Published online May 29, 2020. doi: 10.5500/wjt.v10.i5.117
Peer-review started: December 31, 2019
First decision: March 26, 2020
Revised: April 7, 2020
Accepted: May 5, 2020
Article in press: May 5, 2020
Published online: May 29, 2020
Processing time: 149 Days and 11 Hours
As prevalence of nonalcoholic fatty liver disease increases in the population, livers with steatosis will continue to infiltrate the donor pool. Safe utilization of these extended criteria grafts is paramount given the increased risk associated with their use in transplantation. Prognostic factors that can predict liver dysfunction immediately after transplantation with macrosteatotic grafts are lacking.
To understand the relationship between interleukin-33 (IL-33) and complement in recipients immediately following liver reperfusion as a marker of liver dysfunction.
Cohort consisted of patients who received a liver transplant from September 2016–September 2019 at our institution. Clinical variables were retrospectively extracted from the electronic medical record. Back-table donor biopsies were obtained with donor steatosis percentage retrospectively determined by a board-certified pathologist. Blood samples were available immediately following liver transplantation. Quantification of plasma IL-33 and complement proteins, C3a and C5a, were determined by enzyme-linked immunosorbent assay. For mRNA expression, RNA was extracted from donor biopsies and used against a 780 gene panel.
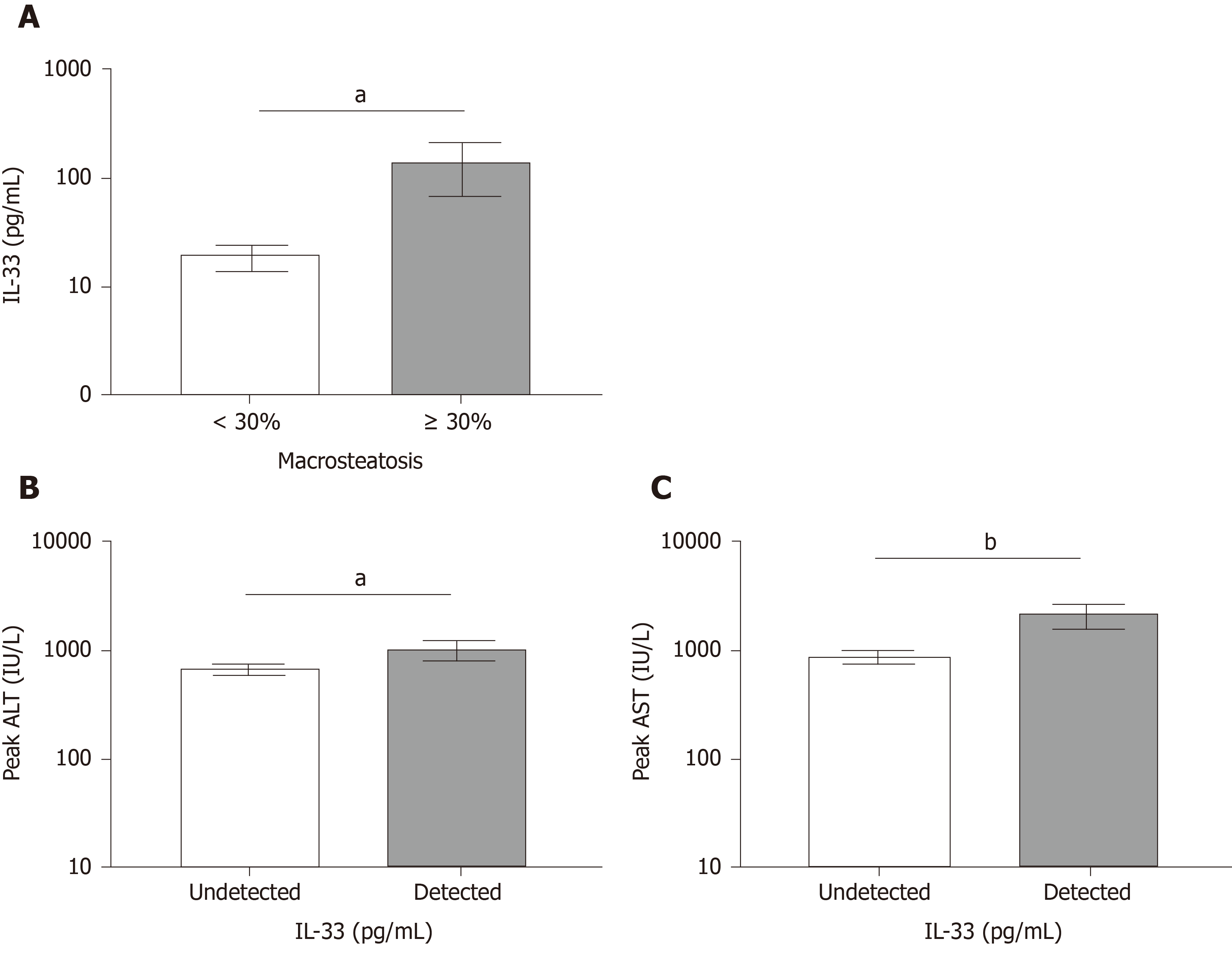
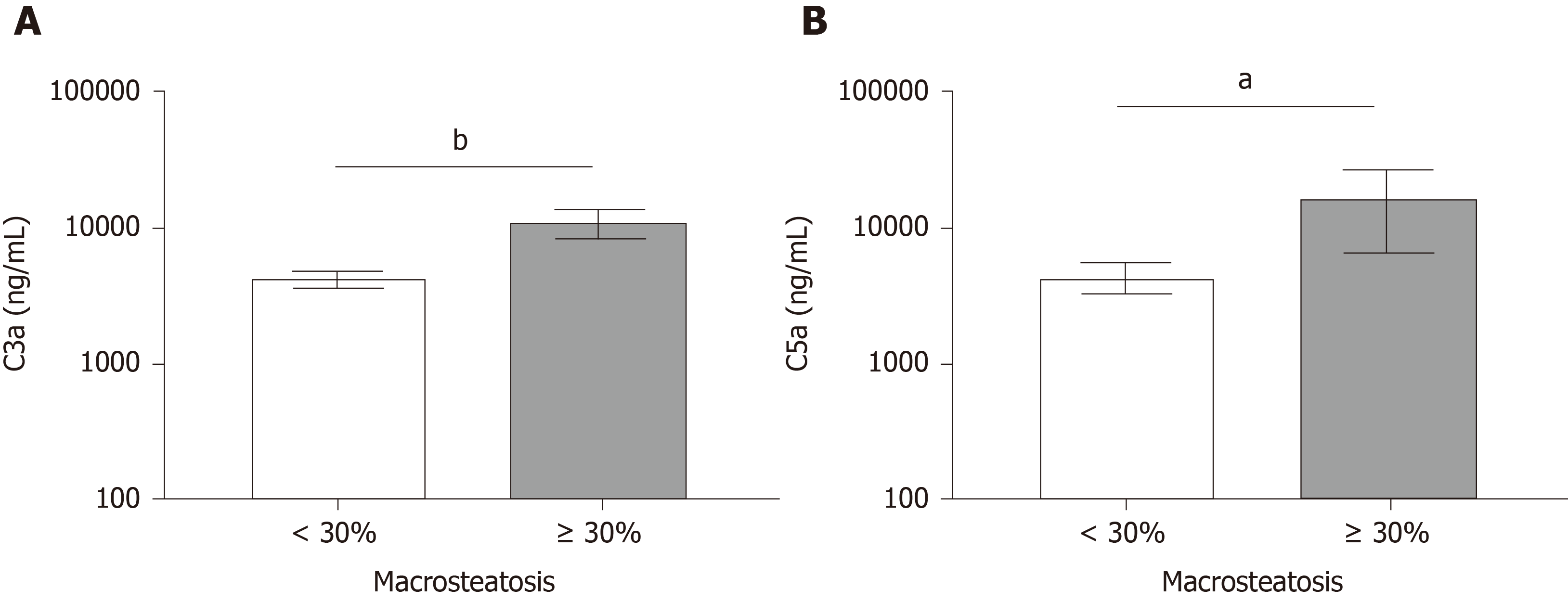
Cohort consisted of 99 donor and recipients. Donor median age was 45 years and 55% male. Recipients had a median age of 59 years with 62% male. The main etiologies were alcoholic hepatitis, nonalcoholic steatohepatitis, and hepatocellular carcinoma. Median MELD-Na at transplant was 21. Donors were grouped based on moderate macrosteatosis (≥ 30%). Recipients implanted with moderate macrosteatotic grafts had significantly higher peak alanine aminotransferase/aspartate aminotransferase (P < 0.001 and P < 0.004), and increased incidence of early allograft dysfunction (60% compared to 18%). Circulating IL-33 levels were significantly elevated in recipients of ≥ 30% macrosteatotic grafts (P < 0.05). Recipients with detectable levels of circulating IL-33 immediately following reperfusion had significantly higher alanine aminotransferase/aspartate aminotransferase (P < 0.05 and P < 0.01). Activated complement (C3a and C5a) were elevated in recipients implanted with moderate macrosteatotic grafts. RNA expression analysis of donor biopsies revealed moderate steatotic grafts upregulated genes inflammatory processes while downregulated hepatocyte-produced complement factors.
