Copyright
©The Author(s) 2016.
World J Transplant. Jun 24, 2016; 6(2): 411-422
Published online Jun 24, 2016. doi: 10.5500/wjt.v6.i2.411
Published online Jun 24, 2016. doi: 10.5500/wjt.v6.i2.411
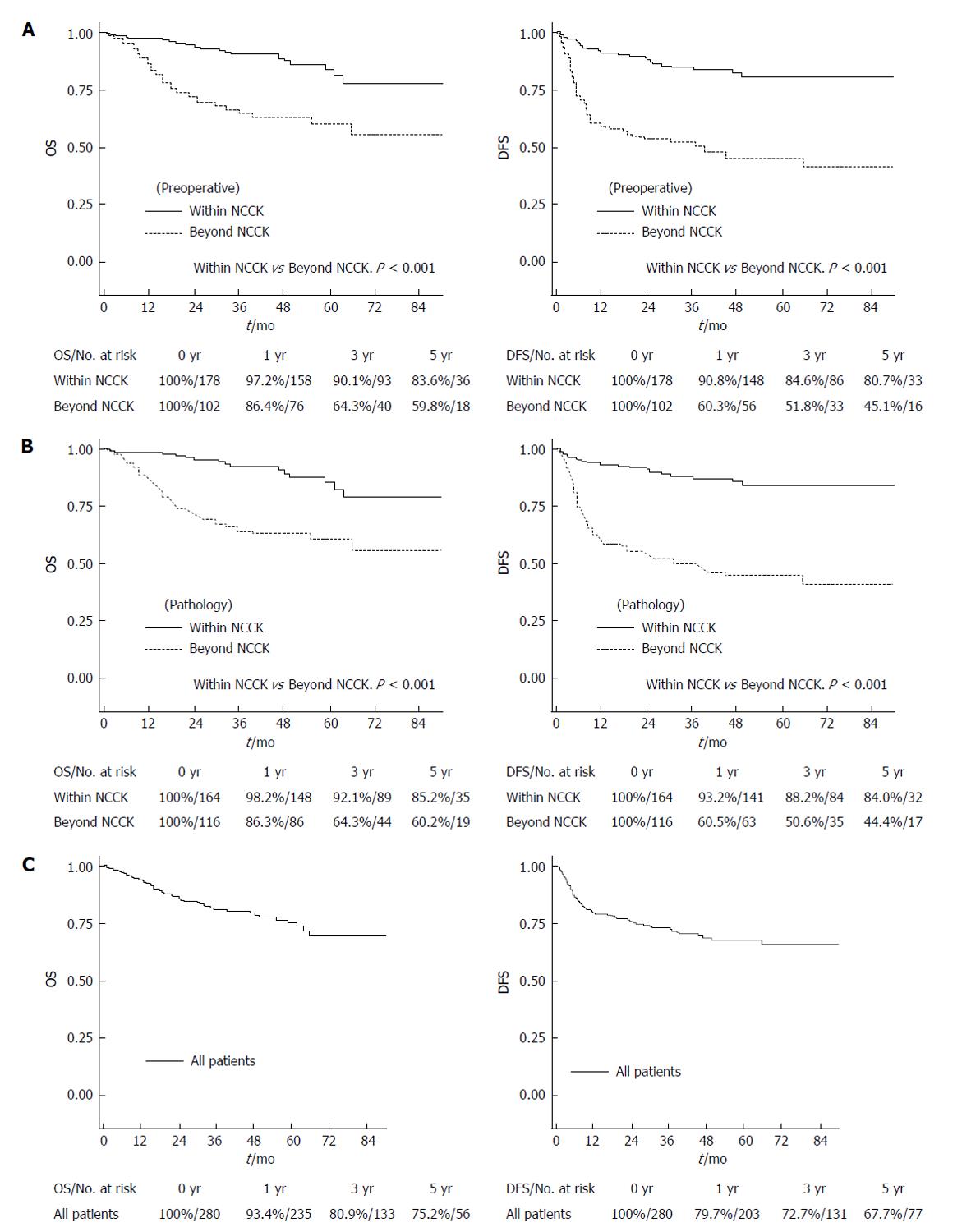
Figure 1 Overall and disease-free survival rates according to the National Cancer Center Korea criteria.
