Published online Dec 28, 2020. doi: 10.5500/wjt.v10.i12.404
Peer-review started: June 22, 2020
First decision: July 25, 2020
Revised: August 18, 2020
Accepted: October 9, 2020
Article in press: October 9, 2020
Published online: December 28, 2020
Processing time: 184 Days and 9.6 Hours
To summarize the long-term complications after pancreas transplantation that affect graft function, a literature search was carried out on the long-term complications of pancreatic transplantation, namely, complications from postoperative 3rd mo onwards, in terms of loss of graft function, late infection and vascular complications as pseudoaneurysms. The most relevant reviews and studies were selected to obtain the current evidence on these topics. The definition of graft failure varies among different studies, so it is difficult to evaluate, a standardized definition is of utmost importance to know the magnitude of the problem in all worldwide series. Chronic rejection is the main cause of long-term graft failure, occurring in 10% of patients. From the 3rd mo of transplantation onwards, the main risk factor for late infections is immunosuppression, and patients have opportunistic infections like: Cytomegalovirus, hepatitis B and C viruses, Epstein-Barr virus and varicella-zoster virus; opportunistic bacteria, reactivation of latent infections as tuberculosis or fungal infections. Complete preoperative studies and serological tests should be made in all recipients to avoid these infections, adding perioperative prophylactic treatments when indicated. Pseudoaneurysm are uncommon, but one of the main causes of late bleeding, which can be fatal. The treatment should be performed with radiological endovascular approaches or open surgery in case of failure. Despite all therapeutic options for the complications mentioned above, transplantectomy is a necessary option in approximately 50% of relaparotomies, especially in life-threatening complications. Late complications in pancreatic transplantation threatens long-term graft function. An exhaustive follow-up as well as a correct immunosuppression protocol are necessary for prevention.
Core Tip: Late complications after pancreas transplant (> 3 mo after surgery) may occur, endangering loss of graft function. Chronic rejection is the main cause of long-term graft failure, occurring in 10% of patients, so targeted immunosuppressive therapy is important to prevent it; however, it predisposes to opportunistic viral, bacterial and fungal infections, and even the reactivation of latent infections, which should be prevented with perioperative prophylaxis and treated when necessary. Pseudoaneurysm should be early diagnosed, and treated by endovascular approach when possible, to prevent bleeding. Nonetheless, in some late complications, transplantectomy is a necessary option, especially in life-threatening complications.
- Citation: Maupoey Ibáñez J, Boscà Robledo A, López-Andujar R. Late complications of pancreas transplant. World J Transplant 2020; 10(12): 404-414
- URL: https://www.wjgnet.com/2220-3230/full/v10/i12/404.htm
- DOI: https://dx.doi.org/10.5500/wjt.v10.i12.404
Pancreas transplantation is currently the most effective method to establish durable normoglycemia for patients with diabetes mellitus[1], and therefore achieves the benefits demonstrated with intensive insulin therapy, but without hypoglycemic complications derived from that treatment. Pancreatic graft loss may occur due to several complications, such as technical failure in the early postoperative period, but also late complications (which occur from postoperative 3rd mo onwards), among which the most relevant are chronic rejection, late infections and vascular complications as pseudoaneurysms. In recent years, pancreatic graft long-term survival has improved, with a half-life longer than 14 years in simultaneous pancreas and kidney (SPK) transplantation, which is the most frequent modality of pancreas transplantation as it is associated with better allograft survival[2]. In this review, we will describe the late complications of pancreas transplant and recent trends in their prevention, diagnosis and treatment.
The Organ Procurement and Transplantation Network/United Network for Organ Sharing (OPTN/UNOS) Pancreas Transplantation Committee approved precise definitions of pancreas graft failure in early 2018. Some of the definitions are concrete, such as a recipient’s transplanted pancreas is removed, a recipient re-registers for pancreas transplant, a recipient registers for an islet transplant after undergoing pancreas transplant, or a recipient dies. Pancreas graft failure can also be defined if a recipient’s total insulin use is greater than or equal to 0.5 units/kg/d for a consecutive 90 d. The latter definition may be problematic if the recipient’s starting insulin dose is less than 0.5 units/kg/d[2].
Recently the consensus report from the International Pancreas & Islet Transplant Association and European Pancreas & Islet Transplantation Association[3] defined the outcomes of beta–cell (b-cell) replacement therapy: Optimal b-cell graft function is defined by near-normal glycemic control (HbA1c ≤ 6.5% [48 mmol/mol]) without severe hypoglycemia or requirement for insulin or other antihyperglycemic therapy, and with an increase over pretransplant measurement of C-peptide. Good b-cell graft function requires HbA1c < 7.0% (53 mmol/mol) without severe hypoglycemia and with a significant (> 50%) reduction in insulin requirements and restoration of clinically significant C-peptide production. Marginal b-cell graft function is defined by failure to achieve HbA1c < 7.0% (53 mmol/mol), the occurrence of any severe hypoglycemia, or less than 50% reduction in insulin requirements when there is restoration of clinically significant C-peptide production documented by improvement in hypoglycemia awareness/severity, or glycemic variability/lability. A failed b-cell graft is defined by the absence of any evidence for clinically significant C-peptide production. Optimal and good functional outcomes are considered successful clinical outcomes.