Circulating IL-33 and activated complement levels immediately following liver reperfusion in recipients of moderate macrosteatotic grafts may identify which patients are at risk of early allograft dysfunction.
Core tip: As nonalcoholic fatty liver disease incidence continues to rise, steatosis, both micro- and macro- will continue to infiltrate the donor pool. While many transplant centers have success utilizing these extended criteria donor grafts, macrosteatotic grafts remain at increased risk. In this study, elevations in interleukin-33 and activated complement (C3a and C5a) in recipients immediately following reperfusion indicate that patients receiving macrosteatotic grafts may have more injury post-transplant. Through mRNA expression of donor biopsies, we identify potential target genes present in macrosteatotic grafts that could aid in deciphering which macrosteatotic grafts are safe for implantation.
- Citation: Núñez K, Hamed M, Fort D, Bruce D, Thevenot P, Cohen A. Links between donor macrosteatosis, interleukin-33 and complement after liver transplantation. World J Transplant 2020; 10(5): 117-128
- URL: https://www.wjgnet.com/2220-3230/full/v10/i5/117.htm
- DOI: https://dx.doi.org/10.5500/wjt.v10.i5.117
Liver transplantation remains the only curative option for patients with end-stage liver disease. In order to address the deficit between donor availability and liver transplant demand, transplant centers have increasingly utilized extended criteria donor (ECD) grafts. ECD grafts include those of advanced age, history of viral hepatitis, long cold ischemia times, or simple steatosis[1]. Research into safe utilization of ECD grafts has identified several thresholds, specific to each extended criterion, which can minimize the risk of primary non-function. The rising incidence of nonalcoholic fatty liver disease is widespread throughout the world increasing the number of potential grafts with steatosis. The safe utilization of ECD grafts with steatosis would significantly increase the donor pool and decrease wait times for liver transplantation.