A: By preoperative imaging; B: By explant pathology; C: OS and DFS rates for all patients. OS: Overall survival; DFS: Disease-free survival; NCCK: National Cancer Center Korea.
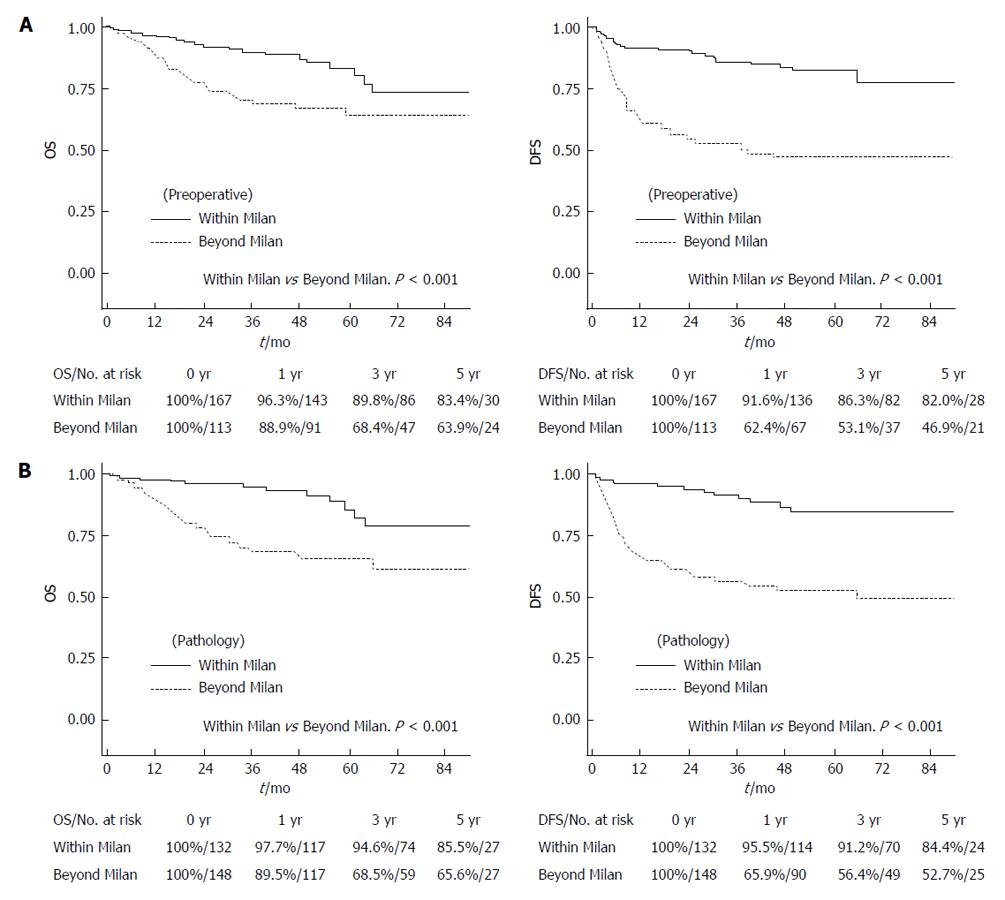
Figure 2 Overall and disease-free survival rates according to the Milan criteria.
A: By preoperative imaging; B: By explant pathology. OS: Overall survival; DFS: Disease-free survival.
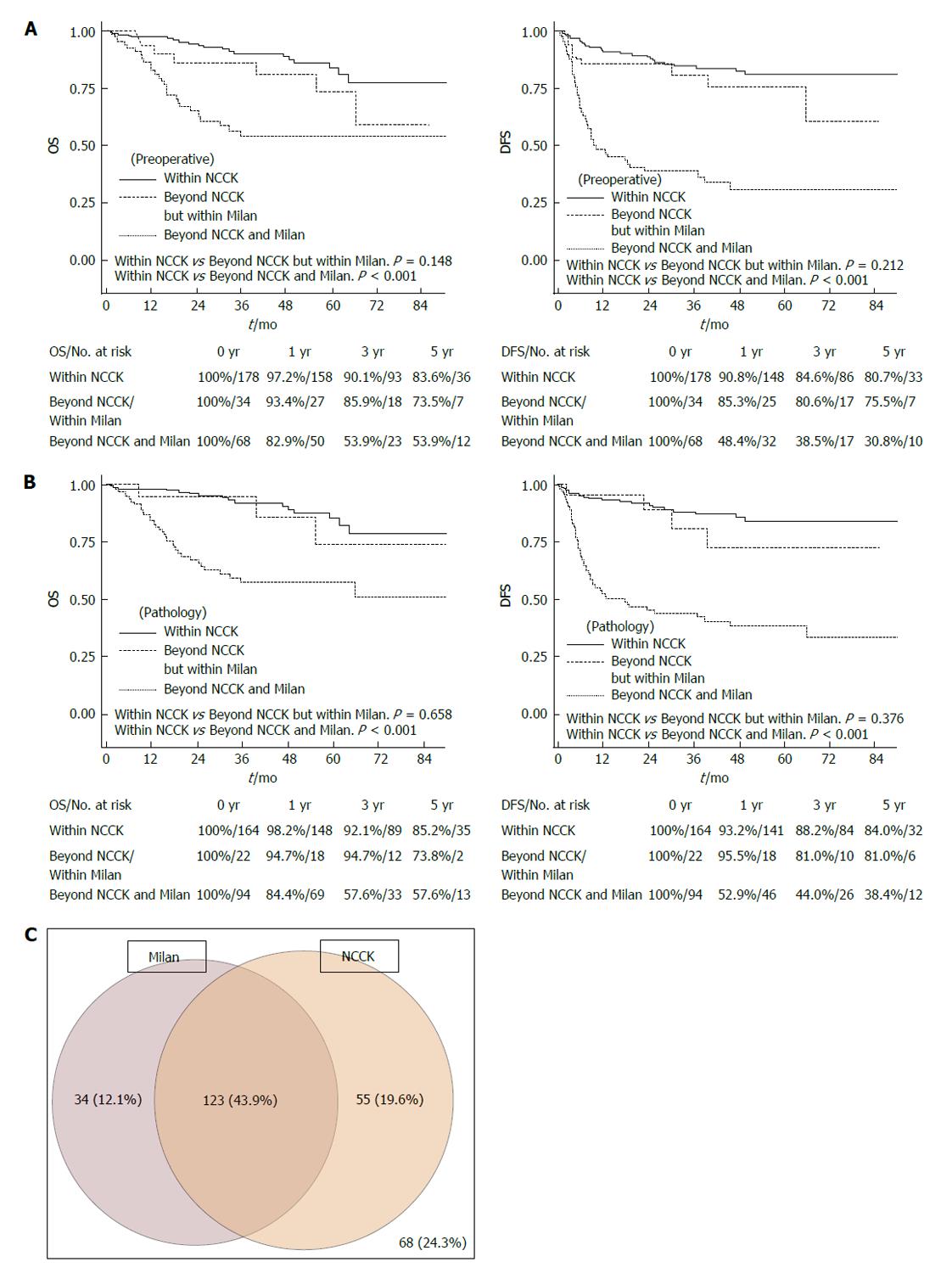
Figure 3 Overall and disease-free survival rates according to three groups (within the National Cancer Center Korea criteria, Beyond the National Cancer Center Korea but within the Milan criteria, Beyond both the National Cancer Center Korea and Milan criteria).
A: By preoperative imaging; B: By explant pathology; C: The diagram of the portion of patients in Milan and NCCK criteria by preoperative imaging. OS: Overall survival; DFS: Disease-free survival; NCCK: National Cancer Center Korea.
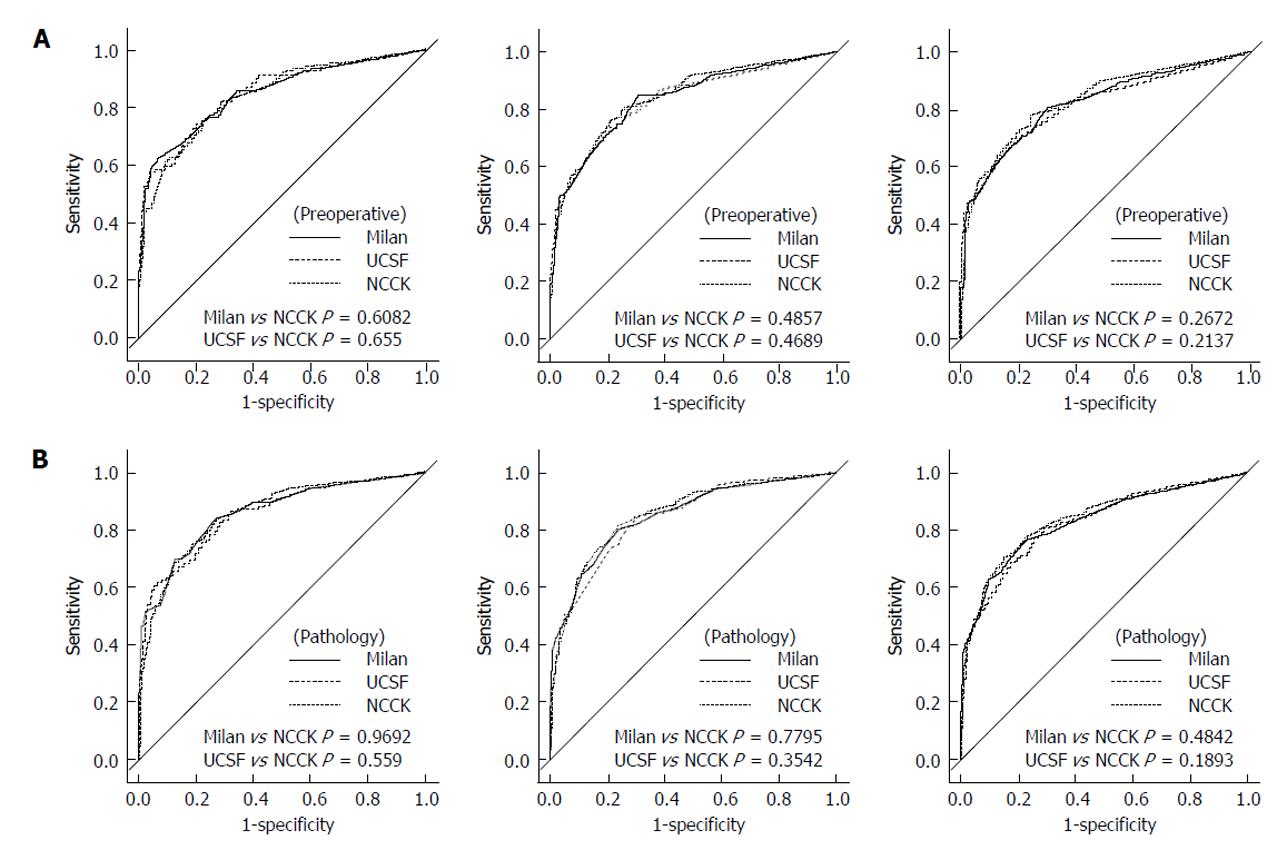
Figure 4 Receiver operating characteristic curves of three criteria (the National Cancer Center Korea, Milan and University of California, San Francisco) at 1, 3, and 5 years.
A: By preoperative imaging; B: By explant pathology. UCSF: University of California, San Francisco; NCCK: National Cancer Center Korea.
- Citation: Lee SD, Lee B, Kim SH, Joo J, Kim SK, Kim YK, Park SJ. Proposal of new expanded selection criteria using total tumor size and 18F-fluorodeoxyglucose - positron emission tomography/computed tomography for living donor liver transplantation in patients with hepatocellular carcinoma: The National Cancer Center Korea criteria. World J Transplant 2016; 6(2): 411-422
- URL: https://www.wjgnet.com/2220-3230/full/v6/i2/411.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i2.411