Published online Dec 28, 2020. doi: 10.5500/wjt.v10.i12.381
Peer-review started: June 18, 2020
First decision: July 25, 2020
Revised: August 4, 2020
Accepted: October 5, 2020
Article in press: October 5, 2020
Published online: December 28, 2020
Processing time: 186 Days and 11.5 Hours
In pancreas transplantation, complications can arise at each step of the process, from the initial selection of donors and recipients through the surgical technique itself and the post-operative period, when lifelong immunosuppression is required. In the early steps, careful retrieval and preservation of the pancreas are crucial for the viability of the organ and ultimate success of the transplant. The pancreas is a low-flow gland, making it highly sensitive to transplantation conditions and presenting risk of pancreatitis due to periods of ischemia. The two groups of donors - after brain death (DBD) or after cardiac arrest (DCD) - require different strategies of retrieval and preservation to avoid or reduce the risk of complications developing during and after the transplantation. For DBD donor transplantation, multiorgan retrieval and cold preservation is the conventional technique. Asystole donor (DCD) transplantation, in contrast, can benefit from the newest technologies, such as hypothermic and especially normothermic preservation machines (referred to as NECMO), to optimize organ preservation. The latter has led to an increase in the pool of donors by facilitating recuperation of organs for transplantation that would have been discarded otherwise.
Core Tip: The retrieval and preservation steps of pancreas transplantation are critical factors for graft and patient survival. The most frequent complications of these steps are pancreatitis, graft thrombosis, fistula, and infectious collections. Therefore, it is very important to design and carry out a careful surgical technique for retrieval and a rigorous method of preservation for optimal organ integrity.
- Citation: Casanova D, Gutierrez G, Gonzalez Noriega M, Castillo F. Complications during multiorgan retrieval and pancreas preservation. World J Transplant 2020; 10(12): 381-391
- URL: https://www.wjgnet.com/2220-3230/full/v10/i12/381.htm
- DOI: https://dx.doi.org/10.5500/wjt.v10.i12.381
The selection requirements for accepting a pancreatic graft are very strict, with the transplant and patient outcomes depending largely on such[1-3]. The most important risk criteria for recipients are age over 55 years, body mass index over 30%, creatinine level over 1.5, preservation period over 20 h, prolonged periods of cardiac arrest and hypotension, and donor factors of atherosclerosis as cause of brain death, presence of arteriosclerosis of the celiac axis, presence of thrombophilia, and history of cardiac arrest[4,5]. The complications that occur during the earliest steps (retrieval and preservation) are determinant factors of the transplant outcome. Early recognition of complications and initiation of preventive measures, therefore, guide the decision-making process for moving forward with any transplantation.
The potential complications are numerous but the most common are postoperative bleeding, graft thrombosis, peripancreatic collections and abscesses, duodenal fistulas, pancreatitis, pseudocyst formation, compartment syndrome, and long-term formation of fungal aneurysms[6-8]. These recipient complications present equal risk with regard to donor status at time of harvesting: Brain dead donor (DBD) or asystole (DCD). These two forms of donor status are determined by the circumstances of the donation situation. DBD, which uses cold-temperature preservation, occurs under the following three scenarios: A1, with unidentified vascular anomalies during the retrieval; A2, with iatrogenic lesions during the retrieval; and A3, with anomalous factors related to the perfusion and preservation. DCD, on the other hand, is classified as either B1, with super-fast retrieval and cold preservation, or B2, with preservation with normothermic preservation machines (NECMOs).
In these scenarios, the pancreatic retrieval technique can be performed by classic dissection, obtaining the pancreas in combination with the liver, or by a technique of removal of the entire abdominal block (liver, pancreas, and kidneys). In most pancreas transplant programs, the type of graft used is the pancreatic-duodenal form obtained from DBDs; although, in some centers, DCDs are used. Moreover, at the end of the century (1980s and 1990s), some centers used segmental grafts, including those obtained from living donors.
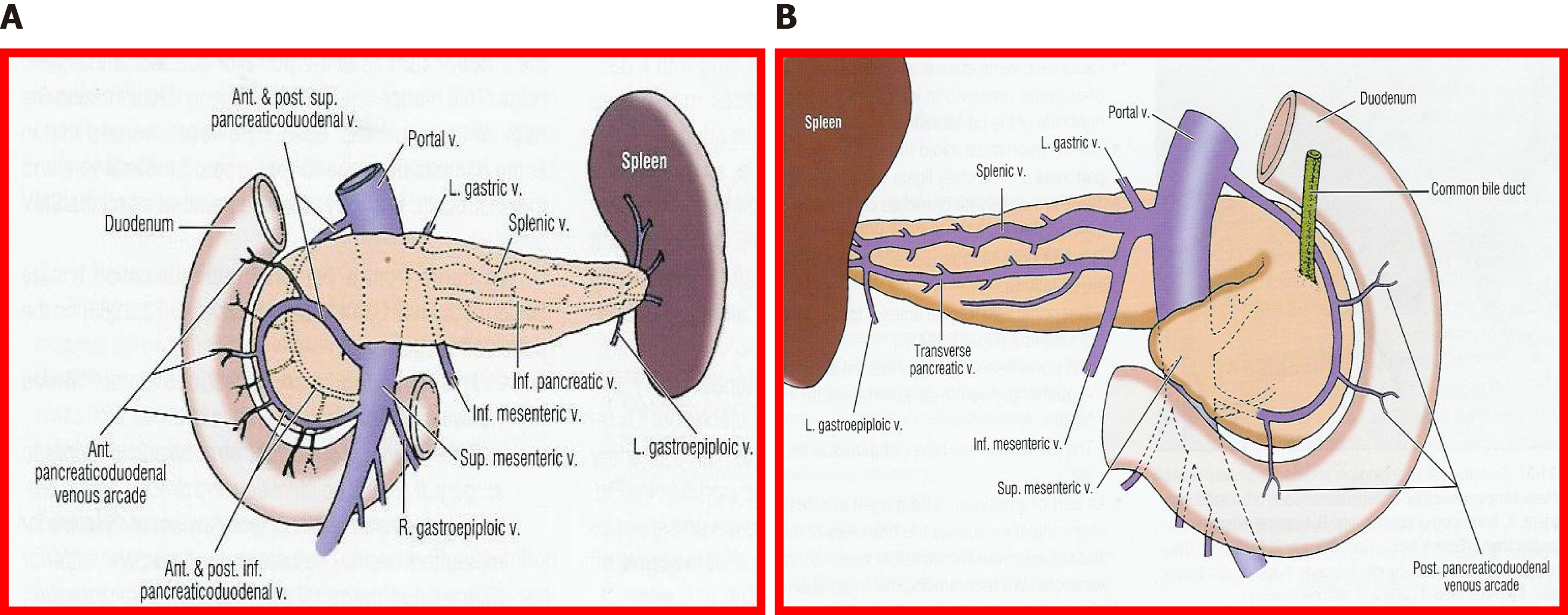
Since pancreas donors are often also liver donors, it is mandatory to share both arterial and venous vascularization[9-11]. As such, there are a number of specific factors that determine the viability of a pancreatic graft, such as vascular abnormalities, especially of the hepatic artery, edema or fat infiltration of the pancreas, injuries during harvesting, injuries to the surface of the pancreas, hematomas, etc[12-14].
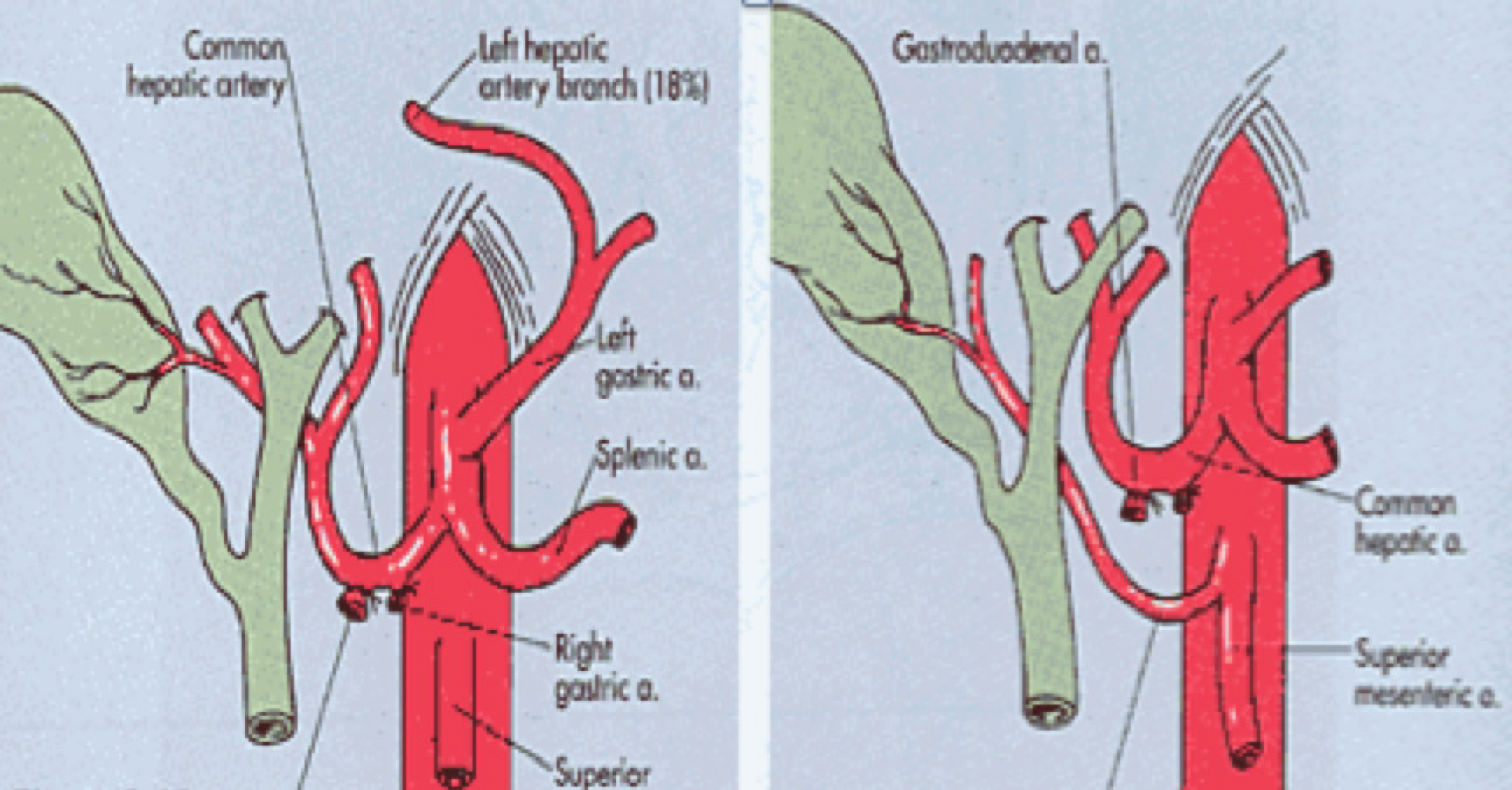
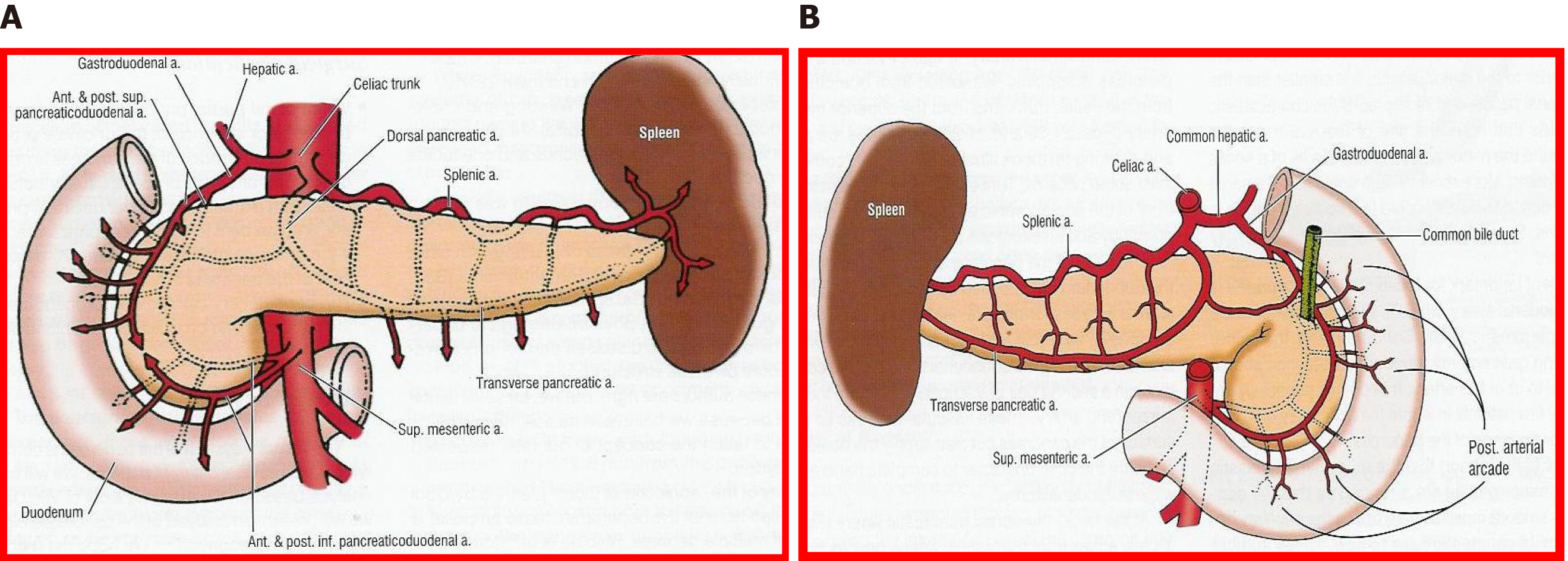
The fundamental axis of the arterial circulation is the celiac axis, and the system consists of three main arteries: The splenic artery, the gastroduodenal artery, and the superior mesenteric artery. One of the most frequent vascular anomalies encountered is a right hepatic artery from the upper mesenteric artery (Figures 1 and 2). This anomaly must be detected at the beginning of the retrieval operation, since injury or inadvertent section of this artery compromises the use of one of the associated organs[15,16]. Another frequently encountered anomaly is a dorsal pancreatic artery exiting directly into the celiac trunk or just before the splenic artery (Figure 3); injury of this artery during surgical maneuvers results in graft thrombosis[17].
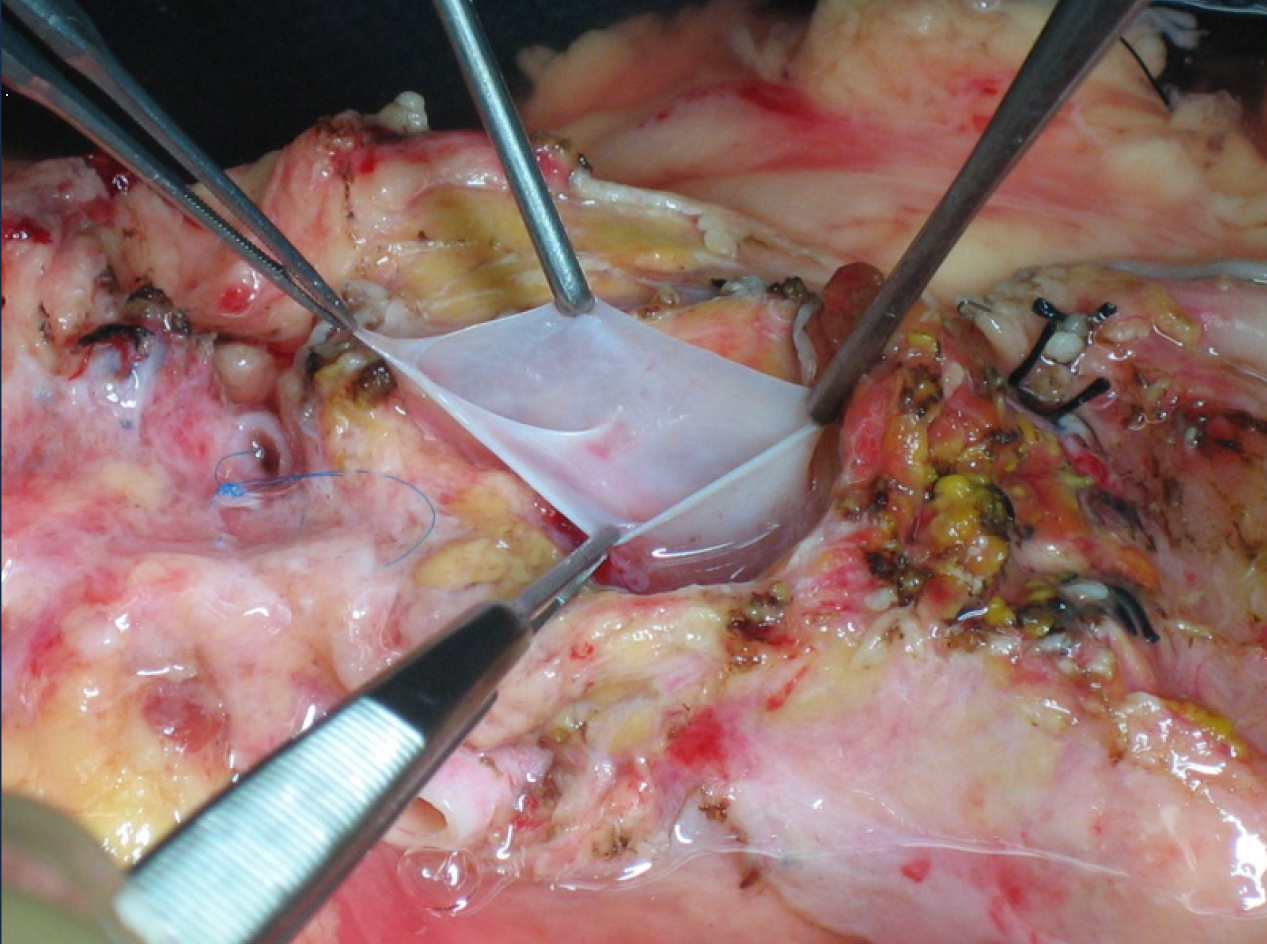
This situation can only be avoided (or risk minimized) by careful performance of the pancreas retrieval technique[18]. In most cases, the situation can be readily recognized by the surgeon during the harvesting procedure and repaired immediately; if it goes undetected until the inspection of the graft during the bench surgery, or even until the time of implantation and revascularization, the consequences can be dire (hematoma and postoperative pancreatitis)[19,20]. From a technical point of view, it is recommended to mobilize the pancreas without touching the gland (as much as possible), holding it through the spleen in order to avoid traction, hematomas and capsule tears (Figure 4).
The most critical injuries are those that involve the vessels of the gland at the pancreas head and duodenum; although, those involving the pancreatic body and tail are not trifling. One of the complications that can occur is related to the anatomical vascular anomalies already mentioned, such as the existence of a right hepatic artery as the first branch of the superior mesenteric artery or the section or injury of a segment of the splenic artery or the dorsal pancreatic artery. If this has occurred and tail perfusion is compromised, at most centers the graft is usually discarded for vascularized transplantation, leaving open the option of use for islet isolation. However, in some emergent situations, such as a rescue surgery, a distal resection could be performed in order to use the graft for vascularized transplant.
It is convenient to place some sutures that identify the edge of the splenic artery, since it can retract, and also to identify the section edge of the portal vein, given its small size. It is important to check that the lower pancreaticoduodenal artery is located on the pancreatic side entirely; this is especially important during preparation of the duodenal segment of the graft, and during distal section of the mesenteric vessels.
Static cold storage is the preservation technique used in most programs of pancreas transplants. It is based on the principle of reducing cell metabolism by lowering the temperature. This decreases the consumption of adenosine triphosphate and inhibits the activity of intracellular enzymes, with consequent reduction in cell degradation by hydrolysis of phospholipids. Under the hypothermic condition, metabolism activity drops to 10% but, over time, it produces ischemic lesions. This is particularly detrimental to graft survival, and preservative solutions are designed to minimize the detrimental effects of cold ischemic injury[21,22]. This remains an essential aspect for the surgeon’s attention, however, since the pancreas is a gland that is very sensitive to edema.
The perfusion technique must be carried out with any preservation solution [e.g., electrolyte mimicking intracellular, i.e., UW, or extracellular, i.e., IGL, solutions, and mannitol-containing solutions, i.e., Celsior and histidine-tryptophan-ketoglutarate (commonly referred to as HTK), etc.] through arterial cannulas at low pressure (< 60 cm) in order to minimize edema[23-27]. In general, it is recommended to perform perfusion through the aorta[9], sectioning the portal vein above the pancreas after having perfused the 1st L of solution, in order to facilitate drainage of the pancreas and avoid edema.
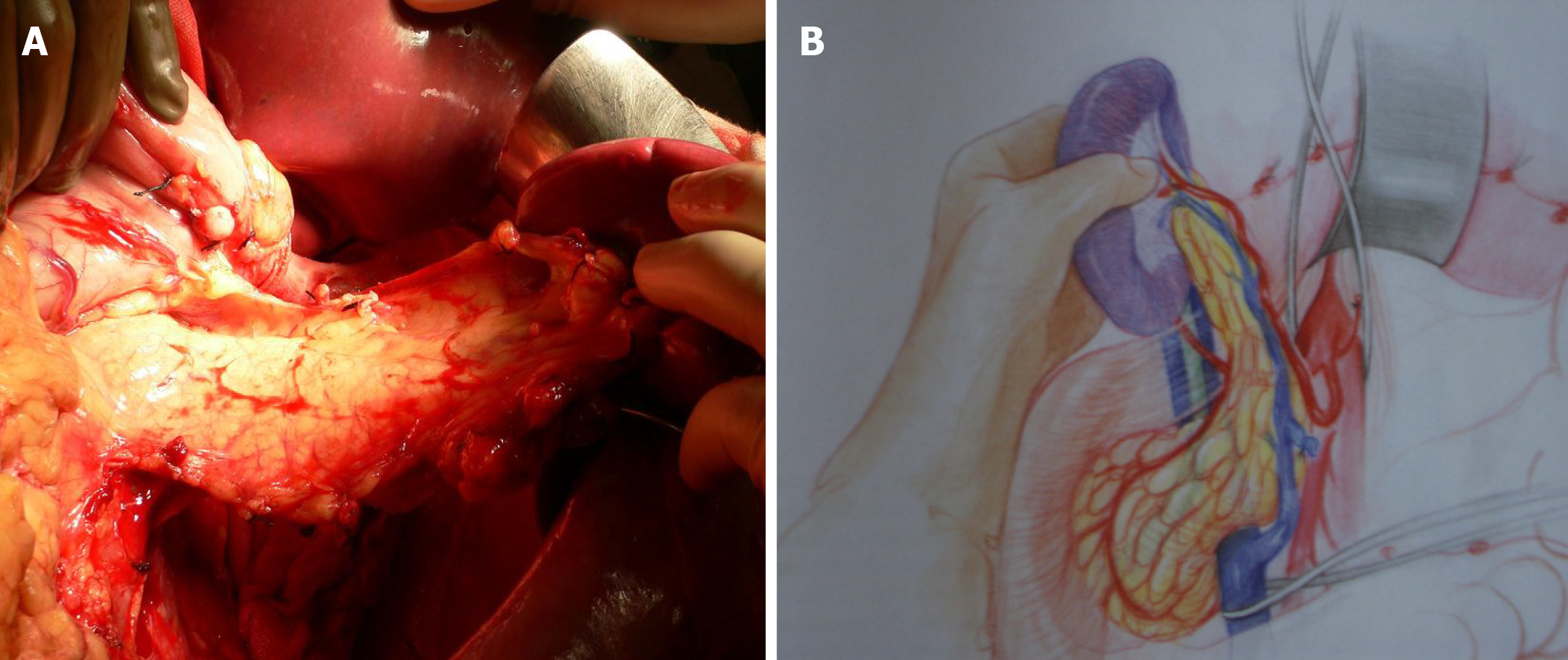
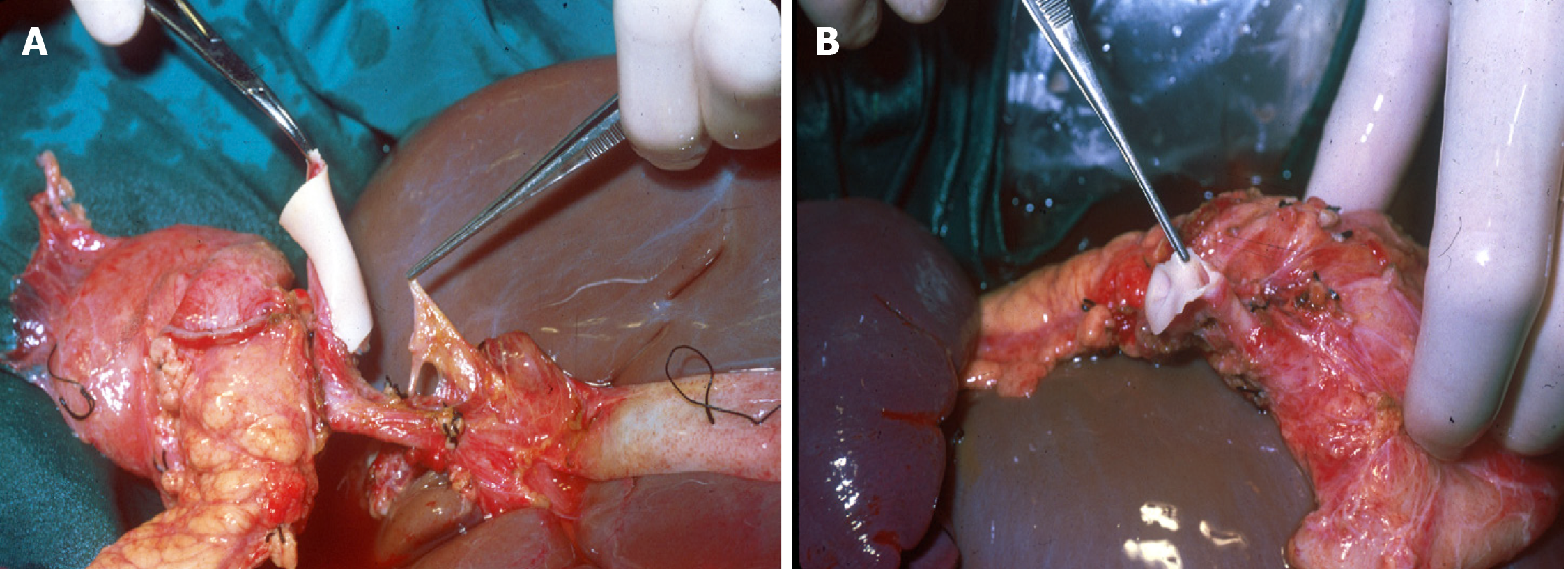
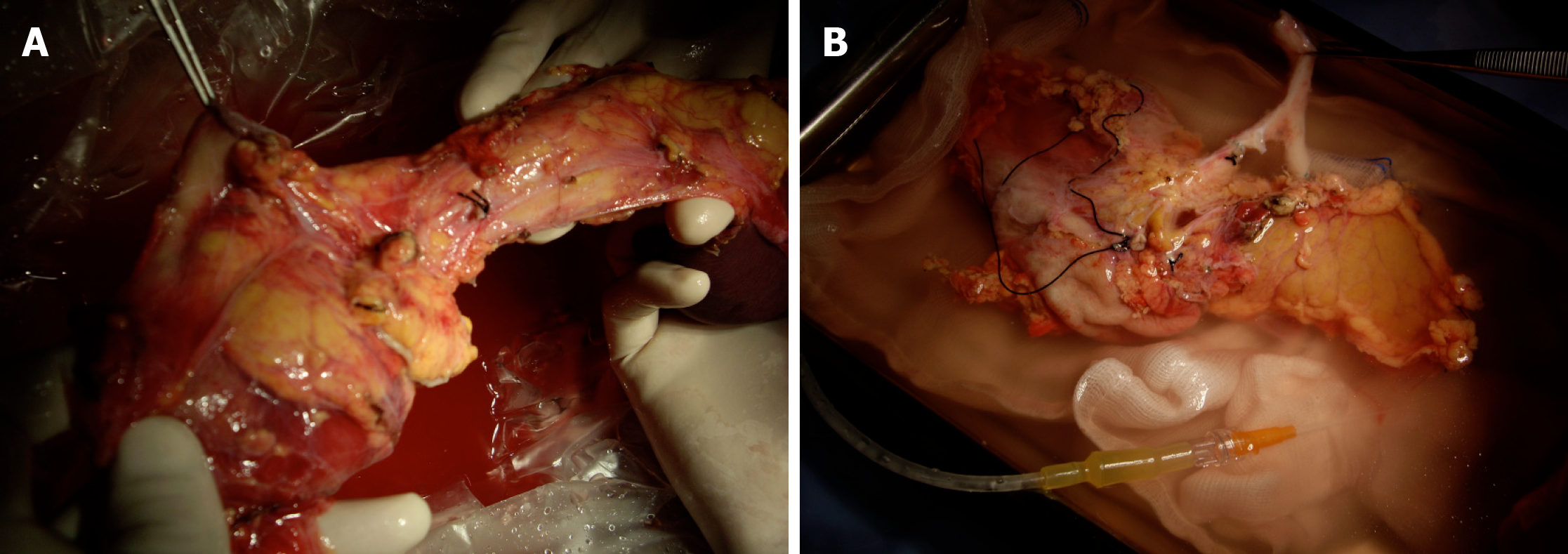
In order to maintain arterial and venous perfusion for the liver, the portal vein proximal to the liver may be cannulated and perfusion continued. Arterial perfusion should also be continued to maintain the perfusion of the liver and kidneys. A maximum of 4 L of perfusion solution is recommended for a 70 kg donor. Once the pancreas has cooled through the perfusion and via contact with ice, it can be retrieved separately or together with the liver (Figures 5 and 6). There is good evidence that both liver and pancreas function better when retrieved en bloc and separated on the back table[10].
In bench surgery, the integrity of the gland and its vessels must be checked. In addition to confirming that the capsule is intact, it is important to infuse preservation solution at low pressure through the cut ends of the splenic and superior mesenteric arteries, to verify that the effluent flows properly through the end of the graft’s portal vein. It can also be left without ligating the stump of the gastroduodenal artery to check the permeability of the arterial tree at that level. The venous drainage of the pancreas is made up of vessels that connect to the splenic vein and the superior mesenteric vein, giving way to the portal vein (Figures 7 and 8).
If the perfusion is not satisfactory or the portal effluent remains bloody with small clots, the perfusion is deemed inadequate and a risk for venous thrombosis of the graft[28].
Shortage of conventional pancreas donors has led to a significant increase in DCD donations. Indeed, asystole donors have constituted a very important group in recent years. The largest studies comparing the outcomes of pancreas transplantation from DCDs with those from DBDs have shown comparable results. However, it is important to remember that the selection of DCDs is often more rigorous and DCDs tend to be younger, with a lower body mass index; and, recipients of such are also at lower-risk immunological level, further favoring the results from DCD transplants[29-32]. Given that the use of these donors implies an increase in resources, we must be extreme in the selection criteria, in order to identify the risk factors and therefore the complications that these donors may present (Table 1). Although there are some differences in the screening and control criteria of these donors in different occidental countries, in general, the 5-min non-touch period is accepted. The technique chosen for organ retrieval depends on whether NECMO technology is available; alternatively, a super-fast cold technique must be used.
| No. of risk factors | Tecnical failure (%) | Graft survival (%) |
| 0 | 7.5 | 100 |
| 1 | 12.8 | 92.5 |
| 2 | 26.7 | 75.9 |
| 3 | 42.9 | 57.1 |
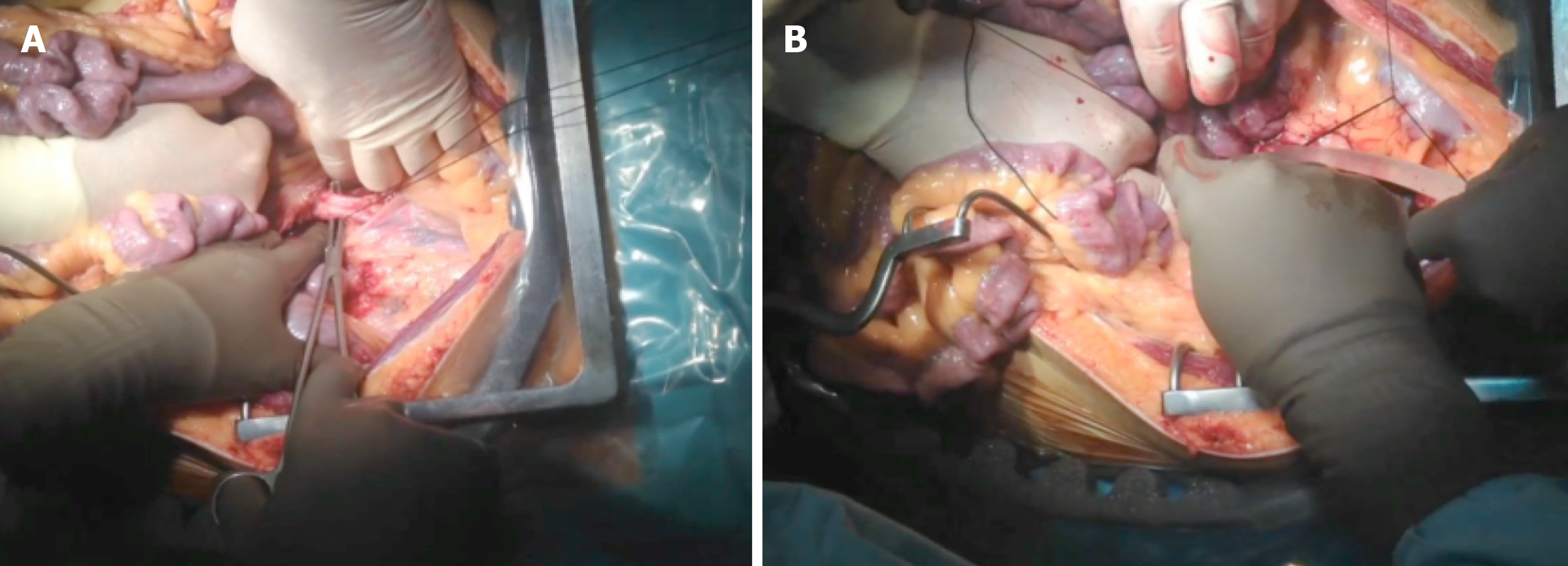
Super-fast retrieval and cold dissection technique was introduced as a means of rapid procurement of all abdominal organs. This approach was initially used with unstable donors but has since been applied successfully to multiorgan retrievals. In such cases, extreme care must be taken when performing the surgery to avoid iatrogenic injuries. It is very important to check for anatomical anomalies and carry out the surgical technique in conjunction with an early abdominal perfusion through the aorta. After the non-touch period, wide-access laparotomy and rapid access to the aorta should be performed to insert the perfusion cannula[33-35] (Figure 9).
Warm ischemic time has two components - the time from when the patient is hypotensive after the start of life-support limitation, and the time from asystole to cannulation of the abdominal aorta and the start of organ-preserving perfusion. Once the entire abdominal block has been perfused, it should be explanted en bloc. Subsequently, the dissection and identification of the different anatomical structures can be performed in the bench surgery. It is convenient to reperfuse the pancreas through the splenic and upper mesenteric arteries, until the effluent becomes clear.
The asystole donor is considered to be at higher thrombotic risk. Pre-transplant anticoagulation with low molecular weight heparin (40 mg at 6 h before surgery) should be performed, followed by heparinization with 2500 U of unfractionated heparin before vascular clamping in the recipient.
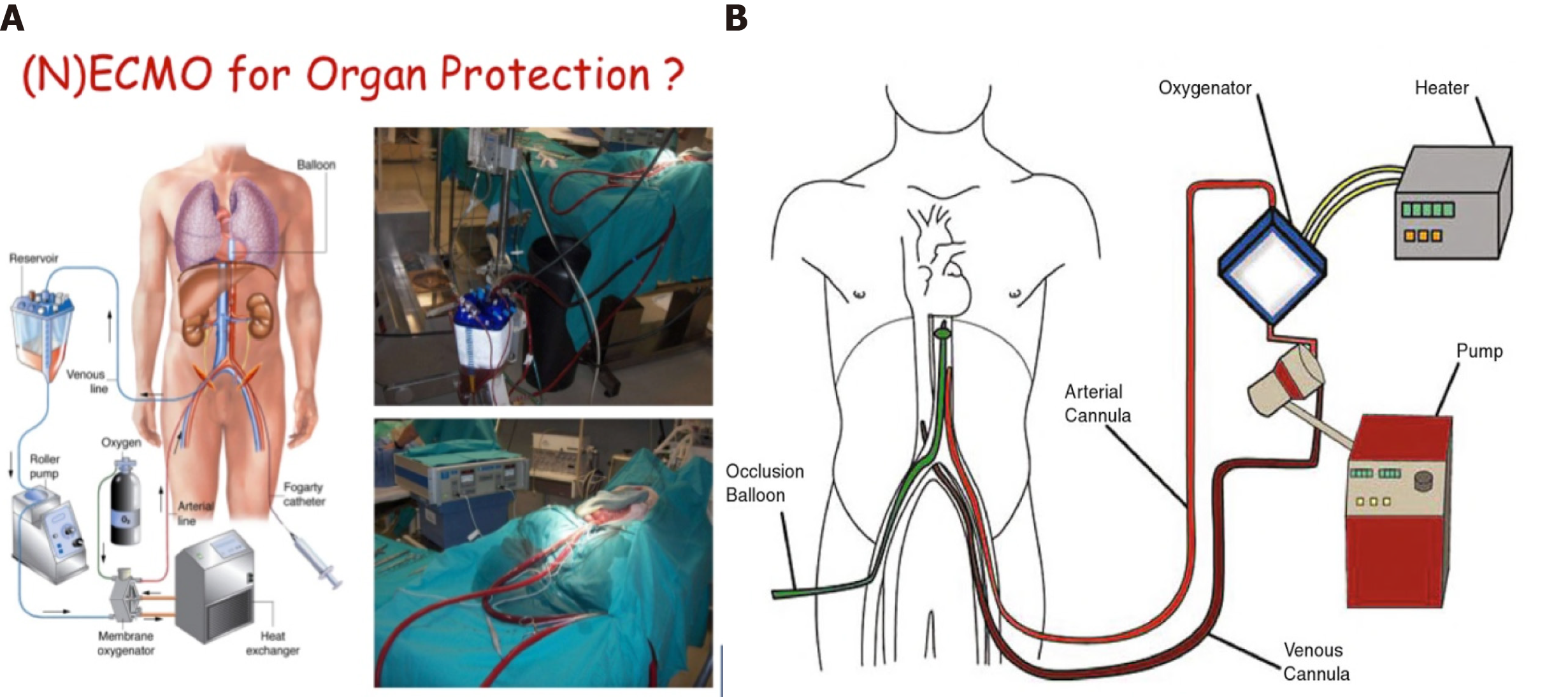
NECMO technology is relatively new but has emerged as a successful approach for the retrieval and preservation of organs from controlled asystole Maastricht type III donors[36-40]. Normothermal perfusion has the potential to decrease or ameliorate ischemic injury and also facilitates the testing of graft viability, reducing the percentage of organs discarded prior to transplantation. Its use in DCDs has been proposed as superior to super-fast extraction, as it overcomes the risk of ischemia effects and improves the graft outcome in recipients. The demonstrated advantages are better immediate graft function, fewer post-transplant complications, shorter hospital stay, and better graft survival. These differences are especially significant in the case of liver and pancreas transplants[41].
One of the important advantages in Spain, in particular, is that it is legally authorized to start anticoagulation maneuvers and placement of cannulas after consent for donation. This process consists of the administration of heparin (600 U/kg) and the cannulation of the femoral vessels before the withdrawal of life-sustaining therapies. The femoral artery and vein are percutaneously cannulated in the intensive care unit, using the Seldinger technique. An aortic occlusion balloon is placed through the femoral vessels in the contralateral groin to prevent cerebral and coronary perfusion during the normothermal recirculation. It is also essential to maintain a pump flow of 2-2.4 L/min[42,43] (Figure 10).
The functional warm ischemic time (commonly known as f-WIT) for abdominal grafts is defined as the time from systolic blood pressure < 60 mmHg to the start of normothermal recirculation, including the 5-min non-touch period. A continuous pressure of 60-65 mmHg should be maintained at the femoral artery cannula, along with a temperature of 37 °C. Bicarbonate is administered immediately after the start of recirculation, to maintain pH of 7.35-7.45. Hematocrit is maintained > 25%. To avoid low blood flow in the pump due to the absence of venous return from the chest and head, 1-1.5 L of saline is perfused to the DCD just prior to vena cava ligation. The potential advantage is that it provides a continuous circulation that is able to improve the metabolic support during perfusion, preserving the microcirculation of the organs and thereby improving preservation. Another important aspect is that it allows the quality of the organs to be evaluated before transplantation, especially in those organs coming from donors with expanded criteria. Finally, it reduces the risk of delayed graft function and improves the survival of the graft.
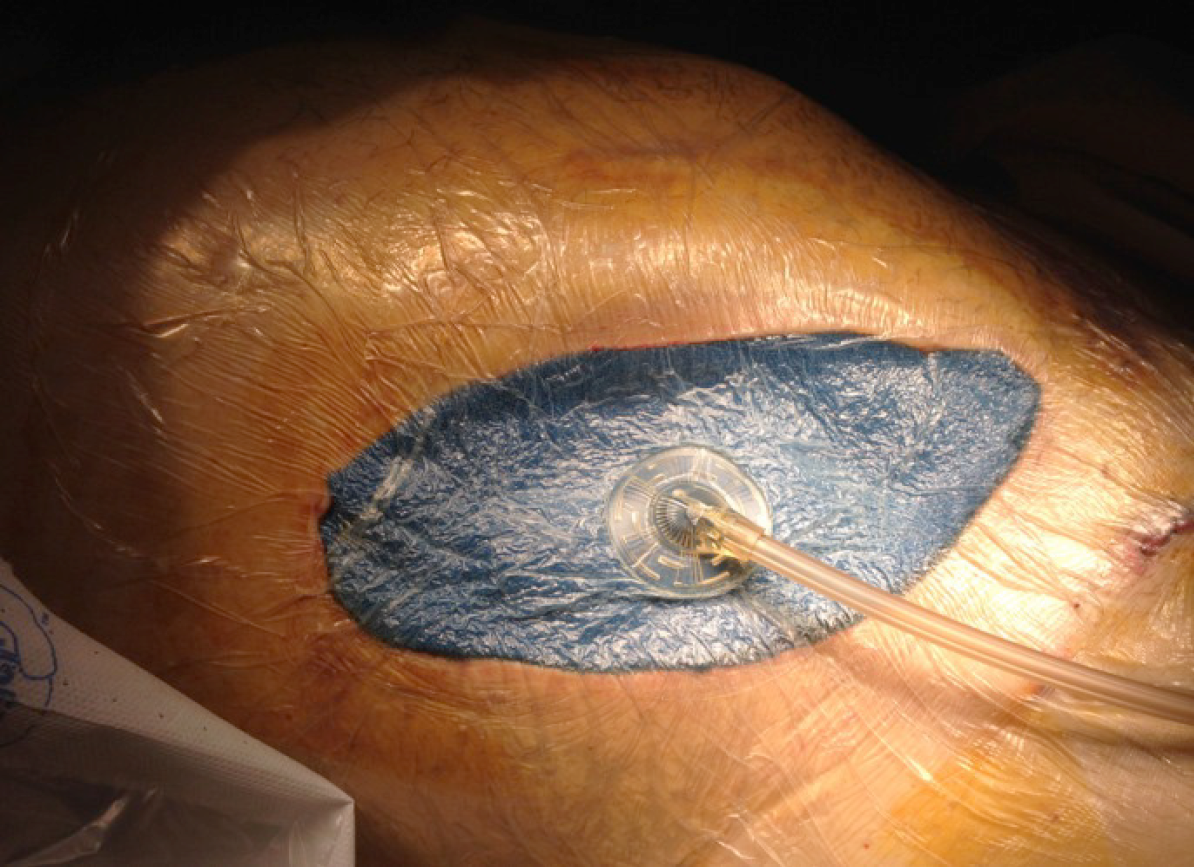
One of the complications related to the severe anticoagulation, to which these donors are subjected, is the risk of postoperative bleeding, along with the consequently required reoperation. Other complications observed include the existence of peripancreatic hematomas with risk of abdominal compartmental syndrome, especially with the use of a retroperitoneal graft technique. A compartment syndrome exists when increased pressure in a closed anatomic space threatens the viability of the tissue within the compartment[44-47]. In these cases, the entire wound must be opened to drain the hematoma and liberate the graft from the existing pressure, since otherwise the ischemia and thrombosis of the graft is the rule. Subsequently, a negative pressure closure technique is recommended (Figure 11).
Negative-pressure wound therapy is a frequently applied open abdomen treatment. There are only few experimental data published in support of this method and describing the optimal settings and pressure distribution in the abdominal cavity during this procedure[48-51]. The retrieval and preservation of the pancreas for transplantation is a surgical procedure that requires very high level anatomical and technical knowledge. It is necessary to remember that the pancreas is the most frequently discarded organ during donation.
Pancreas transplant is a milestone in the treatment of diabetes mellitus, for selected patients. Despite the improvement of its results in terms of patient and graft survival (similar to the transplantation of other solid organs), its complications are important and numerous, often compromising the viability of the transplant. For this reason, we must be very careful with the selection of donors and recipients, as well as with the technical aspects related to the retrieval, preservation and transplantation of the pancreas. The new techniques of retrieval and preservation of the pancreas in asystole donors have allowed us to increase the pool of donors while maintaining safety guidelines for patients.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: AEC (Spanish Association Surgeons), No. 0323.
Specialty type: Transplantation
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Novita BD, Pranata R S-Editor: Huang P L-Editor: A P-Editor: Wang LL
| 1. | Krieger NR, Odorico JS, Heisey DM, D'Alessandro AM, Knechtle SJ, Pirsch JD, Sollinger HW. Underutilization of pancreas donors. Transplantation. 2003;75:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Lam HD, Schaapherder AF, Kopp WH, Putter H, Braat AE, Baranski AG. Professionalization of surgical abdominal organ recovery leading to an increase in pancreatic allografts accepted for transplantation in the Netherlands: a serial analysis. Transpl Int. 2017;30:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Wullstein C, Woeste G, de Vries E, Persijn GG, Bechstein WO. Acceptance criteria of pancreas grafts: how do surgeons decide in Europe? Transplant Proc. 2005;37:1259-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Maglione M, Ploeg RJ, Friend PJ. Donor risk factors, retrieval technique, preservation and ischemia/reperfusion injury in pancreas transplantation. Curr Opin Organ Transplant. 2013;18:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Gruessner AC, Sutherland DE, Gruessner RW. Pancreas transplantation in the United States: a review. Curr Opin Organ Transplant. 2010;15:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Troppmann C. Complications after pancreas transplantation. Curr Opin Organ Transplant. 2010;15:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Goodman J, Becker YT. Pancreas surgical complications. Curr Opin Organ Transplant. 2009; 14:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Humar A, Ramcharan T, Kandaswamy R, Gruessner RW, Gruessner AC, Sutherland DE. Technical failures after pancreas transplants: why grafts fail and the risk factors--a multivariate analysis. Transplantation. 2004;78:1188-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Brockmann JG, Vaidya A, Reddy S, Friend PJ. Retrieval of abdominal organs for transplantation. Br J Surg. 2006;93:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Marsh CL, Perkins JD, Sutherland DE, Corry RJ, Sterioff S. Combined hepatic and pancreaticoduodenal procurement for transplantation. Surg Gynecol Obstet. 1989;168:254-258. [PubMed] |
| 11. | Dunn DL, Morel P, Schlumpf R, Mayoral JL, Gillingham KJ, Moudry-Munns KC, Krom RA, Gruessner RW, Payne WD, Sutherland DE. Evidence that combined procurement of pancreas and liver grafts does not affect transplant outcome. Transplantation. 1991;51:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Sanseverino R, Martin X, Caldara R, Faure JL, Lefrancois N, Dubernard JM. Technique of pancreas revascularization after combined liver and pancreas harvesting in the same cadaveric donor. Clin Transplant. 1991;5:55-59. [PubMed] |
| 13. | Fridell JA, Powelson JA, Kubal CA, Burke GW, Sageshima J, Rogers J, Stratta RJ. Retrieval of the pancreas allograft for whole-organ transplantation. Clin Transplant. 2014;28:1313-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Fernández ED, Schmid M, Schlosser K, Mauer D; Working Group of the Organ Procurement Central Region of the German Foundation for Organ Transplantation (DSO). Technical complications in organ procurement. Transplant Proc. 2007;39:2975-2976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Molmenti EP, Klein AS, Henry ML. Procurement of liver and pancreas allografts in donors with replaced/accessory right hepatic arteries. Transplantation. 2004;78:770-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Ames SA, Kisthard JK, Smith JL, Piper JB, Corry RJ. Successful combined hepatic and pancreatic allograft retrieval in donors with a replaced right hepatic artery. Surg Gynecol Obstet. 1991;173:216-222. [PubMed] |
| 17. | Baranski AG, Lam HD, Braat AE, Schaapherder AF. The dorsal pancreatic artery in pancreas procurement and transplantation: anatomical considerations and potential implications. Clin Transplant. 2016;30:1360-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Marang-van de Mheen PJ, Hilling DE, Dirkes MC, Baranski AG. Surgical injuries of pancreatic allografts during procurement. Clin Transplant. 2011;25:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Fridell JA, Powelson JA, Sanders CE, Ciancio G, Burke GW 3rd, Stratta RJ. Preparation of the pancreas allograft for transplantation. Clin Transplant. 2011;25:E103-E112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Wright FH, Wright C, Ames SA, Smith JL, Corry RJ. Pancreatic allograft thrombosis: donor and retrieval factors and early postperfusion graft function. Transplant Proc. 1990;22:439-441. [PubMed] |
| 21. | Humar A, Kandaswamy R, Drangstveit MB, Parr E, Gruessner AG, Sutherland DE. Prolonged preservation increases surgical complications after pancreas transplants. Surgery. 2000;127:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Sollinger HW, Vernon WB, D'Alessandro AM, Kalayoglu M, Stratta RJ, Belzer FO. Combined liver and pancreas procurement with Belzer-UW solution. Surgery. 1989;106:685-690; discussion 690. [PubMed] |
| 23. | Boggi U, Vistoli F, Del Chiaro M, Signori S, Croce C, Pietrabissa A, Berchiolli R, Marchetti P, Del Prato S, Mosca F. Pancreas preservation with University of Wisconsin and Celsior solutions: a single-center, prospective, randomized pilot study. Transplantation. 2004;77:1186-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Parsons RF, Guarrera JV. Preservation solutions for static cold storage of abdominal allografts: which is best? Curr Opin Organ Transplant. 2014;19:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Voigt MR, DeLario GT. Perspectives on abdominal organ preservation solutions: a comparative literature review. Prog Transplant. 2013;23:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Potdar S, Malek S, Eghtesad B, Shapiro R, Basu A, Patel K, Broznick B, Fung J. Initial experience using histidine-tryptophan-ketoglutarate solution in clinical pancreas transplantation. Clin Transplant. 2004;18:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Agarwal A, Powelson JA, Goggins WC, Milgrom ML, Fridell JA. Organ preservation with histidine-tryptophan ketogluatarate solution in clinical pancreas transplantation: an update of the indiana university experience. Transplant Proc. 2008;40:498-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Baertschiger RM, Berney T, Morel P. Organ preservation in pancreas and islet transplantation. Curr Opin Organ Transplant. 2008;13:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Muthusamy AS, Mumford L, Hudson A, Fuggle SV, Friend PJ. Pancreas transplantation from donors after circulatory death from the United Kingdom. Am J Transplant. 2012; 12:2150-2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Qureshi MS, Callaghan CJ, Bradley JA, Watson CJ, Pettigrew GJ. Outcomes of simultaneous pancreas-kidney transplantation from brain-dead and controlled circulatory death donors. Br J Surg. 2012;99:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Salvalaggio PR, Davies DB, Fernandez LA, Kaufman DB. Outcomes of pancreas transplantation in the United States using cardiac-death donors. Am J Transplant. 2006;6:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Fernandez LA, Di Carlo A, Odorico JS, Leverson GE, Shames BD, Becker YT, Chin LT, Pirsch JD, Knechtle SJ, Foley DP, Sollinger HW, D'Alessandro AM. Simultaneous pancreas-kidney transplantation from donation after cardiac death: successful long-term outcomes. Ann Surg. 2005;242:716-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Boggi U, Vistoli F, Del Chiaro M, Signori S, Pietrabissa A, Costa A, Bartolo TV, Catalano G, Marchetti P, Del Prato S, Rizzo G, Jovine E, Pinna AD, Filipponi F, Mosca F. A simplified technique for the en bloc procurement of abdominal organs that is suitable for pancreas and small-bowel transplantation. Surgery. 2004; 135:629-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Jeon H, Ortiz JA, Manzarbeitia CY, Alvarez SC, Sutherland DE, Reich DJ. Combined liver and pancreas procurement from a controlled non-heart-beating donor with aberrant hepatic arterial anatomy. Transplantation. 2002;74:1636-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | de Boer JD, Kopp WH, Ooms K, Haase-Kromwijk BJ, Krikke C, de Jonge J, van Heurn LW, Baranski AG, van der Vliet JA, Braat AE. Abdominal organ procurement in the Netherlands - an analysis of quality and clinical impact. Transpl Int. 2017; 30:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Reich DJ, Mulligan DC, Abt PL, Pruett TL, Abecassis MM, D'Alessandro A, Pomfret EA, Freeman RB, Markmann JF, Hanto DW, Matas AJ, Roberts JP, Merion RM, Klintmalm GB; ASTS Standards on Organ Transplantation Committee. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 37. | Hessheimer AJ, Billault C, Barrou B, Fondevila C. Hypothermic or normothermic abdominal regional perfusion in high-risk donors with extended warm ischemia times: impact on outcomes? Transpl Int. 2015;28:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Miñambres E, Rubio JJ, Coll E, Domínguez-Gil B. Donation after circulatory death and its expansion in Spain. Curr Opin Organ Transplant. 2018;23:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 39. | Brogi E, Circelli A, Gamberini E, Russo E, Benni M, Pizzilli G, Agnoletti V. Normothermic Regional Perfusion for Controlled Donation After Circulatory Death: A Technical Complication During Normothermic Regional Perfusion. ASAIO J. 2020;66:e19-e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Oniscu GC, Randle LV, Muiesan P, Butler AJ, Currie IS, Perera MT, Forsythe JL, Watson CJ. In situ normothermic regional perfusion for controlled donation after circulatory death--the United Kingdom experience. Am J Transplant. 2014;14:2846-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 41. | Barlow AD, Hosgood SA, Nicholson ML. Current state of pancreas preservation and implications for DCD pancreas transplantation. Transplantation. 2013; 95:1419-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Miñambres E, Suberviola B, Dominguez-Gil B, Rodrigo E, Ruiz-San Millan JC, Rodríguez-San Juan JC, Ballesteros MA. Improving the Outcomes of Organs Obtained From Controlled Donation After Circulatory Death Donors Using Abdominal Normothermic Regional Perfusion. Am J Transplant. 2017;17:2165-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 43. | Butler AJ, Randle LV, Watson CJ. Normothermic regional perfusion for donation after circulatory death without prior heparinization. Transplantation. 2014;97:1272-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | 44 Pineda-Solís K, Xie WY, McAlister V, Sener A, Luke PP. Retroperitoneal Compartment Syndrome in Renal Transplantation: How to Salvage the Graft? Urology 2017; 107: 268 . [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Ortiz J, Parsikia A, Horrow MM, Khanmoradi K, Campos S, Zaki R. Risk factors for renal allograft compartment syndrome. Int Surg. 2014;99:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Handschin AE, Weber M, Renner E, Clavien PA. Abdominal compartment syndrome after liver transplantation. Liver Transpl. 2005;11:98-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Michalska M, Wen K, Pauly RP. Acute Kidney Injury in Pregnant Patient With Pancreas-Kidney Transplant Caused by Abdominal Compartment Syndrome: A Case Presentation, Review of Literature, and Proposal of Diagnostic Approach. Can J Kidney Health Dis. 2019; 6:2054358119861942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Wang TY, Elliott R, Low DW. Damage control abdomen: single-stage reconstruction using a vicryl mesh buttress. Ann Plast Surg. 2013;70:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Ghazi B, Deigni O, Yezhelyev M, Losken A. Current options in the management of complex abdominal wall defects. Ann Plast Surg. 2011;66:488-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 50. | Smith CT, Katz MG, Foley D, Welch B, Leverson GE, Funk LM, Greenberg JA. Incidence and risk factors of incisional hernia formation following abdominal organ transplantation. Surg Endosc. 2015;29:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 51. | Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51:301-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 313] [Article Influence: 28.5] [Reference Citation Analysis (2)] |