Although these newer definitions have not yet been reflected, reported early overall rate of pancreas graft failure (within the first 90 d) in 2018 was 5.9%, and 5-year pancreas graft survival rates were 73% for SPK, 65% for pancreas after kidney (PAK), and 53% for pancreas transplant alone (PTA)[2]. These results are similar in Spain, with a 5-year graft survival rate of 72%-74% for SPK[4].
Adverse technical and immunological events within the 1st year need to be avoided under all circumstances; what happens in the 1st year posttransplant has a very strong impact on long-term graft function[5]. The main cause of pancreas graft loss within the first 3 mo usually is technical failure: Graft thrombosis (10%-35%) followed by intraabdominal infections and pancreatitis[6]. An adequate immunosuppression therapy with tight control is recommended to avoid pancreas allograft rejection, since it is also one of the main causes of graft failure. Acute rejection usually develops 1 wk to 3 mo after transplantation but can occur earlier or later, it is difficult to diagnose, but may be suspected when loss of allograft function (hyperglycemia) is associated with high level of serum amylase and/or lipase. Early detection is essential to institute antirejection treatment and avert graft failure. Incidence in OPTN/UNOS data of a first rejection episode is improving over time, with low rates respect previous data for all categories of pancreas transplant, in 2016-2017 in the United States it was: 11.7%, 19.2%, and 12.4% following PAK, PTA and SPK respectively[2]. In the same way, global incidence of rejection in Spain series was 10.9% in SPK during last decade[4]. These low rejection rates clearly reflect ongoing improvements in immunosuppression protocols. In fact, the avoidance of acute rejection episodes is probably the single highest impact on excellent long-term function[5].
There is some controversy regarding immunosuppressive treatment in pancreas transplantation. Standard protocols include use of induction therapy followed by maintenance immunosuppression, but the amount, frequency and duration of each therapy, especially the induction treatment has not been clearly defined, which consist in T-cell depleting agent (e.g., antithymocyte globulin and alemtuzumab), even though it has been adopted by most centers (> 80%). Maintenance immunosuppressive therapy for pancreatic transplantation is similar for that used for kidney transplantation. A combination of a calcineurin inhibitor (predominantly tacrolimus), an antimetabolite (mycophenolate mofetil or mycophenolate sodium), is associated to low-dose corticosteroids therapy in approximately 60% of the centers[2]. However, taking into account the metabolic side effects as well as the increased risk of infection associated with the use of steroids[7], some studies have suggested early steroid withdrawal or steroid free regimens in these patients, particularly after the introduction of induction therapy with T-cell depleting agents, but there is currently insufficient evidence for the benefits and harms of this therapy[8]. There is also a trend to incorporate mammalian target of rapamycin (mTOR) inhibitors over mycophenolate in combination with tacrolimus into immunosuppressive protocol, because rapamycin appears to be more effective in preventing acute rejection, but it must be weighed against its potential negative metabolic consequences and the accentuation of the nephrotoxicity of the calcineurin inhibitor and wound-healing complications[9-11], so at the moment it has not been widely adopted. Future research should focus on developing personalized immune assessment of transplant patients, through non-invasive tests of immunological biomarkers to monitor the recipient’s immune status[12]. These tests will permit recipient-specific tailored immunosuppressive protocols, based on their immunity response and individual risk, minimizing the complications of excessive immunosuppression while maintaining a low incidence of rejection.
Chronic rejection remains the main cause of long-term graft failure after 1 year, occurring in 10% of patients[13,14]. It may be the result of recurrent episodes of acute rejection with posterior fibrosis, atrophy, and eventual loss of graft function. Therefore, effective immunosuppression protocols and close monitoring of pancreas and kidney allograft function are the best way to prevent it. The image tests findings of acute and chronic rejections are relatively nonspecific and unreliable, making it difficult to diagnose[15]. Diagnostic confirmation must be performed by needle core pancreas allograft biopsy, preferably percutaneous ultrasound-guided biopsy[16]. The Banff schema for grading pancreas allograft rejection based on pathological findings, differentiate the type (T-cell-mediated rejection or antibody-mediated pancreas allograft rejection) and the grade (mild, moderate, and severe) with a high diagnostic accuracy, helping to select the appropriate treatment in each case[17,18].
Given the possibility of chronic rejection, type 1 diabetes mellitus recurrence should also be suspected, which is an infrequent entity, appearing approximately in 3%-7% of properly immunosuppressed patients[19]. It occurs due to the presence of antibodies against pancreatic beta cells in the recipient (type 1 diabetes-associated autoantibodies to the autoantigens GAD65, IA-2, and ZnT8) causing destruction of the pancreatic islets (insulitis). Usually the antibody conversion precede hyperglycemia by a variable length of time, and negative autoimmunity prior to transplantation does not ensure that autoimmune diabetes will not recur. Unlike rejection, serum amylase and lipase levels usually do not rise, and there is no effective treatment, as the increase in immunosuppression does not improve the progression of islet autoimmunity. Diagnostic confirmation must be performed by percutaneous biopsy, in which a variable degree of insulitis and loss of insulin staining must be seen, sometimes associated to minimal to moderate and rarely severe pancreas and/or kidney transplant rejection[20].