Liver steatosis is classified as either macro- or micro- depending on the intracellular fat droplet size. Macrosteatosis (MaS) can be defined as cytoplasmic lipid droplets large enough to displace the nucleus with microsteatosis defined as smaller droplets without nuclear displacement. MaS is most accurately determined through histological analysis by a pathologist and can be mild (< 30%), moderate (30%-60%), or severe (> 60%). The range of steatosis deemed acceptable for transplantation remains up to the discretion of each transplant center. Ploeg et al[2] found biopsy-proven donor MaS of moderate or severe were at increased risk of initial poor function. There are conflicting studies on the safe utilization of moderate steatotic grafts with studies demonstrating no difference in patient survival[3-5] and others showing large differences[6,7]. A large retrospective review of over 5000 donor biopsies from the United Network for Organ Sharing Standard Transplant Analysis and Research files, demonstrated that moderate MaS grafts were associated with graft failure at 1 year post-transplantation[8]. Identifying other critical co-variates in the setting of steatosis which more clearly identify primary nonfunction risk remain elusive.
Recently, our group identified interleukin-33 (IL-33) as a steatosis-driven biomarker that was predictive of liver damage in an animal model of ischemia-reperfusion injury[9]. Animals with severe MaS had decreased intrahepatic levels of IL-33 which occurs when hepatocytes undergo cellular damage[10]. Release of IL-33 immediately following reperfusion in recipients has not been investigated yet. In addition to alarmin release upon liver damage, during ischemia reperfusion injury, all three complement pathways become activated resulting in the cleavage product C3a and C5a. Furthermore, complement pathway may play a role in steatosis progression as observed in animal models[11,12].
MaS grafts are under an additional stress from steatosis, yet some moderate MaS grafts are implanted without significant problems. The purpose of this study was to understand the relationship between post-transplant IL-33 and complement anaphylatoxins (C3a and C5a) in recipients immediately following liver reperfusion with peak alanine aminotransferase/aspartate aminotransferase as an early marker of liver dysfunction. Additionally, we utilized mRNA expression analysis of pre-transplant donor biopsies to identify target genes in moderate MaS donors that may identify which moderate MaS grafts were safe for implantation.
This study was approved from the Ochsner Clinic Foundation Institutional Review Board (#2010.179). A total of 123 adults undergoing orthotopic liver transplantation (OLT) at our institution between September 2016–September 2019 were prospectively enrolled. Exclusion criteria included all living donors, recipients < 18 years old, donation after circulatory death and re-transplants. All grafts were from deceased brain dead donors. Donor graft data, recipient demographics, and post-operative variables were extracted from the electronic medical record. Standard surgical technique was used for orthotopic liver transplantations with caval replacement without veno-veno by-pass. Back-table liver biopsy was utilized to determine percentage MaS and microsteatosis, with analysis performed by a board-certified pathologist. Early allograft dysfunction was determined using the method of Olthoff et al[13]. This study was conducted according to the Declaration of Helsinki Principles.
An additional back-table punch biopsy (< 25 mg) was obtained from each donor graft, treated with RNAlater (ThermoFisher, Waltham, MA, United States) and stored. Samples were stored immediately in liquid nitrogen then transferred to -80 °C. Blood was collected in EDTA tubes (Becton Dickinson, Franklin Lakes, NJ) from recipients immediately following reperfusion. Samples were processed according to manufacturer’s protocol. Plasma was stored at -80 °C.
Recipient post-transplant plasma aliquots were recovered from long term cryostorage and batch analyzed for IL-33 by enzyme-linked immunosorbent assay (ThermoFisher, Waltham, MA, United States). Analyte values falling below the lowest standard were reported as the assay limit of detection (0.2 pg/mL). For binary analysis, values above the limit of detection were considered positive for IL-33 with values at or below the limit of detection considered negative for IL-33. Activated complement C3a and C5a were determined by enzyme-linked immunosorbent assay (ThermoFisher, Waltham, MA, United States).
Total RNA was extracted from back-table donor liver biopsies using Qiagen miRNeasy mini kit (Qiagen, Hilden, Germany). Extracted RNA was normalized to 20 ng/L, frozen at -80°C and shipped to NanoString Technologies (Seattle, WA, United States) for analysis. The mRNA was analyzed using the nCounter Human Organ Transplant Panel. The panel consisted of 758 genes spanning core pathways and processes involved in rejection of transplanted grafts plus 12 reference genes. Data analysis was performed using nSolverTM version 4.0. Gene expression was corrected for background and normalized to the mean expression of panel reference genes. Fold difference was obtained by dividing RNA counts. Cutoff for fold difference included highest fold changed with minimum mRNA count > 500.
Variables were reported as median and range for continuous variables, and percentage for categorical variables. χ2 test was used to analyze categorical variables. For continuous variables, Welch’s test was used for unequal variance and Mann-Whitney for non-parametric. Logistic regression was used to analyze continuous and categorical variables. Univariate analysis was performed to determine differences of donor and recipient demographics and variables between absent/mild and moderate macrosteatotic grafts. Data is shown as means ± standard error of the means. Limit of statistical significance was set at P < 0.05. Analysis was performed with SAS JMP version 13.0 (SAS Institute, Cary, NC, United States).
Donor demographics and graft variables are shown in Table 1. A total of 99 donor-recipient pairs met inclusion/exclusion criteria for the study. Donors were 55% male with a median age of 45 years. Graft MaS ranged from 0-45% with microsteatosis from 0-90%. Median cold and warm ischemia were 265 and 29 min.
| Characteristic | n = 99 | |
| Donor | ||
| Age/yr | Median (range) | 45 (6-78) |
| Gender/male | n (%) | 53 (55) |
| Warm ischemia time/min | Median (range) | 29 (24-79) |
| Cold ischemia time/min | Median (range) | 265 (129-453) |
| Graft | ||
| Macrosteatosis | Median % (range) | 0 (0-40%) |
| Microsteatosis | Median % (range) | 0 (0-90%) |
Transplant recipients were 62% male with a median age of 59 years (Table 2). The predominant liver etiologies were alcoholic hepatitis (EtOH, 26%), nonalcoholic steatohepatitis (NASH, 23%), and hepatocellular carcinoma (HCC, 18%). Post-operative peak alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels within the first week post-operative was 631 and 506 IU/L, respectively. Seven days post-transplant, median INR and bilirubin levels were 1.1 and 1.9 mg/dL, respectively. Median MELD-Na at the time of transplant was 21.
| Characteristic | n = 99 | |
| Recipient | ||
| Age/yr | Median (range) | 59 (18-72) |
| Gender/male | n (%) | 61 (62) |
| MELD at transplant | Median (range) | 21 (6-38) |
| Etiology | ||
| EtOH | n (%) | 25 (26) |
| NASH | n (%) | 23 (23) |
| HCC | n (%) | 18 (18) |
| HCV | n (%) | 9 (9) |
| Other | n (%) | 24 (24) |
| Peak AST (Day 1-7)/(IU/L) | Median (range) | 631 (144-13645) |
| Peak ALT (Day 1-7)/(IU/L) | Median (range) | 506 (87-5002) |
| INR (Day 7) | Median (range) | 1.1 (0.9-2.5) |
| Bilirubin (Day 7)/(mg/dL) | Median (range) | 1.9 (0.5-16.8) |
The cohort was sub-grouped and analyzed based on MaS for differences in donor demographics and graft variables, shown in Table 3. Ninety-four donor grafts had absent or mild MaS (< 30%) with 5 grafts have moderate MaS (≥ 30%). Differences in donor age and gender did not reach significance. Microsteatosis was significantly higher in moderate MaS grafts (median 0% MaS < 30% compared to 50% MaS ≥ 30%). Warm and cold ischemia time differed slightly between the two groups but did not reach significance.
| Characteristic | < 30% | ≥ 30% | P value | |
| Donor macrosteatosis | ||||
| Cohort | n (%) | 94 | 5 | |
| Age/yr | Median (range) | 45 (6-78) | 28 (21-52) | 0.21 |
| Gender/male | n (%) | 51 (55) | 2 (40) | 0.50 |
| Warm ischemia time/min | Median (range) | 29 (24-79) | 32 (28-37) | 0.60 |
| Cold ischemia time/min | Median (range) | 265 (129-453) | 307 (248-422) | 0.06 |
| Graft | ||||
| Macrosteatosis | Median % (range) | 0 (0-10%) | 30 (30-40%) | |
| Microsteatosis | Median % (range) | 0 (0-100%) | 50 (0-70%) | 0.001c |
Recipients variables were similarly sub-grouped based on absent-mild MaS (< 30%) or moderate MaS (≥ 30%). Recipient demographics and post-operative lab variables are shown in Table 4. Graft with moderate MaS were given to younger recipients (39 vs 59 years, P = 0.01). MELD-Na at the time of transplant did not significantly differ between the two groups. Moderate MaS grafts were utilized in NASH recipients at a higher frequency (60%) compared to absent-mild MaS grafts (21%). Post-operative peak ALT and AST levels were significantly higher in recipients of moderate MaS grafts (P < 0.004 and P < 0.001). Bilirubin levels on post-operative day 7 were also significantly higher in recipients of moderate MaS grafts (P < 0.02). A significant difference in early allograft dysfunction was observed in recipients of moderate MaS grafts (60% Olthoff EAD compared to 18% Olthoff EAD).
| Characteristic | < 30% | ≥ 30% | P value | |
| Donor macrosteatosis | ||||
| Recipient Cohort | 94 | 5 | ||
| Age/yr | Median (range) | 59 (21-72) | 39 (18-69) | 0.01b |
| Gender/male | n (%) | 57 (61) | 4 (80) | 0.36 |
| MELD at transplant | Median (range) | 21 (6-38) | 14 (8-34) | 0.90 |
| Etiology | ||||
| EtOH | n (%) | 24 (26) | 1 (20) | |
| NASH | n (%) | 20 (21) | 3 (60) | |
| HCC | n (%) | 18 (19) | ||
| HCV | n (%) | 9 (10) | ||
| Other | n (%) | 23 (24) | 1 (20) | |
| Peak AST (Day 1-7)/(IU/L) | Median (range) | 617 (144-13645) | 5195 (321-9829) | 0.001c |
| Peak ALT (Day 1-7)/(IU/L) | Median (range) | 494 (87-4253) | 1327 (398-5002) | 0.004b |
| INR (Day 7) | Median (range) | 1.1 (0.9-2.5) | 1.1 (1.0-1.5) | 0.60 |
| Bilirubin (Day 7)/(mg/dL) | Median (range) | 1.9 (0.5-11) | 4.6 (1.3-16.8) | 0.03a |
| No EAD | n (%) | 77 (82) | 2 (40) | |
| EAD | n (%) | 17 (18) | 3 (60) | 0.04a |
We next investigated the hypothesis that moderate MaS grafts released IL-33 and whether moderate MaS donor-derived products increase complement activation immediately following organ reperfusion. IL-33 was above the limit of detection in 45% of specimens analyzed. Figure 1A shows a significant increase in circulating IL-33 in recipients receiving moderate MaS grafts compared to those receiving grafts with absent-mild MaS. We next compared whether detectable IL-33 post-reperfusion was associated with higher peak ALT and AST post-OLT Figure 1B and 1C. Recipients with detectable IL-33 had significantly higher peak ALT and AST within the first 7 d post-transplant (P < 0.05 and P < 0.01). Both C3a and C5a were significantly elevated in recipients of moderate MaS grafts (Figure 2, P < 0.01 and P < 0.02), however regression of these values with peak AST and ALT did not reach significance (data not shown).
The prevailing hypothesis for organ dysfunction when utilizing moderate-severe MaS donors is the hypoxia-reoxygenation stress on lipid-laden hepatocytes leading to necrosis triggering a sterile immune response leading to further hepatocyte loss and ultimately primary dysfunction or nonfunction. As IL-33 (damage-associated molecular pattern) and C3a/C5a (complement anaphylatoxins) were elevated in the blood following transplantation of moderate MaS organs, we next investigated a 758 gene panel associated with organ rejection in absent-mild verses moderate MaS donors following procurement, perfusion and storage in preservation solution, and flushing of preservation solution immediately prior to transplantation. A sub-cohort of 22 donor biopsies were utilized for analysis, with donor and recipient demographics displayed in Table 5 after grouping for moderate MaS.
| Characteristic | < 30% | ≥ 30% | P value | |
| Donor | ||||
| Cohort | n (%) | 18 | 4 | |
| Age/yr | median (range) | 46 (6-70) | 27 (21-45) | 0.13 |
| Gender/male | n (%) | 11 (61) | 2 (50) | 0.68 |
| Warm ischemia time/min | Median (range) | 29 (24-63) | 33 (28-37) | 0.93 |
| Cold ischemia time/min | Median (range) | 270 (203-296) | 341 (248-422) | 0.003b |
| Graft | ||||
| Macrosteatosis | Median % (range) | 2 (0-10%) | 30 (30-40%) | |
| Microsteatosis | Median % (range) | 7.5 (0-80%) | 45 (0-70%) | 0.07 |
| Recipient | ||||
| Age/yr | Median (range) | 58 (35-69) | 40 (18-69) | 0.07 |
| Gender/male | n (%) | 12 (67) | 4 (100) | 0.09 |
| MELD-Na at transplant | Median (range) | 21 (8-29) | 14 (8-34) | 0.66 |
| Etiology | ||||
| EtOH | n (%) | 3 (17) | 1 (25) | |
| NASH | n (%) | 7 (39) | 3 (75) | |
| HCC | n (%) | 5 (28) | ||
| HCV | n (%) | 1 (5) | ||
| Other | n (%) | 2 (11) | ||
| Peak AST (Day 1-7)/(IU/L) | Median (range) | 630 (212-12987) | 3143 (321-9232) | 0.12 |
| Peak ALT (Day 1-7)/(IU/L) | Median (range) | 494 (95-4253) | 1018 (398-5002) | 0.16 |
| INR (Day 7) | Median (range) | 1.1 (0.9-1.3) | 1.1 (1.0-1.5) | 0.45 |
| Bilirubin (Day 7)/(mg/dL) | Median (range) | 1.9 (0.6-9.6) | 4.0 (1.3-16.8) | 0.06 |
| OLTHOFF EAD Index | ||||
| No EAD | n (%) | 16 (89) | 2 (50) | |
| EAD | n (%) | 2 (11) | 2 (50) | 0.10 |
Eighteen samples were from grafts with absent-mild MaS with four containing > 30% MaS. Univariate analysis revealed only cold ischemia time different between the two groups with moderate MaS grafts having a longer time (P < 0.003). Genes which reached preset thresholds for fold change are listed in Table 6. The largest mRNA fold difference was observed in inflammatory mediator C-C Motif Chemokine Ligand 20 (CCL20), also known as liver activation regulated chemokine, with a 4.8-fold increase in ≥ 30% MaS grafts. This was followed by a selection of S100 proteins (S100A12, -8, and -9) with fold increases of > 2 in ≥ 30% MaS grafts. Decreased fold differences were observed in a number of genes with the largest differences in complement 3 (C3), insulin-like growth factor binding protein 1, fibronectin 1, followed by complement factor properdin (CFB), and C-reactive protein.
| Gene name | Accession number | Counts in ≥ 30% | Count in < 30% | Fold difference |
| CCL20 | NM_004591.1 | 1,722 | 357 | +4.8 |
| S100A12 | NM_005621.1 | 1,052 | 327 | +3.2 |
| S100A8 | NM_002964.4 | 5,919 | 2,487 | +2.4 |
| S100A9 | NM_002965.3 | 8,959 | 4,030 | +2.2 |
| C3 | NM_000064.2 | 5,543 | 8,484 | -1.5 |
| IGFBP1 | NM_000596.2 | 2,048 | 3,093 | -1.5 |
| FN1 | NM_212482.1 | 1,833 | 2,736 | -1.5 |
| CFB | NM_001710.5 | 8,096 | 11,359 | -1.4 |
| CRP | NM_000567.2 | 33,132 | 45,455 | -1.4 |
While extended criteria grafts provide a valuable expansion to the available donor pool, safe utilization remains paramount, particularly since these grafts are often utilized in ideal recipients for which excellent outcomes would be anticipated with normal criteria for liver transplantation. The rising incidence of nonalcoholic fatty liver disease is anticipated to have a dramatic influence on MaS prevalence in the donor pool. Several studies from the early 1990s into 2000s demonstrated the risk of primary dysfunction and non-function associated with moderate-severe MaS (generally defined as ≥ 30% with severe MaS ≥ 60%), manifested through increased susceptibility to ischemia reperfusion injury resulting in elevated post-operative AST and ALT levels[14]. More recently, a study of 5000 donors from the Scientific Registry of Transplant Recipients revealed moderate MaS grafts (≥ 30%) were at increased risk of graft failure at 1 year compared to grafts with < 15% MaS[8]. The collective effect of these studies was cautious utilization of livers with MaS ≥ 30% with an elevated focus on identifying ideal recipients, evidenced by several reports demonstration success when transplanting ideal recipients with moderate to severe MaS grafts[3,4]. Ideal recipients of moderate MaS grafts tend to have lower MELD-Na, no prior surgeries, no anticipated intraoperative issues.
Recently our group and others have focused on a damage-associated molecular pattern signature, which may identify MaS livers more susceptible to ischemia-reperfusion injury. Mechanistic animal studies have demonstrated that damage-associated molecular pattern release during necrosis precedes and directly promotes sterile inflammation, through processes involving complement activation and subsequently complement component deposition which further propagates hepatocyte loss. In this study we utilized a prospective cohort from a high-volume transplant center to determine whether levels of IL-33 and activated C3a/C5a were detectable immediately following liver reperfusion and their relationship with donor moderate MaS. Further, we aimed to define the relationship between post-transplant IL-33 and complement anaphylotoxins with peak alanine aminotransferase/aspartate aminotransferase as an early marker of liver dysfunction. Finally, we utilized mRNA analysis of pre-transplant donor biopsy to identify target genes in moderate MaS donors which could ultimately help distinguish high and low risk moderate MaS grafts.
Despite allocation strategies to reduce the risk of primary dysfunction, recipients receiving moderate MaS grafts had higher peak post-operative AST and ALT levels and EAD incidence (Table 4). EAD incidence was 18% in recipients of none-mild steatotic grafts while 60% in those receiving moderate steatotic grafts. This is in agreement with a study of 165 consecutive liver transplants found a 53% incidence of EAD in moderate MaS grafts[15]. Donor graft analysis revealed younger median age, greater cold ischemia time (CIT), and a higher degree of microsteatosis in the moderate MaS group compared to absent-mild MaS. To avoid stacking risk factors, moderate MaS grafts were implanted in lower risk recipients, reflected by younger age with similar CIT and MELD scores at transplant. While the median CIT between the two groups differed by 42 minutes, we do not believe this impacted the greater risk of EAD observed within moderate MaS group. A recent 2018 study showed grafts with CIT > 12 h had increased risk of early allograft dysfunction[16]. Another study demonstrated grafts with > 9 h of CIT were associated with graft loss[17]. Within our cohort, median CIT was under 5 h with the longest CIT at 7.5 h, well below the range described in the literature for increased risk. Additionally, Westerkamp et al[18] demonstrated no difference in post-transplant outcomes between moderate MaS and nonsteatotic grafts with median CIT below 8 h. Liver steatosis is often found as a mixture of both MaS and microsteatosis. Recently, Croome et al[19] showed grafts with microsteatosis were at increased risk of early allograft dysfunction compared to grafts without steatosis. While CIT may not have attributed to the increased incidence of early allograft dysfunction observed in our cohort, microsteatosis could have contributed along with MaS.
Survival outcomes within the cohort were as follows: six patients (6%) experienced graft failure at a median time of 139 d post-transplant with four of these patients having graft-related death (4%). None of the patients were transplanted with moderate MaS grafts experienced graft failure or death, despite 60% meeting criteria for EAD. While recipients with EAD had significantly longer length of stay (P < 0.01) with mean of 22 d for those with EAD compared to 10.5 d for those without EAD.
IL-33 in a nuclear cytokine constitutively expressed in hepatocytes but upon liver damage, IL-33 is rapidly released by hepatocytes undergoing cell death and can act as an alarmin to alert the immune system of tissue damage[10]. During procurement, donor grafts undergo ischemia reperfusion injury and could result in release of IL-33 as an alarmin. In recipients of moderate MaS livers, we find a significant increase in circulating IL-33 immediately following reperfusion. IL-33 released from liver endothelial cells during ischemia reperfusion injury was found to promote neutrophil infiltration[20], which could increase immune-mediated damage to the donor graft.
We also observed a marked increase in C3a/C5a in recipients of livers with moderate MaS immediately following reperfusion, suggesting that donor-derived factors are immediately triggering complement activation in the setting of moderate MaS. Several mechanistic animal studies have demonstrated the role of the complement cascade in response to hepatocyte necrosis during ischemia reperfusion injury[21,22]. While all three pathways are activated, all converge on the cleavage products C3a and C5a (see reviews[23,24]). Evidence suggests that MaS exacerbates complement activation during ischemia-reperfusion[12]. Serum C3 becomes progressively elevated in nonalcoholic fatty liver disease[25,26], and animal models of NAFLD have shown that C3 depletion improves survival following ischemia reperfusion injury[12]. Several clinical trials are underway targeting complement using inhibitors to lessen ischemia reperfusion injury during liver transplantation[27]. Currently, a clinical trial (NCT03468140) is underway using a C5 inhibitor, Eculizumab, in macrosteatotic grafts to decrease post-transplant liver dysfunction. Our results provide further justifications into clinical trials targeting complement in macrosteatotic grafts.
Release of intrahepatic IL-33 with C3a and C5a activation provides an early indication of dysfunction immediately post-transplant. With the goal of identifying driver biomarkers and pathways, we performed panel mRNA analysis on the donor organs from samples obtained prior to transplantation (Table 6).
These results identified a signature of inflammation and possibly mildly impaired hepatic function in moderate MaS organs. CC chemokine ligand 20 (CCL20), a chemokine released to attract lymphocytes, had a 4.8-fold upregulation in moderate steatotic grafts. In the setting of cirrhotic progression of NAFLD, CCL20 was shown to be highly upregulated at both the protein and mRNA level[28]. Using the hepatocellular carcinoma cell line, HepG2, the authors demonstrated that CCL20 expression could be trigger by in vitro lipid loading[28]. In conjunction with CCL20, the other major gene signature in moderate MaS was centered on the S100A protein family. S100A proteins are a family of proteins with pro-inflammatory, calcium-binding properties and overexpressed during inflammation[29]. Both animal models of NASH and samples derived from patients with NASH have shown elevations in S100A9 and S100A8[30-32]. Collectively, these gene targets may identify a steatosis-associated inflammatory background contributing to increased transplant risk in moderate MaS and warrants further analysis after controlling for moderate MaS and transplant outcomes.
The expression of several liver-derived transcripts were found to be downregulated in moderate MaS. C3, CFB, and C-reactive protein are all components of the complement system. While both C3 and CFB were found to be elevated in the serum of NASH patients[33], both were downregulated in the liver, which given the organ status of post-cold storage, could indicate impaired hepatic function prior to transplantation.
As nonalcoholic fatty liver disease incidence continues to rise, steatosis, both micro- and macro- will continue to infiltrate the donor pool. While many transplant centers have success utilizing these extended criteria donor grafts, MaS grafts remain at increased risk. In this study, we identified elevations in IL-33 and activated complement (C3a and C5a) in recipients immediately following reperfusion indicating patients receiving MaS grafts may have more injury post-transplant. Furthermore, through mRNA expression of donor biopsies, we identify potential target genes present in MaS grafts. This data could aid in deciphering which MaS grafts are safe for implantation.
Due to the rise in nonalcoholic fatty liver disease, grafts with macrosteatosis will become more frequent in the donor pool. The use of macrosteatotic donor grafts for transplantation are associated with increased risk of graft failure and patient mortality. Factors that predict which macrosteatotic grafts are safe for transplantation remain limited.
The motivation for this study was to identify biomarkers immediately following reperfusion during transplantation with macrosteatotic grafts that predict increased injury post-transplant.
The objective of this study was to investigate the relationship between interleukin-33 and activated complement (C3a and C5a) with liver dysfunction in recipients immediately following liver reperfusion transplanted with either < 30% or ≥ 30% macrosteatotic grafts.
The cohort consisted of recipients transplanted with either < 30% or ≥ 30% macrosteatotic grafts. Blood was collected immediately following reperfusion with quantification of interleukin-33 and activated complement (C3a and C5a) levels. Punch biopsies in a subset of donor grafts (n = 22) were used for microRNA expression analysis.
Recipients transplanted with ≥ 30% macrosteatotic grafts had significantly higher ALT and AST levels, increased risk of early allograft dysfunction, and higher levels of interleukin-33 and activated complement (C3a and C5a) post-transplant compared to recipients transplanted with < 30% macrosteatotic grafts. Additionally, upregulation of pro-inflammatory genes were found in macrosteatotic grafts.
Quantification of interleukin-33 and activated complement (C3a and C5a) immediately following reperfusion during transplantation can provide insight into which recipients are at increased risk of early allograft dysfunction.
This study provides additional justification for targeting activated complement in macrosteatotic grafts prior to transplantation.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Ho CM, Zheng H S-Editor: Wang J L-Editor: A E-Editor: Liu MY
| 1. | Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 497] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 2. | Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 804] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 3. | McCormack L, Petrowsky H, Jochum W, Mullhaupt B, Weber M, Clavien PA. Use of severely steatotic grafts in liver transplantation: a matched case-control study. Ann Surg. 2007;246:940-946; discussion 946-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 4. | Wong TC, Fung JY, Chok KS, Cheung TT, Chan AC, Sharr WW, Dai WC, Chan SC, Lo CM. Excellent outcomes of liver transplantation using severely steatotic grafts from brain-dead donors. Liver Transpl. 2016;22:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Ahmed EA, El-Badry AM, Mocchegiani F, Montalti R, Hassan AEA, Redwan AA, Vivarelli M. Impact of Graft Steatosis on Postoperative Complications after Liver Transplantation. Surg J (N Y). 2018;4:e188-e196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | de Graaf EL, Kench J, Dilworth P, Shackel NA, Strasser SI, Joseph D, Pleass H, Crawford M, McCaughan GW, Verran DJ. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the Donor Risk Index. J Gastroenterol Hepatol. 2012;27:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Ureña MA, Ruiz-Delgado FC, González EM, Segurola CL, Romero CJ, García IG, González-Pinto I, Gómez Sanz R. Assessing risk of the use of livers with macro and microsteatosis in a liver transplant program. Transplant Proc. 1998;30:3288-3291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Spitzer AL, Lao OB, Dick AA, Bakthavatsalam R, Halldorson JB, Yeh MM, Upton MP, Reyes JD, Perkins JD. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl. 2010;16:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 255] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Núñez KG, Frank A, Gonzalez-Rosario J, Galliano G, Bridle K, Crawford D, Seal J, Abbruscato F, Vashistha H, Thevenot PT, Cohen AJ. Interleukin-33/ Cyclin D1 imbalance in severe liver steatosis predicts susceptibility to ischemia reperfusion injury. PLoS One. 2019;14:e0216242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Gao Y, Liu Y, Yang M, Guo X, Zhang M, Li H, Li J, Zhao J. IL-33 treatment attenuated diet-induced hepatic steatosis but aggravated hepatic fibrosis. Oncotarget. 2016;7:33649-33661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Bavia L, Cogliati B, Dettoni JB, Ferreira Alves VA, Isaac L. The complement component C5 promotes liver steatosis and inflammation in murine non-alcoholic liver disease model. Immunol Lett. 2016;177:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | He S, Atkinson C, Evans Z, Ellett JD, Southwood M, Elvington A, Chavin KD, Tomlinson S. A role for complement in the enhanced susceptibility of steatotic livers to ischemia and reperfusion injury. J Immunol. 2009;183:4764-4772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 874] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 14. | Perez-Daga JA, Santoyo J, Suárez MA, Fernández-Aguilar JA, Ramírez C, Rodríguez-Cañete A, Aranda JM, Sánchez-Pérez B, Montiel C, Palomo D, Ruiz M, Mate A. Influence of degree of hepatic steatosis on graft function and postoperative complications of liver transplantation. Transplant Proc. 2006;38:2468-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Deroose JP, Kazemier G, Zondervan P, Ijzermans JN, Metselaar HJ, Alwayn IP. Hepatic steatosis is not always a contraindication for cadaveric liver transplantation. HPB (Oxford). 2011;13:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Sibulesky L, Li M, Hansen RN, Dick AA, Montenovo MI, Rayhill SC, Bakthavatsalam R, Reyes JD. Impact of Cold Ischemia Time on Outcomes of Liver Transplantation: A Single Center Experience. Ann Transplant. 2016;21:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Pan ET, Yoeli D, Galvan NTN, Kueht ML, Cotton RT, O'Mahony CA, Goss JA, Rana A. Cold ischemia time is an important risk factor for post-liver transplant prolonged length of stay. Liver Transpl. 2018;24:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Westerkamp AC, de Boer MT, van den Berg AP, Gouw AS, Porte RJ. Similar outcome after transplantation of moderate macrovesicular steatotic and nonsteatotic livers when the cold ischemia time is kept very short. Transpl Int. 2015;28:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Croome KP, Lee DD, Croome S, Nakhleh RE, Abader Sedki Senada P, Livingston D, Yataco M, Taner CB. Does Donor Allograft Microsteatosis Matter? Comparison of Outcomes in Liver Transplantation With a Propensity-Matched Cohort. Liver Transpl. 2019;25:1533-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Yazdani HO, Chen HW, Tohme S, Tai S, van der Windt DJ, Loughran P, Rosborough BR, Sud V, Beer-Stolz D, Turnquist HR, Tsung A, Huang H. IL-33 exacerbates liver sterile inflammation by amplifying neutrophil extracellular trap formation. J Hepatol. 2017;S0168-8278:32291-32292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Diepenhorst GM, de Graaf W, Niessen HW, van Vliet AK, Hack CE, van Gulik TM. Immunoglobulin M, C-reactive protein and complement activation in rat hepatic ischemia-reperfusion injury. Eur Surg Res. 2014;52:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Heijnen BH, Straatsburg IH, Padilla ND, Van Mierlo GJ, Hack CE, Van Gulik TM. Inhibition of classical complement activation attenuates liver ischaemia and reperfusion injury in a rat model. Clin Exp Immunol. 2006;143:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1248] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 24. | Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33:479-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 575] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 25. | Jia Q, Li C, Xia Y, Zhang Q, Wu H, Du H, Liu L, Wang C, Shi H, Guo X, Liu X, Sun S, Wang X, Zhou M, Zhao H, Song K, Wu Y, Niu K. Association between complement C3 and prevalence of fatty liver disease in an adult population: a cross-sectional study from the Tianjin Chronic Low-Grade Systemic Inflammation and Health (TCLSIHealth) cohort study. PLoS One. 2015;10:e0122026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Xu C, Chen Y, Xu L, Miao M, Li Y, Yu C. Serum complement C3 levels are associated with nonalcoholic fatty liver disease independently of metabolic features in Chinese population. Sci Rep. 2016;6:23279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Núñez K, Thevenot P, Alfadhli A, Cohen A. Complement Activation in Liver Transplantation: Role of Donor Macrosteatosis and Implications in Delayed Graft Function. Int J Mol Sci. 2018;19:1750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Chu X, Jin Q, Chen H, Wood GC, Petrick A, Strodel W, Gabrielsen J, Benotti P, Mirshahi T, Carey DJ, Still CD, DiStefano JK, Gerhard GS. CCL20 is up-regulated in non-alcoholic fatty liver disease fibrosis and is produced by hepatic stellate cells in response to fatty acid loading. J Transl Med. 2018;16:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Foell D, Frosch M, Sorg C, Roth J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta. 2004;344:37-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 30. | Liu X, Wang Y, Ming Y, Song Y, Zhang J, Chen X, Zeng M, Mao Y. S100A9: A Potential Biomarker for the Progression of Non-Alcoholic Fatty Liver Disease and the Diagnosis of Non-Alcoholic Steatohepatitis. PLoS One. 2015;10:e0127352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Mukai K, Miyagi T, Nishio K, Yokoyama Y, Yoshioka T, Saito Y, Tanaka S, Shigekawa M, Nawa T, Hikita H, Sakamori R, Yoshihara H, Imai Y, Hiramatsu N, Tatsumi T, Takehara T. S100A8 Production in CXCR2-Expressing CD11b+Gr-1high Cells Aggravates Hepatitis in Mice Fed a High-Fat and High-Cholesterol Diet. J Immunol. 2016;196:395-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Serhal R, Hilal G, Boutros G, Sidaoui J, Wardi L, Ezzeddine S, Alaaeddine N. Nonalcoholic Steatohepatitis: Involvement of the Telomerase and Proinflammatory Mediators. Biomed Res Int. 2015;2015:850246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Segers FM, Verdam FJ, de Jonge C, Boonen B, Driessen A, Shiri-Sverdlov R, Bouvy ND, Greve JW, Buurman WA, Rensen SS. Complement alternative pathway activation in human nonalcoholic steatohepatitis. PLoS One. 2014;9:e110053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |