Copyright
©The Author(s) 2016.
World J Transplant. Mar 24, 2016; 6(1): 183-192
Published online Mar 24, 2016. doi: 10.5500/wjt.v6.i1.183
Published online Mar 24, 2016. doi: 10.5500/wjt.v6.i1.183
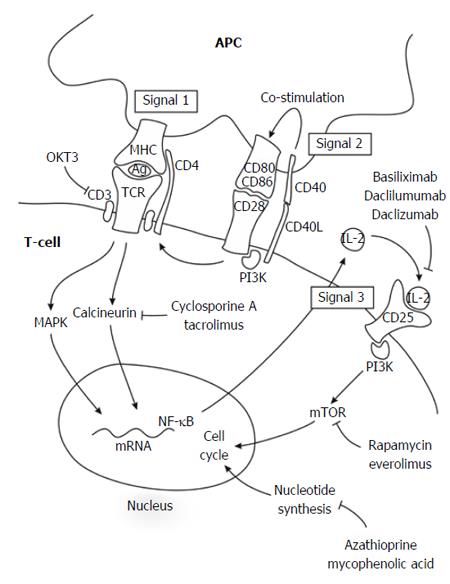
Figure 1 Three-signal pathway of lymphocyte activation and targets of inhibitory agents.
The elucidation of lymphocyte activation pathways has facilitated the development of novel immunosuppressive drugs. At the molecular level, T-cell-mediated rejection is explained by the three-signal model of lymphocyte activation. Signal 1 occurs when alloantigen-bearing APCs engage alloantigen-reactive naïve and memory T-cells and trigger their activation; alloantigen recognition is transduced through the TCR-CD3 complex. Signal 2 occurs when CD80 and CD86 on the surface of APCs engage CD28 on T-lymphocytes, providing T-lymphocyte co-stimulation. Together, signals 1 and 2 activate several signal transduction pathways, including the calcium-calcineurin pathway, the MAPK pathway, and the NF-κB pathway, which in turn, trigger the expression of many cytokines. Several of these cytokines (IL-2, IL-4, IL-7, IL-15, and IL-21) induce proliferation (signal 3) through PI3K and mTOR pathways. Ag: Antigen; APC: Antigen-presenting cell; MAPK: Mitogen-activated protein kinase; MHC: Major histocompatibility complex; mTOR: Mechanistic target of rapamycin; NF-κB: Nuclear factor kappa B; PI3K: Phosphatidylinositol 3-kinase; TCR: T-cell receptor.
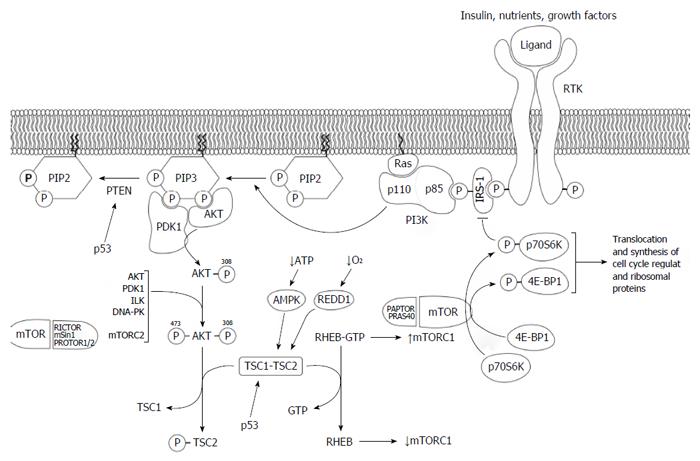
Figure 2 PI3K/AKT/mTOR signaling pathways.
PI3K is activated by growth factor stimulation through RTK. The regulatory subunit of PI3K, p85, binds directly to phosphotyrosine residues on RTK and/or adaptors, such as the IRS-1. This binding relieves the intermolecular inhibition of the p110 catalytic subunit of PI3K by p85 and allows it to move toward PI3K to the plasma membrane where its substrate, PIP2, resides. The catalytic subunit can also be activated by activated RAS, which binds directly to p110, and by G protein-coupled receptors. PI3K phosphorylates PIP2 to produce PIP3. In addition, the tumor suppressor PTEN dephosphorylates PIP3 to PIP2, thereby regulating PI3K-dependent signaling in a negative manner. Following PIP3 formation, PDK1 and AKT bind to PIP3 through its pleckstrin homology domains into close proximity at the cell plasma membrane. PDK1 activates AKT by phosphorylating AKT at threonine 308. After phosphorylation, AKT is fully activated by the subsequent phosphorylation at serine 473 by several protein kinases such as PDK1, the complex mTORC2, or AKT itself. AKT phosphorylates TSC2, thereby inhibiting the GTPase activity of the TSC1-TSC2 dimer, and the GTP-binding protein RHEB remains in its active GTP-bound state, causing a rise in mTORC1. In the mTORC1 complex, mTOR phosphorylates p70S6K and 4E-BP1, leading to an increase in the translation and synthesis of cell cycle-regulating and ribosomal proteins. Activated p70S6K also participates in a negative feedback loop, reducing the activation of the PI3K pathway through the phosphorylation and subsequent inhibition of ISR-1. 4E-BP1: eIF4E-binding protein; AMPK: AMP-activated kinase; AKT: Protein kinase B; IRS-1: Insulin-like growth factor-1; p70S6K: 70 kDa ribosomal protein S6 kinase; PDK1: Phosphoinositide-dependent kinase 1; PI3K: Phosphatidylinositol 3-kinase; PIP2: Phosphatidylinositol 4,5-bisphosphate; PIP3: Phosphatidylinositol 3,4,5-trisphosphate; PTEN: Phosphatase and tensin homolog; mTOR: Mechanistic target of rapamycin; RAPTOR: Regulatory-associated protein of mTOR; REDD1: Factor protein regulated in the development of DBA damage response 1; RHEB: RAS homolog enriched in brain; RICTOR: Rapamycin-insensitive companion of mTOR; RTK: Receptor tyrosine kinase; TSC1-TSC2: Tuberous sclerosis protein 1 and 2.
- Citation: Baroja-Mazo A, Revilla-Nuin B, Ramírez P, Pons JA. Immunosuppressive potency of mechanistic target of rapamycin inhibitors in solid-organ transplantation. World J Transplant 2016; 6(1): 183-192
- URL: https://www.wjgnet.com/2220-3230/full/v6/i1/183.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i1.183