The 5-year mortality for SPK in OPTN/UNOS data[2] and Spanish series[4] ranges over 7% to 8%. Long-term mortality data reflect the high cardiovascular comorbidity in this population, with 10-year mortality 26.8%, 20.1%, and 25.3% for PAK, PTA, and SPK, respectively. Despite effective glycemia control after pancreas transplantation delays progression of microvascular complications and improves survival in diabetic patients, cardiovascular comorbidity is the main factor that threatens long-term survival of pancreas transplant patients[5], so its optimal control is another of the priority objectives in the follow up of this patients.
There has been controversy regarding transplant center volume and the risk of short and long-term pancreas graft failure, so it has been analyzed by several studies, highlighting that patient and graft survival after pancreas transplantation are superior in higher volume centers (> 13 transplants/year) than in lower volume centers[21,22], or at least equal even though they transplant organs with the highest pancreas donor risk index[23]. The explanation for inferior outcomes in low-volume centers is likely to be complex, but center volume could be a surrogate marker for adequate recipient selection, surgical expertise, multidisciplinary preoperative, and postoperative inpatient and outpatient care, and appropriate long-term follow-up. Actually, approximately two-thirds of pancreas transplants are performed at programs that perform more than 10 transplants per year, and these numbers have not changed substantially over the past decade[2], this may have contributed to explain the improvement of global pancreas allograft and patient survival throughout last years, demonstrating a better management in these demanding patients over time.
Approximately 63% of pancreas transplant recipients develop a serious infection during the first 5 years of follow-up[24]. Many of the infections in pancreas transplantation follow a typical temporal pattern. Three periods have been described based on the predominance of certain infections: In the first period (1st mo), bacterial infections derived from the surgical procedure are typical. In the second period (2-6 mo), the main risk factor is immunosuppression, and patients basically have de novo opportunistic infections or reactivation of latent diseases: Cytomegalovirus (CMV), herpes simplex virus type 6 (VHV), hepatitis B and C viruses (HBV/HCV), Epstein-Barr virus (EBV) and varicella-zoster virus (VZV); and opportunistic bacteria: Pneumocystis jiroveci pneumonia, Nocardia, Listeria monocytogenes, Toxoplasma gondii, and fungal infections, among others. During the third period (after the 6th mo), in patients with good allograft function, the majority of infections are those acquired in the community, similar to the general population, although opportunistic infections may also occur, by reactivation of certain latent viruses (BK virus, CMV…)[7]. In addition, tumors related to viral infections such as vulgar warts and post-transplant lymphoproliferative diseases, especially due to EBV, may develop in this period[25].
Prophylaxis before transplant: Prevention of infection begins with the adequate selection of the donor and a rigorous evaluation of the recipient with an exhaustive physical examination and study of viral serological markers: CMV, EBV, VZV, HBV, HCV, HIV toxoplasmosis, Treponema pallidum serology, and some others that can vary according to the epidemiology of each geographic area. This permits us to diagnose and treat active infections in the candidates, make decisions about their acceptance or exclusion, and identify the risk factors. Moreover, vaccinations must be completed before the transplantation according to the protocol of each center and geographical area[24].
Perioperative prophylaxis: At the time of the transplant surgery, prophylactic administration of antibiotics like cephalosporins, or ampicillin-sulbactam as an alternative, every 2 h during surgery, and maintenance until 48 h after surgery is recommended. Antifungals (fluconazole) are also associated in a single dose during surgery, and in postoperative prophylaxis regimen up to 14 d in cases of increased risk of infection[26]. The choice of therapeutic agent, both for prophylaxis and for empirical treatment, will also depend on the incidence and type of microorganisms isolated in each center.
The use of cotrimoxazole at low doses until the 6th mo post-transplant has decreased the incidence of Pneumocystis pneumonia[27]. Likewise, it constitutes an excellent prophylaxis to avoid infection by intracellular bacteria, such as Listeria monocytogenes and Nocardia spp.
CMV prophylaxis and monitoring after transplantation: CMV infections are one of the most frequent complications affecting solid organ transplant recipients, conveying higher risk of graft loss, morbidity and mortality. Given that the CMV serostatus of donor and recipient (D/R) are key predictors of the risk of CMV after transplant, and guide decisions on antiviral prophylaxis or preemptive treatment. A measurement of CMV-specific IgG should be performed, and if the donor or recipient is seronegative during the pretransplant evaluation, serology should be repeated at the time of the transplantation. According to the guidelines of the latest international consensus of 2018 on the management of CMV in solid organ transplants[28], in pancreas transplantation, prophylactic treatment with valganciclovir is recommended based on the risk of CMV infection (Table 1). Other valid option is the preemptive therapy, which consists in the monitoring of CMV viral load in blood at regular intervals (weekly) to detect early viral replication. Once a predetermined assay threshold is achieved (optimally prior to the development of symptoms), antiviral treatment is begun, which should prevent progression to clinical disease. Moreover, there are specific situations that increase the risk of CMV infection, such as in cases where antilymphocyte antibodies for induction are administered, plasmapheresis or HIV infection patients. In these cases, it is also mandatory to administer a prophylactic treatment with valganciclovir, and duration will depend on the degree of immunosuppression of the patient. However, there are resistances associated with currently available therapeutics, as there are series with pancreas-kidney transplant high risk patients (D+/R-) who developed CMV infection/disease despite correct CMV prophylaxis in up to 44%[29]. Novel antivirals therapies are emerging including letermovir, maribavir, and brincidofovir at various states of research development. Future research are needed to evaluate combination therapies for prophylaxis and treatment as well as adjunctive therapies[30].
| Serostatus | Risk level | Recommended | Alternate |
| D-/R- | Low | Monitoring for clinical symptoms; consider antiviral prophylaxis against other herpes infections | Preemptive therapy (if higher risk, i.e. significant transfusions) |
| D+/R- | High | 3-6 mo of VGCV | Preemptive therapy |
| R+ | Intermediate | 3 mo of VGCV or preemptive therapy |
Furthermore, there is moderate to high-quality evidence of a lower risk of CMV infection in transplant recipients with mTOR inhibitor-based immunosuppressive therapy compared with calcineurin inhibitor-based regimens, therefore a combination of a mTOR inhibitor and a reduced dose of calcineurin inhibitor could be a good alternative in high risk CMV infection patients[31,32].
Prevention of tuberculosis infection: In the pre-transplant evaluation, the possibility of latent tuberculosis infection should be assessed by performing the tuberculin skin test (TST), and/or tests based on the secretion of interferon gamma by memory T cells in response to mycobacterial antigens (interferon gamma release assays) as QuantiFERON TM-TB Gold test. If the result is positive in any of these tests, a prophylactic treatment with isoniazid should be administered, and active tuberculosis infection should also be ruled out. If tuberculosis infection is confirmed, the transplant would be contraindicated until the infection is cured.
According to the guidelines of the European Society for Clinical Microbiology and Infectious Diseases[33], if the TST or QuantiFERON are positive, or the chest X-ray shows lesions of previous tuberculosis, and there is no evidence that the patient have received a correct treatment, chemoprophylaxis with isoniazid for 9 mo should be administered.
Pseudoaneurysms (PA) occur due to a laceration or disruption of the arterial wall caused by chemical damage due to the exposure to enzymes in the event of pancreatic fistulas or infections with peripancreatic collections, chronic rejections, surgical trauma or biopsies, causing a bleeding into an external fibrous compartment that will contain the hematoma. Vascular anastomoses are the most frequent areas affected by PA, but those caused by biopsies usually occur in the pancreatic parenchyma, and can lead to arteriovenous fistulas.
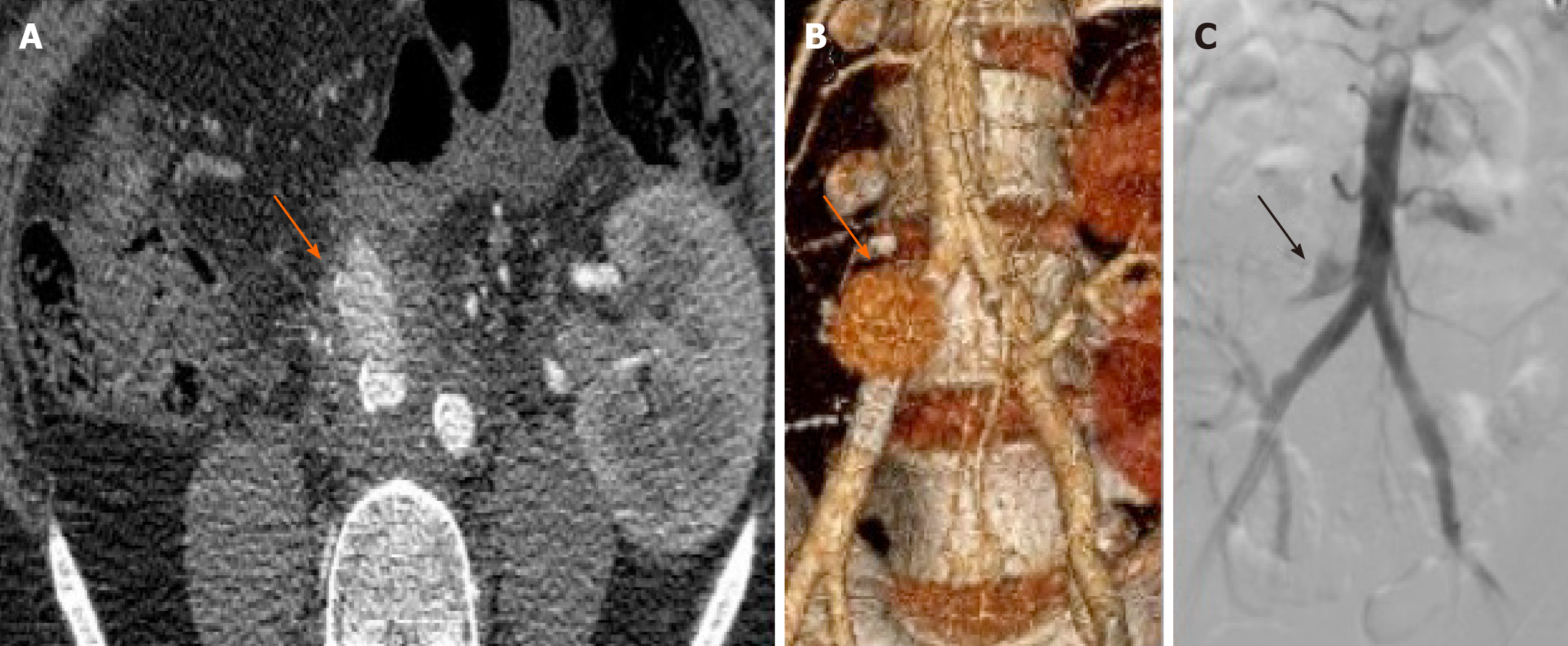
Although PA are infrequent entities, they are one of the main causes of late bleeding, sometimes years after transplantation, which can be lethal. PA tend to debut first with mild digestive hemorrhages, called sentinel bleeding, which can progress to an arteriovenous or arterioenteric fistula[34], preceding of fatal hemorrhage. PA are difficult to detect before the development of massive hemorrhage due to the absence or mildness of the previous symptoms. For this reason, graft control with doppler ultrasonography in the follow up should be performed[35]. Once PA is suspected or demonstrated at ultrasonography, the full anatomy of the PA is best displayed with volumetric high-spatial-resolution CT scanning (angio-CT) or magnetic resonance angiography[14]. Such imaging display is required before planning the treatment (Figure 1).
Current treatment of PA firstly should be performed with radiological endovascular approaches, when possible. Although there is still a lack of reliable data due to the relatively little experience of this approach in pancreas transplant complications, endovascular procedures are becoming more frequently used as they are safe and feasible, but technically demanding. One of the main risks of this approach is the renal function damage caused by contrast agent, but the risk of kidney graft function deterioration, as well as of bleeding due to the high dose of heparin used, seems to be lower than with open surgery[36].
When a PA is diagnosed, elective treatment should be performed to prevent the potential life- and graft-threatening bleeding. The choice between minimally invasive and open surgical repair should be individualized depending on the site of the lesion, the patient status and the multidisciplinary team experience[37,38]. Nevertheless, at the time of acute bleeding, immediate treatment is essential: Endovascular approach with a covered stenting of the involved artery to exclude the PA may provide immediate vascular control in these situations. But if an arteriovenous fistula exists, embolization with microparticles is the treatment of choice[39]. These procedures can be either a definitive treatment[40] or a bridge therapy to posterior open repair[41,42], since rebleeding or necrosis and posterior loss of graft function are not uncommon. For this reason, on many occasions (especially in infections) allograft transplantectomy including the graft vascular anastomosis is performed, requiring sometimes an extra anatomic bypass to maintain blood flow of the lower limb[43].
Surgical complications after pancreas transplantation and later relaparotomies are frequent (25%-30%) and the main cause are thrombosis, bleeding, infection, pancreatitis and bowel obstruction. Unfortunately, it implies allograft pancreatectomy in 50% of cases, and occasionally patient death[44]. The management of such complications has evolved from a low threshold to remove the graft, to considering options for graft preservation through the new antimicrobial and immunosuppressive therapies, as well as the imaging techniques that permit an early diagnosis and image-guided invasive procedures. However, the need to perform a transplantectomy is a necessary situation in some occasions, especially in life-threatening complications. Currently there are no guidelines or consensus that clearly indicate to the surgeon when and how it should be performed, but as a general rule, safety of the recipient should be considered as the main factor to take into account.
Thrombosis-related graft necrosis, severe pancreatitis, and uncontrolled duodenal leak constitute the main indications for allograft pancreatectomy during the first 4 wk after transplantation[44]. And late allograft pancreatectomy is usually reserved for recipients with failed grafts, predominantly from chronic rejection who have abdominal and flank pain, gastrointestinal symptoms and/or fever[45]. The rate of allograft pancreatectomy for late graft failure ranges from 25% to 50%, and in these cases the symptoms associated with the failed pancreas graft, risk of formation of arterioenteric fistula, and candidacy for potential retransplantation need to be cautiously evaluated in order to appropriately select the timing and the surgical approach for allograft pancreatectomy[46]. Recent studies show a marked fall in morbidity and mortality of allograft pancreatectomy, and emphasize the benefits of early retransplantation in appropriate candidates[47]. However, late pancreas retransplantation is also possible, although it has been associated with poorer allograft survival[48], in recent series technical failure and patient death for primary vs pancreas retransplants are similar in highly selected patients, if they are carried out in experienced centers[49-51].
In cases of early transplantectomy, adhesions are not usually a problem. In order to gain rapid exposure of the vascular anastomoses, it is helpful to divide the enteric anastomosis first using a gastrointestinal anastomosis stapler by resecting the jejunum segment together with the duodenum (in enteric drainage transplants). The portal vein and iliac artery of the graft are clamped, divided, and oversewn. And then, the intestinal transit is reestablished with a jejunal anastomosis.
In cases of late transplantectomy, it can be performed in isolation or associated with a pancreas retransplantation. The operative field can be complex due to the adhesions, and in many occasions the graft will be shrunken, fibrosed, and poorly perfused, and there may be extensive adhesions to the retroperitoneum, making it difficult to identify. When graft pancreatectomy is performed as an isolated operation, the complete exposure of the recipient vasculature is often unnecessary. But when a failed allograft is removed at the time of retransplantation, the initial pancreas graft must be carefully explored and dissected away from the recipient iliac vessels and the inferior vena cava, in order to identify appropriate sites for implantation of the new pancreas graft. The allograft portal vein and iliac artery, once identified, are divided and oversewn taking care to avoid compromising the recipient arterial lumen. In cases where is not possible to preserve the recipient iliac vessels blood flow due to their involvement, as in PA or arterioenteric fistulas, an endovascular placement of a covered stent can be placed at the level of the anastomotic site. Alternatively, if it is not effective, an angioplasty with bovine pericardial patch can be done in cases of small defects of the vessel wall. Otherwise, a reconstruction with a synthetic prosthesis or cadaver graft if available in cases of major vessel damage is a valid option[52].
Pancreas transplantation is a complex and technically demanding procedure. Early complications may occur, mostly due to technical failure as thrombosis, intraabdominal infections and pancreatitis. But late complications also entail a high percentage of graft losses, particularly secondary to chronic rejection or even late infections. PA are uncommon, but one of the main causes of late bleeding, which can be fatal. A high index of suspicion is needed to detect it, and once PA is diagnosed, the treatment should be performed, preferably with radiological endovascular approaches or open surgery in case of failure. Despite all therapeutic options for the complications mentioned above, transplantectomy is necessary in approximately 50% of relaparotomies, especially in life-threatening complications. Although pancreas retransplantation can be performed later in selected patients. Close monitoring of graft function and clinical and radiological surveillance, as well as new advances in the immunosuppression protocols, individualized according to the patient´s situation are crucial to prevent rejection, improving pancreas graft survival, as well as the optimal control of cardiovascular comorbidity to achieve better patient long-term survival.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Akhtar M, Ciancio G S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LL
| 1. | Dean PG, Kukla A, Stegall MD, Kudva YC. Pancreas transplantation. BMJ. 2017;357:j1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Kandaswamy R, Stock PG, Gustafson SK, Skeans MA, Urban R, Fox A, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2018 Annual Data Report: Pancreas. Am J Transplant. 2020;20 Suppl s1:131-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Rickels MR, Stock PG, de Koning EJP, Piemonti L, Pratschke J, Alejandro R, Bellin MD, Berney T, Choudhary P, Johnson PR, Kandaswamy R, Kay TWH, Keymeulen B, Kudva YC, Latres E, Langer RM, Lehmann R, Ludwig B, Markmann JF, Marinac M, Odorico JS, Pattou F, Senior PA, Shaw JAM, Vantyghem MC, White S. Defining outcomes for β-cell replacement therapy in the treatment of diabetes: a consensus report on the Igls criteria from the IPITA/EPITA opinion leaders workshop. Transpl Int. 2018;31:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Jiménez-Romero C, Marcacuzco Quinto A, Manrique Municio A, Justo Alonso I, Calvo Pulido J, Cambra Molero F, Caso Maestro Ó, García-Sesma Á, Moreno González E. Simultaneous pancreas-kidney transplantation. Experience of the Doce de Octubre Hospital. Cir Esp. 2018;96:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Gruessner AC, Gruessner RW. Long-term outcome after pancreas transplantation: a registry analysis. Curr Opin Organ Transplant. 2016;21:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Grochowiecki T, Gałązka Z, Madej K, Frunze S, Nazarewski S, Jakimowicz T, Paczek L, Durlik M, Szmidt J. Multivariate analysis of complications after simultaneous pancreas and kidney transplantation. Transplant Proc. 2014;46:2806-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Herrero-Martínez JM, Lumbreras C, Manrique A, San-Juan R, García-Reyne A, López-Medrano F, Lizasoain M, de Dios B, Andrés A, Jiménez C, Gutiérrez E, Moreno E, Aguado JM. Epidemiology, risk factors and impact on long-term pancreatic function of infection following pancreas-kidney transplantation. Clin Microbiol Infect. 2013;19:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Montero N, Webster AC, Royuela A, Zamora J, Crespo Barrio M, Pascual J. Steroid avoidance or withdrawal for pancreas and pancreas with kidney transplant recipients. Cochrane Database Syst Rev. 2014;: CD007669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Heilman RL, Mazur MJ, Reddy KS. Immunosuppression in simultaneous pancreas-kidney transplantation: progress to date. Drugs. 2010;70:793-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Ciancio G, Sageshima J, Chen L, Gaynor JJ, Hanson L, Tueros L, Montenora-Velarde E, Gomez C, Kupin W, Guerra G, Mattiazzi A, Fornoni A, Pugliese A, Roth D, Wolf M, Burke GW 3rd. Advantage of rapamycin over mycophenolate mofetil when used with tacrolimus for simultaneous pancreas kidney transplants: randomized, single-center trial at 10 years. Am J Transplant. 2012;12:3363-3376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Marcella-Neto R, de Sá JR, Melaragno CS, Gonzalez AM, Salzedas-Neto A, Linhares MM, Medina-Pestana JO, Rangel ÉB. Late Conversion to Sirolimus or Everolimus After Pancreas Transplant. Transplant Proc. 2020;52:1376-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Kudva YC, Erickson JR, Parsaik A, Rostambeigi N, Thapa P, Abraham RS. Comprehensive immune monitoring reveals profound immunological changes in pancreas after kidney (PAK) transplant recipients. Hum Immunol. 2013;74:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Humar A, Khwaja K, Ramcharan T, Asolati M, Kandaswamy R, Gruessner RW, Sutherland DE, Gruessner AC. Chronic rejection: the next major challenge for pancreas transplant recipients. Transplantation. 2003;76:918-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Wakil K, Sugawara Y, Kokudo N, Kadowaki T. Causes of graft failure in simultaneous pancreas-kidney transplantation by various time periods. Clin Transpl. 2013: 23-30. [PubMed] |
| 15. | O'Malley RB, Moshiri M, Osman S, Menias CO, Katz DS. Imaging of Pancreas Transplantation and Its Complications. Radiol Clin North Am. 2016;54:251-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Lee BC, McGahan JP, Perez RV, Boone JM. The role of percutaneous biopsy in detection of pancreatic transplant rejection. Clin Transplant. 2000;14:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Drachenberg CB, Odorico J, Demetris AJ, Arend L, Bajema IM, Bruijn JA, Cantarovich D, Cathro HP, Chapman J, Dimosthenous K, Fyfe-Kirschner B, Gaber L, Gaber O, Goldberg J, Honsová E, Iskandar SS, Klassen DK, Nankivell B, Papadimitriou JC, Racusen LC, Randhawa P, Reinholt FP, Renaudin K, Revelo PP, Ruiz P, Torrealba JR, Vazquez-Martul E, Voska L, Stratta R, Bartlett ST, Sutherland DE. Banff schema for grading pancreas allograft rejection: working proposal by a multi-disciplinary international consensus panel. Am J Transplant. 2008;8:1237-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Drachenberg CB, Torrealba JR, Nankivell BJ, Rangel EB, Bajema IM, Kim DU, Arend L, Bracamonte ER, Bromberg JS, Bruijn JA, Cantarovich D, Chapman JR, Farris AB, Gaber L, Goldberg JC, Haririan A, Honsová E, Iskandar SS, Klassen DK, Kraus E, Lower F, Odorico J, Olson JL, Mittalhenkle A, Munivenkatappa R, Paraskevas S, Papadimitriou JC, Randhawa P, Reinholt FP, Renaudin K, Revelo P, Ruiz P, Samaniego MD, Shapiro R, Stratta RJ, Sutherland DE, Troxell ML, Voska L, Seshan SV, Racusen LC, Bartlett ST. Guidelines for the diagnosis of antibody-mediated rejection in pancreas allografts-updated Banff grading schema. Am J Transplant. 2011;11:1792-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Argente-Pla M, Martínez-Millana A, Del Olmo-García MI, Espí-Reig J, Pérez-Rojas J, Traver-Salcedo V, Merino-Torres JF. Autoimmune Diabetes Recurrence After Pancreas Transplantation: Diagnosis, Management, and Literature Review. Ann Transplant. 2019;24:608-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Burke GW 3rd, Vendrame F, Virdi SK, Ciancio G, Chen L, Ruiz P, Messinger S, Reijonen HK, Pugliese A. Lessons From Pancreas Transplantation in Type 1 Diabetes: Recurrence of Islet Autoimmunity. Curr Diab Rep. 2015;15:121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Kopp W, van Meel M, Putter H, Samuel U, Arbogast H, Schareck W, Ringers J, Braat A. Center Volume Is Associated With Outcome After Pancreas Transplantation Within the Eurotransplant Region. Transplantation. 2017;101:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Alhamad T, Malone AF, Brennan DC, Stratta RJ, Chang SH, Wellen JR, Horwedel TA, Lentine KL. Transplant Center Volume and the Risk of Pancreas Allograft Failure. Transplantation. 2017;101:2757-2764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Kim Y, Dhar VK, Wima K, Jung AD, Xia BT, Hoehn RS, Diwan TS, Shah SA. The center volume-outcome effect in pancreas transplantation: a national analysis. J Surg Res. 2017;213:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Rostambeigi N, Kudva YC, John S, Mailankody S, Pedersen RA, Dean PG, Prieto M, Cosio FG, Kremers WK, Walker RC, Abraham RS, Stegall MD. Epidemiology of infections requiring hospitalization during long-term follow-up of pancreas transplantation. Transplantation. 2010;89:1126-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Helfrich M, Dorschner P, Thomas K, Stosor V, Ison MG. A retrospective study to describe the epidemiology and outcomes of opportunistic infections after abdominal organ transplantation. Transpl Infect Dis. 2017;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Anesi JA, Blumberg EA, Abbo LM. Perioperative Antibiotic Prophylaxis to Prevent Surgical Site Infections in Solid Organ Transplantation. Transplantation. 2018;102:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 27. | Wang EH, Partovi N, Levy RD, Shapiro RJ, Yoshida EM, Greanya ED. Pneumocystis pneumonia in solid organ transplant recipients: not yet an infection of the past. Transpl Infect Dis. 2012;14:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102:900-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 832] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 29. | Fallatah SM, Marquez MA, Bazerbachi F, Schiff JR, Cattral MS, McGilvray ID, Norgate A, Selzner M, Rotstein C, Husain S. Cytomegalovirus infection post-pancreas-kidney transplantation--results of antiviral prophylaxis in high-risk patients. Clin Transplant. 2013;27:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Kotton CN. Updates on antiviral drugs for cytomegalovirus prevention and treatment. Curr Opin Organ Transplant. 2019;24:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Mallat SG, Tanios BY, Itani HS, Lotfi T, McMullan C, Gabardi S, Akl EA, Azzi JR. CMV and BKPyV Infections in Renal Transplant Recipients Receiving an mTOR Inhibitor-Based Regimen Versus a CNI-Based Regimen: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Clin J Am Soc Nephrol. 2017;12:1321-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Karpe KM, Talaulikar GS, Walters GD. Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients. Cochrane Database Syst Rev. 2017;7:CD006750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Meije Y, Piersimoni C, Torre-Cisneros J, Dilektasli AG, Aguado JM; ESCMID Study Group of Infection in Compromised Hosts. Mycobacterial infections in solid organ transplant recipients. Clin Microbiol Infect. 2014;20 Suppl 7:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Bratton CF, Hamid A, Selby JB, Baliga PK. Case report: gastrointestinal hemorrhage caused by a pancreas transplant arteriovenous fistula with large psuedoanuerysm 9 years after transplantation. Transplant Proc. 2011;43:4039-4043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Morelli L, Di Candio G, Campatelli A, Vistoli F, Del Chiaro M, Balzano E, Croce C, Moretto C, Signori S, Boggi U, Mosca F. Role of color Doppler sonography in post-transplant surveillance of vascular complications involving pancreatic allografts(). J Ultrasound. 2008;11:18-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Surowiecka-Pastewka A, Matejak-Górska M, Frączek M, Sklinda K, Walecki J, Durlik M. Endovascular Interventions in Vascular Complications After Simultaneous Pancreas and Kidney Transplantations: A Single-Center Experience. Ann Transplant. 2019;24:199-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Yadav K, Young S, Finger EB, Kandaswamy R, Sutherland DER, Golzarian J, Dunn TB. Significant arterial complications after pancreas transplantation-A single-center experience and review of literature. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Montenovo M, Vaidya S, Bakthavatsalam R, Halldorson J. Pseudoaneurysm after combined kidney/pancreas transplantation presenting with sentinel bleeding: a case report and review. Ann Transplant. 2014;19:317-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Buttarelli L, Capocasale E, Marcato C, Mazzoni MP, Iaria M, Rossi C. Embolization of pancreatic allograft arteriovenous fistula with the Amplatzer Vascular Plug 4: case report and literature analysis. Transplant Proc. 2011;43:4044-4047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Huurman VAL, Lardenoye JHP. Pancreas graft salvage after successful endovascular treatment of Y graft pseudoaneurysm. J Surg Case Rep. 2019;2019:rjz124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Kurose S, Inoue K, Yoshino S, Nakayama K, Yamashita S, Yoshiya K, Yoshiga R, Morisaki K, Kaku K, Okabe Y, Furuyama T, Maehara Y. Successful Bridge Therapy with Initial Endovascular Repair for Arterioenteric Fistula Resulting from Pseudoaneurysm Rupture with Massive Gastrointestinal Hemorrhage after Pancreas Transplantation. Ann Vasc Surg 2019; 58: 379.e15-379. e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Arantes RM, Pantanali CA, Santos VR, Carneiro D'Albuquerque LA. Arterial Pseudoaneurysm Associated with Pancreas and Kidney Transplantation: A Case Report. Am J Case Rep. 2017;18:198-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Patrono D, Verhelst R, Buemi A, Darius T, Godefroid N, Mourad M. Presentation and management of mycotic pseudoaneurysm after kidney transplantation. Transpl Infect Dis. 2015;17:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Manrique A, Jiménez C, López RM, Cambra F, Morales JM, Andrés A, Gutiérrez E, Ortuño T, Calvo J, Sesma AG, Moreno E. Relaparotomy after pancreas transplantation: causes and outcomes. Transplant Proc. 2009;41:2472-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Parajuli S, Odorico J, Astor BC, Djamali A, Sollinger H, Redfield R, Kaufman D, Mandelbrot DA. Incidence and Indications for Late Allograft Pancreatectomy While on Continued Immunosuppression. Transplantation. 2017;101:2228-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Nagai S, Powelson JA, Taber TE, Goble ML, Mangus RS, Fridell JA. Allograft Pancreatectomy: Indications and Outcomes. Am J Transplant. 2015;15:2456-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Hollinger EF, Powelson JA, Mangus RS, Kazimi MM, Taber TE, Goble ML, Fridell JA. Immediate retransplantation for pancreas allograft thrombosis. Am J Transplant. 2009;9:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Siskind E, Maloney C, Jayaschandaran V, Kressel A, Akerman M, Shen A, Amodu L, Platz J, Ricci JP, Bhaskaran M, Basu A, Molmenti E, Ortiz J. Pancreatic retransplantation is associated with poor allograft survival: an update of the United Network for Organ Sharing database. Pancreas. 2015;44:769-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Perosa M, Sergi F, Noujaim H. Outcomes after pancreas retransplantation: is the juice worth the squeeze? Curr Opin Organ Transplant. 2018;23:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Gasteiger S, Cardini B, Göbel G, Oberhuber R, Messner F, Resch T, Bösmüller C, Margreiter C, Schneeberger S, Maglione M. Outcomes of pancreas retransplantation in patients with pancreas graft failure. Br J Surg. 2018;105:1816-1824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Rudolph EN, Finger EB, Chandolias N, Kandaswamy R, Sutherland DE, Dunn TB. Outcomes of pancreas retransplantation. Transplantation. 2015;99:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Laurence JM, Cattral MS. Techniques of pancreas graft salvage/indications for allograft pancreatectomy. Curr Opin Organ Transplant. 2016;21:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |