Published online Nov 28, 2020. doi: 10.5500/wjt.v10.i11.297
Peer-review started: May 18, 2020
First decision: June 3, 2020
Revised: June 9, 2020
Accepted: September 22, 2020
Article in press: September 22, 2020
Published online: November 28, 2020
Processing time: 188 Days and 17.1 Hours
Liver transplantation (LT) is one of the leading curative therapies for hepatocellular carcinoma (HCC). Despite recent optimization of transplant selection criteria, including alpha-feto protein, HCC recurrence after LT is still the leading cause of death in these patients. During the last decades, effective systemic treatments for HCC, including tyrosine kinase inhibitors and immunotherapy, have been approved. We describe the clinical scenario of a patient with recurrence of HCC five years after LT, who received lenvatinib as first-line systemic therapy to introduce systemic treatment options in this clinical setting. In this opinion review, we detail first and second-line systemic treatment options, focusing on those feasible for patients with recurrent HCC after LT. Several trials have evaluated new drugs to treat HCC patients in first and second-line therapy, but patients with recurrent HCC after LT have been excluded from these trials. Consequently, most of the evidence comes from observational retrospective studies. Whether tyrosine kinase inhibitors will remain the primary therapeutic approach in these patients, due to a relative contraindication for immunotherapy, may be clarified in the near future.
Core Tip: Post-transplant hepatocellular carcinoma (HCC) recurrence is a significant negative predictor of survival. There is no consensus on the treatment of recurrence. If possible, resection should be attempted. The use of systemic chemotherapy after transplant is limited to small retrospective cohort studies. Immunotherapy with checkpoint inhibitors in the post-transplant setting is challenging due to the potentially increased risk of allograft rejection. This opinion review illustrates a late post-transplant HCC recurrence treated with lenvatinib, with good tolerance and overall survival after lung and adrenal metastasis resections in a patient previously intolerant to sorafenib.
- Citation: Piñero F, Thompson M, Marín JI, Silva M. Lenvatinib as first-line therapy for recurrent hepatocellular carcinoma after liver transplantation: Is the current evidence applicable to these patients? World J Transplant 2020; 10(11): 297-306
- URL: https://www.wjgnet.com/2220-3230/full/v10/i11/297.htm
- DOI: https://dx.doi.org/10.5500/wjt.v10.i11.297
Hepatocellular carcinoma (HCC) recurrence is a dramatic event with a dismal prognosis after liver transplantation (LT)[1]. Despite recent optimization of LT selection criteria, HCC recurrence still develops in approximately 8%-20% of these patients[1,2], being the most frequent cause of death among LT HCC patients[3-6].
Although transplant consensus recommends surveillance for recurrent HCC after LT[7,8], its prompt diagnosis after LT has not been associated with improved survival or reduced cancer-related mortality. This scenario is probably related to the lack of curative treatments in these patients.
Moreover, recurrence occurring during the first 2 years after transplant has been associated with even worse post-recurrence survival (PRS)[6,9-11]. Early recurrences may represent an aggressive HCC with worse biological behavior after transplantation.
The cost-effectiveness of surveillance for recurrent HCC remains uncertain. Several groups have proposed risk-based surveillance. The RETREAT score incorporates three variables in its model: Explant tumor burden, microvascular invasion, and α-fetoprotein levels determine whether HCC surveillance after LT is warranted and identifies patients who may benefit from future adjuvant therapies[12,13].
To date, it is unknown whether early recurrences are associated with a continuum of metastatic disease, not adequately assessed or unknown before LT, or with aggressive biological behavior. Indeed, early recurrences have not been linked to any known associated pre-LT or explant-based risk variable[14,15] and may have completely different oncogenic mutations or activated pathways than the original location[16].
On the other hand, there is no efficient or specific treatment for HCC recurrence. Indeed, heterogeneous data have been published, including locoregional therapies, liver resection, endovascular and systemic therapies[17]. The efficacy of each therapy for post-LT recurrence is not well defined, and most of the evidence comes from retrospective uncontrolled published data. Despite the fact that some authors have proposed locoregional approaches, even in the setting of extrahepatic metastasis[11], whether these therapies or in combination with systemic treatment are effective is still uncertain[18]. Indeed, most of these patients have been excluded from randomized controlled trials (RCTs) assessing the efficacy of systemic therapies. However, recurrent HCC is an excellent opportunity to address and evaluate precision oncology as tumor pathology is available at explant analysis and with metastatic tumor recurrence. Consequently, targeted therapies may be the future in these patients[19,20].
We describe the clinical scenario of a real-world patient with recurrence of HCC five years after LT, who received lenvatinib as first-line systemic therapy to introduce systemic treatment options in this clinical setting. A non-cirrhotic male patient with chronic hepatitis C infection with sustained viral response after treatment with Peg-Interferon and ribavirin, developed a large HCC of 8 cm in diameter during 2011. He underwent a right lobe hepatectomy and was enrolled in a double-blind RCT evaluating the effect of adjuvant therapy with sorafenib over placebo[21]. He developed intolerance to adjuvant therapy (unknown arm). Eighteen months later, a multinodular recurrent HCC was diagnosed with two liver nodules smaller than 2 cm, both treated with radiofrequency ablation. Ten months later, another single intrahepatic HCC recurrence was observed, and a second ablation therapy was performed, achieving a complete response. However, a year later, he presented with two new intrahepatic recurrences smaller than 2 cm; thus, liver transplantation was proposed after excluding vascular or extrahepatic disease, and without any tumor progression during a waitlist observation period of 6 mo. Liver transplantation was performed in September 2013 (at the age of 66). There were two nodules at explant pathology, one nodule with complete necrosis and the other was a well-differentiated HCC nodule 6 mm in diameter without microvascular invasion.
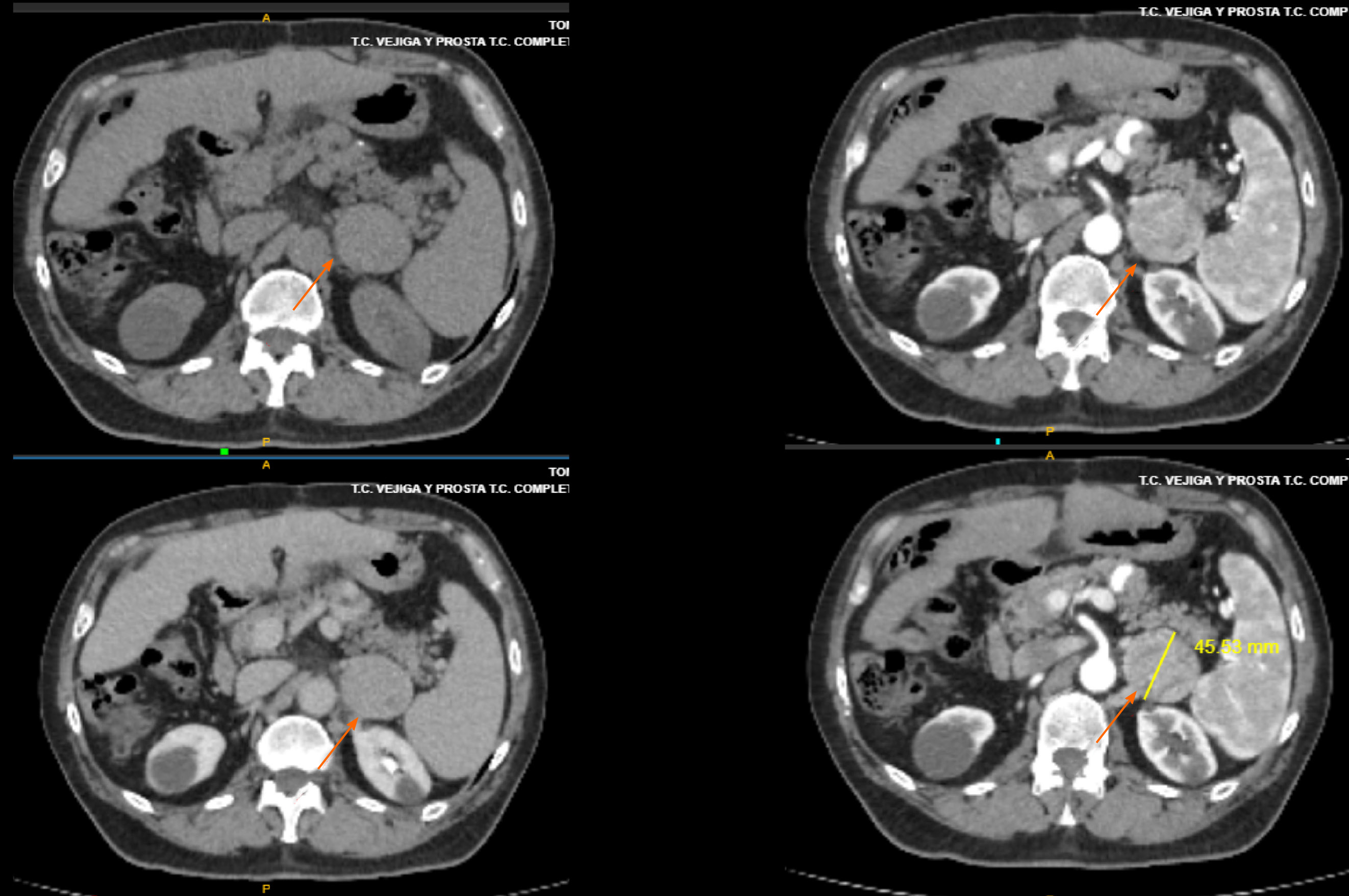
The patient was followed with biannual computed tomography scans and serum alpha-feto protein (AFP) evaluation and received immunosuppressive treatment with tacrolimus. Five years after LT, a single pulmonary nodule was observed, and video-assisted thoracoscopic surgery was performed. A lung HCC metastasis was histologically confirmed with complete resection. He was offered sorafenib, but he refused due to prior intolerance. No other metastatic sites were observed, and a strict follow-up was proposed. Immunosuppression continued with tacrolimus monotherapy with blood trough levels between 3-4 ng/mL. Ten months after pulmonary resection, a small tumor was observed near the left suprarenal gland (Figure 1), which was resected and HCC was confirmed by pathology examination. He started lenvatinib 12 mg/day on May 20th 2019, as tumor bleeding was observed during adrenal gland resection. The patient showed adequate tolerance without any significant adverse events, and there was no need for tacrolimus dose adjustments. Only high blood pressure was observed, which was well controlled with amlodipine 5 mg/day. The patient is still receiving lenvatinib (September 14, 2020).
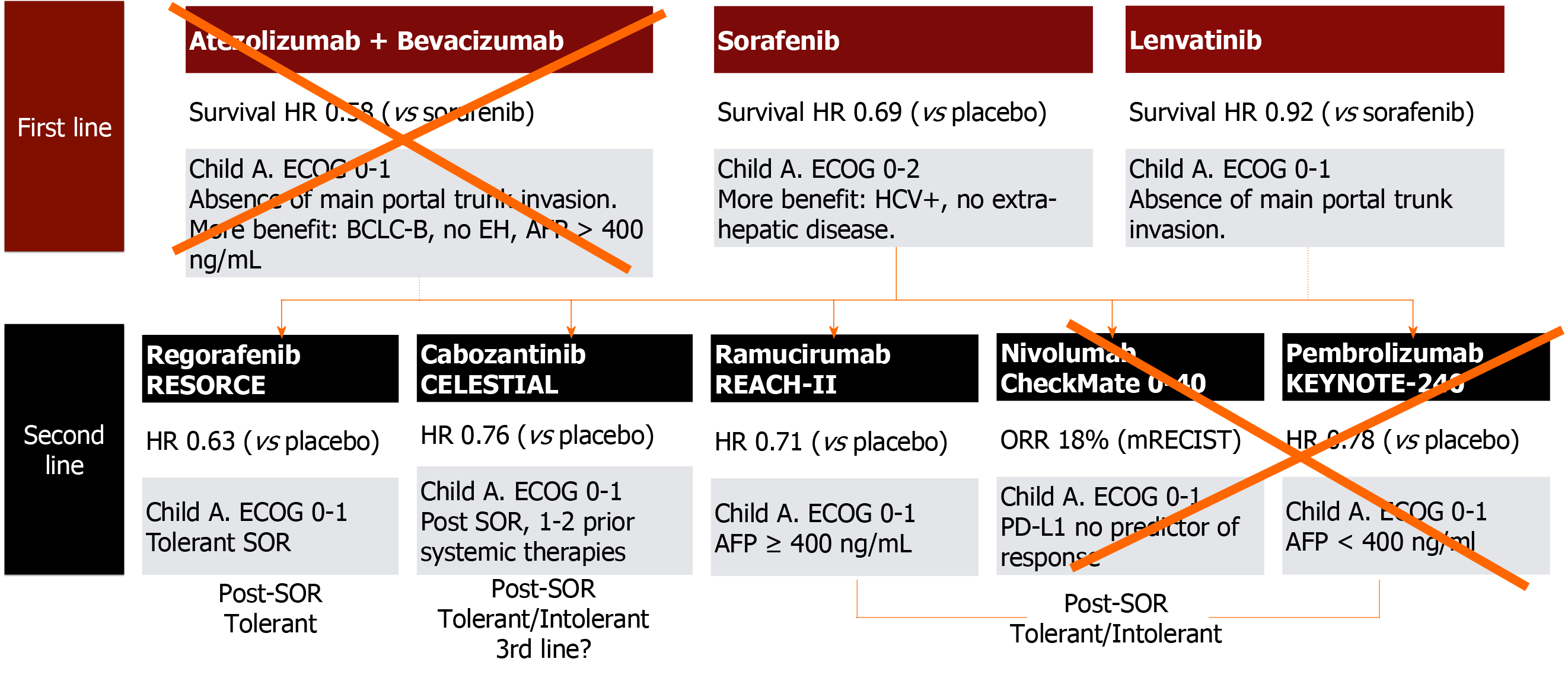
During the last decade, enormous improvement in the treatment of advanced HCC has been achieved, with unthinkable survival rates years ago (Figure 2)[22]. Sorafenib, a tyrosine multikinase inhibitor was the first drug to show a survival benefit over placebo (SHARP and Asia-Pacific trials)[23,24]. High serum AFP values (> 200 ng/mL), macroscopic vascular invasion, and a low neutrophil/leukocyte ratio are baseline variables associated with poor prognosis in these patients, but even in these subgroups, sorafenib showed a survival benefit vs placebo[25].
In several retrospective cohort studies, sorafenib has shown a heterogeneous effect on PRS[18]. In some series, treatment effects were assessed without a control group or adjustment for prognostic baseline variables. Whether the same prognostic factors in the non-transplant setting apply to the post-LT setting, such as hepatitis C, no-extrahepatic disease or a low neutrophil to lymphocyte ratio[25], is unknown. Nevertheless, these patients lead with other prognostic issues that may confound or modify each treatment effect.
Firstly, performance status may be better or even worse after LT than in the non-transplant setting. This depends on LT complications, over-immunosuppression, and opportunistic infections. In this regard, retrospective cohort studies reporting sorafenib after LT have not entirely addressed liver function and performance status at HCC recurrence as prognostic variables[6,9-11]. Nevertheless, poor nutritional status has been suggested to be a surrogate marker of shorter PRS[26].
Secondly, time to recurrence (TTR) is thought to be an independent prognostic factor that may modify the treatment effect. Indeed, in several retrospective cohort studies, early recurrences (during the first year after LT) have been associated with poorer PRS[6,9-11]. The problem with TTP in the context of LT is that it has been reported with different information and selection bias, as there is no standardized surveillance policy for HCC recurrence. Besides, the diagnosis of HCC recurrence either with imaging alone or with tumor biopsy confirmation has not been homogenously reported. Despite these methodological issues, it seems that in the post-LT setting, TTR correlates with PRS[6,9-11].
Other prognostic variables have been reported at the time of HCC recurrence following LT. Tumor location and serum AFP value at recurrence have been associated with PRS[9-11,27,28]. Multi-organ sites at recurrence or bone metastasis have been associated with poorer PRS in some studies[9,10,27], but not in others[6]. Serum AFP values at HCC recurrence diagnosis were also reported to be associated with PRS. Although Harimoto et al[28] did not report the adjusted effect in a multivariable model[28], Sapisochin et al[11] reported that AFP values higher than 100 ng/mL were independently associated with higher rate of death after recurrence[11].
Finally, these prognostic factors should adjust the treatment effect associated with reported therapies for post-transplant HCC recurrence. Adjusted effects were reported in some studies, but not in all, particularly considering TTR as the most critical confounder factor or effect modifier[9-11,27,28]. Moreover, in most available observational studies, the treatment was not allocated randomly, and baseline prognostic factors should have adjusted the effect on PRS. None of these studies have addressed this issue, not even with propensity score matching analysis[29].
Table 1 describes studies reporting the effect of sorafenib in recurrent HCC after LT. Long PRS has been reported, with significant confounding effects regarding TTR[30]. Indeed, early recurrent HCC may not have the expected PRS as those with longer TTR. Whether this represents a selection bias or better tumor behavior is uncertain.
| Ref. | Design | Population | SOR/BSC | mTOR | SOR duration(mo) | Adverse events with SOR | PRS with SOR (mo) |
| Bhoori et al[16] | Case report | Single patient with HCC recurrence | 1 (1/-) | 1/1 | 4 | HFS | 8 |
| Yoon et al[47] | Retrospective cohort | HCC-R with SOR median TTR (12.3 mo) | 13 (13/-) | 1/13 | 2.4 | HFS | 5.4 |
| Kim et al[48] | Retrospective cohort | HCC-R with SOR | 9 (9/-) | 7/9 | 2.8 | - | - |
| Staufer et al[49] | Retrospective cohort | HCC-R with SOR | 20 (13/7) | 9/18 | 5.5 | Grade 3-4 92%. Diarrhea 77% Discontinuation | 19 |
| Gomez-Martín et al[50] | Retrospective cohort | HCC-R with SOR + mTOR median TTR (22.6 mo) | 31 (31/-) | 31/31 | - | Diarrhea | 19 |
| Weinmann et al[51] | Retrospective cohort | HCC-R with SOR median TTR (37.5 mo) | 11 (11/-) | 9/11 | 8.9 | Diarrhea | 20 |
| Vitale et al[52] | Retrospective cohort | HCC-R with SOR median TTR (7 mo) | 27 (10/-) | 10/27 | 10 | Diarrhea 30%, Discontinuation | 18 |
| Zavaglia et al[53] | Retrospective cohort | HCC-R with SOR | 11 (11/-) | 7/11 | 2.2 | Fatigue | 5 |
| Waghrayet al[54] | Retrospective cohort | HCC-R | 34 (17/17) | 10/34 | 10.2 | Diarrhea | 7 |
| Sposito et al[30] | Retrospective cohort | HCC-R with SOR | 39 (15/24) | 7/39 | 6.9 | HFS | 10.6 vs 2.2 median TTR 20.6 vs 15.5 mo |
| Alsina et al[55] | Retrospective cohort | HCC-R with/without SOR | 22 (9/13) | 10.2 | Rash | 42 vs 16 since LT |
Hepatitis C, absence of extrahepatic disease, and low neutrophil/lymphocyte ratio (< 3) have been linked to predictive factors of better outcomes with sorafenib[25]. Although dermatological events during the first 60 d of treatment were associated with better overall survival (OS) in the non-LT setting, this must be confirmed in post-LT patients[31]. Better PRS predictive factors after treatment with sorafenib are also lacking in the post-LT setting.
The REFLECT phase III, open-label RCT, showed non-inferior survival of lenvatinib (8 mg/day if < 60 kg or 12 mg/day if > 60 kg) vs sorafenib[32]. This tyrosine kinase inhibitor blocks VEGF as well as FGF and PDGF pathways. In this trial, eligibility criteria excluded patients with main portal trunk tumor invasion and those with > 50% of total liver volume involvement[32,33]. Median survival was 13.6 mo with lenvatinib vs 12.3 mo with sorafenib [HR: 0.92 (CI: 0.79-1.06)][32]. TTP, as well as higher rates of partial response and objective response rates were observed with lenvatinib. Higher rates of severe adverse events were observed in the lenvatinib arm (57% vs 49%), mainly hypertension, hypothyroidism, and proteinuria.
The REFLECT trial modified the future therapeutic options in patients with advanced HCC. It remains unclear which subgroup of patients will obtain benefits by being treated with lenvatinib or sorafenib. Indeed, similar prognostic and predictive variables for lenvatinib have recently been published[34,35].
Unfortunately, there are no reported data regarding lenvatinib in the post-LT setting. To date, this is the first reported case treated with lenvatinib, at least in the non-Asian population. Our patient reported similar adverse events to those originally reported in the REFLECT trial, with initial hypertension during the first weeks of therapy and hypothyroidism presenting at week 4 of treatment and 13-mo therapy. There were no severe events, tolerance was appropriate and we did not observe liver function test abnormalities. In addition, blood tacrolimus levels were stable during the entire follow-up period. Although in this particular case, the real benefit on post-recurrence survival of lenvatinib vs surgical resection is still uncertain, and prognosis might have been associated with a more extended TTR presentation.
Three potential scenarios can develop during first-line systemic treatment, which determines the subsequent patient’s management: (1) Tolerance or intolerance; (2) Radiological progression; and (3) Symptomatic progression[22]. In HCC recurrence after LT, higher discontinuation rates and lower tolerance were reported with sorafenib (Table 1). However, this figure was not reported in a recently published study of sequential systemic therapy with sorafenib-regorafenib in the post-LT setting[36]. Whether adverse events are higher in the post-LT setting with lenvatinib is unknown.
More recently, immunotherapy has evolved as a potential first-line systemic option. Nivolumab was tested against sorafenib in the first-line setting (Check-Mate 459 study; NCT02576509) and failed in both co-primary endpoints. Another phase III, open-label, randomized trial evaluating atezolizumab, another immune-checkpoint inhibitor, with bevacizumab, an anti-VEGF monoclonal antibody, was superior to sorafenib in both co-primary endpoints of OS and progression free survival (PFS)[37]. Nevertheless, this therapy may not be applicable for post-LT patients as a higher risk of graft rejection has been reported[38,39] (Figure 2).
Currently, regorafenib[40], cabozantinib (CELESTIAL phase III RCT)[41] and ramucirumab (REACH I and REACH II phase III RCTs)[42] have demonstrated second-line efficacy. Neither pembrolizumab nor nivolumab, immune-checkpoint inhibitors, are recommended in the post-LT setting as previously mentioned[43,44]. The RESORCE phase III RCT included patients with advanced HCC who were tolerant and progressed under sorafenib[40]. The median OS was 10.6 mo (CI: 9.1-12.1) for regorafenib and 7.8 mo (CI: 6.3-8.8) for placebo, with a HR of 0.62 (95%CI: 0.50-0.79)[40]. Likewise, regorafenib was beneficial for TTP[40]. Overall, 93% of the patients receiving regorafenib developed AEs (i.e., high blood pressure, fatigue, diarrhea and hand–foot skin reaction), 46% grade III, and 4% grade IV, with drug discontinuation due to intolerance in 10% of the patients[40].
There is no reported data regarding the safety and efficacy of these second line therapies in patients with recurrent HCC after LT except for regorafenib[36]. Iavarone et al[36] reported the safety and outcomes of 28 patients treated with sequential systemic sorafenib-regorafenib after LT. Almost all patients developed adverse events, with 43% being severe events and 68% needing dose reductions[36]. The most common grade 3/4 adverse events were fatigue and hand-foot skin reaction. Interaction between CYP3A4 metabolism was reported with higher plasma levels of immunosuppressive drugs. The median regorafenib duration of treatment was 6.5 mo, with a median survival after regorafenib therapy of 12.9 mo and was 38.4 mo after sorafenib-regorafenib sequential treatment. This latter outcome is longer than previously reported in a retrospective analysis from the RESORCE trial[45]. However, it should be noted that these post-LT outcomes were assessed in a population with a better prognosis compared to patients with early recurrence. Indeed, the median TTR in that study was 26.4 mo[36].
Finally, neither neo-adjuvant nor adjuvant therapies have decreased the incidence of HCC recurrence following LT[46]. Whether sorafenib or other systemic therapy may be effective as adjuvant post-LT therapy is uncertain[47-55]. In the non-transplant setting, the STORM trial has not shown the benefit of sorafenib in decreasing the risk of HCC recurrence[21]; other trials evaluating immunotherapy in this setting are ongoing.
Recurrent HCC after LT has been associated with a dismal prognosis. Particularly in patients with recurrences during the first year after LT. Attempts have been made with radical therapies, some of them associated with better PRS. However, evidence supporting such radical therapies is low to very low quality. This is similar to the reported outcomes with systemic therapies.
Moreover, there are no appropriate adjustment treatment effects with other prognostic variables, such as TTR. Whether more efficient therapies are yet to be identified and applied to these patients remains unknown. In this scenario, surveillance for HCC recurrence is still controversial due to a lack of reduction in mortality rates. However, after LT, surveillance for recurrence is demanded from a social point of view, triggering areas of improvement in the LT selection processes.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jiménez Pérez M, Sun HC S-Editor: Zhang L L-Editor: Webster JR P-Editor: Wang LL
| 1. | Toso C, Mentha G, Majno P. Liver transplantation for hepatocellular carcinoma: five steps to prevent recurrence. Am J Transplant. 2011;11:2031-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Piñero F, Carrihlo FJ, Silva MO. Predictive models for recurrence risk of hepatocellular carcinoma after liver transplantation: Still an unmet need. Liver Int. 2017;37:648-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Piñero F, Tisi Baña M, de Ataide EC, Hoyos Duque S, Marciano S, Varón A, Anders M, Zerega A, Menéndez J, Zapata R, Muñoz L, Padilla Machaca M, Soza A, McCormack L, Poniachik J, Podestá LG, Gadano A, Boin IS, Duvoux C, Silva M; Latin American Liver Research; Education and Awareness Network (LALREAN). Liver transplantation for hepatocellular carcinoma: evaluation of the alpha-fetoprotein model in a multicenter cohort from Latin America. Liver Int. 2016;36:1657-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 4. | Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J, Dharancy S, Gugenheim J, Bernard PH, Adam R, Radenne S, Muscari F, Conti F, Hardwigsen J, Pageaux GP, Chazouillères O, Salame E, Hilleret MN, Lebray P, Abergel A, Debette-Gratien M, Kluger MD, Mallat A, Azoulay D, Cherqui D; Liver Transplantation French Study Group. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012; 143: 986-994. quiz e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 721] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 5. | Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, Bongini M, Cucchetti A. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 466] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 6. | Toso C, Cader S, Mentha-Dugerdil A, Meeberg G, Majno P, Morard I, Giostra E, Berney T, Morel P, Mentha G, Kneteman NM. Factors predicting survival after post-transplant hepatocellular carcinoma recurrence. J Hepatobiliary Pancreat Sci. 2013;20:342-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Kneteman N, Livraghi T, Madoff D, de Santibañez E, Kew M. Tools for monitoring patients with hepatocellular carcinoma on the waiting list and after liver transplantation. Liver Transpl. 2011;17 Suppl 2:S117-S127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Geissler EK, Schnitzbauer AA, Zülke C, Lamby PE, Proneth A, Duvoux C, Burra P, Jauch KW, Rentsch M, Ganten TM, Schmidt J, Settmacher U, Heise M, Rossi G, Cillo U, Kneteman N, Adam R, van Hoek B, Bachellier P, Wolf P, Rostaing L, Bechstein WO, Rizell M, Powell J, Hidalgo E, Gugenheim J, Wolters H, Brockmann J, Roy A, Mutzbauer I, Schlitt A, Beckebaum S, Graeb C, Nadalin S, Valente U, Turrión VS, Jamieson N, Scholz T, Colledan M, Fändrich F, Becker T, Söderdahl G, Chazouillères O, Mäkisalo H, Pageaux GP, Steininger R, Soliman T, de Jong KP, Pirenne J, Margreiter R, Pratschke J, Pinna AD, Hauss J, Schreiber S, Strasser S, Klempnauer J, Troisi RI, Bhoori S, Lerut J, Bilbao I, Klein CG, Königsrainer A, Mirza DF, Otto G, Mazzaferro V, Neuhaus P, Schlitt HJ. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016;100:116-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 348] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 9. | Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 344] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 10. | Roh Y-N, David Kwon CH, Song S, Shin M, Man Kim J, Kim S, Joh J-W, Lee S-K. The prognosis and treatment outcomes of patients with recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2013;28:141–7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Sapisochin G, Goldaracena N, Astete S, Laurence JM, Davidson D, Rafael E, Castells L, Sandroussi C, Bilbao I, Dopazo C, Grant DR, Lázaro JL, Caralt M, Ghanekar A, McGilvray ID, Lilly L, Cattral MS, Selzner M, Charco R, Greig PD. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann Surg Oncol. 2015;22:2286-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 13. | Mehta N, Dodge JL, Roberts JP, Yao FY. Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. Am J Transplant. 2018;18:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Decaens T, Roudot-Thoraval F, Badran H, Wolf P, Durand F, Adam R, Boillot O, Vanlemmens C, Gugenheim J, Dharancy S, Bernard PH, Boudjema K, Calmus Y, Hardwigsen J, Ducerf C, Pageaux GP, Hilleret MN, Chazouillères O, Cherqui D, Mallat A, Duvoux C. Impact of tumour differentiation to select patients before liver transplantation for hepatocellular carcinoma. Liver Int. 2011;31:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Costentin CE, Amaddeo G, Decaens T, Boudjema K, Bachellier P, Muscari F, Salame E, Bernard PH, Francoz C, Dharancy S, Vanlemmens C, Radenne S, Dumortier J, Hilleret MN, Chazouilleres O, Pageaux GP, Calderaro J, Laurent A, Roudot-Thoraval F, Group CDFLTFS. Prediction of hepatocellular carcinoma recurrence after liver transplantation: comparison of 4 explant-based prognostic models. Liver Int. 2017;37:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Bhoori S, Toffanin S, Sposito C, Germini A, Pellegrinelli A, Lampis A, Mazzaferro V. Personalized molecular targeted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol. 2010;52:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | de'Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J Gastroenterol. 2015;21:11185-11198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 18. | Mancuso A, Mazzola A, Cabibbo G, Perricone G, Enea M, Galvano A, Zavaglia C, Belli L, Cammà C. Survival of patients treated with sorafenib for hepatocellular carcinoma recurrence after liver transplantation: a systematic review and meta-analysis. Dig Liver Dis. 2015;47:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Kudo M. Targeted and immune therapies for hepatocellular carcinoma: Predictions for 2019 and beyond. World J Gastroenterol. 2019;25:789-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 118] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (2)] |
| 20. | De Mattia E, Cecchin E, Guardascione M, Foltran L, Di Raimo T, Angelini F, D'Andrea M, Toffoli G. Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma. World J Gastroenterol. 2019;25:3870-3896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 21. | Bruix J, Takayama T, Mazzaferro V, Chau G-Y, Yang J, Kudo M, Cai J, Poon RT, Han K-H, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Le Berre Marie-Aude, Meinhardt G, Llovet JM, investigators S. Articles Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16: :1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 777] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 22. | Piñero F, Silva M, Iavarone M. Sequencing of systemic treatment for hepatocellular carcinoma: Second line competitors. World J Gastroenterol. 2020;26:1888-1900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10225] [Article Influence: 601.5] [Reference Citation Analysis (2)] |
| 24. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4632] [Article Influence: 272.5] [Reference Citation Analysis (0)] |
| 25. | Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. 2017;67:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 450] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 26. | Nagai S, Mangus RS, Kubal CA, Ekser B, Fridell JA, Klingler KR, Maluccio MA, Tector AJ. Prognosis after recurrence of hepatocellular carcinoma in liver transplantation: predictors for successful treatment and survival. Clin Transplant. 2015;29:1156-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Shin WY, Suh KS, Lee HW, Kim J, Kim T, Yi NJ, Uk Lee K. Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2010;16: :678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 28. | Harimoto N, Shirabe K, Nakagawara H, Toshima T, Yamashita Y, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y. Prognostic factors affecting survival at recurrence of hepatocellular carcinoma after living-donor liver transplantation: with special reference to neutrophil/Lymphocyte ratio. Transplantation. 2013;96:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Austin PC, Fine JP. Propensity-score matching with competing risks in survival analysis. Stat Med. 2019;38:751-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 30. | Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib vs best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Díaz-González Á, Sanduzzi-Zamparelli M, Sapena V, Torres F, LLarch N, Iserte G, Forner A, da Fonseca L, Ríos J, Bruix J, Reig M. Systematic review with meta-analysis: the critical role of dermatological events in patients with hepatocellular carcinoma treated with sorafenib. Aliment Pharmacol Ther. 2019;49:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3774] [Article Influence: 539.1] [Reference Citation Analysis (1)] |
| 33. | Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda M, Okusaka T, Tamai T, Suzuki T, Hisai T, Hayato S, Okita K, Kumada H. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52:512-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 34. | Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Yasuda S, Toyoda H, Fukunishi S, Ohama H, Kawata K, Nakamura S, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Arai T, Imai M, Joko K, Koizumi Y, Hiasa Y. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int. 2020;40:968-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 35. | Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Toyoda H, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Imai M, Joko K, Koizumi Y, Hiasa Y. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: A multicenter analysis with propensity score matching. Hepatol Res. 2020;50:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 36. | Iavarone M, Invernizzi F, Czauderna C, Sanduzzi-Zamparelli M, Bhoori S, Amaddeo G, Manini MA, López MF, Anders M, Pinter M, Rodríguez MJB, Cristóbal MR, Soteras GA, Piñero F, Villadsen GE, Weinmann A, Crespo G, Mazzaferro V, Regnault H, Giorgio M, González-Diéguez ML, Donato MF, Varela M, Wörns MA, Bruix J, Lampertico P, Reig M. Preliminary experience on safety of regorafenib after sorafenib failure in recurrent hepatocellular carcinoma after liver transplantation. Am J Transplant. 2019;19:3176-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 37. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4596] [Article Influence: 919.2] [Reference Citation Analysis (2)] |
| 38. | Gassmann D, Weiler S, Mertens JC, Reiner CS, Vrugt B, Nägeli M, Mangana J, Müllhaupt B, Jenni F, Misselwitz B. Liver Allograft Failure After Nivolumab Treatment-A Case Report With Systematic Literature Research. Transplant Direct. 2018;4:e376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 39. | Aguirre LE, Guzman ME, Lopes G, Hurley J. Immune Checkpoint Inhibitors and the Risk of Allograft Rejection: A Comprehensive Analysis on an Emerging Issue. Oncologist. 2019;24:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 40. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2692] [Article Influence: 336.5] [Reference Citation Analysis (0)] |
| 41. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1749] [Article Influence: 249.9] [Reference Citation Analysis (0)] |
| 42. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1236] [Article Influence: 206.0] [Reference Citation Analysis (0)] |
| 43. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3279] [Article Influence: 409.9] [Reference Citation Analysis (1)] |
| 44. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1877] [Article Influence: 268.1] [Reference Citation Analysis (0)] |
| 45. | Finn RS, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Gerolami R, Caparello C, Cabrera R, Chang C, Sun W, LeBerre MA, Baumhauer A, Meinhardt G, Bruix J. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 46. | Lin HS, Wan RH, Gao LH, Li JF, Shan RF, Shi J. Adjuvant chemotherapy after liver transplantation for hepatocellular carcinoma: a systematic review and a meta-analysis. Hepatobiliary Pancreat Dis Int. 2015;14:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Yoon DH, Ryoo BY, Ryu MH, Lee SG, Hwang S, Suh DJ, Lee HC, Kim TW, Ahn CS, Kim KH, Moon DB, Kang YK. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010;40:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Kim R, Aucejo F. Radiologic complete response with sirolimus and sorafenib in a hepatocellular carcinoma patient who relapsed after orthotopic liver transplantation. J Gastrointest Cancer. 2011;42:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Staufer K, Fischer L, Seegers B, Vettorazzi E, Nashan B, Sterneck M. High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transpl Int. 2012;25:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Gomez-Martín C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, Herrero I, Matilla A, Sangro B. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012;18:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 51. | Weinmann A, Niederle IM, Koch S, Hoppe-Lotichius M, Heise M, Düber C, Schuchmann M, Otto G, Galle PR, Wörns MA. Sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Dig Liver Dis. 2012;44:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Vitale A, Boccagni P, Kertusha X, Zanus G, D'Amico F, Lodo E, Pastorelli D, Ramirez Morales R, Lombardi G, Senzolo M, Burra P, Cillo U. Sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation? Transplant Proc. 2012;44:1989-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Zavaglia C, Airoldi A, Mancuso A, Vangeli M, Viganò R, Cordone G, Gentiluomo M, Belli LS. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: experience at a single center and review of the literature. Eur J Gastroenterol Hepatol. 2013;25:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Waghray A, Balci B, El-Gazzaz G, Kim R, Pelley R, Narayanan Menon KV, Estfan B, Romero-Marrero C, Aucejo F. Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2013;27:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Alsina AE, Makris A, Nenos V, Sucre E, Arrobas J, Franco E, Kemmer N. Can sorafenib increase survival for recurrent hepatocellular carcinoma after liver transplantation? Am Surg. 2014;80:680-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |