Published online May 29, 2020. doi: 10.5500/wjt.v10.i5.90
Peer-review started: January 10, 2020
First decision: February 25, 2020
Revised: March 26, 2020
Accepted: April 23, 2020
Article in press: April 23, 2020
Published online: May 29, 2020
Processing time: 139 Days and 11.9 Hours
In recent years, pharmacogenetics has emerged as an important tool for choosing the right immunosuppressant drug and its appropriate dose. Indeed, pharmacogenetics may exert its action on immunosuppressant drugs at three levels. Pharmacogenetics identifies and studies the genes involved in encoding the proteins involved in drug pharmacokinetics and in encoding the enzymes involved in drug degradation. Pharmacogenetics is also relevant in encoding the enzymes and proteins involved in codifying the transmembrane proteins involved in transmembrane passage favoring the absorption and intracellular action of several immunosuppressants. Pharmacogenetics concern the variability of genes encoding the proteins involved as immunosuppressant triggers in the pharmacodynamic pathways. Of course, not all genes have been discovered and studied, but some of them have been clearly examined and their relevance together with other factors such as age and race has been defined. Other genes on the basis of relevant studies have been proposed as good candidates for future studies. Unfortunately, to date, clear conclusions may be drawn only for those drugs that are metabolized by CYP3A5 and its genotyping before kidney, heart and lung transplantation is recommended. The conclusions of the studies on the recommended candidate genes, together with the development of omics techniques could in the future allow us to choose the right dose of the right immunosuppressant for the right patient.
Core tip: The most common factors associated with drug response include age, sex, ancestry, concomitant drugs and liver or kidney diseases and drug pharmacogenetics. In general pharmacogenetics is the study of the variability of the response of a drug related to the complex gene arrays. More recently, the term pharmacogenomics has been introduced. This term in particular is related to omics studies. In recent years, pharmacogenetics that evaluates the drug response to genetic variations has emerged as an important tool for choosing the right therapeutic dose. According to the aim of the Precision Medicare Initiative, pharmacogenomics may contribute to providing the right drug at the right dose for the right patient.
- Citation: Salvadori M, Tsalouchos A. Pharmacogenetics of immunosuppressant drugs: A new aspect for individualized therapy. World J Transplant 2020; 10(5): 90-103
- URL: https://www.wjgnet.com/2220-3230/full/v10/i5/90.htm
- DOI: https://dx.doi.org/10.5500/wjt.v10.i5.90
Immunosuppressant drugs are known to have a very narrow therapeutic range that characterizes the fact that immunosuppression easily ranges between efficacy and toxicity[1]. As a consequence, therapeutic drug monitoring is recommended during the use of immunosuppressants principally after solid organ transplantation.
The most common factors associated with drug response include age, sex, ancestry, concomitant drugs and liver or kidney diseases and drug pharmacogenetics.
For a better understanding of the content of this review, the terminology used should be better defined.
In general pharmacogenetics is the study of the variability of the response of a drug related to the complex gene arrays. More recently, the term pharmacogenomics has been introduced. This term in particular is related to omics studies. Both terms are often used to highlight the same issue, which is the influence of the genomic pattern on the fate of a drug.
The term pharmacokinetics (PK) is related to the studies that refer to how an organism affects a drug (i.e., by different enzymes), while the term pharmacodynamics (PD) is related to the studies that consider how a drug affects an organism influencing targets and metabolic pathways. PK and PD are deeply influenced by the gene variability of an organism.
In recent years, pharmacogenetics that evaluates the drug response to genetic variations has emerged as an important tool for choosing the right therapeutic dose. According to the aim of the Precision Medicare Initiative, pharmacogenomics may contribute to providing the right drug at the right dose for the right patient[2].
Pharmacogenomic studies principally follow two different approaches.
First, the selection of a candidate gene is developed on the basis of the knowledge of the PK and PD pathways[3].
Second, pharmacogenomic studies are based on genome-wide associations, according to which, several single-nucleotide polymorphisms (SNPs) associated with different phenotypes are tested on a large number of patients[4].
In particular, in genome-wide association studies, the association between hundreds of thousands to millions of SNPs and the complex phenotypes is tested in hundreds to thousands of persons. In this way, genome-wide association studies have proved successful in identifying genetic associations with complex traits. This approach was able to identify associations between SNPs and several phenotypes. When a genetic association is found, the next step is to validate the association in an independent study cohort.
Pharmacogenetics may exert an action on immunosuppressant drugs at three levels.
First, pharmacogenetics identifies and studies the genes involved in encoding the proteins involved in drug PK and in encoding the enzymes involved in drug degradation.
Second, pharmacogenetics is relevant in encoding the enzymes and proteins involved in codifying the transmembrane proteins involved in transmembrane passage favoring the absorption and intracellular action of several immuno- suppressants[5].
Finally, a third field of pharmacogenetics concerns the variability of genes encoding the proteins involved as immunosuppressant triggers in the PD pathways[6].
A systematic search of the literature was performed to identify relevant published studies on the pharmacogenetics of the therapeutic drugs in the field of organ transplantation. All the studies were retrieved from PubMed. The key words used to retrieve the articles were pharmacokinetics, pharmacodynamics, pharmacogenetics, pharmacogenomics, transplantation and immunosuppressant drugs. The eligibility criteria related to the international relevance of the journal were studies published in the past 10 years. Where possible controlled studies and systematic reviews were selected. The references in these studies were also searched for other relevant articles to be included in this review. The main limitations were that few controlled studies and few randomized clinical trials were identified.
The research was limited to drugs currently used in transplantation. As a consequence drugs rarely or no longer used as well as drugs not-longer on the market were excluded.
The aim of this review is to summarize what is actually known about the pharmacogenetics of immunosuppressant drugs which will serve as a clinical tool for physicians involved in the care of transplanted patients.
Both the calcineurin inhibitors (CNIs), cyclosporine (CsA) and tacrolimus (TAC), are metabolized by gastrointestinal and hepatic cytochrome P450 (CYP) 3A isoenzymes, principally CYP3A4 and CYP3A5, while other enzymes of the same family have a reduced influence, such as CYP3A7 and CYP3A43. The main enzyme involved in TAC pharmacokinetics (PK) is CYP3A5, with a lower relevance for CYP3A4[7]. In contrast, CYP3A4 principally metabolizes CsA[8]. Genetic variants concerning genes coding such enzymes have an influence on drug levels and their activity. The main variant is a SNP concerning the intron 3 g CYP3A5 (6986A>G; rs776746 SNP), better known as CYPA5*3. The variant codes an enzyme with reduced activity. As a consequence patients homozygous for this variant require a dose lower of TAC than approximately 50%[9-11].
Genotyping performed at the time of transplantation for the CYPA5*3 allele is recommended both by the Clinical Pharmacogenetics Implementation Consortium (CPIC)[12] and the study by Haufroid et al[13]. The CYP3A4 polymorphism may influence the PK of TAC. The SNPCYP3A*22 (rs35599367; c.522-191C>T) variant in intron 6 has a lower mRNA expression of CYP3A4 with a lower activity[14,15].
CsA is extensively metabolized by CYP3A4. Only one frequent variant in the CYP3A4 locus, namely, *22 (rs35599367), is associated with reduced enzyme activity[16]. Interestingly, CYP3A4*22 does not significantly affect kidney graft survival but is associated with a reduced risk of cancer[17].
Several recent studies have shown an association between nephrotoxicity and the graft genotype variant c.3435TT[18].
Two recent meta-analyses[19,20] documented a higher risk of biopsy-proven acute rejection (BPAR) in patient carriers of the CYP3A5* allele treated with TAC, while this risk was not observed in patients treated with CsA[21].
The fact that patient carriers of the CYP3A5* allele treated with TAC could have a higher risk of BPAR could look paradoxical. Possible explanations for this are that this derives from meta-analyses and not from controlled studies. In such cases, the TAC dose reduction could be higher than due principally if such reductions are not controlled by TAC blood dosing. In addition, these studies do not take in account other factors influencing the PK of TAC such as age, ethnicity and time from transplantation. Overall, the message from these observations is that it is possibly safer to treat patients carrying these alleles with CsA instead of TAC.
Another study suggested that patients with the CYP3A4*22 allele and treated with CsA were at higher risk for delayed graft function and worse renal function than noncarriers[22].
In addition to CYP3A polymorphisms, P450 oxidoreductase (POR) polymorphisms may modify the PK of CNIs. Individuals carrying at least one POR*28 allele need a higher TAC dose than those not carrying the POR*28 allele. POR homozygosity is associated with higher CYP3A4 activity, but the POR*28 allele does not seem to be associated with BPAR after TAC or CsA therapy[23].
Two different SNPs in peroxisome proliferator-activated receptor alpha influence CYP3A4 activity[24]. However, the influence of both SNPs seems to be limited to TAC metabolism.
ABCB1 (also called MDR1) is the gene that encodes the P-glycoprotein, which is a transmembrane pump that facilitates drug passage into the cells. In so doing, it facilitates the absorption and metabolism of a drug. The most common and studied SNPs of ABCB1 are rs1128503 (1236C>T, Gly412Gly), rs2032582 (2677G>T/A, Ala893Ser/Thr), and rs1045642 (3435C>T, Ile1145Ile)[25].
Another interesting polymorphism is that concerning the ABCB11199G>A encoding a SNP located in exon 11 (rs2229109). The role of the SNPs of ABCB1 on the PK of TAC is not clear[16,26,27]. In the case of CsA, lymphomonocytes in peripheral blood demonstrated that patient carriers of the allele variant TT and the SNPs C2435T, G2677T and C1236T have lower ABCB1 activity than noncarriers and this fact could determine a higher intracellular level with a higher risk of drug-related toxicity[28].
Several studies have documented the relationship between ABCB1 SNPs and long-term graft survival. In particular, the allele variant TT in the 3435th position of the ABCB1 gene is associated with reduced graft function[29,30]. Additionally, the ABCB1 1199G>A SNP is associated with better renal function[31].
Recent studies have explored the impact of genetic variants in proteins of the PD pathways targeted by CNIs.
The question of whether genetic variants in the calcineurin pathway have any impact on clinical outcomes has been examined in a study conducted on 381 renal transplant patients[32]. The authors genotyped 13 genes encoding proteins expressed on T cells, in particular, cyclophilin A, FKBP12, calmodulin 1-3 isoforms, calcineurin A (α and β subunits, PPP3CA, PPP3CB) and β/α subunit (PP3R1), nuclear factor of activated T cells (NFAT 1,2,3), interleukin-2 (IL-2) and the IL-2α-chain receptor (IL-2RA). The multivariate analyses did not identify any associations between genetic variants and clinical outcomes. In a further study[6], the same authors performed a review of all the available studies on this issue. The authors concluded that some of these studies are interesting, but their clinical utility remains unclear. Nevertheless, from all the studies analyzed, the authors recognized seven “highly recommended” genes that merit further analysis as potential candidates (Table 1).
| Drugs | Gene | SNP | MAF | QOE | LOR |
| PPIA | rs8177826, rs6850 | G = 0.033, G = 0.384 | C, D | 3, 4 | |
| PPP3CA | rs45441997, rs3804358rs | G = 0.268, G = 0.133 | A, A | 1, 1 | |
| PPP3CB | rs376679 | T = 0.066 | A | 1 | |
| PPP3R1 | rs3039851, rs1868402 | NA, G = 0.301 | B, A | 2, 1 | |
| CALM1 | rs12885713 | T = 0.400 | A | 1 | |
| CALM3 | rs150954567, rs3814843, rs3814843 | NA, C = 0.358, C = 0.018 | A, C, C | 1, 3, 3 | |
| IL2 | rs2069762, rs2069763, rs6822844 | G = 0.232, A = 0.400, T = 0.146 | A, B, C | 4, 1, 3 |
Table 1 shows the SNPs recommended to be studied in further analyses or because they are linked to transplant outcomes or to other relevant disease.
In order to facilitate further research, the authors have graded the quality of evidence based on the reviews considered. In addition a level of recommendation has been carried out dividing the SNPs in “highly recommended”, “recommended”, and “potential” candidates for future studies.
rs8177826 and rs6850 should be studied as their variants are related to myocardial infarction due to their atherogenic activity. In addition, rs8177826 variants are associated with nephrotoxicity. rs45441997 is frequent among drug abusers. SNP rs1868402 variants have been found to be linked to a protein characteristic of Alzheimer’s disease. rs12885713 is a SNP linked to calmodulin variants and has been studied in several bone diseases. rs3814843 in the G variant significantly increases the risk of ischemic stroke. The SNP promoters for IL-2, rs2069762, rs2069763 and rs6822844 have been found to be associated with several outcomes including type 1 diabetes mellitus[6].
TAC is one of the most commonly used immunosuppressants as it provides improved graft survival with respect to CsA[33]. Thus, the vast majority of PK studies have analyzed this drug.
Two large studies have been conducted in France (Tactique trial) and the Netherlands[34,35] to optimize the initial TAC dose using pharmacogenetic testing.
The French randomized trial was conducted on 280 kidney transplant recipients. Patients were divided into two groups: The standard dose group that received 0.2 mg/kg. and the genotype-adapted dose group that received different TAC doses according to the CYP3A5 variants.
The aim of the study was to obtain a TAC trough concentration in the target range (10-15 μg/L) after 6 doses.
The study concluded that significantly more patients in the genotype-adapted dose arm were in the target range.
A further study from Pallet et al[36] examined the long-term clinical outcome of the French study. After 5 years no difference was found in graft survival, BPAR, renal function or cardiovascular event rates.
The Netherlands trial was conducted on 240 kidney transplants from living donors. In this study, the two groups also received a standard dose or a genotype-adapted dose.
In the Netherlands study[35], the target dose was reached by both groups, and the incidence of BPAR was also similar.
Both studies had several limitations and several strengths (principally in the French study) as referred by the authors themselves (Table 2).
| Limitations | Strengths |
| TAC initiated on day 7 (French); Single SNP studied (CYP3A5*3); Limited genotypic diversity with few CYP3A5*1/1 carriers (French); Used same dose for CYP3A5*1/1 and *1/*3 carriers (French); Genotype-based dosing did not account for clinical factors; Low risk populations and underpowered for AR; Dosing regimens designed to achieve target of 10-15 ng/mL | Established the safety of genotype directed dosing (both trials); Genotype dosing reduced time to therapeutic (French); Genotype dosing had greater proportion of troughs in range at day 3 and 10 (French); Fewer dose adjustments (French) |
The main limitations in both studies were that only a single SNP was studied, the same dose of TAC was used in both arms and that the studies were conducted in a low risk population.
The most important strength of both studies was that they were able to establish the safety of genotype-directed dosing.
Both studies, although they had some weaknesses (Table 2), should be considered as essential to our knowledge of TAC pharmacogenetics and for clinical practice. Indeed, as a consequence of these studies, the CPIC published the guidelines for TAC as shown in Table 3[12]. The CPIC summarized the evidence from the published literature and, taking into account the French and Netherlands studies, provided dosing recommendations for TAC, according to the CYP3A5 genotype.
| CYP3A5 phenotype | Implications for tacrolimus pharmacologic measures | Therapeutic recommendations | Classification of recommendation |
| Extensive metabolizer (CYP3A5 expresser) | Lower dose-adjusted trough concentrations of TAC and decreased chance of achieving target TAC concentrations | Increase starting dose to 1.5-2 times recommended starting dose. Use therapeutic drug monitoring to guide dose adjustments | Strong |
| Intermediate metabolizer (CYP3A5 expresser) | Lower dose-adjusted trough concentrations of TAC and decreased chance of achieving target TAC concentrations | Increase starting dose to 1.5-2 times recommended starting dose. Use therapeutic drug monitoring to guide dose adjustments | Strong |
| Poor metabolizer (CYP3A5 non expresser) | Higher dose-adjusted trough concentrations of TAC and increased chance of achieving target TAC concentrations | Initiate therapy with standard recommended dose. Use therapeutic drug monitoring to guide dose adjustments | Strong |
The recommendations are strong as the evidence from these studies is of high quality and the desirable effects clearly outweigh the undesirable effects.
Other factors, such as age, have been documented to influence TAC trough levels and, as a consequence, TAC dosing.
In a study, conducted on more than 2000 patients, Jacobson et al[37] demonstrated that older recipients had higher normalized TAC troughs than younger adults receiving similar TAC doses. Additionally, after normalization for dose and weight, CNI troughs were more than 50% higher in older recipients.
A different approach to analyze the variability of troughs and doses is genomic-wide association studies[38]. Using this approach, Oetting et al[39] analyzed TAC trough levels in Afro-American patients according to the clinical variables and variants in CYPA4/5 genes. Accordingly, they found that the variations explained by the model increased when considering the clinical variables and the different gene variability (Table 4).
| Model | Variation of tacrolimus troughs | Variation explained by model |
| Simple time-trend model | 0.3114 | - |
| Clinical variables | 0.2497 | 19.8% |
| Clinical variables + rs776746 | 0.1929 | 39.1% |
| Clinical variables + rs10264272 | 0.2495 | 19.9% |
| Clinical variables + rs41303343 | 0.2310 | 25.8% |
| Clinical variables + rs776746 + rs10264272 | 0.1845 | 40.7% |
| Clinical variables + rs776746 + rs41303343 | 0.1553 | 50.1% |
| Clinical variables + rs776746 + rs10264272 + rs41303343 | 0.1436 | 53.9% |
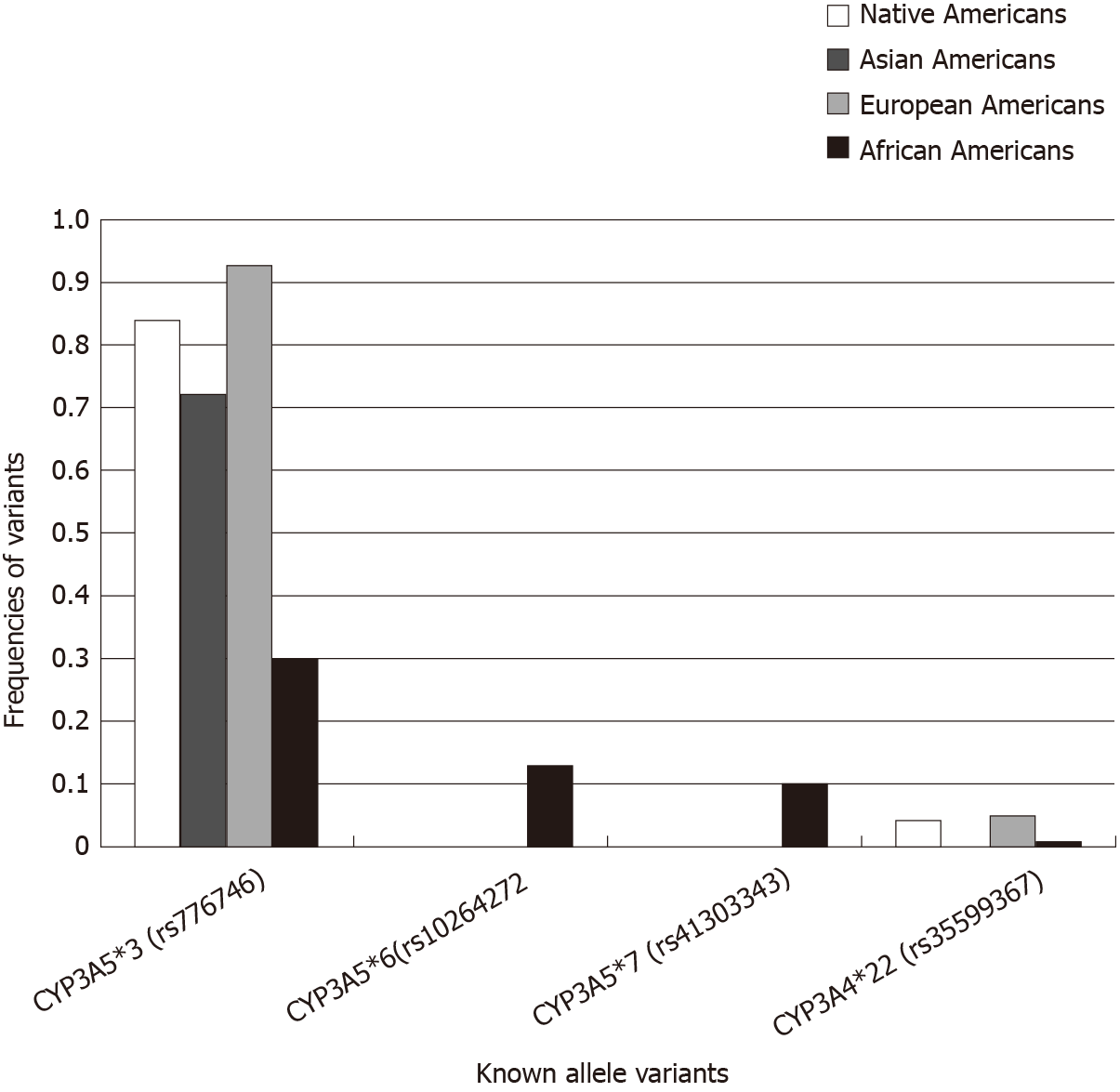
In a very recent study, Mohamed et al[40] conducted an observational, prospective, multicenter study on 2595 kidney transplant recipients of European, African, Native Americans and Asian ancestry, and studied TAC troughs, doses and genetic determinants of metabolism. In particular, they studied well-known variants and conducted a CYP3A4/5 genome-wide analysis to identify new variants.
Daily doses and dose-normalized troughs were significantly different across the groups and this was related to the genetic variants (Figure 1, Table 5)[40].
| Native American, n = 77 | Asian ancestry, n = 91 | European ancestry, n = 1966 | African American, n = 461 | P value | |
| Trough concentration (ng/mL) | 8.3 | 8.4 | 8.4 | 6.9 | < 0.0001 |
| Total daily dose (mg) | 5.0 | 6.0 | 5.0 | 8.0 | < 0.0001 |
| Dose-normalized trough concentration (ng/mL per total daily dose in mg) | 1.73 | 1.50 | 1.56 | 0.78 | 0.0001 |
Genetic modification of the P-glycoprotein also plays an important role in the PK of CNIs. Modifications of the ABCB1 gene are associated with reduced or improved renal function[29-31].
Studies on the relevance of genes encoding the trigger proteins of CNIs are interesting, but their clinical utility remains unclear.
The two main drugs belonging to this group, namely, sirolimus and everolimus, are principally metabolized by the enzymes encoded by the CYP3A family genes[41]. In particular, a low ratio between mammalian target of rapamycin inhibitors (mTORI) concentrations and doses has been observed in patients with CYP3A5*1 (CYP3A5 expressers) with respect to patients with CYP3A5*3/*3 (nonexpressers) suggesting that the latter need lower mTORI doses[41,42]. Carriers of CYP3A5*1/*1 have a faster hepatic metabolism and need higher doses than noncarriers[43,44]. An important limitation of these studies is that the results described have been found only for sirolimus and only for patients not on CNI therapy[45].
Recently, other polymorphisms have been described such as CYP3A4*22, POR*28 and peroxisome proliferator-activated receptor alpha, but none of these appear to have any impact on mTORI metabolism[46].
Considering all the mentioned studies, no association between the genotypes and clinical outcomes was found. In summary, these reports suggest that the CYP3A5*3 genotype has a potential role in the PK of mTORIs, but there is no evidence to justify prospective genotyping of CYP3A5 in renal transplantation.
Similar results are seen with the CYP2C8 polymorphism. CYP2C8 is expressed on hepatocytes, but it seems to have only a minor role in the PK of mTORIs.
ABCB1 influences mTORIs intestinal absorption and passage into T lymphocytes.
Studies on the effect of ABCB1 SNPs on mTORI concentrations are contradictory. Two studies on patients with renal transplantation[43,47] did not show any association between the ABCB1 c.3435C>T SNP and the mTORI dose. Another study[48] found that Chinese patients carrying the ABCB1CGC/CGC haplotype require a lower dose of sirolimus. ABCB1 does not seem to exert any pharmacogenetic effect on the PK of mTORIs. The drawback of these studies is that the mTORI concentrations in T lymphocytes have never been studied.
Among the most relevant genes involved in mTORI activity, MTOR, RAPTOR and RPS6KB1 have been principally studied. These genes encode mTOR, the regulatory associated protein of mTOR (Raptor) and the phosphatidyl-inositol 3 kinase-p70 ribosomal 6S protein kinase (p70S6 kinase).
Variants in mTOR, RPS6KB1 and RAPTOR have been analyzed in one study involving 179 patients[49].
A significant relationship was found between 5 SNPs of mTOR (rs1770345, rs2300095, rs2076655, rs1883965 and rs12732063) and hemoglobin levels. None of these variants was associated with renal outcomes or other mTORI-related side effects.
On the basis of a literature review[6], some of the variants shown on Table 6 are highly recommended candidates for further studies.
| Drugs | Gene | SNP | MAF | QOE | LOR |
| mTOR inhibitors | mTOR | rs2024627 | T = 0.270 | A | 1 |
| rs2295080 | G = 0.308 | A | 1 | ||
| rs1883965 | A = 0.288 | B | 1 | ||
| rs1057079 | G = 0.243 | C | 3 |
rs2024627 variants are associated with a decrease in mTOR mRNA expression as well as rs2295080. Variants of rs1883965 have been found to be associated with decreased hemoglobin levels.
Overall, to date, there are poor data documenting the relevance of the pharmacogenetics of mTORIs on clinical outcomes.
Only CYP3A5*3 seems to have an impact on clinical outcomes, but only in renal transplant patients not treated with CNI.
Mycophenolate mofetil (MMF) is a prodrug that is rapidly transformed into mycophenolic acid (MPA), which acts as a selective, powerful, noncompetitive inhibitor of the enzyme inosine monophosphate dehydrogenase (IMPDH), which has a key role in the de novo synthesis of guanosine nucleotides that are essential for B and T lymphocytes.
The hepatic enzyme UDP glucuroniltransferase (UGT), in particular UGT1A9, is principally involved in the PK of MPA.
Several studies have documented that two SNPs, namely, UGT1A9 -2152C>T (rs17868320) and UGT1A9 -275T>A (rs6714486) are associated with reduced MPA levels[50-52].
A further study conducted by van Schaik et al[53] demonstrated that these SNPs were associated with lower MPS levels and a higher risk of acute rejection.
Other studies have documented that another UGT1A9 SNP, -98T>C (or UGT 1A9*3), is associated with higher MPA levels[54,55].
As the SNP UGT1A9*3 is present in less than 5% of patients, these studies should be evaluated with caution as they have been conducted on a very small number of patients.
Other studies have reported the association of the genetic variants of UGT1A8 and UGT2B7 and the incidence of side effects due to MPA such as diarrhea and hematological complications[56,57].
These studies have not been confirmed, and this relationship is still unclear.
Two main genes are responsible for MPA transport in the enterohepatic circulation, ABCC2 and SLCO1B1.
SNPs in ABCC2, in particular -24C>T, have been studied, but no association with the PK of MPA, BPAR or gastrointestinal side-effects has been found[53,54,58-60].
No SLCO1B1 SNPs have been found to be associated with the PK of MPA[61].
In one of these studies, Michelon et al[60] found that recipient carriers of the SLCO1B1*15 (521T>C) allele have fewer MPA-related side effects.
The influence of SLCO genotypes on the PK of MPA has been examined in different studies[61,62]. The results of these studies were different and controversial. To date, the influence of SLCO genotypes on the PK of MPA remains unknown.
The mechanism of action of MPA involves the inhibition of the rate-limiting enzyme in de novo purine synthesis, IMPDH. Two isoforms of IMPDH have been identified, IMPDH1 and IMPDH2[63]. A large number of genetic variants of both enzymes have been identified. Similarly, different SNPs have been identified[64-67]. IMPDH1 is normally expressed in most cells, including lymphocytes. After lymphocyte activation, the IMPDH2 isoform is up-regulated. Although some genetic isoforms occur with a very low frequency and do not seem to have any effect on enzyme activity[68,69], others have been studied more extensively.
Glander et al[70] demonstrated an association between high pretransplant activity of IMPDH and posttransplant acute rejection. These patients require a higher MPA dose to obtain satisfactory immunosuppression. The opposite occurs in patients with lower pretransplant IMPDH activity.
A study by Wang et al[67] in 191 kidney transplant patients showed that SNP rs2278293 and SNP rs2278294 at the level of intron 7 of the IMPDH1 gene are associated with a higher BPAR rate one year posttransplant[71].
In another study on 237 renal transplant patients from the Cyclosporine Avoidance Eliminates Serious Adverse Renal toxicity study (CAESAR)[72], an OR of 3.39 was found for BPAR in carriers of the 3757T>C allele of the IMPDH2 variant rs11706052.
Other studies have not confirmed this finding[73]. The study conducted by Shah et al[74] on 1000 renal transplant patients did not document any association between IMPDH variants, the risk of BPAR and graft survival.
In summary, even if controversial data do exist on the role of IMPDH2 rs11706052, it is still a highly recommended candidate for further studies as it was found to be associated with a risk of BPAR, but this has not been confirmed.
Less clear is the role of IMPDH1 rs2278294 and rs2278293 even if they have been found to be associated with a decreased risk of BPAR. The study by Shah et al[74] suggests that these variants should not be considered in the future (Table 7).
| Drugs | Gene | SNP | MAF | QOE | LOR |
| Mycophenolic acid | IMPDH2 | rs11706052 | G = 0.115 | A | 1 |
| IMPDG1 | rs2278293 | A = 0.431 | C | 4 | |
| IMPDH1 | rs2278294 | A = 0.323 | C | 4 |
A correct understanding of immunosuppressant use has a clear impact on allograft and patient survival rates. Several studies have demonstrated that the immunosuppressant dose has a relevant role in reducing chronic graft damage.
Unfortunately, to date, clear conclusions may be drawn only for those drugs that are metabolized by CYP3A5. The influence of CYP3A5*3 and CYP3A4*22 has been clearly documented for TAC dosing in kidney, liver and heart transplant patients. Even if, in a recent study on TAC, the final multiple linear regression model that included CYP3A5, CYP3A4 and age explained only 18.3% of the interindividual variability of TAC trough concentrations[75], the French National Network of Pharmacogenetics[76] recommends genotyping for CYP3A5 before kidney, heart and lung transplantation.
It should be understood that pharmacogenetics is still in its early stages in the setting of renal transplantation.
Single studies have been able to document a well-defined relationship between several SNPs and clinical outcomes (Table 8)[67,73,77-79]. However, these genes have not been validated by other studies, and these genes as well as other genes that have been described in this review are only recommended candidates for future studies (Table 9)[80].
| Drug | SNPs | Patients (n) | Outcomes | OR | CI | P value |
| CNI | rs2069762TT | 50 | CAN | 4.57 | 1.04-20.11 | 0.044 |
| CNI | rs8177826 | 290 | Nephrotoxicity | 3.49 | 1.47-8.24 | 0.006 |
| CNI | rs2069762 | 90 | AR | 6.3 | 1.8-22.15 | 0.005 |
| EC-MPS | rs11706052 | 237 | AR | 3.39 | 1.42-8.09 | 0.006 |
| EC-MPS | rs2278293 | 191 | AR | 0.34 | 0.15-0.76 | 0.008 |
| EC-MPS | rs2278294 | 191 | AR | 0.40 | 0.18-0.89 | 0.02 |
| Drug | Phase I enzymes | Phase II enzymes | Uptake transporters | ABC transporters | |||
| CYP3A4 | CYP3A5 | UGT1A9 | OATP1B1/3 | ABCB1 | ABCC2 | IMPDH I/II | |
| rs35599367 | rs776746, rs10264272 | rs17868320, rs6714486 | rs41490556, rs4149117 | rs1128503, rs2032582, rs1045642 | rs717620 | rs2278293, rs2278294, rs11706052 | |
| Mycophenolic acid | - | - | + | (+) | - | (+) | + |
| Cyclosporine | (+) | - | - | - | (+) | - | - |
| Tacrolimus | + | ++ | - | - | (+) | - | - |
| Sirolimus | (+) | - | - | - | - | - | - |
The development of omics techniques could, in the future, have a relevant role in choosing the right dose for the right patient.
More work is required in organ transplant populations, other than the kidney transplant population, with a particular focus on high-risk populations such as African Americans.
The use of a dose algorithm should be performed taking in account all the relevant variants such as age or race. Indeed, the future of pharmacogenetics will rely on models in which patient characteristics will be combined with the polymorphisms in multiple genes. This will give more information than the few genes studies performed up to now.
Concerning the future of pharmacogenomics in transplantation, the following suggestions should be included in relation to care: (1) More work should be done in other organ transplant populations, principally liver; (2) More light should be shed on high risk populations such as African Americans; (3) A careful account of all the relevant race specific variants should be carried out; and (4) Use of genetic variants known to be important for therapy management beyond immunosuppression.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gonzalez FM, Hibberd AD, Sureshkumar K, Uygun I S-Editor: Zhang L L-Editor: Webster JR E-Editor: Li X
| 1. | Casati C, Menegotto A, Querques ML, Ravera F, Colussi G. [Immunosuppression in kidney transplantation: a way between efficacy and toxicity]. G Ital Nefrol. 2017;34:29-39. [PubMed] |
| 2. | Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3287] [Cited by in RCA: 3217] [Article Influence: 321.7] [Reference Citation Analysis (0)] |
| 3. | Roden DM, Altman RB, Benowitz NL, Flockhart DA, Giacomini KM, Johnson JA, Krauss RM, McLeod HL, Ratain MJ, Relling MV, Ring HZ, Shuldiner AR, Weinshilboum RM, Weiss ST; Pharmacogenetics Research Network. Pharmacogenomics: challenges and opportunities. Ann Intern Med. 2006;145:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010;363:166-176. [PubMed] |
| 5. | Evans WE, McLeod HL. Pharmacogenomics--drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1091] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 6. | Pouché L, Stojanova J, Marquet P, Picard N. New challenges and promises in solid organ transplantation pharmacogenetics: the genetic variability of proteins involved in the pharmacodynamics of immunosuppressive drugs. Pharmacogenomics. 2016;17:277-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Kamdem LK, Streit F, Zanger UM, Brockmöller J, Oellerich M, Armstrong VW, Wojnowski L. Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem. 2005;51:1374-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Dai Y, Iwanaga K, Lin YS, Hebert MF, Davis CL, Huang W, Kharasch ED, Thummel KE. In vitro metabolism of cyclosporine A by human kidney CYP3A5. Biochem Pharmacol. 2004;68:1889-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Thervet E, Anglicheau D, King B, Schlageter MH, Cassinat B, Beaune P, Legendre C, Daly AK. Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation. 2003;76:1233-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Jacobson PA, Oetting WS, Brearley AM, Leduc R, Guan W, Schladt D, Matas AJ, Lamba V, Julian BA, Mannon RB, Israni A; DeKAF Investigators. Novel polymorphisms associated with tacrolimus trough concentrations: results from a multicenter kidney transplant consortium. Transplantation. 2011;91:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Haufroid V, Mourad M, Van Kerckhove V, Wawrzyniak J, De Meyer M, Eddour DC, Malaise J, Lison D, Squifflet JP, Wallemacq P. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 360] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 12. | Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, Wang D, Vinks AA, He Y, Swen JJ, Leeder JS, van Schaik R, Thummel KE, Klein TE, Caudle KE, MacPhee IA. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther. 2015;98:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 527] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 13. | Haufroid V, Wallemacq P, VanKerckhove V, Elens L, De Meyer M, Eddour DC, Malaise J, Lison D, Mourad M. CYP3A5 and ABCB1 polymorphisms and tacrolimus pharmacokinetics in renal transplant candidates: guidelines from an experimental study. Am J Transplant. 2006;6:2706-2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Wang D, Guo Y, Wrighton SA, Cooke GE, Sadee W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenomics J. 2011;11:274-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 395] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 15. | Elens L, Bouamar R, Hesselink DA, Haufroid V, van der Heiden IP, van Gelder T, van Schaik RH. A new functional CYP3A4 intron 6 polymorphism significantly affects tacrolimus pharmacokinetics in kidney transplant recipients. Clin Chem. 2011;57:1574-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Wang D, Sadee W. CYP3A4 intronic SNP rs35599367 (CYP3A4*22) alters RNA splicing. Pharmacogenet Genomics. 2016;26:40-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Traynor C, Conlon P, Phelan PJ, O'Kelly P, Elens L, McCormack M, Cavalleri G, Comber H, van Schaik RH, Conlon PJ. Association of CYP3A variants with kidney transplant outcomes. Ren Fail. 2015;37:562-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Woillard JB, Rerolle JP, Picard N, Rousseau A, Guillaudeau A, Munteanu E, Essig M, Drouet M, Le Meur Y, Marquet P. Donor P-gp polymorphisms strongly influence renal function and graft loss in a cohort of renal transplant recipients on cyclosporine therapy in a long-term follow-up. Clin Pharmacol Ther. 2010;88:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Tang HL, Xie HG, Yao Y, Hu YF. Lower tacrolimus daily dose requirements and acute rejection rates in the CYP3A5 nonexpressers than expressers. Pharmacogenet Genomics. 2011;21:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Rojas L, Neumann I, Herrero MJ, Bosó V, Reig J, Poveda JL, Megías J, Bea S, Aliño SF. Effect of CYP3A5*3 on kidney transplant recipients treated with tacrolimus: a systematic review and meta-analysis of observational studies. Pharmacogenomics J. 2015;15:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Tang HL, Ma LL, Xie HG, Zhang T, Hu YF. Effects of the CYP3A5*3 variant on cyclosporine exposure and acute rejection rate in renal transplant patients: a meta-analysis. Pharmacogenet Genomics. 2010;20:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Elens L, Bouamar R, Hesselink DA, Haufroid V, van Gelder T, van Schaik RH. The new CYP3A4 intron 6 C>T polymorphism (CYP3A4*22) is associated with an increased risk of delayed graft function and worse renal function in cyclosporine-treated kidney transplant patients. Pharmacogenet Genomics. 2012;22:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Elens L, Hesselink DA, Bouamar R, Budde K, de Fijter JW, De Meyer M, Mourad M, Kuypers DR, Haufroid V, van Gelder T, van Schaik RH. Impact of POR*28 on the pharmacokinetics of tacrolimus and cyclosporine A in renal transplant patients. Ther Drug Monit. 2014;36:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Klein K, Thomas M, Winter S, Nussler AK, Niemi M, Schwab M, Zanger UM. PPARA: a novel genetic determinant of CYP3A4 in vitro and in vivo. Clin Pharmacol Ther. 2012;91:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 25. | Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 893] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 26. | Mourad M, Wallemacq P, De Meyer M, Brandt D, Van Kerkhove V, Malaise J, Chaïb Eddour D, Lison D, Haufroid V. The influence of genetic polymorphisms of cytochrome P450 3A5 and ABCB1 on starting dose- and weight-standardized tacrolimus trough concentrations after kidney transplantation in relation to renal function. Clin Chem Lab Med. 2006;44:1192-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Kuypers DR, Naesens M, de Jonge H, Lerut E, Verbeke K, Vanrenterghem Y. Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther Drug Monit. 2010;32:394-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Crettol S, Venetz JP, Fontana M, Aubert JD, Ansermot N, Fathi M, Pascual M, Eap CB. Influence of ABCB1 genetic polymorphisms on cyclosporine intracellular concentration in transplant recipients. Pharmacogenet Genomics. 2008;18:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Naesens M, Lerut E, de Jonge H, Van Damme B, Vanrenterghem Y, Kuypers DR. Donor age and renal P-glycoprotein expression associate with chronic histological damage in renal allografts. J Am Soc Nephrol. 2009;20:2468-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Hesselink DA, Bouamar R, van Gelder T. The pharmacogenetics of calcineurin inhibitor-related nephrotoxicity. Ther Drug Monit. 2010;32:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | De Meyer M, Haufroid V, Elens L, Fusaro F, Patrono D, De Pauw L, Kanaan N, Goffin E, Mourad M. Donor age and ABCB1 1199G>A genetic polymorphism are independent factors affecting long-term renal function after kidney transplantation. J Surg Res. 2012;178:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Pouché L, Koitka M, Stojanova J, Woillard JB, Monchaud C, Villeneuve C, Essig M, Abraham J, Le Meur Y, Rerolle JP, Kamar N, Rostaing L, Merville P, Gandia P, Bouchet S, Petersen BS, Marquet P, Picard N. A candidate gene approach of the calcineurin pathway to identify variants associated with clinical outcomes in renal transplantation. Pharmacogenomics. 2016;17:375-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Ekberg H, Bernasconi C, Tedesco-Silva H, Vítko S, Hugo C, Demirbas A, Acevedo RR, Grinyó J, Frei U, Vanrenterghem Y, Daloze P, Halloran P. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009;9:1876-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 236] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 34. | Thervet E, Loriot MA, Barbier S, Buchler M, Ficheux M, Choukroun G, Toupance O, Touchard G, Alberti C, Le Pogamp P, Moulin B, Le Meur Y, Heng AE, Subra JF, Beaune P, Legendre C. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Shuker N, Bouamar R, van Schaik RH, Clahsen-van Groningen MC, Damman J, Baan CC, van de Wetering J, Rowshani AT, Weimar W, van Gelder T, Hesselink DA. A Randomized Controlled Trial Comparing the Efficacy of Cyp3a5 Genotype-Based With Body-Weight-Based Tacrolimus Dosing After Living Donor Kidney Transplantation. Am J Transplant. 2016;16:2085-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 36. | Pallet N, Etienne I, Buchler M, Bailly E, Hurault de Ligny B, Choukroun G, Colosio C, Thierry A, Vigneau C, Moulin B, Le Meur Y, Heng AE, Legendre C, Beaune P, Loriot MA, Thervet E. Long-Term Clinical Impact of Adaptation of Initial Tacrolimus Dosing to CYP3A5 Genotype. Am J Transplant. 2016;16:2670-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Jacobson PA, Schladt D, Oetting WS, Leduc R, Guan W, Matas AJ, Israni A. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am J Transplant. 2012;12:3326-3336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Birdwell KA, Chung CP. The Potential of Pharmacogenomics to Advance Kidney Disease Treatment. Clin J Am Soc Nephrol. 2017;12:1035-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Oetting WS, Schladt DP, Guan W, Miller MB, Remmel RP, Dorr C, Sanghavi K, Mannon RB, Herrera B, Matas AJ, Salomon DR, Kwok PY, Keating BJ, Israni AK, Jacobson PA; DeKAF Investigators. Genomewide Association Study of Tacrolimus Concentrations in African American Kidney Transplant Recipients Identifies Multiple CYP3A5 Alleles. Am J Transplant. 2016;16:574-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 40. | Mohamed ME, Schladt DP, Guan W, Wu B, van Setten J, Keating BJ, Iklé D, Remmel RP, Dorr CR, Mannon RB, Matas AJ, Israni AK, Oetting WS, Jacobson PA; DeKAF Genomics and GEN03 Investigators. Tacrolimus troughs and genetic determinants of metabolism in kidney transplant recipients: A comparison of four ancestry groups. Am J Transplant. 2019;19:2795-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Sattler M, Guengerich FP, Yun CH, Christians U, Sewing KF. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos. 1992;20:753-761. [PubMed] |
| 42. | Caletti C, Granata S, Tomei P, Dalla Gassa A, Lupo A, Zaza G. [Pharmacogenetics: a promising tool to personalize immunosuppressive therapy in renal transplantation]. G Ital Nefrol. 2015;32. [PubMed] |
| 43. | Anglicheau D, Le Corre D, Lechaton S, Laurent-Puig P, Kreis H, Beaune P, Legendre C, Thervet E. Consequences of genetic polymorphisms for sirolimus requirements after renal transplant in patients on primary sirolimus therapy. Am J Transplant. 2005;5:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Le Meur Y, Djebli N, Szelag JC, Hoizey G, Toupance O, Rérolle JP, Marquet P. CYP3A5*3 influences sirolimus oral clearance in de novo and stable renal transplant recipients. Clin Pharmacol Ther. 2006;80:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Mourad M, Mourad G, Wallemacq P, Garrigue V, Van Bellingen C, Van Kerckhove V, De Meyer M, Malaise J, Eddour DC, Lison D, Squifflet JP, Haufroid V. Sirolimus and tacrolimus trough concentrations and dose requirements after kidney transplantation in relation to CYP3A5 and MDR1 polymorphisms and steroids. Transplantation. 2005;80:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Picard N, Rouguieg-Malki K, Kamar N, Rostaing L, Marquet P. CYP3A5 genotype does not influence everolimus in vitro metabolism and clinical pharmacokinetics in renal transplant recipients. Transplantation. 2011;91:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Miao LY, Huang CR, Hou JQ, Qian MY. Association study of ABCB1 and CYP3A5 gene polymorphisms with sirolimus trough concentration and dose requirements in Chinese renal transplant recipients. Biopharm Drug Dispos. 2008;29:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Lee J, Huang H, Chen Y, Lu X. ABCB1 haplotype influences the sirolimus dose requirements in Chinese renal transplant recipients. Biopharm Drug Dispos. 2014;35:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Woillard JB, Kamar N, Rousseau A, Rostaing L, Marquet P, Picard N. Association of sirolimus adverse effects with m-TOR, p70S6K or Raptor polymorphisms in kidney transplant recipients. Pharmacogenet Genomics. 2012;22:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Girard H, Court MH, Bernard O, Fortier LC, Villeneuve L, Hao Q, Greenblatt DJ, von Moltke LL, Perussed L, Guillemette C. Identification of common polymorphisms in the promoter of the UGT1A9 gene: evidence that UGT1A9 protein and activity levels are strongly genetically controlled in the liver. Pharmacogenetics. 2004;14:501-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 51. | Kuypers DR, Naesens M, Vermeire S, Vanrenterghem Y. The impact of uridine diphosphate-glucuronosyltransferase 1A9 (UGT1A9) gene promoter region single-nucleotide polymorphisms T-275A and C-2152T on early mycophenolic acid dose-interval exposure in de novo renal allograft recipients. Clin Pharmacol Ther. 2005;78:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 52. | Hesselink DA, van Gelder T. Genetic and nongenetic determinants of between-patient variability in the pharmacokinetics of mycophenolic acid. Clin Pharmacol Ther. 2005;78:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | van Schaik RH, van Agteren M, de Fijter JW, Hartmann A, Schmidt J, Budde K, Kuypers D, Le Meur Y, van der Werf M, Mamelok R, van Gelder T. UGT1A9 -275T>A/-2152C>T polymorphisms correlate with low MPA exposure and acute rejection in MMF/tacrolimus-treated kidney transplant patients. Clin Pharmacol Ther. 2009;86:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 54. | Johnson LA, Oetting WS, Basu S, Prausa S, Matas A, Jacobson PA. Pharmacogenetic effect of the UGT polymorphisms on mycophenolate is modified by calcineurin inhibitors. Eur J Clin Pharmacol. 