Copyright
©The Author(s) 2018.
World J Transplantation. Oct 22, 2018; 8(6): 203-219
Published online Oct 22, 2018. doi: 10.5500/wjt.v8.i6.203
Published online Oct 22, 2018. doi: 10.5500/wjt.v8.i6.203
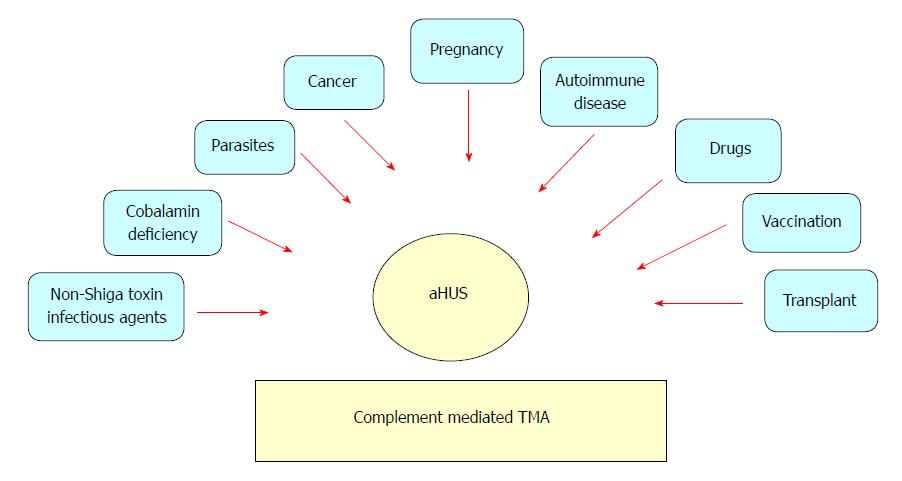
Figure 1 Heterogeneity of atypical hemolytic uremic syndrome.
Adapted from Salvadori et al[1]. TMA: Thrombotic microangiopathy; aHUS: Atypical hemolytic uremic syndrome.
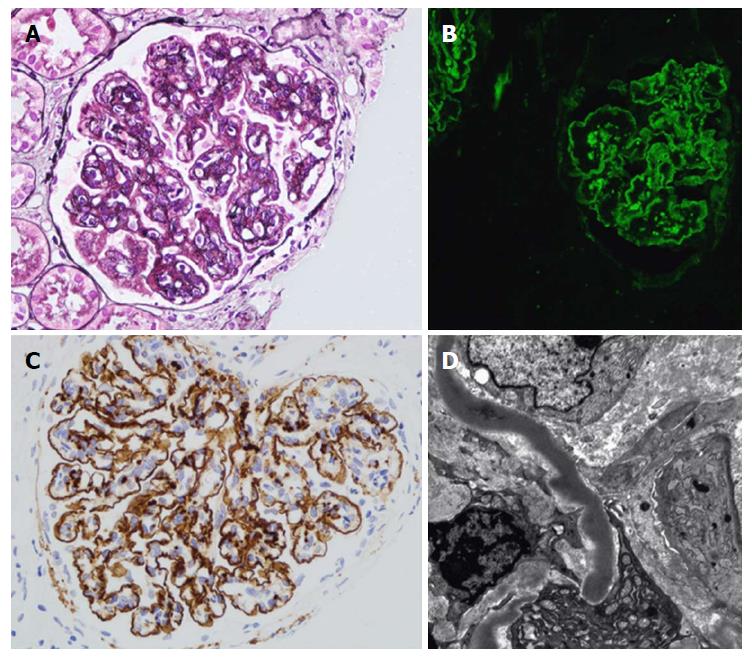
Figure 2 Renal histology in individuals with dense deposit disease.
A: Light microscopy with silver stain showing a membranoproliferative glomerulonephritis pattern with double contours of the glomerular basement membrane; B: Immunofluorescence; C: Immunohistochemistry with immunoperoxidase showing strong capillary wall staining of C3 and some granular mesangial C3; D: Characteristic sausage-like, intramembranous, osmiophilic deposits on electron microscopy. Adapted from Barbour et al[11].
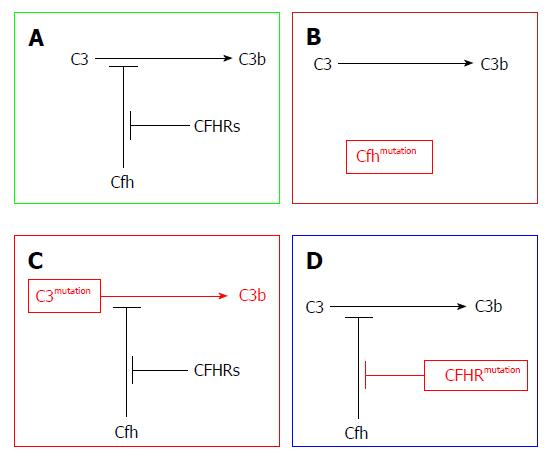
Figure 3 Disease mechanisms in C3 glomerulopathy, based on genetic defects identified in family studies.
A: Physiological regulation of C3 activation to C3b via the alternative pathway is mediated by complement factor H (CFH) (Cfh). Competitive inhibition of CFH by CFHR proteins is termed CFH deregulation; B: Homozygous deficiency or dysfunction of CFH results in excessive C3 activation; C: Hyper-functional C3 produces excessive C3 activation despite normal CFH activity; D: Abnormal CFHR proteins enhance CFH deregulation, leading to excessive C3 activation. Adapted from Barbour et al[11].
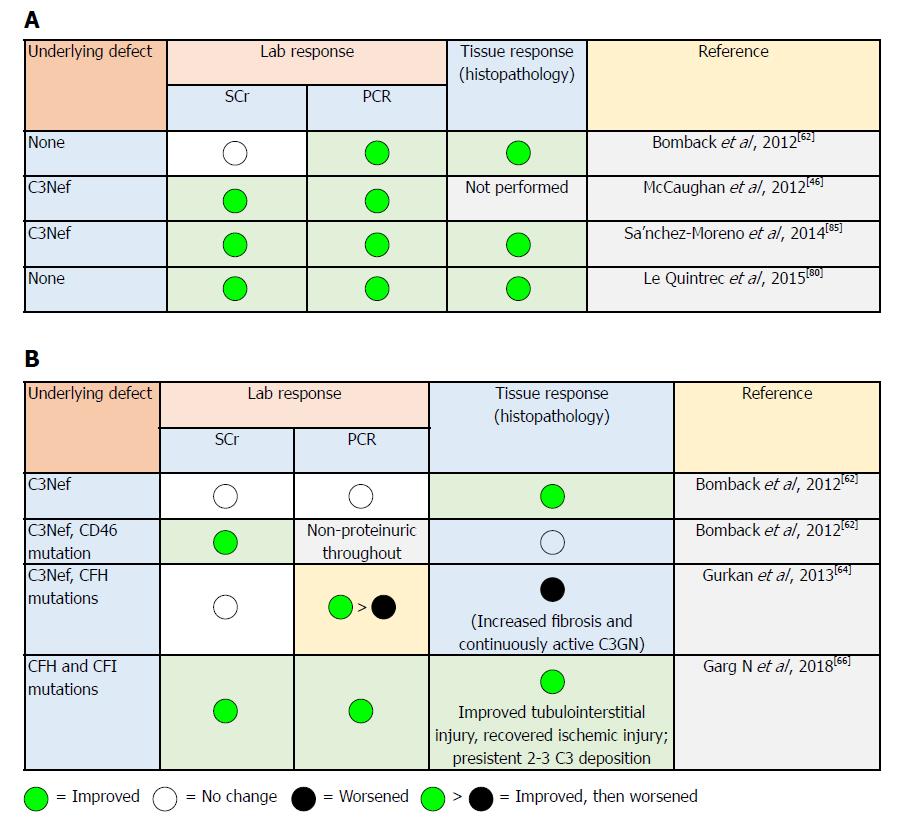
Figure 4 Response of complement 3 glomerulopathy subtypes to eculizumab therapy based on laboratory parameters and tissue (histopathological) response.
A: Dense deposit disease response to eculizumab therapy[66]; B: Complement 3 glomerulonephritis response to eculizumab therapy[66]. CFH: Complement factor H; CFI: Complement factor I; C3Nef: C3 nephritic factor.
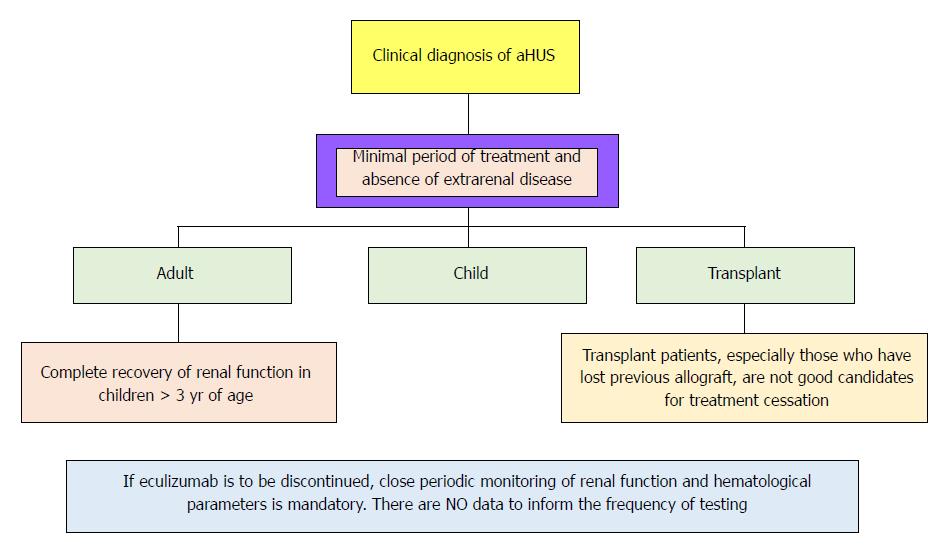
Figure 5 Recommendations for cessation of treatment with complement inhibitors.
There are no prospective controlled studies in patients with atypical hemolytic uremic syndrome (aHUS) to define criteria for discontinuation of eculizumab therapy. This flow diagram is based on expert opinion[134-137]. Discontinuation can be considered on a case-by-case basis in patients after at least 6-12 mo of treatment and at least 3 mo of normalization (or stabilization in the case of residual chronic kidney disease) of kidney function. Earlier cessation (at 3 mo) may be considered in patients (especially children) with pathogenic variants in membrane cofactor protein if there has been rapid remission and recovery of renal function. Patients on dialysis or eculizumab should be maintained for at least 4 to 6 mo before discontinuation. In this setting, assessment of fibrotic changes in kidney biopsy may be helpful. In transplant patients, especially patients who have lost previous allografts, discontinuation is not recommended. Adapted from Goodship et al[12].
- Citation: Abbas F, El Kossi M, Kim JJ, Shaheen IS, Sharma A, Halawa A. Complement-mediated renal diseases after kidney transplantation - current diagnostic and therapeutic options in de novo and recurrent diseases. World J Transplantation 2018; 8(6): 203-219
- URL: https://www.wjgnet.com/2220-3230/full/v8/i6/203.htm
- DOI: https://dx.doi.org/10.5500/wjt.v8.i6.203