Published online May 29, 2020. doi: 10.5500/wjt.v10.i5.138
Peer-review started: February 22, 2020
First decision: April 9, 2020
Revised: April 22, 2020
Accepted: May 5, 2020
Article in press: May 5, 2020
Published online: May 29, 2020
Processing time: 90 Days and 12.9 Hours
Although ABO-nonidentical and ABO-incompatible liver transplantation (LT) are other options for end-stage liver disease treatment, the development of antibodies against blood group antigens (anti-A/B antibodies) is still a challenge in managing and follow-up of the recipients.
A 56-year-old male with end-stage liver disease with rapid deterioration and poor prognosis was considered to receive a deceased ABO-nonidentical liver graft. All required tests were performed according to our pre-LT diagnostic protocol. The orthotopic LT procedure involving O+ donor and A1B+ recipient was performed. Our treatment strategy to overcome the antibody‐mediated rejection included a systemic triple immunosuppressive regimen: methylprednisolone, mycophenolate mofetil, and tacrolimus. The immunological desensitization consisted of the chimeric anti-CD20 monoclonal antibody rituximab and intravenous immunoglobulins. The patient was also on antibiotic treatment with amoxicillin/clavulanate, cefotaxime, and metronidazole. On the 10th postoperative day, high titers of IgG anti-A and anti-B antibodies were found in the patient’s plasma. We performed a liver biopsy, which revealed histological evidence of antibody-mediated rejection, but the rejection was excluded according to the Banff classification. The therapy was continued until the titer decreased significantly on the 18th postoperative day. Despite the antibiotic, antifungal, and antiviral treatment, the patient deteriorated and developed septic shock with anuria and pancytopenia. The conservative treatment was unsuccessful, which lead to the patient’s fatal outcome on the 42nd postoperative day.
We present a patient who underwent ABO-nonidentical LT from a deceased donor. Even though we implemented the latest technological advancements and therapeutic approaches in the management of the patient and the initial results were promising, due to severe infectious complications, the outcome was fatal.
Core tip: Living and deceased donor liver transplantation (LT) may apply for both urgent and elective LT, especially for those patients on a long waiting list with rapidly deteriorating liver function. The main threat after ABO-nonidentical LT is the antibody-mediated rejection due to anti-A/B antibodies as a result of passenger lymphocyte syndrome. Our case demonstrated that a proper treatment protocol, including immunosuppression, anti-CD20 monoclonal antibodies, intravenous immunoglobulin, anti-infectious agents, etc, is potent to maintain and even lower the isotiters of antibodies after ABO-nonidentical LT. However, due to other complications, the outcome for the patient was unfavorable.
- Citation: Peruhova M, Georgieva V, Yurukova N, Sekulovska M, Panayotova G, Mihova A, Terzieva V, Velikova TV. ABO-nonidentical liver transplantation from a deceased donor and clinical outcomes following antibody rebound: A case report. World J Transplant 2020; 10(5): 138-146
- URL: https://www.wjgnet.com/2220-3230/full/v10/i5/138.htm
- DOI: https://dx.doi.org/10.5500/wjt.v10.i5.138
Liver transplantation (LT) is considered as a sole option treatment for end-stage liver disease. Due to the limited donor pool, and especially the shortage of ABO-identical donors, the utilization of the ABO blood type nonidentical or incompatible donors may be the only option for unfavorably ill patients with MELD ≥ 30[1,2]. ABO identical LT is when the donor and the recipient are the exact same ABO blood type. ABO compatible-nonidentical is when blood type O could be a donor for blood type A, B or AB recipient, as well as when a blood type AB recipient receives a liver from blood type O, A or B donor. ABO-incompatible LT includes all other possible blood type combinations[2]. When ABO-nonidentical and ABO-incompatible LT is performed, immunological phenomena may occur based on the donor B lymphocytes of the graft. These B lymphocytes may produce antibodies against antigens on the recipient’s erythrocytes, the so-called passenger lymphocyte syndrome (PLS)[3]. Living and deceased donor LT may apply for both urgent and elective LT, especially for many patients included on a long waiting list with rapidly deteriorating liver function[4,5].
The full potential of LT was appreciated when considering the annual liver waitlist mortality, estimated at about 15% for pediatric populations as a result of the lack of size-matched allografts. In line with this and taking into account the donor shortage worldwide, ABO-nonidentical and ABO-incompatible LT remains an option to expand the utilization of organs in cases of life-threatening conditions for the patients[6].
Although the liver is contemplated as an immune-privileged organ where humoral rejection is rare unlike the kidney and the heart, initial transplantation experience with ABO-nonidentical and ABO-incompatible liver allografts confirmed the elevated risk for certain complications[1]. The major risks related to these types of LT are cellular and antibody-mediated rejection (AMR), followed by hepatic artery and biliary complications, such as thrombosis, liver necrosis, and sepsis[6,7]. Another possible complication is hemolytic anemia due to PLS[3]. Furthermore, graft survival after ABO-nonidentical LT was significantly reduced compared to ABO-identical before the introduction of the novel therapeutic strategies.
However, ABO-nonidentical and ABO-incompatible LT have been reported in many countries in Europe, Asia, and North America, with increased prevalence in Eastern countries that face donation problems[7]. Nevertheless, these types of transplantation remain a controversial approach compared to ABO-identical LT[1].
To achieve better results, an effective desensitization protocol for successful ABO-nonidentical/incompatible LT has been developed recently. These treatment options that rely on B cell desensitization are very demanding. They may include total plasma exchange, plasmapheresis, splenectomy, anti-CD20 monoclonal antibodies (i.e., rituximab), mycophenolate mofetil, intravenous immunoglobulin (IVIG), local graft infusion therapy, etc.[1]. Since the introduction of rituximab in 2003, the treatment has a significant decrease in the incidence of AMR by blocking B cells involved in acute rejection[8]. ABO-nonidentical/ incompatible LT is no longer a contraindication for LT due to the significantly improved life expectancy of the recipients.
Here, we present a case report of ABO-nonidentical LT, O+ donor and A1B+ recipient, as the patient developed anti-A/B antibodies after transplantation.
A 56-year-old male presented to the Surgery Department for LT from a deceased donor. The patient had alcoholic liver cirrhosis with MELD score 33 (52.6% estimated 3-mo mortality), which placed him first on the LT waiting list. Due to the severely impaired general condition, the rapid deterioration, and the poor prognosis of the patient, it was decided to perform LT from a donor with ABO type O+, and the recipient was A1B+.
The patient was diagnosed in 2015 with alcoholic liver cirrhosis because of bleeding from esophageal varices with hematemesis and melaena. Endoscopic band ligation of the varices was performed twice. Subsequently, he developed severe and frequent episodes of hepatic encephalopathy, hepatorenal syndrome, and refractory ascites. In 2018 a large pleural effusion on the right side was established, and an unsuccessful attempt for pleurodesis was made in 2019.
In 2001 and 2003, he underwent endoprosthesis of both hip joints. In 2009 percutaneous nephrolithotomy was performed due to calculi in the left kidney.
Upon admission, the patient was in a severely impaired general condition with bradypsychia. He was with jaundice, palmar erythema, and spider angiomas. The patient’s temperature was normal, heart rate was 64 beats per minute, respiratory rate was 18 breaths per minute, blood pressure was 100/70 mmHg, and oxygen saturation at room air was 90%. On auscultation, he had weakened to lacking vesicular breath sounds in the right lung. He had physical evidence of ascites as well as edema of the lower legs.
Upon admission, the complete blood count revealed macrocytic anemia with hemoglobin of 109 g/L, thrombocytopenia, and normal white blood cell count. His coagulation was severely impaired with international normalized ratio of 4.1 and prothrombin time of 18 s. The patient presented with mildly elevated hepatocellular and cholestatic enzymes with total bilirubin of 118 µmol/L and direct bilirubin of 56 µmol/L. He had worsening kidney function with creatinine of 109 µmol/L and urea of 9.9 mmol/L. The A1B+ blood type of the patient was determined with commercially available antisera reagents with standard immunohematologic techniques. Before transplantation, no alloantibodies or anti-erythrocyte antibodies were found.
Imaging evaluation with whole-body computed tomography scan with intravenous contrast medium administration before the LT revealed a pleural effusion on the left side, liver cirrhosis with portal hypertension, massive ascites, splenomegaly, and cholelithiasis. A gastroscopy was performed, and esophageal varices and portal hypertensive gastropathy were observed.
Further tests were performed according to our pre-LT diagnostic protocol. A full panel of serum tumor markers was performed and alpha-fetoprotein (2 ng/mL), carcinoembryonic antigen (5 ng/mL), cancer antigen 19-9 (25 U/mL), prostate-specific antigen (2.33 ng/mL) were without deviations. Serological tests for hepatitis A, B, and C were negative [HAV IgM (-), HAV IgG (-), HBsAg (-), Anti-HBc total (-)]. Human immunodeficiency virus 1 and 2, herpes simplex virus 1 and 2, Herpes Zoster virus, Epstein-Barr virus, cytomegalovirus, toxoplasma, aspergillus, and syphilis were also ruled out. A pleural puncture was performed as the results showed that the effusion is a transudate without bacterial growth, and the adenosine deaminase was in the reference range. QuantiFERON-TB Gold test was negative. The echocardiography showed that there are no myocardial hypertrophy and no significant lesions of the valves. The spirometry showed mild restriction. The levels of immunoglobulins IgG and IgM were normal (IgG: 25 g/L, IgM: 2.3 g/L), and there was a slight elevation of IgA (5.2 g/L). The anti-nuclear antibodies, the anti-mitochondrial antibodies, and anti-smooth muscle antibodies were within reference values.
On an interdisciplinary LT expert board, it was decided to perform LT from a deceased ABO-nonidentical donor because of the patient’s end-stage liver disease and the poor chances for ABO-identical transplantation.
It was established that the patient was with alcoholic liver cirrhosis, Child C (12), MELD 33 without contraindications to undergo LT.
After the extended multidisciplinary LT expert board decision, the orthotopic LT procedure was done according to our standardized surgical protocol. The donor was ABO blood type O+, and the recipient was A1B+. A thoracentesis was performed with the evacuation of 5000 mL of transparent pleural effusion, and a thoracic drain was placed.
Our treatment strategy to overcome the blood type barrier included a systemic triple immunosuppressive regimen: methylprednisolone, mycophenolate mofetil, and tacrolimus. The immunological desensitization consisted of rituximab (chimeric anti-CD20 monoclonal antibody) and IVIG.
Intraoperatively the patient received methylprednisolone (10 mg/kg) after reperfusion and tapered from 160 mg to 40 mg over 10 d then switched to 20 mg of oral prednisolone. Tacrolimus was initiated on a postoperative day 1 with a standard dosing regimen twice a day with a target of 10-12 ng/mL[7]. The antimetabolite mycophenolate mofetil was started in the 1st post-LT wk with a dose 1 mg bid.
We added rituximab to the therapy according to the data in the literature for the great progress on outcomes of ABO-nonidentical/incompatible donor LT[9]. In our case, a regular dose of rituximab (500 mg) was administered once a week in the postoperative period.
On the 10th postoperative day, there was laboratory evidence of anemia and acute hemolysis [hemoglobin of 48 g/L, extreme elevation of total bilirubin > 422 µmol/L, lactate dehydrogenase of 360 U/L (normal range < 240 U/L)]. High titers of IgG anti-A and anti-B antibodies were established in the patient’s plasma (titer IgG anti-A 1:16, titer IgG anti-B 1:8) (Table 1). We performed a liver biopsy, which revealed histological evidence of AMR. The rejection was excluded according to the Banff classification[10]. Therefore, we decided to initiate treatment with high‐dose IVIG (0.5 mg/kg per day). The therapy was continued until the titer decreased to IgG anti-A 1:8 and IgG anti-B 0 (on 18th postoperative day) as well as normalization of the hematological parameters[11]. The patient received daily leukoreduced red blood cells units (donor blood type O), platelet concentrate, and fresh frozen plasma blood type AB.
| Postoperative day | 8th | 10th | 12th | 18th |
| Allo-anti-erythrocyte antibodies | Negative | Negative | Negative | Negative |
| Direct Coombs (fixed antibodies) | Positive | Positive | Positive | Weak positive |
| Polyspecific | 4+ | 3, 5+ | 2, 5+ | 1+ |
| Anti-IgG | 3, 5+ | 3+ | 1, 5+ | 1+ |
| Anti-IgM | /-/ | /-/ | /-/ | /-/ |
| Anti-Complement | 1.5+ | 1.5+ | 1+ | |
| Auto-anti-A IgG | 1:4 | 1:16 | 1:8 | 1:8 |
| Auto-anti-A IgM | Negative | 1:8 | 1:2 | 1:1 |
| Auto-anti-B IgG | 1:2 | 1:2 | 1:1 | 0 |
| Auto-anti-B IgM | Negative | 1:2 | 1:1 | 1:1 |
| Conclusion | Observation GVHD | Increase in auto-antibody titers | Decrease in auto-antibody titers | Significant reduction in auto-antibody titers |
Bacterial, viral, fungal, or parasitic infections are prevalent post-transplant complications[3]. Thus, our patient received antibiotic treatment with amoxicillin/clavulanate, cefotaxime, and metronidazole. Antifungal prophylaxis included nystatin. Valganciclovir was administered against cytomegalovirus-infection.
The evaluation of cellular immunity is not a routine practice in liver transplanted patients, but we checked up the current status of lymphocyte populations after LT. We measured the percentage of T cells, helper CD4+ T cells, regulatory and activated T cells, and the degree of immune activation by the expression of HLA-DR. We found a preserved population of CD3+ T cells (79.1%, within the reference range of 49%-84%) with a slight increase of CD4+ helper cells (71.5%, reference ranges of 28%-63%). The degree of T cell activation was low as determined by HLD-DR expression on CD3+ cells and helper T cells (6% and 3.3%, respectively), probably as a consequence of the immunosuppressive therapy. At the same time, the percentage of regulatory T cells was within the reference range (4.77%, our established reference ranges of 4%-8%)[12] being less affected by the therapy. Our previous study showed that regulatory T cells have a particular dynamic during the early post-LT period[13].
Оn the 39th postoperative day there was laboratory evidence of infection. C-reactive protein and procalcitonin were high with positive blood cultures and cultures of fluid collected through the abdominal drainage catheters for Enterococcus faecalis, Klebsiella pneumoniae, and Pseudomonas aeruginosa with > 105 CFU/mL. The blood tests showed severe pancytopenia with hemoglobin: 45 g/L, red blood cells: 2.1 T/L, white blood cells: 0.8 G/L, and platelet: 13 G/L. We accepted the thesis that the bone marrow failure is a result of the septic condition of the patient, valganciclovir, and the triple immunosuppressive regimen. For this reason, we did not perform a trephine biopsy to verify the bone marrow aplasia.
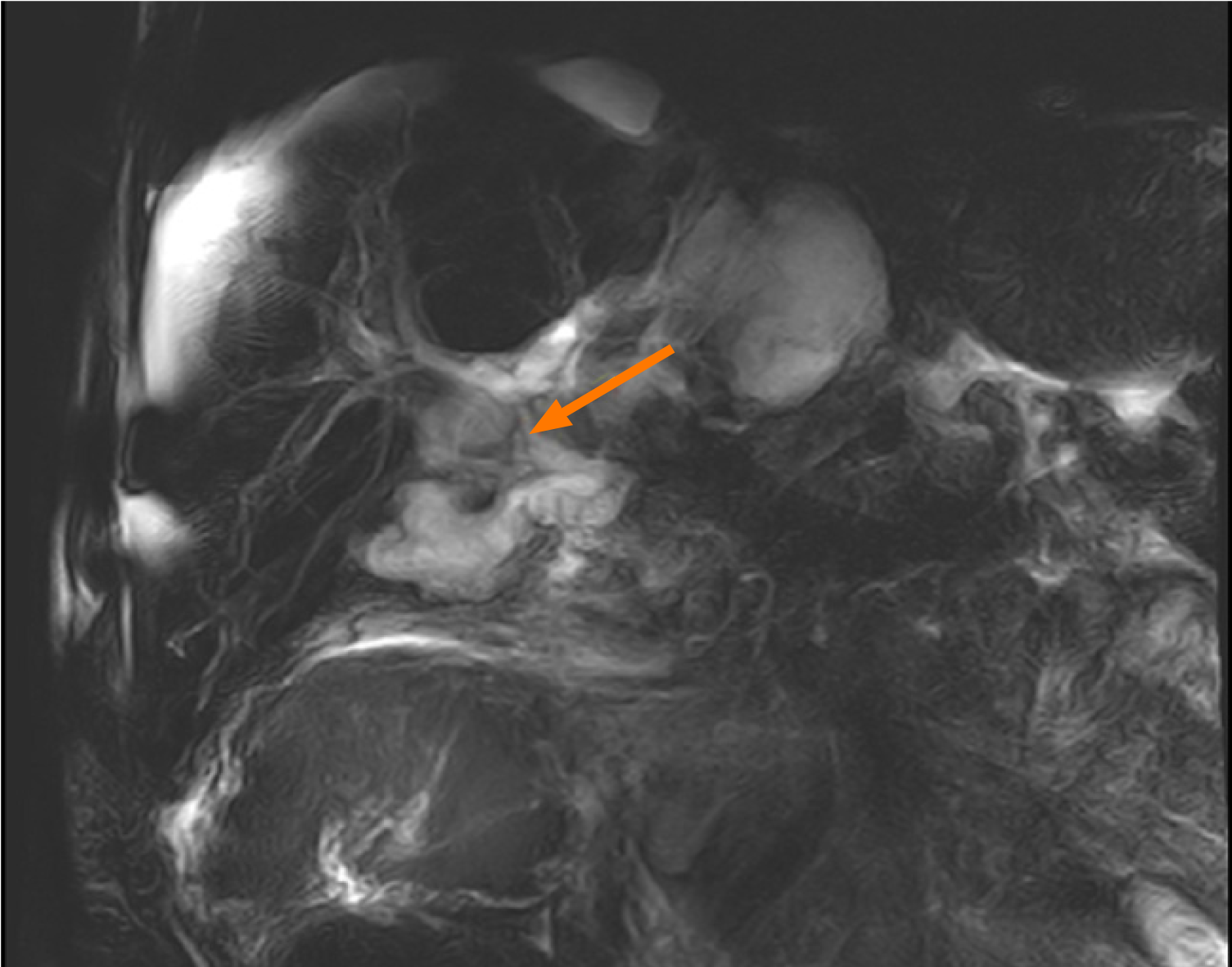
Оn the 40th postoperative day there was ultrasound evidence of fluid with heterogenic echogenicity around the graft and in the abdominal cavity as well as abnormal folding of the common bile duct. Magnetic resonance cholangiopancreatography was performed, which showed that the fluid collection is with high signal intensity on T2W TSE and is with a biliary origin (Figure 1 and Figure 2). The patient underwent emergency surgical revision. Intraoperatively a large abscess adjacent to the graft was established, which was drained. T-tube drain was placed in the common bile duct. Forty-eight hours after the surgery, the patient developed septic shock with anuria. The conservative treatment was unsuccessful, which lead to the patient’s fatal outcome on the 42nd postoperative day.
In cases of acutely ill patients, when a ABO-identical graft is not available, the only viable option is to perform LT using ABO‐nonidentical or incompatible grafts. When the donor and the recipient are ABO-nonidentical/incompatible, there is a high risk for hemolytic anemia due to PLS as well as AMR[6]. AMR leads to higher incidence of hepatic artery and biliary complications and decreased graft survival[14,15].
It is well-known that even though the liver is relatively resistant to rejection compared to other solid organs, hyperacute rejection may be observed after LT, especially after ABO-nonidentical/incompatible LT[16]. The issue with antibodies against blood group antigens is a severe risk factor because these antigens are found on the surface of blood cells and also on the vessel endothelium and the cells covering the large bile duct[17]. Moreover, epithelium of biliary tract and endothelium continue to express blood group antigens of the donor up to 5 mo after transplantation leading to increased risk of certain immunological complications related to the bile duct injury and susceptibility to hepatic artery thrombosis[15].
We have to emphasize that the safety of ABO-nonidentical/incompatible LT is different depending on the patient’s age. For pediatric populations, especially for children under 2 years of age, it is considered safer mainly because of the relatively immature immune system and the more significant potential of accommodating the liver graft[18]. However, although it is a debatable topic among the transplant community, ABO-mismatched LT is also performed in adults due to the shortage of organ donation. This explains the lower rate of adult ABO-incompatible LT compared to pediatric (0.8% vs 4% of all LT)[6].
In line with this, the survival of liver allografts followed by ABO-noni-dentical/incompatible transplantation depends on three factors according to Warner et al[19]. The first condition for the successful ABO-mismatched LT is the low expression of antigens on the graft cells. The second is low titer of anti-donor ABO antibodies in the recipient before transplantation. For our patient before LT, the isotiters were undetectable. The better survival rate was proved in ABO-incompatible pediatric patients (younger than 1 year) compared to recipients older than 16 years (76% vs 22%, respectively)[5]. However, as expected, we found anti-erythrocyte IgG antibodies in patient plasma and antibodies against anti-A and anti-B and complement fixed on the recipient erythrocytes at the end of 2nd wk after transplantation.
The third, and probably the most critical factor related to graft survival after ABO-nonidentical/incompatible LT, is the capacity to sustain low titers of donor ABO antibodies in the recipient after the transplantation for at least the 1st couple of weeks (preferably from week 3 to week 6)[19]. Usually, after the early days following ABO-nonidentical/incompatible transplantation, the isotiters increase[20], as we observed in our patient after the transplantation. Furthermore, except for the natural rebound, there are also de novo alloantibodies developed. Therefore, the critical period for AMR and risk of graft loss in the 1st 2 wk is due to this alloimmune reaction[21]. After this period, it is assumed that the graft is better adapted to the new environment, the abovementioned process of accommodation. In addition, we must mention that the target titer for anti-erythrocyte IgG and IgM antibodies after ABO-nonidentical/incompatible LT varies from center to center[3]. However, in our patient, after implementing a reliable treatment protocol, we observed a decrease in isotiters at the end of week 3 after the transplantation.
In such cases, when stimulated donor B lymphocytes produce antibodies against antigens on the recipient’s red blood cells after the transplantation, utilizing B lymphocyte targeted therapy (rituximab) is proven to be beneficial[11]. Furthermore, the risk of hepatic necrosis and biliary complications (due to AMR) is dramatically reduced[10]. Due to the rituximab-based protocol, ABO-mismatched LT became a feasible option for patients with end-stage liver disease. However, we have to keep in mind that even current treatment protocols cannot completely prevent the development of early complications, such as AMR and PLS[22].
In 90% of all post-transplantation cases, the rebound of anti-A/B antibodies, even in low titers, leads to rejection. Although the use of plasma treatment procedures, conventional immunosuppression regimes, and anti-CD20 monoclonal antibodies, the depletion of B cells that are responsible for the production of these isoantibodies is only transient, often leading to fatal outcomes due to AMR. However, other scenarios like severe cell-mediated rejection, vascular thrombosis, acute liver necrosis, bile duct complications, and sepsis are also possible[5]. In our case, the cause for the fatal outcome was the development of sepsis and not the AMR. We did not obtain data for AMR contribution to patient death.
The use of anti-CD20 antibodies or splenectomy along with immunosuppression to prevent post-transplantation antibody rebound is still debated. We have to consider the possibility of over-immunosuppression and severe systemic infections[5,8]. The mechanism of action of anti-CD20 monoclonal antibodies is to eliminate CD20-positive B cells for up to half a year. However, this therapy regimen does not contribute directly to reducing antibody-producing plasma cells. Thus, even on such treatment, low titers of isoantibodies are possible. The major advantage of anti-CD20 therapy is the delayed production of de novo antibodies due to the depletion of B cells[23-25].
Recent reports did not show any benefit of splenectomy in ABO-incompatible LT. No significant differences in anti-A/B IgM and IgG antibody titers were determined between the groups of patients with and without splenectomy[26]. Our team of surgeons did not consider splenectomy for our patient due to the risk of developing other possible complications, such as over-immunosuppression, perioperative risk of pancreatic fistula, morbidity, and sepsis.
On the other hand, we decided to add IVIG preparations to the patient’s treatment taking the advantages of their potent immunoregulatory qualities[27]. One of the most essential and desirable immunomodulating properties of IVIG is the mechanism of idiotypic-anti-idiotypic antibody interaction. However, we have to keep in mind that IVIG can passively infuse anti-A/B antibodies.
As the meta-analysis of Wu et al[28] in 2011 confirmed, the ABO-noni-dentical/incompatible LT has not been a contraindication in cases of life-saving conditions and shortage of organs. Furthermore, the graft and patient survival rates were similar to ABO-identical LT when using treatment protocol with plasmapheresis, IVIG, immunosuppressive therapy (tacrolimus, mycophenolate mofetil, steroids, rituximab), etc. The proper therapeutic regimen can limit the direct immunological mechanisms related to expressed A and B blood group antigens on the bile duct epithelium.
Our patient developed hemolytical anemia due to the PLS with elevated anti-A/B antibodies. However, with the proper therapeutic regimen, the ABO-mismatch could be overcome. However, 42 d after the surgical procedure of LT, the patient passed away due to biliary leakage, formation of abdominal abscess, and development of septic shock.
In our case, we presented a patient who underwent ABO-nonidentical LT from a deceased donor as an option for curative treatment of end-stage liver disease. We implemented the latest technological advancements and therapeutic approaches in the management of the patient to improve the clinical outcomes following transplantation and the antibody rebound. However, despite the satisfactory initial results in enhancing the patient’s condition and the decrease in antibody titers, the outcome was not favorable due to severe infectious complications and sepsis.
We want to thank Madarjieva, MD, Prof. Iskra Altankova, MD and Prof. Lyubomir Spassov, MD.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Islek A, Marino IR S-Editor: Dou Y L-Editor: Filipodia E-Editor: Liu MY
| 1. | Yadav DK, Hua YF, Bai X, Lou J, Que R, Gao S, Zhang Y, Wang J, Xie Q, Edoo MIA, Chutturghoon VK, Liang T. ABO-Incompatible Adult Living Donor Liver Transplantation in the Era of Rituximab: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2019;2019:8589402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Lai JC, Roberts JP. ABO-Nonidentical Liver Transplantation in the United States. Am J Transplant. 2016;16:2430-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Romero S, Solves P, Lancharro A, Cano I, Moscardó F, Carpio N, Sanz MÁ. Passenger lymphocyte syndrome in liver transplant recipients: a description of 12 cases. Blood Transfus. 2015;13:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | Rummler S, Bauschke A, Bärthel E, Jütte H, Maier K, Ziehm P, Malessa C, Settmacher U. Current techniques for AB0-incompatible living donor liver transplantation. World J Transplant. 2016;6:548-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Fisher RA. Living donor liver transplantation: eliminating the wait for death in end-stage liver disease? Nat Rev Gastroenterol Hepatol. 2017;14:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Rummler S, Bauschke A, Baerthel E, Juette H, Maier K, Malessa C, Barz D, Settmacher U. ABO-Incompatible Living Donor Liver Transplantation in Focus of Antibody Rebound. Transfus Med Hemother. 2017;44:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Goss MB, Rana A. ABO-incompatible liver transplantation: Is it a viable option with modern innovation? Clin Liver Dis (Hoboken). 2017;10:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Monteiro I, McLoughlin LM, Fisher A, de la Torre AN, Koneru B. Rituximab with plasmapheresis and splenectomy in abo-incompatible liver transplantation. Transplantation. 2003;76:1648-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Egawa H, Umeshita K, Uemoto S. The Best Regimen With Rituximab for ABO-Incompatible Living Donor Liver Transplantation [abstract]. Am J Transplant. 2015;15 Suppl 3. Available from: https://atcmeetingabstracts.com/abstract/the-best-regimen-with-rituximab-for-abo-incompatible-living-donor-liver-transplantation. |
| 10. | Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, Horsfield C, Loupy A, Mengel M, Perkowska-Ptasińska A, Rabant M, Racusen LC, Solez K, Becker JU. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation. 2018;102:1795-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 542] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 11. | Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, Ha TY, Jung DH, Park GC, Kim WJ, Sin MH, Yoon YI, Kang WH, Kim SH, Tak EY. ABO-Incompatible Adult Living Donor Liver Transplantation Under the Desensitization Protocol With Rituximab. Am J Transplant. 2016;16:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Terzieva V, Velikova T, Mihova A, Uzunova Y, Goncharov A, Yurukova N, Georgieva V, Sekulosky M, Mutafov Y, Chalamanov O, Altankova I, Spassov L. The Importance of Regulatory T Cells for the Tolerance Maintaining After Liver Transplantation. M J Immu. 2018;2:7. [DOI] [Full Text] |
| 13. | Terzieva V, Mihova A, Altankova I, Velikova T, Donchev D, Uzunova J, Goncharov A, Jurukova N, Georgieva V, Yordanova E, Sekulovski M, Chalamanov O, Spassov L. The Dynamic Changes in Soluble CD30 and Regulatory T Cells Before and After Solid Organ Transplantations: A Pilot Study. Monoclon Antib Immunodiagn Immunother. 2019;38:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, Esquivel C, Shapiro R, Zjako A, Markus B. Antibody mediated rejection of human liver allografts: transplantation across ABO blood group barriers. Transplant Proc. 1989;21:2217-2220. [PubMed] |
| 15. | Sanchez-Urdazpal L, Batts KP, Gores GJ, Moore SB, Sterioff S, Wiesner RH, Krom RA. Increased bile duct complications in liver transplantation across the ABO barrier. Ann Surg. 1993;218:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 141] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Hanto DW, Fecteau AH, Alonso MH, Valente JF, Whiting JF. ABO-incompatible liver transplantation with no immunological graft losses using total plasma exchange, splenectomy, and quadruple immunosuppression: evidence for accommodation. Liver Transpl. 2003;9:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Ravn V, Dabelsteen E. Tissue distribution of histo-blood group antigens. APMIS. 2000;108:1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 229] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Yandza T, Lambert T, Alvarez F, Gauthier F, Jacolot D, Huault G, Fabre M, Valayer J. Outcome of ABO-incompatible liver transplantation in children with no specific alloantibodies at the time of transplantation. Transplantation. 1994;58:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Warner PR, Nester TA. ABO-incompatible solid-organ transplantation. Am J Clin Pathol. 2006;125 Suppl:S87-S94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Toso C, Al-Qahtani M, Alsaif FA, Bigam DL, Meeberg GA, James Shapiro AM, Bain VG, Kneteman NM. ABO-incompatible liver transplantation for critically ill adult patients. Transpl Int. 2007;20:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Raut V, Uemoto S. Management of ABO-incompatible living-donor liver transplantation: past and present trends. Surg Today. 2011;41:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Honda M, Sugawara Y, Kadohisa M, Shimata K, Sakisaka M, Yoshii D, Uto K, Hayashida S, Ohya Y, Yamamoto H, Yamamoto H, Inomata Y, Hibi T. Long-term Outcomes of ABO-incompatible Pediatric Living Donor Liver Transplantation. Transplantation. 2018;102:1702-1709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Uchiyama H, Mano Y, Taketomi A, Soejima Y, Yoshizumi T, Ikegami T, Shirabe K, Maehara Y. Kinetics of anti-blood type isoagglutinin titers and B lymphocytes in ABO-incompatible living donor liver transplantation with rituximab and plasma exchange. Transplantation. 2011;92:1134-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Tydén G, Donauer J, Wadström J, Kumlien G, Wilpert J, Nilsson T, Genberg H, Pisarski P, Tufveson G. Implementation of a Protocol for ABO-incompatible kidney transplantation--a three-center experience with 60 consecutive transplantations. Transplantation. 2007;83:1153-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Schukfeh N, Lenz V, Metzelder ML, Paul A, Mathe Z, Kathemann S, Hoyer PF, Dohna-Schwake C, Gerner P. First case studies of successful ABO-incompatible living-related liver transplantation in infants in Germany. Eur J Pediatr Surg. 2015;25:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Raut V, Mori A, Kaido T, Ogura Y, Taku I, Nagai K, Sasaki N, Endo K, Hata T, Yagi S, Egawa H, Uemoto S. Splenectomy does not offer immunological benefits in ABO-incompatible liver transplantation with a preoperative rituximab. Transplantation. 2012;93:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Sewell WA, Jolles S. Immunomodulatory action of intravenous immunoglobulin. Immunology. 2002;107:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Wu J, Ye S, Xu X, Xie H, Zhou L, Zheng S. Recipient outcomes after ABO-incompatible liver transplantation: a systematic review and meta-analysis. PLoS One. 2011;6:e16521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |