Published online Mar 12, 2016. doi: 10.5499/wjr.v6.i1.16
Peer-review started: October 3, 2015
First decision: November 24, 2015
Revised: December 23, 2015
Accepted: January 5, 2016
Article in press: January 7, 2016
Published online: March 12, 2016
Processing time: 170 Days and 13.5 Hours
IgG4-related disease (IgG4-RD) is a systemic fibro-inflammatory disease with multiple organ disorders. Recently, in IgG4-RD, increased circulating plasmablasts have been found. The subsets of plasmablasts are negative for RP105 (CD180). A large population of B cells lacking RP105 (RP105-negative B cells) are found in patients with active with systemic lupus erythematosus and other systemic autoimmune diseases, including dermatomyositis, and Sjögren’s syndrome. In other conditions, such as neuromyelitis optica, Kawasaki’s disease, primary biliary cirrhosis and aging, RP105 expression on B cells and monocytes also alters. We review the basic science and clinical significance of RP105-negative B cells including plasmablasts in various immune-based diseases. RP105-negative B cells, especially plasmablasts, play crucial roles in both systemic and organ-specific autoimmune and inflammatory disorders.
Core tip: RP105 (CD180) is associated with B cell function, survival and death. RP105-negative B cells, especially plasmablasts, take part in pathophysiology of various immune-based diseases.
- Citation: Koarada S, Tada Y. Roles of plasmablasts in IgG4-related disease and various immune-based diseases. World J Rheumatol 2016; 6(1): 16-22
- URL: https://www.wjgnet.com/2220-3214/full/v6/i1/16.htm
- DOI: https://dx.doi.org/10.5499/wjr.v6.i1.16
IgG4-related disease (IgG4-RD) is a novel systemic fibro-inflammatory disease with multiple organ disorders[1,2]. IgG4-RD affects the various organs, including pancreas, kidney, aorta, lung, lymph node, salivary gland, lacrimal gland, prostate, pericardium, and so on. The elevated serum IgG4 levels are associated with the pathophysiology of IgG4-RD. B cell depletion therapy using rituximab (RTX) is an effective and alternative therapy of refractory IgG4-RD[3]. These results suggest that B cells play important immunological roles in the disease. The diagnosis of IgG4-RD is performed by biopsy-proven characteristic histology and immunohistochemistry features. Although, to date, the etiology and B cell biology in IgG4-RD have not been fully elucidated, recent studies suggest that late B cells, especially plasmablasts, play a pivotal role[4,5]. In patients with IgG4-RD, increased circulating plasmablasts and IgG4+ plasmablasts were found[6].
Toll-like receptors (TLRs) are important components of innate immune system that trigger antimicrobial responses. TLRs recognize various pathogens such as lipopolysaccharides (LPS), lipopeptides and CpG-DNA. RP105 [radioprotective, 105 kDa (MW); CD180], TLR associated molecule, is principally expressed on mature B cells[7]. Interestingly, a large population of B cells lacking RP105 (RP105-negative B cells) are found in patients with active systemic lupus erythematosus (SLE)[8] and other systemic autoimmune diseases, including dermatomyositis (DM), Sjögren’s syndrome (SS) and so on[9]. Moreover, in organ-specific autoimmune diseases, for example, in neuromyelitis optica (NMO), an inflammatory disease affecting the optic nerve and spinal cord, increased circulating RP105-negative B cells were reported[10]. Recently, in IgG4-RD, increased RP105-negative B cells, especially the subsets of plasmablasts, have been described[11-13]. Moreover, in various conditions, such as Kawasaki disease (KD), primary biliary cirrhosis (PBC) and aging, altered RP105 expression on B cells and monocytes was found. We review the basic science and clinical significance of plasmablasts and RP105-negative B cells in various immune-based diseases.
RP105 is a pathogen receptor of the leucine-rich repeat (LRR) family with homology to TLR-4. It was first reported that RP105 is mainly expressed on murine naïve and memory B cells[7]. The human homologue of RP105 was identified in 1998[7,14]. Although RP105 was originally discovered as a surface marker of B cells both in mice and humans, the molecule is also expressed on monocytes, macrophages, and DCs.
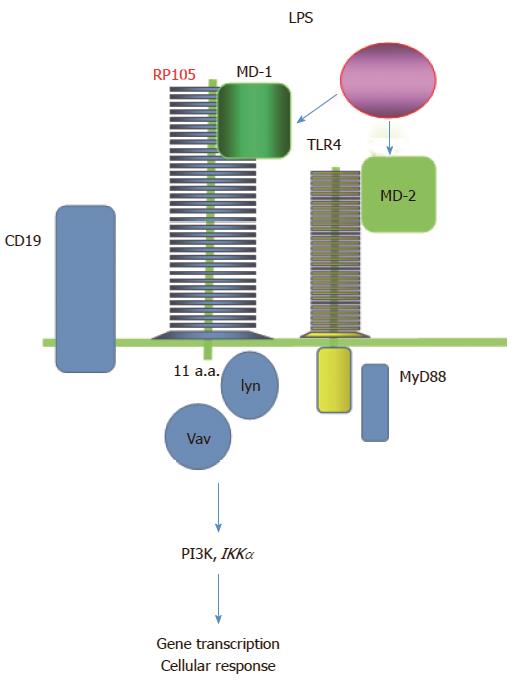
Virtually, all human B cells express RP105 strongly but not on plasma cells[15]. RP105 consists of extracellular LRRs and a short cytoplasmic tail (Figure 1). The LRRs involve in protein-protein interaction[16]. Extracellular LRR motifs of RP105 are similar to the other TLRs. RP105 forms a heterodimer complex with MD-1[17-19]. In the same manner as MD-2 for TLR-4, MD-1 is essential for expression of RP105 on the cell surface. Because RP105 has a very short cytoplasmic tail, 11-amino-acids, RP105 lacks the conserved intracellular signaling domain, Toll-IL-1 receptor (TIR) domain. TIR domain is required for TLR-signal transduction via adapters such as MyD88. Therefore, RP105 may be associated with a coreceptor transducing a signal into the cell.
The molecules with LRRs take part in the recognition of exogenous pathogens and activation of the immune system[20,21]. Historically, TLRs were first identified in Drosophila[22]. The molecules having LRRs are also important in the defense against pathogens in humans. The structural similarity of the extracellular LRRs of RP105 to TLRs suggests that RP105 also senses pathogen invasion, such as LPS[18].
Although signaling molecules binding to cytoplasmic tail of RP105 are not fully identified yet, there are multiple signaling pathways of RP105. RP105 signals separate from MyD88 and use CD19 as a coreceptor to signal through lyn, Vav, phosphoinositide 3-kinase (PI3K), AKT and IκB kinase α[23,24]. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and PI3K inhibitors control TLR4/RP105/LPS signaling in the CD19+ B cells and pan PI3K inhibitors reverse the lymphoproliferative phenotype in vivo[25].
The differential function of RP105 on macrophages/monocytes and B cells has been reported. RP105 has a negative regulatory function for TLR-4/MD-2 signal in macrophages and monocytes[26,27]. Recently, the unique role of RP105 in macrophages to TLR ligands has been reported. The function of TLR2 and TLR4 in activated macrophages could be associated with RP105[28,29]. In B cells, RP105 may have enhancing role of TLR-signals. Anti-RP105 monoclonal antibodies induce polyclonal B cell proliferation and immunoglobulin production of IgG1 and IgG3[30]. RP105 may regulate signals and functions of TLR-7 and TLR-9 to limit activation of autoreactive B cells[31]. In mice, RP105 plays a role in regulation of B cell growth and death. Although, in humans, the function of RP105 in B cells is still controversial and undefined, RP105 affects activation and regulatory function of B cells.
Cross-linking of anti-RP105 antibodies transmits an activation signal leading to B cell proliferation strongly, provides resistance against radio- and glucocorticoid-induced apoptosis, and expresses CD86, a co-stimulatory molecule, in mice[19]. RP105/MD-1 is functioning in concert with TLR4, controlling B cell recognition and signaling of LPS from Gram-negative bacteria[14].
RP105 physically interacts with TLR2, and both RP105 and TLR2 are required for macrophage activation by Mycobacterium tuberculosis lipoproteins[32]. RP105 is also involved in activation of macrophages by gram-positive bacteria, Staphylococcus aureus[28] and by Pam3CSK4 through TLR2 signaling[29]. In activation of macrophages by LPS and Pam3CSK4, TLR2 signaling overcomes RP105-mediated regulation of TLR4 signaling[29].
In RP105- and MD-1-deficient mice, activating function of B cells, including antibody production, CD86 expression and proliferative response to LPS, was reduced. However, because RP105 or MD-1-deficient mice do not lack LPS responsiveness completely, there may be functional associations between TLR4/MD-2 and RP105/MD-1[17].
On the other hand, because, unlike the TLRs, RP105 has a short cytoplasmic protein and lacks an important signaling domain, RP105 may function as a competitive negative regulator of TLR signals structurally. RP105 plays a physiological role of negative regulation of TLR-4 signaling in dendritic cells (DCs) and macrophages[25-27]. We have also investigated the inhibitory role of RP105 in the development of collagen-induced arthritis (CIA)[33]. Onset and severity of arthritis were accelerated in RP105-deficient DBA/1 mice. In this model, RP105 regulates the antigen-presenting cell function and regulatory T cell (Treg) development. As a result, RP105 induces the attenuation of the cell-mediated immune responses and suppression of the development of CIA.
RP105-activated B cells after cross-linking of surface IgM show growth arrest and apoptosis[24,34]. This result suggests that RP105 can function as a negative regulator of B cell activation. RP105 regulates proliferation and survival of B cells in response to various stimulation.
Up to the present time, expression of RP105 on B cells and monocytes from patients with various diseases has been examined (Table 1). The numbers of RP105-negative B cells vary considerably according to the diseases. Especially, RP105-negative B cells are increased in SLE, SS, DM and IgG4-RD in which pathophysiologically B cells are significantly involved[1,11]. Also, in NMO, an organ-specific autoimmune disease, increased RP105-negative B cells were found[10]. Some NMO patients have elevated serum anti-nuclear and anti-SS-A/SS-B antibodies, and then NMO might share common pathological mechanism with systemic autoimmune diseases to some extent.
| Human | Disease | Ref. |
| Increased RP105-negative B cells | ||
| SLE | [8] | |
| ANA-negative SLE | [48] | |
| Sjögren’s syndrome | [56,57] | |
| Dermatomyositis | [58] | |
| IgG4-related disease | [13] | |
| ANCA-associated vasculitis | [submitted] | |
| Neuromyelitis optica | [10] | |
| Aging | [64] | |
| Increased RP105-negative B cells; low levels | ||
| Rheumatoid arthritis | [56] | |
| Systemic sclerosis | [56] | |
| Behçet's disease | [56] | |
| Mixed connective tissue disease | [56] | |
| Polymyositis | [56] | |
| Increased RP105 on B cells | ||
| Kawasaki disease | [63] | |
| Decreased RP105 on stimulated monocytes | ||
| Primary biliary cirrhosis | [65] | |
| BWF1 | [52] |
Although normal mature B cells express RP105, RP105-negative B cells are dramatically increased in active SLE patients[8]. The disease activity of SLE, SLE Disease Activity Index (SLEDAI) scores, is correlated with the percentages of RP105-negative B cells. Also, serial analysis of the ratio of RP105-negative B cells from the same SLE patients was performed individually and RP105-negative B cells decreased as the disease turned inactive. The serum IgG levels were also correlated with the percentages of RP105-negative B cells. These results suggest that RP105-negative B cells in the peripheral blood are closely associated with activity and function of B cells of SLE. Being similar to RP105-negative B cells, CD27highCD38+ B cells producing high-affinity IgG are increased in the peripheral blood of SLE patients with correlation to disease activity[35,36]. RP105-negative B cells and CD27highCD38+ B cells should be phenotypically identical[9].
RP105-negative B cells disappeared in the peripheral blood from patients treated with corticosteroids and seem to be more sensitive to corticosteroids than RP105-positive B cells in vivo. The effect of dexamethasone on apoptosis of RP105-negative B cells was confirmed in vitro. Although RP105-negative B cells underwent spontaneous apoptosis more easily compared to RP105-positive B cells, dexamethasone induced apoptosis of RP105-negative B cells, but not RP105-positive B cells. This result illustrates the rapid clearance of RP105-negative B cells from peripheral blood by the treatment with corticosteroids in SLE patients.
Because, in patients with SLE, antinuclear antibody (ANA) in serum is a primary hallmark, ANA-negative SLE is very rare[37]. Although, in clinical practice, ANA-negative SLE patients exist as a subpopulation of SLE, the diagnosis of seronegative SLE can be difficult in patients showing no immunological abnormalities[38-46]. The numbers of RP105-negative B cells were increased and correlated with disease activity even in ANA-negative SLE patients[47]. Without significant serological markers for SLE, examination of B cell population may be useful in evaluation of activity. Later, these patients turned out to be serologically positive, including ANA, anti-dsDNA and anti-Sm antibodies.
RP105-negative B cells produce autoantibodies, including IgG and IgM class anti-dsDNA and single stranded DNA antibodies in vitro[48]. Especially, IgG class anti-dsDNA antibodies are specific and profoundly associated with pathogenesis of SLE. RP105-negative B cells have characteristic phenotype compared to RP105-positive conventional B cells[49,50]. Collectively, RP105-negative B cells are assigned as autoantibody-producing pathogenic B cells.
In addition, recently RP105-negative B cells have been found in a murine lupus model, the first filial generation of New Zealand Black (NZB) and White (NZW) mice (BWF1)[51]. Although the parental strains (NZB and NZW mice) do not show the phenotype of SLE, BWF1 mice develop autoimmunity with diffuse proliferative nephritis and production of anti-DNA antibodies. In BWF1 mice, splenic or peripheral RP105-negative B cells are increased with progression of renal lesions and aging.
NMO is an inflammatory neurological disorder with recurrent attacks of severe optic neuritis and myelitis[52]. In NMO, anti-aquaporin-4 (AQP4) water channel protein antibodies are pathogenic autoantibodies and can be used as a disease marker[52,53]. Because anti-AQP4 antibodies alone do not cause the disease, cellular immunity works in concert with anti-AQP4 antibodies in pathophysiology in NMO[54]. Although RP105-negative (CD19intCD27highCD38highRP105-) B cells are increased in the peripheral blood of anti-AQP4 antibody-positive NMO patients compared to normal subjects or patients with conventional form of multiple sclerosis (CMS), the frequencies of naïve and memory B cells are not changed. The frequency of RP105-negative B cells is correlated with the serum levels of anti-AQP4 antibodies[10]. Serial analysis of paired samples from the same NMO patients during relapse and in remission shows that RP105-negative B cells increased during relapse.
Among various systemic rheumatic diseases, RP105-negative B cells are also increased in SS[55,56], DM[57], IgG4-RD[13] and ANCA-associated vasculitis [submitted]. In the patients with rheumatoid arthritis, systemic sclerosis, angiitis syndromes except for granulomatosis with polyangiits, Behçet’s disease, mixed connective tissue disease, and polymyositis (PM), the numbers of RP105-negative B cells are increased compared to normal subjects. However, the levels are not very high[55].
DM and PM are clinically similar diseases each other. Difference between two diseases is not only the presence of skin manifestations, but also etiological findings, the involvement of humoral immune mechanism in DM and cellular immunity in PM. The proportion of RP105-negative B cells is increased in patients with DM compared to PM patients or normal subjects[57]. The increase of RP105-negative B cells reflects B cell activation in DM but not in PM. This finding is similar to the difference between NMO and CMS, as increased RP105-negative B cells are only found in NMO but not in CMS[10].
The different distribution of RP105-negative B cells between in the peripheral blood and the target organ is also interesting. Bronchoalveolar lavage fluid from a DM patient contained larger number of RP105-negative B cells than the peripheral blood. RP105-negative B cells may be preferentially located in the impaired organs, such as lung.
In SS patients, polyclonal hyperactivation of B cells exists[58]. Increased RP105-negative B cells are also found in SS patient. RP105-negative B cells from SS patients produced IgG and IgM spontaneously in vitro[56]. In some of salivary glands with lymphoid follicles in SS, germinal centers mainly consisted of RP105-negative B cells. B cells infiltrating the area other than lymphoid follicles were RP105-negative. RP105-negative B cells may be associated with the inflammation and tissue damage of the target organs in SS.
IgG4-RD is a rare and novel systemic inflammatory disease characterized by tumefactive lesions with infiltrating IgG4-positive plasma cells[1,2]. IgG4-RD affects various organs. The elevated serum concentration of IgG4 has been believed as a hallmark of IgG4-RD. B cell depletion therapy using RTX is an effective and alternative approach in refractory IgG4-RD[3]. B cells play an important role in the pathophysiology of IgG4-RD.
RP105-negative B cells increase in IgG4-RD[11]. Because RP105-negative B cells consist of mainly plasmablasts and early plasma cells, precursors of plasma cells are increased in peripheral blood in IgG4-RD. Serial analysis showed that RP105-negative B cells decreased in parallel with disease activity.
Wallace et al[12] reported that plasmablast is a biomarker for IgG4-RD, independent of serum IgG4 concentrations. Patients with active, untreated IgG4-RD have elevations in their circulating plasmablast counts. Increased RP105-negatvie plasmablasts are associated with disease activity and the number of organ involvement[11,12]. Existence of RP105-negative B cells may reflect the dysregulation of differentiation and localization of late B cells in patients with IgG4-RD. Moreover, in patients with IgG4-RD, CXCR5 is expressed on the later B cell subsets.
KD is one of the vasculitis syndromes in childhood and an acute febrile illness with the formation of aneurysms in coronary arteries[59]. The percentages of RP105-positive B cells are higher in patients with KD than normal subjects. The levels of RP105 expression are also high in children with KD. RP105 expression at both protein and messenger RNA levels was enhanced in B cells stimulated with poly inosinic-cytidyric acid [poly(IC)], a synthetic double-stranded RNA in vitro. Similar mechanism may be involved in the up-regulation of RP105 expression on B cells in KD and viral infections.
In the elderly people, RP105-negative B cells are increased compared to the young[60]. In normal young persons, RP105-negative B cells are seldom (1.7% ± 1.1%)[8].
Altered monocyte response to ligands for TLRs was reported in patients with PBC[61]. Peripheral blood mononuclear cells and monocytes from PBC patients were stimulated with LPS. The level of TLR4 expression was increased with LPS stimulation on PBC monocytes compared to controls. Conversely, the expression of RP105 on PBC monocytes was decreased in comparison with controls.
RP105 molecule is deeply associated with B cell function, survival and death. RP105-negative B cells produce autoantibodies and take part in pathophysiology in various diseases. RP105-negative B cells play a crucial role and are useful as a disease marker in both systemic and organ-specific immune-based diseases. As RP105 has complicated function, different mechanisms of the increase in RP105-negative B cells may function in each disease. To clarify these mechanism, further studies should be required.
P- Reviewer: Cordero OJ, Jin B, Rothschild BM, Weissert R S- Editor: Qi Y L- Editor: A E- Editor: Jiao XK
| 1. | Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1870] [Article Influence: 143.8] [Reference Citation Analysis (83)] |
| 2. | Umehara H. A new clinical entity: IgG4-related disease (IgG4-RD) discovered in the 21st century. Intern Med. 2012;51:821-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 2010;62:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 4. | Fox RI, Fox CM. IgG4 levels and plasmablasts as a marker for IgG4-related disease (IgG4-RD). Ann Rheum Dis. 2015;74:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Koarada S, Tashiro S, Tokuda Y, Ono Y, Sadanaga Y, Suematsu R, Ono N, Ohta A, Tada Y. Persistent expression of CXCR5 on plasmablasts in IgG4-related disease. Ann Rheum Dis. 2015;74:e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Wallace ZS, Deshpande V, Mattoo H, Mahajan VS, Kulikova M, Pillai S, Stone JH. IgG4-Related Disease: Clinical and Laboratory Features in One Hundred Twenty-Five Patients. Arthritis Rheumatol. 2015;67:2466-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 460] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 7. | Miura Y, Miyake K, Yamashita Y, Shimazu R, Copeland NG, Gilbert DJ, Jenkins NA, Inazawa J, Abe T, Kimoto M. Molecular cloning of a human RP105 homologue and chromosomal localization of the mouse and human RP105 genes (Ly64 and LY64. Genomics. 1996;38:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Koarada S, Tada Y, Ushiyama O, Morito F, Suzuki N, Ohta A, Miyake K, Kimoto M, Nagasawa K. B cells lacking RP105, a novel B cell antigen, in systemic lupus erythematosus. Arthritis Rheum. 1999;42:2593-2600. [PubMed] |
| 9. | Koarada S, Tada Y. RP105-negative B cells in systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:259186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Chihara N, Aranami T, Sato W, Miyazaki Y, Miyake S, Okamoto T, Ogawa M, Toda T, Yamamura T. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc Natl Acad Sci USA. 2011;108:3701-3706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 353] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 11. | Koarada S, Tashiro S, Nagao N, Suematsu R, Ohta A, Tada Y. Increased RP105-Negative B Cells in IgG4-Related Disease. Open Rheumatol J. 2013;7:55-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Wallace ZS, Mattoo H, Carruthers M, Mahajan VS, Della Torre E, Lee H, Kulikova M, Deshpande V, Pillai S, Stone JH. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74:190-195. [RCA] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 319] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 13. | Koarada S, Tashiro S, Tokuda Y, Ono Y, Sadanaga Y, Suematsu R, Ono N, Ohta A, Tada Y. Subsets of RP105-negative plasmablasts in IgG4-related disease. Ann Rheum Dis. 2014;73:e65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Miura Y, Shimazu R, Miyake K, Akashi S, Ogata H, Yamashita Y, Narisawa Y, Kimoto M. RP105 is associated with MD-1 and transmits an activation signal in human B cells. Blood. 1998;92:2815-2822. [PubMed] |
| 15. | Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009;182:890-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Ishii A, Matsuo A, Sawa H, Tsujita T, Shida K, Matsumoto M, Seya T. Lamprey TLRs with properties distinct from those of the variable lymphocyte receptors. J Immunol. 2007;178:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Kimoto M, Nagasawa K, Miyake K. Role of TLR4/MD-2 and RP105/MD-1 in innate recognition of lipopolysaccharide. Scand J Infect Dis. 2003;35:568-572. [PubMed] |
| 18. | Miyake K, Yamashita Y, Ogata M, Sudo T, Kimoto M. RP105, a novel B cell surface molecule implicated in B cell activation, is a member of the leucine-rich repeat protein family. J Immunol. 1995;154:3333-3340. [PubMed] |
| 19. | Miyake K, Yamashita Y, Hitoshi Y, Takatsu K, Kimoto M. Murine B cell proliferation and protection from apoptosis with an antibody against a 105-kD molecule: unresponsiveness of X-linked immunodeficient B cells. J Exp Med. 1994;180:1217-1224. [PubMed] |
| 20. | Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. [PubMed] |
| 21. | Roshak AK, Anderson KM, Holmes SD, Jonak Z, Bolognese B, Terrett J, Marshall LA. Anti-human RP105 sera induces lymphocyte proliferation. J Leukoc Biol. 1999;65:43-49. [PubMed] |
| 22. | Higgs R, Cormican P, Cahalane S, Allan B, Lloyd AT, Meade K, James T, Lynn DJ, Babiuk LA, O’farrelly C. Induction of a novel chicken Toll-like receptor following Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006;74:1692-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Yazawa N, Fujimoto M, Sato S, Miyake K, Asano N, Nagai Y, Takeuchi O, Takeda K, Okochi H, Akira S. CD19 regulates innate immunity by the toll-like receptor RP105 signaling in B lymphocytes. Blood. 2003;102:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Ogata H, Su I, Miyake K, Nagai Y, Akashi S, Mecklenbräuker I, Rajewsky K, Kimoto M, Tarakhovsky A. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J Exp Med. 2000;192:23-29. [PubMed] |
| 25. | Singh AR, Peirce SK, Joshi S, Durden DL. PTEN and PI-3 kinase inhibitors control LPS signaling and the lymphoproliferative response in the CD19+ B cell compartment. Exp Cell Res. 2014;327:78-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A, Finberg RW, Tarakhovsky A, Vogel SN, Belkaid Y. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol. 2005;6:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 315] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 27. | Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A, Finberg RW, Tarakhovsky A, Vogel SN, Belkaid Y. Inhibition of TLR-4/MD-2 signaling by RP105/MD-1. J Endotoxin Res. 2005;11:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Liu B, Fu Y, Feng S, Zhang X, Liu Z, Cao Y, Li D, Liang D, Li F, Zhang N. Involvement of RP105 and toll-like receptors in the activation of mouse peritoneal macrophages by Staphylococcus aureus. Scand J Immunol. 2013;78:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Liu B, Zhang N, Liu Z, Fu Y, Feng S, Wang S, Cao Y, Li D, Liang D, Li F. RP105 involved in activation of mouse macrophages via TLR2 and TLR4 signaling. Mol Cell Biochem. 2013;378:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Chaplin JW, Kasahara S, Clark EA, Ledbetter JA. Anti-CD180 (RP105) activates B cells to rapidly produce polyclonal Ig via a T cell and MyD88-independent pathway. J Immunol. 2011;187:4199-4209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Means TK. Toll-like receptors in SLE. San Diego: Academic Press 2011; 292-306. |
| 32. | Blumenthal A, Kobayashi T, Pierini LM, Banaei N, Ernst JD, Miyake K, Ehrt S. RP105 facilitates macrophage activation by Mycobacterium tuberculosis lipoproteins. Cell Host Microbe. 2009;5:35-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Tada Y, Koarada S, Morito F, Mitamura M, Inoue H, Suematsu R, Ohta A, Miyake K, Nagasawa K. Toll-like receptor homolog RP105 modulates the antigen-presenting cell function and regulates the development of collagen-induced arthritis. Arthritis Res Ther. 2008;10:R121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Yamashita Y, Miyake K, Miura Y, Kaneko Y, Yagita H, Suda T, Nagata S, Nomura J, Sakaguchi N, Kimoto M. Activation mediated by RP105 but not CD40 makes normal B cells susceptible to anti-IgM-induced apoptosis: a role for Fc receptor coligation. J Exp Med. 1996;184:113-120. [PubMed] |
| 35. | Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 920] [Cited by in RCA: 845] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 36. | Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR, Lipsky PE, Radbruch A, Dörner T. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000;165:5970-5979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 488] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 37. | Maddison PJ, Provost TT, Reichlin M. Serological findings in patients with “ANA-negative” systemic lupus erythematosus. Medicine (Baltimore). 1981;60:87-94. [PubMed] |
| 38. | Maraina CH, Kamaliah MD, Ishak M. ANA negative (Ro) lupus erythematosus with multiple major organ involvement: a case report. Asian Pac J Allergy Immunol. 2002;20:279-282. [PubMed] |
| 39. | Sugisaki K, Takeda I, Kanno T, Nogai S, Abe K, Sakuma H, Kasukawa R. An anti-nuclear antibody-negative patient with systemic lupus erythematosus (SLE) accompanied with anti-ribosomal P antibody (anti-P). Intern Med. 2002;41:1047-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Reichlin M. ANA negative systemic lupus erythematosus sera revisited serologically. Lupus. 2000;9:116-119. [PubMed] |
| 41. | Khajehdehi P, Islam SF, Salinas-Madrigal L, Bastani B. Lupus nephritis in an anti-nuclear antibody-negative young male. The simultaneous presence of class III and class V renal lesions. Clin Nephrol. 1999;51:379-382. [PubMed] |
| 42. | Zoli A, Altomonte L, Galossi A, Taranto A, Mirone L, Magaró M. Neurobehavioural and psychiatric manifestations in a case of ANA-negative SLE with antiphospholipid antibodies. Clin Rheumatol. 1998;17:68-70. [PubMed] |
| 43. | Morris CN, Calobrisi SD, Matteson EL. Antinuclear antibody negative lupus associated with dystrophic calcification. J Rheumatol. 1998;25:825-826. [PubMed] |
| 44. | Blaustein DA, Blaustein SA. Antinuclear antibody negative systemic lupus erythematosus presenting as bilateral facial paralysis. J Rheumatol. 1998;25:798-800. [PubMed] |
| 45. | Sircar S, Taneja VA, Kansra U. ANA-negative SLE presenting with nephritis and oculomotor palsy--a case report. Indian J Pathol Microbiol. 1997;40:539-542. [PubMed] |
| 46. | Caltik A, Demircin G, Bülbül M, Erdogan O, Akyüz SG, Arda N. An unusual case of ANA negative systemic lupus erythematosus presented with vasculitis, long-standing serositis and full-house nephropathy. Rheumatol Int. 2013;33:219-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Koarada S, Ide M, Haruta Y, Tada Y, Ushiyama O, Morito F, Ohta A, Nagasawa K. Two cases of antinuclear antibody negative lupus showing increased proportion of B cells lacking RP105. J Rheumatol. 2005;32:562-564. [PubMed] |
| 48. | Kikuchi Y, Koarada S, Tada Y, Ushiyama O, Morito F, Suzuki N, Ohta A, Miyake K, Kimoto M, Horiuchi T. RP105-lacking B cells from lupus patients are responsible for the production of immunoglobulins and autoantibodies. Arthritis Rheum. 2002;46:3259-3265. [PubMed] |
| 49. | Koarada S, Tada Y, Suematsu R, Soejima S, Inoue H, Ohta A, Nagasawa K. Phenotyping of P105-negative B cell subsets in patients with systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:198206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Koarada S, Tada Y, Sohma Y, Haruta Y, Suematsu R, Mitamura M, Inoue H, Ehara H, Tokoro Y, Ohta A. Autoantibody-producing RP105(-) B cells, from patients with systemic lupus erythematosus, showed more preferential expression of BCMA compared with BAFF-R than normal subjects. Rheumatology (Oxford). 2010;49:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Fujita K, Akasaka Y, Kuwabara T, Wang B, Tanaka K, Kamata I, Yokoo T, Kinoshita T, Iuchi A, Akishima-Fukasawa Y. Pathogenesis of lupus-like nephritis through autoimmune antibody produced by CD180-negative B lymphocytes in NZBWF1 mouse. Immunol Lett. 2012;144:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1489] [Cited by in RCA: 1601] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 53. | Jarius S, Paul F, Franciotta D, Waters P, Zipp F, Hohlfeld R, Vincent A, Wildemann B. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008;4:202-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 256] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 54. | Hinson SR, McKeon A, Fryer JP, Apiwattanakul M, Lennon VA, Pittock SJ. Prediction of neuromyelitis optica attack severity by quantitation of complement-mediated injury to aquaporin-4-expressing cells. Arch Neurol. 2009;66:1164-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Koarada S, Tada Y, Kikuchi Y, Ushiyama O, Suzuki N, Ohta A, Nagasawa K. CD180 (RP105) in rheumatic diseases. Rheumatology (Oxford). 2001;40:1315-1316. [PubMed] |
| 56. | Kikuchi Y, Koarada S, Nakamura S, Yonemitsu N, Tada Y, Haruta Y, Morito F, Ohta A, Miyake K, Horiuchi T. Increase of RP105-lacking activated B cells in the peripheral blood and salivary glands in patients with Sjögren’s syndrome. Clin Exp Rheumatol. 2008;26:5-12. [PubMed] |
| 57. | Kikuchi Y, Koarada S, Tada Y, Ushiyama O, Morito F, Suzuki N, Ohta A, Horiuchi T, Miyake K, Nagasawa K. Difference in B cell activation between dermatomyositis and polymyositis: analysis of the expression of RP105 on peripheral blood B cells. Ann Rheum Dis. 2001;60:1137-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Anaya JM, Talal N. Sjogren’s syndrome and connective tissue diseases associated with other immunologic disorders. Arthritis and Allied Conditions: A Textbook of Rheumatology 13th edition. Baltimore: Williams and Wilkins 1997; 1561-1580. |
| 59. | Imayoshi M, Yamamoto S, Watanabe M, Nishimura S, Tashiro K, Zaitsu M, Tasaki H, Kimoto M, Hamasaki Y, Ishii E. Expression of CD180, a toll-like receptor homologue, is up-regulated in children with Kawasaki disease. J Mol Med (Berl). 2006;84:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Buffa S, Pellicanò M, Bulati M, Martorana A, Goldeck D, Caruso C, Pawelec G, Colonna-Romano G. A novel B cell population revealed by a CD38/CD24 gating strategy: CD38(-)CD24 (-) B cells in centenarian offspring and elderly people. Age (Dordr). 2013;35:2009-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Honda Y, Yamagiwa S, Matsuda Y, Takamura M, Ichida T, Aoyagi Y. Altered expression of TLR homolog RP105 on monocytes hypersensitive to LPS in patients with primary biliary cirrhosis. J Hepatol. 2007;47:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |