size alone in making decisions about which kidney to donate.
The key findings in this study include the following: Equal sex distribution among potential donors whose mean ages and GFRs were comparable; weak correlation between difference in length and divided function of kidney pairs (CT-measured kidney length provided a stronger correlation than US-measured length); the proportion of donors with a difference in length above 1 cm (34/285) was statistically significantly different from those with a differential function of 10% or higher (73/285); and of 73 with a split function greater than 10, 18 (24.7%) had no difference in kidney length; 54 (74%) had a difference of < 2 cm and only one of > 2 cm. Furthermore, using a difference in length of 2 cm as cut off for performing split function would lead to false reassurance in 72 patients (25%).
This study highlights the significant potential for making wrong/harmful decision (removing the significantly better functioning kidney) if kidney length alone is used for decision making. A wrong decision would be made possible in 65/285 (23%) if the trigger for measuring split function were a difference in length of 1 cm; and in 72/285 (25%) if 2 cm were used in the presence of significantly different divided function. Length difference between kidney pairs alone is not sufficient to replace measurement of divided function. The findings of this study have important practical implications for clinical practice in avoiding potential harm to living kidney donors. This issue requires a randomised controlled trial to resolve it.
This study has shown unequivocally that kidney length alone is not sufficient to determine which kidney to donate. It raises the question about the role of kidney volume measurement. The literature suggests that renal volume is the most precise predictor of kidney size. It has been shown by other workers that renal volume calculation using the ellipsoid method (length x antero-posterior diameter x lateral diameter x π/6) compares favourably with volume determined using volumetric software. CT based volume measurements of kidneys, (particularly cortical volumetry) correlates well with split renal function, raising the possibility that CT volumetric measurement of kidney size could replace the need for split function assessment. This issue is of vital importance and requires a randomised controlled trial to resolve whether CT-measured split cortex volume, for example is equivalent to MAG3-measured split renal function.
We acknowledge the meticulous and accurate records of potential donors maintained by Mrs Sara L Stacey, Living Donor Co-ordinator, South West Transplant Centre. We are grateful to Leanne Savage for her help in obtaining data particularly for donors out of our area.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Parajuli S, Sureshkumar K S-Editor: Wang J L-Editor: A E-Editor: Wu YXJ
| 1. | Mjøen G, Hallan S, Hartmann A, Foss A, Midtvedt K, Øyen O, Reisæter A, Pfeffer P, Jenssen T, Leivestad T, Line PD, Øvrehus M, Dale DO, Pihlstrøm H, Holme I, Dekker FW, Holdaas H. Long-term risks for kidney donors. Kidney Int. 2014;86:162-167. [PubMed] [DOI] [Full Text] |
| 2. | British Transplantation Society/Renal Association. Guidelines for Living Donor Kidney Transplantation 2018. 4th ed. Available from: https://bts.org.uk/wp-content/uploads/2018/07/FINAL_LDKT-guidelines_June-2018.pdf/. |
| 3. | Nunes-Carneiro D, Marques-Pinto A, Veiga C, Braga I, Cabral JF, Almeida M, Cavadas V, Castro-Henriques A, Almeida R, Fraga A, Silva-Ramos M. Which One Is the Best for Living Donation: A Multiple-Artery Left Kidney Nephrectomy or a Right Kidney Nephrectomy? Transplant Proc. 2019;51:1559-1562. [PubMed] [DOI] [Full Text] |
| 4. | Akoh JA, Rana TA, Stacey SL. Isotope Differential Renal Function Versus Ultrasound Measured Kidney Size in Assessing Potential Living Donors. Dialysis Transplant. 2010;39:23-26. [DOI] [Full Text] |
| 5. | Ninan VT, Koshi KT, Niyamthullah MM, Jacob CK, Gopalakrishnan G, Pandey AP, Shastry JC. A comparative study of methods of estimating renal size in normal adults. Nephrol Dial Transplant. 1990;5:851-854. [PubMed] [DOI] [Full Text] |
| 6. | Widjaja E, Oxtoby JW, Hale TL, Jones PW, Harden PN, McCall IW. Ultrasound measured renal length versus low dose CT volume in predicting single kidney glomerular filtration rate. Br J Radiol. 2004;77:759-764. [PubMed] [DOI] [Full Text] |
| 7. | Kang KY, Lee YJ, Park SC, Yang CW, Kim YS, Moon IS, Koh YB, Bang BK, Choi BS. A comparative study of methods of estimating kidney length in kidney transplantation donors. Nephrol Dial Transplant. 2007;22:2322-2327. [PubMed] [DOI] [Full Text] |
| 8. | Huntington DK, Hill SC, Hill MC. Sonographic manifestations of medical renal disease. Semin Ultrasound CT MR. 1991;12:290-307. [PubMed] |
| 9. | Yano M, Lin MF, Hoffman KA, Vijayan A, Pilgram TK, Narra VR. Renal measurements on CT angiograms: correlation with graft function at living donor renal transplantation. Radiology. 2012;265:151-157. [PubMed] [DOI] [Full Text] |
| 10. | Paleologo G, Abdelkawy H, Barsotti M, Basha A, Bernabini G, Bianchi A, Caprio F, Emad A, Grassi G, Nerucci B, Tregnaghi C, Rizzo G, Donadio C. Kidney dimensions at sonography are correlated with glomerular filtration rate in renal transplant recipients and in kidney donors. Transplant Proc. 2007;39:1779-1781. [PubMed] [DOI] [Full Text] |
| 11. | Sanusi AA, Arogundade FA, Famurewa OC, Akintomide AO, Soyinka FO, Ojo OE, Akinsola A. Relationship of ultrasonographically determined kidney volume with measured GFR, calculated creatinine clearance and other parameters in chronic kidney disease (CKD). Nephrol Dial Transplant. 2009;1690-1694. [PubMed] [DOI] [Full Text] |
| 12. | Hugen CM, Polcari AJ, Farooq AV, Fitzgerald MP, Holt DR, Milner JE. Size does matter: donor renal volume predicts recipient function following live donor renal transplantation. J Urol. 2011;185:605-609. [PubMed] [DOI] [Full Text] |
| 13. | Herts BR, Sharma N, Lieber M, Freire M, Goldfarb DA, Poggio ED. Estimating glomerular filtration rate in kidney donors: a model constructed with renal volume measurements from donor CT scans. Radiology. 2009;252:109-116. [PubMed] [DOI] [Full Text] |
| 14. | Song T, Fu L, Huang Z, He S, Zhao R, Lin T, Wei Q. Change in renal parenchymal volume in living kidney transplant donors. Int Urol Nephrol. 2014;46:743-747. [PubMed] [DOI] [Full Text] |
| 15. | Moorthy HK, Venugopal P. Measurement of renal dimensions in vivo: A critical appraisal. Indian J Urol. 2011;27:169-175. [PubMed] [DOI] [Full Text] |
| 16. | Lee JH, Won JH, Oh CK. Impact of the ratio of graft kidney volume to recipient body surface area on graft function after live donor kidney transplantation. Clin Transplant. 2011;25:E647-E655. [PubMed] [DOI] [Full Text] |
| 17. | Halleck F, Diederichs G, Koehlitz T, Slowinski T, Engelken F, Liefeldt L, Friedersdorff F, Fuller TF, Magheli A, Neumayer HH, Budde K, Waiser J. Volume matters: CT-based renal cortex volume measurement in the evaluation of living kidney donors. Transpl Int. 2013;26:1208-1216. [PubMed] [DOI] [Full Text] |
| 18. | Diez A, Powelson J, Sundaram CP, Taber TE, Mujtaba MA, Yaqub MS, Mishler DP, Goggins WC, Sharfuddin AA. Correlation between CT-based measured renal volumes and nuclear-renography-based split renal function in living kidney donors. Clinical diagnostic utility and practice patterns. Clin Transplant. 2014;28:675-682. [PubMed] [DOI] [Full Text] |
| 19. | Gardan E, Jacquemont L, Perret C, Heudes PM, Gourraud PA, Hourmant M, Frampas E, Limou S. Renal cortical volume: High correlation with pre- and post-operative renal function in living kidney donors. Eur J Radiol. 2018;99:118-123. [PubMed] [DOI] [Full Text] |
| 20. | Breau RH, Clark E, Bruner B, Cervini P, Atwell T, Knoll G, Leibovich BC. A simple method to estimate renal volume from computed tomography. Can Urol Assoc J. 2013;7:189-192. [PubMed] [DOI] [Full Text] |
| 21. | Habbous S, Woo J, Lam NN, Lentine KL, Cooper M, Reich M, Garg AX. The Efficiency of Evaluating Candidates for Living Kidney Donation: A Scoping Review. Transplant Direct. 2018;4:e394. [PubMed] [DOI] [Full Text] |
| 22. | Taherimahmoudi M, Mehrsai A, Nikoobakht M, Saraji A, Emamzadeh A, Pourmand G. Does donor nephron mass have any impact on graft survival? Transplant Proc. 2007;39:914-916. [PubMed] [DOI] [Full Text] |
| 23. | Poggio ED, Hila S, Stephany B, Fatica R, Krishnamurthi V, del Bosque C, Goldfarb D, Herts B, Dennis VW, Heeger PS, Braun W. Donor kidney volume and outcomes following live donor kidney transplantation. Am J Transplant. 2006;6:616-624. [PubMed] [DOI] [Full Text] |
| 24. | Saxena AB, Busque S, Arjane P, Myers BD, Tan JC. Preoperative renal volumes as a predictor of graft function in living donor transplantation. Am J Kidney Dis. 2004;44:877-885. [PubMed] [DOI] [Full Text] |