2008;64:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Lévesque E, Delage R, Benoit-Biancamano MO, Caron P, Bernard O, Couture F, Guillemette C. The impact of UGT1A8, UGT1A9, and UGT2B7 genetic polymorphisms on the pharmacokinetic profile of mycophenolic acid after a single oral dose in healthy volunteers. Clin Pharmacol Ther. 2007;81:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 56. | Woillard JB, Rerolle JP, Picard N, Rousseau A, Drouet M, Munteanu E, Essig M, Marquet P, Le Meur Y. Risk of diarrhoea in a long-term cohort of renal transplant patients given mycophenolate mofetil: the significant role of the UGT1A8 2 variant allele. Br J Clin Pharmacol. 2010;69:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Prausa SE, Fukuda T, Maseck D, Curtsinger KL, Liu C, Zhang K, Nick TG, Sherbotie JR, Ellis EN, Goebel J, Vinks AA. UGT genotype may contribute to adverse events following medication with mycophenolate mofetil in pediatric kidney transplant recipients. Clin Pharmacol Ther. 2009;85:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Barraclough KA, Lee KJ, Staatz CE. Pharmacogenetic influences on mycophenolate therapy. Pharmacogenomics. 2010;11:369-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Picard N, Yee SW, Woillard JB, Lebranchu Y, Le Meur Y, Giacomini KM, Marquet P. The role of organic anion-transporting polypeptides and their common genetic variants in mycophenolic acid pharmacokinetics. Clin Pharmacol Ther. 2010;87:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 60. | Michelon H, König J, Durrbach A, Quteineh L, Verstuyft C, Furlan V, Ferlicot S, Letierce A, Charpentier B, Fromm MF, Becquemont L. SLCO1B1 genetic polymorphism influences mycophenolic acid tolerance in renal transplant recipients. Pharmacogenomics. 2010;11:1703-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Miura M, Satoh S, Inoue K, Kagaya H, Saito M, Inoue T, Suzuki T, Habuchi T. Influence of SLCO1B1, 1B3, 2B1 and ABCC2 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. Eur J Clin Pharmacol. 2007;63:1161-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 62. | Miura M, Kagaya H, Satoh S, Inoue K, Saito M, Habuchi T, Suzuki T. Influence of drug transporters and UGT polymorphisms on pharmacokinetics of phenolic glucuronide metabolite of mycophenolic acid in Japanese renal transplant recipients. Ther Drug Monit. 2008;30:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 63. | Natsumeda Y, Ohno S, Kawasaki H, Konno Y, Weber G, Suzuki K. Two distinct cDNAs for human IMP dehydrogenase. J Biol Chem. 1990;265:5292-5295. [PubMed] |
| 64. | Digits JA, Hedstrom L. Species-specific inhibition of inosine 5'-monophosphate dehydrogenase by mycophenolic acid. Biochemistry. 1999;38:15388-15397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | McPhillips CC, Hyle JW, Reines D. Detection of the mycophenolate-inhibited form of IMP dehydrogenase in vivo. Proc Natl Acad Sci USA. 2004;101:12171-12176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Roberts RL, Gearry RB, Barclay ML, Kennedy MA. IMPDH1 promoter mutations in a patient exhibiting azathioprine resistance. Pharmacogenomics J. 2007;7:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Wang J, Yang JW, Zeevi A, Webber SA, Girnita DM, Selby R, Fu J, Shah T, Pravica V, Hutchinson IV, Burckart GJ. IMPDH1 gene polymorphisms and association with acute rejection in renal transplant patients. Clin Pharmacol Ther. 2008;83:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | Wu TY, Peng Y, Pelleymounter LL, Moon I, Eckloff BW, Wieben ED, Yee VC, Weinshilboum RM. Pharmacogenetics of the mycophenolic acid targets inosine monophosphate dehydrogenases IMPDH1 and IMPDH2: gene sequence variation and functional genomics. Br J Pharmacol. 2010;161:1584-1598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Gensburger O, Van Schaik RH, Picard N, Le Meur Y, Rousseau A, Woillard JB, Van Gelder T, Marquet P. Polymorphisms in type I and II inosine monophosphate dehydrogenase genes and association with clinical outcome in patients on mycophenolate mofetil. Pharmacogenet Genomics. 2010;20:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Glander P, Hambach P, Braun KP, Fritsche L, Giessing M, Mai I, Einecke G, Waiser J, Neumayer HH, Budde K. Pre-transplant inosine monophosphate dehydrogenase activity is associated with clinical outcome after renal transplantation. Am J Transplant. 2004;4:2045-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 71. | Kagaya H, Miura M, Saito M, Habuchi T, Satoh S. Correlation of IMPDH1 gene polymorphisms with subclinical acute rejection and mycophenolic acid exposure parameters on day 28 after renal transplantation. Basic Clin Pharmacol Toxicol. 2010;107:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Sombogaard F, van Schaik RH, Mathot RA, Budde K, van der Werf M, Vulto AG, Weimar W, Glander P, Essioux L, van Gelder T. Interpatient variability in IMPDH activity in MMF-treated renal transplant patients is correlated with IMPDH type II 3757T > C polymorphism. Pharmacogenet Genomics. 2009;19:626-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Grinyó J, Vanrenterghem Y, Nashan B, Vincenti F, Ekberg H, Lindpaintner K, Rashford M, Nasmyth-Miller C, Voulgari A, Spleiss O, Truman M, Essioux L. Association of four DNA polymorphisms with acute rejection after kidney transplantation. Transpl Int. 2008;21:879-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 74. | Shah S, Harwood SM, Döhler B, Opelz G, Yaqoob MM. Inosine monophosphate dehydrogenase polymorphisms and renal allograft outcome. Transplantation. 2012;94:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Bruckmueller H, Werk AN, Renders L, Feldkamp T, Tepel M, Borst C, Caliebe A, Kunzendorf U, Cascorbi I. Which Genetic Determinants Should be Considered for Tacrolimus Dose Optimization in Kidney Transplantation? A Combined Analysis of Genes Affecting the CYP3A Locus. Ther Drug Monit. 2015;37:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 76. | Woillard JB, Chouchana L, Picard N, Loriot MA; French Network of Pharmacogenetics (RNPGX). Pharmacogenetics of immunosuppressants: State of the art and clinical implementation - recommendations from the French National Network of Pharmacogenetics (RNPGx). Therapie. 2017;72:285-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Satoh S, Saito M, Inoue K, Miura M, Komatsuda A, Habuchi T. Association of cytokine polymorphisms with subclinical progressive chronic allograft nephropathy in Japanese renal transplant recipients: preliminary study. Int J Urol. 2007;14:990-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Moscoso-Solorzano GT, Ortega F, Rodríguez I, García-Castro M, Gómez E, Díaz-Corte C, Baltar JM, Alvarez V, Ortiz A, Coto E. A search for cyclophilin-A gene variants in cyclosporine A-treated renal transplanted patients. Clin Transplant. 2008;22:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Seyhun Y, Ciftci HS, Kekik C, Karadeniz MS, Tefik T, Nane I, Turkmen A, Oguz FS, Aydin F. Genetic association of interleukin-2, interleukin-4, interleukin-6, transforming growth factor-β, tumour necrosis factor-α and blood concentrations of calcineurin inhibitors in Turkish renal transplant patients. Int J Immunogenet. 2015;42:147-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Cascorbi I. The Pharmacogenetics of Immune-Modulating Therapy. Adv Pharmacol. 2018;83:275-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |