Published online Jul 12, 2015. doi: 10.5499/wjr.v5.i2.108
Peer-review started: June 29, 2014
First decision: Steptember 27, 2014
Revised: October 24, 2014
Accepted: October 31, 2014
Article in press: November 3, 2014
Published online: July 12, 2015
Processing time: 375 Days and 13.5 Hours
AIM: To evaluate the effectiveness of the biological disease-modifying antirheumatic drugs (bDMARD) in the treatment of rheumatoid arthritis through a systematic review of observational studies.
METHODS: The studies were searched in the PubMed, EMBASE, Cochrane Controlled Trials Register and LILACS databases (until August 2014), in the grey literature and conducted a manual search. The assessed criteria of effectiveness included the EULAR, the disease activity score (DAS), the Clinical Disease Activity Index, the Simplified Disease Activity Index, the American College of Rheumatology and the Health Assessment Questionnaire. The meta-analysis was performed with Review Manager® 5.2 software using a random effects model. A total of 35 studies were included in this review.
RESULTS: The participants anti-tumor necrosis factor inhibitors (TNF) naïve, who used adalimumab (P = 0.0002) and etanercept (P = 0.0006) exhibited greater good EULAR response compared to the participants who used infliximab. No difference was detected between adalimumab and etanercept (P = 0.05). The participants who used etanercept exhibited greater remission according to DAS28 compared to the participants who used infliximab (P = 0.01). No differences were detected between adalimumab and infliximab (P = 0.12) or etanercept (P = 0.79). Better results were obtained with bDMARD associated with methotrexate than with bDMARD alone. The good EULAR response and DAS 28 was better for combination with methotrexate than bDMARD monotherapy (P = 0.03 e P < 0.00001). In cases of therapeutic failure, the participants who used rituximab exhibited greater DAS28 reduction compared to those who used anti-TNF agents (P = 0.0002). The participants who used etanercept achieved greater good EULAR response compared to those who did not use that drug (P = 0.007). Studies that assessed reduction of the CDAI score indicated the superiority of abatacept over rituximab (12.4 vs +1.7) and anti-TNF agents (7.6 vs 8.3). The present systematic review with meta-analysis found that relative to anti-TNF treatment-naïve patients, adalimumab and etanercept were more effective when combined with methotrexate than when used alone. Furthermore, in case of therapeutic failure with anti-TNF agents; rituximab and abatacept (non anti-TNF) and etanercept (as second anti-TNF) were more effective. However, more studies of effectiveness were found for the rituximab.
CONCLUSION: The best treatment for treatment-naïve patients is adalimumab or etanercept combined with methotrexate. For anti-TNF therapeutic failure, the best choice is rituximab, abatacept or etanercept.
Core tip: Rheumatoid arthritis is a chronic, progressive, systemic inflammatory disease that preferentially affects the synovial membranes of joints, eventually leading to bone and cartilage destruction. Its worldwide prevalence is estimated to be 0.3% to 1%. Observational studies could provide relevant information for deciding the choice of treatments, the elaboration of clinical protocols, and the formulation of health policies. The present systematic review of biological disease-modifying antirheumatic drugs included cohort observational studies that reported treatment results applied in real-life conditions; thus, these studies are able to fill in gaps in knowledge left by clinical trials.
- Citation: Santos JBD, Costa JO, Junior HAO, Lemos LLP, Araújo VE, Machado MA&, Almeida AM, Acurcio FA, Alvares J. What is the best biological treatment for rheumatoid arthritis? A systematic review of effectiveness. World J Rheumatol 2015; 5(2): 108-126
- URL: https://www.wjgnet.com/2220-3214/full/v5/i2/108.htm
- DOI: https://dx.doi.org/10.5499/wjr.v5.i2.108
Rheumatoid arthritis (RA) is a chronic, progressive, systemic inflammatory disease that preferentially affects the synovial membranes of joints, eventually resulting in destruction of bone and cartilage[1]. Its worldwide prevalence is estimated to be 0.3% to 1%[2].
Treatment of RA includes non-steroidal anti-inflammatory drugs, corticoids and synthetic (sDMARD) and biological [biological disease-modifying antirheumatic drugs (bDMARD)] disease-modifying antirheumatic drugs. bDMARD are indicated for individuals with persistent disease activity despite the use of sDMARD[3-5]. Tumor necrosis factor inhibitors (anti-TNF) are inhibitors of tumor necrosis factor alpha, rituximab is depleting B lymphocyte, abatacept is blocking of costimulation of T lymphocyte and tocilizumab is a blocking interleukin-6 receptor. Among the bDMARD, anti-TNF represent the first choice after failure of regimens that included sDMARD, and there is more evidence of the post-marketing efficacy and safety for anti-TNF agents[4,5]. Nevertheless, anti-TNF could eventually exhibit therapeutic failure, in which case another anti-TNF drug or another class of bDMARD might be used[6,7].
Appropriate knowledge of the effectiveness profiles of all of these strategies is relevant for choosing the best option for each patient. In this regard, observational studies are particularly interesting, as they seek to understand treatments in the actual practice setting. Thus, this type of study could contribute to decide the choice of treatments, the elaboration of clinical protocols, and the formulation of health policies. The present systematic review selected cohort observational studies. These types of studies more accurately represent real-life conditions (actual practice setting) and are able to provide complementary data to the results of randomized clinical studies conducted in controlled conditions[8].
The aim of the present study was to assess the effectiveness of the anti-TNFs adalimumab, etanercept, infliximab, golimumab and certolizumab pegol and of the non anti-TNF rituximab, tocilizumab and abatacept, in the treatment of active RA by means of a systematic review with meta-analysis.
This systematic review followed the recommendations in the Cochrane Collaboration Handbook and was elaborated using Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)[9,10].
We included prospective and retrospective cohort studies and database records of patients with RA whose diagnoses were confirmed based on the ACR 1987 and the more recent ACR/EULAR 2010 criteria. Studies that accessed the effectiveness of adalimumab, etanercept, infliximab, golimumab, certolizumab pegol, rituximab, tocilizumab and abatacept between themselves, in monotherapy or combined with sDMARD were evaluated for inclusion.
We performed an electronic search of relevant articles published before August 2014 in the PubMed, EMBASE, Cochrane Controlled Trials Register and LILACS databases. Several combinations of terms corresponding to the disease, interventions and type of study were used in the search strategy (Table 1).
| PubMed |
| (((Arthritis, Rheumatoid[Text Word] or "Arthritis, Rheumatoid"[Mesh]) and (((((((((((rituximab[Text Word] or Mabthera[Text Word]) or Rituxan[Text Word]) or IDEC-C2B8 antibody[Text Word]) or "rituximab"[Supplementary Concept]) or (((((((TNFR-Fc fusion protein[Text Word] or TNR 001[Text Word]) or TNR-001[Text Word]) or TNF receptor type II-IgG fusion protein[Text Word]) or recombinant human dimeric TNF receptor type II-IgG fusion protein[Text Word]) or Enbrel[Text Word]) or etanercept[Text Word]) or "TNFR-Fc fusion protein"[Supplementary Concept])) or ((((infliximab[Text Word] or monoclonal antibody cA2[Text Word]) or MAb cA2[Text Word]) or Remicade[Text Word]) or "infliximab"[Sup plementary Concept])) or ((adalimumab[Text Word] or Humira[Text Word]) or "adalimumab"[Supplementary Concept])) or (((((certolizumab[Text Word] or CDP870[Text Word]) or CDP 870[Text Word]) or Cimzia[Text Word]) or certolizumab pegol[Text Word]) or "certolizumab pegol"[Supplementary Concept])) or ((((((((((((abatacept[Text Word] or BMS 188667[Text Word]) or BMS-188667[Text Word]) or nulojix[Text Word]) or CTLA-4-Ig[Text Word]) or cytotoxic T lymphocyte-associated antigen 4-immunoglobulin[Text Word]) or CTLA4-Fc[Text Word]) or CTLA4-Ig[Text Word]) or LEA29Y[Text Word]) or Orencia[Text Word]) or BELATACEPT[Text Word]) or BMS-224818[Text Word]) or "abatacept" [Supplementary Concept])) or (((tocilizumab[Text Word] or atlizumab[Text Word]) or Actemra[Text Word]) or "tocilizumab"[Supplementary Concept])) or ("golimumab"[Supplementary Concept] or (Simponi[Text Word] or golimumab[Text Word]))))) and (("Cohort Studies"[Mesh]) or (((cohort*[Text Word]) or controlled clinical trial[Publication Type]) or epidemiologic methods)) |
| EMBASE |
| "golimumab"/exp and [embase]/lim or ("cnto$148" and [embase]/lim) or ("simponi" and [embase]/lim) or ("tocilizumab"/exp and [embase]/ lim) or ("actemra" and [embase]/lim) or ("actemra 200" and [embase]/lim) or ("atlizumab" and [embase]/lim) or ("r$1569" and [embase]/lim) or ("roactemra" and [embase]/lim) or ("abatacept"/exp and [embase]/lim) or ("bms$188667" and [embase]/lim) or ("ctla4$ig" and [embase]/ lim) or ("ctla4 immunoglobulin" and [embase]/lim) or ("ctla4 immunoglobulin g" and [embase]/lim) or ("orencia" and [embase]/lim) or ("certolizumab pegol"/exp and [embase]/lim) or ("cdp$870" and [embase]/lim) or ("cimzia" and [embase]/lim) or ("pha$738144" and [embase]/ lim) or ("adalimumab"/exp and [embase]/lim) or ("humira"/exp and [embase]/lim) or ("monoclonal antibody d2e7" and [embase]/lim) or ("trudexa" and [embase]/lim) or ("infliximab"/exp and [embase]/lim) or ("avakine" and [embase]/lim) or ("inflectra" and [embase]/lim) or ("remicade" and [embase]/lim) or ("remsima" and [embase]/lim) or ("revellex" and [embase]/lim) or ("etanercept"/exp and [embase]/lim) or ("embrel" and [embase]/lim) or ("enbrel" and [embase]/lim) or ("recombinant tumor necrosis factor receptor fc fusion protein" and [embase]/lim) or ("tnr$001" and [embase]/lim) or ("tumor necrosis factor receptor fc fusion protein" and [embase]/lim) or ("rituximab"/exp and [embase]/lim) or ("idec c2b8" and [embase]/lim) or ("mabthera" and [embase]/lim) or ("monoclonal antibody idec c2b8" and [embase]/lim) or ("reditux" and [embase]/lim) or ("rituxan" and [embase]/lim) or ("rituxin" and [embase]/lim) and ("rheumatoid arthritis"/exp and [embase]/lim or ("arthritis, rheumatoid" and [embase]/lim)) and ("cohort analysis"/exp and [embase]/lim or ("longitudinal study"/exp and [embase]/lim) or ("prospective study"/exp and [embase]/lim) or ("follow up"/exp and [embase]/lim) or ("cohort$" and [embase]/lim)) |
| Cochrane Controlled Trials Register |
| #1 MeSH descriptor: [Arthritis, Rheumatoid] explode all trees |
| #2 Rheumatoid Arthritis in Trials |
| #3 golimumab in Trials |
| #4 tocilizumab in Trials |
| #5 abatacept in Trials |
| #6 certolizumab pegol in Trials |
| #7 adalimumab in Trials |
| #8 infliximab in Trials |
| #9 etanercept in Trials |
| #10 rituximab in Trials |
| #11 #1 or #2 in Trials |
| #12 #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 in Trials |
| #13 #11 and #12 |
| LILACS |
| (tw:((mh:(arthritis, rheumatoid)) or (tw:(artrite reumatoide)) or (tw:(artritis reumatoide)) )) and (tw:((tw:(adalimumab)) or (tw:(etanercept)) or (tw:(infliximab)) or (tw:(rituximab)) or (tw:(golimumab)) or (tw:(tocilizumab)) or (tw:(abatacept)) or (tw:(certolizumab pegol)))) |
In addition, we conducted a manual search in the 2012 and 2013 editions of four rheumatology journals (Journal Rheumatology, Rheumatology, Rheumatology International and the Brazilian Journal of Rheumatology) and in the abstracts of the ACR and the EULAR meetings. Also, we searched for grey literature in the Digital Library of Theses and Dissertations of University of São Paulo, and ProQuest Dissertation and Theses Database.
We performed the study selection in duplicate by four independent examiners (JBS, JOC, HAOJ, LLPL). The steps included analysis of titles, abstracts, and analysis of the full-texts of articles. Divergences were analyzed by another reviewer (VEA). Data collection was performed by four investigators (JBS, JOC, HAOJ, LLPL). The authors were contacted for additional information whenever needed. We assessed effectiveness as indicated by the rate of response to bDMARD according to the criteria of ACR and EULAR. We also analysed the Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Disease Activity Score (DAS28) and the Health Assessment Questionnaire (HAQ).
The methodological quality of each study was assessed by four examiners (JBS, JOC, HAOJ, LLPL); divergences were solved by consensus. For that purpose, we used the Newcastle-Ottawa scale, as recommended by the Cochrane Collaboration in the case of observational studies[11]. This scale assesses studies in three major domains: selection of the study groups, comparability of groups, and ascertainment of exposure and of results of interest. The maximum total score is nine stars, and scores above six stars are indicative of high methodological quality.
Funding sources were identified to establish potential sources of bias. Publication bias was assessed by funnel plot analysis of the results of EULAR responses and DAS28.
We used the Software Review Manager® 5.2 to perform the meta-analyses. The results are expressed as relative risks (dichotomous variables) or means differences (continuous variables) with the corresponding 95%CIs. Values of I2 > 40% and P < 0.10 on the χ2 test were considered as indicative of significant heterogeneity. The causes of heterogeneity were investigated by excluding one study at a time and checking the changes in I2 and P values.
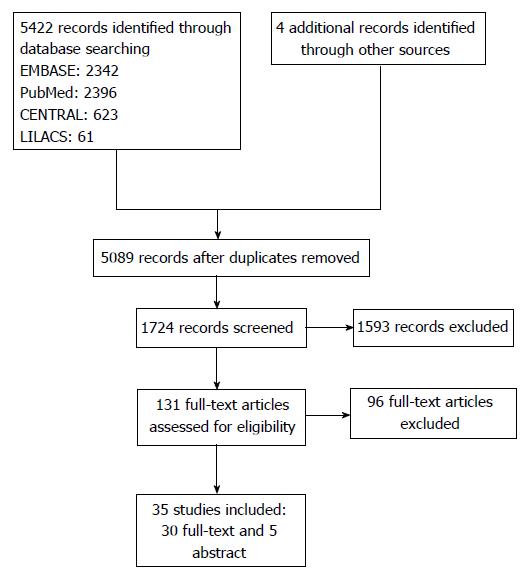
A total of 5422 articles were found in the investigated electronic databases, and a further four after manual search. Following the exclusion of duplicates, 5089 articles were selected for title analysis, from which 1724 were selected for abstract analysis, and finally 131 for full-text reading. Following full-text reading, 35 studies were included in the review, corresponding to 30 full-text articles[12-42] and five abstracts[43-47] (Figure 1). No observational study assessed the medicines golimumab or certolizumab pegol.
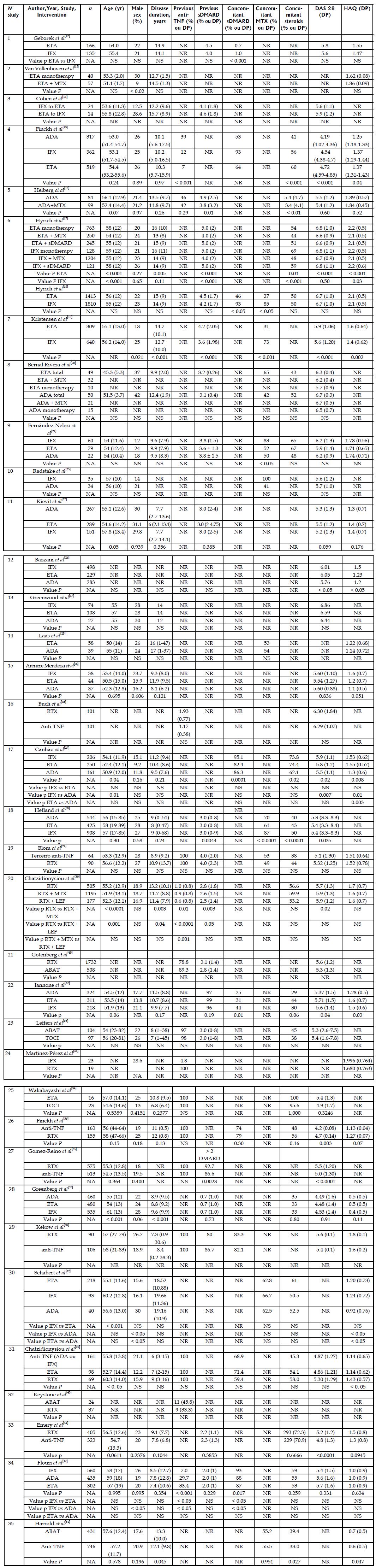
Among the 35 observational studies included, 16 were registry studies and 19 were cohort studies; eight were retrospective, and 27 were prospective. The study duration varied from 15 to 80 mo, though this information was not provided by some authors. The participants were followed from three to 48 mo. Five studies were funded by pharmaceutical companies, two studies were not funded by the pharmaceutical industry, and 16 had mixed funding; in the remainder articles the authors did not disclose the funding source. Nine studies assessed anti-TNF naïve participants, and 11 studies assessed cases of therapeutic failure with at least one anti-TNF agent; the remainder of the studies did not inform whether therapeutic failure had occurred or did not separate patients into subgroups (Table 2). Disease duration varied from 6 to 20 years. Approximately 50% of the participants used glucocorticoids and the use of sDMARD varied from 31% to 100%. In most of the studies, the DAS28 score was > 5.1, which indicates high disease activity. The HAQ score varied from 0.4 to 2.2 (Figure 2).
| Nstudy | Ref. | Type of study | Time horizon | Patient | Intervention | Country conducting the study | Funding Sources | Duration of the study (mo) | Follow-up (mo) |
| 1 | Geborek et al[12] | Cohort | Prospective | Naive | ETA vs IFX vs LEF | Sweden | NR | 24 | 12 |
| 2 | Van Vollenhoven et al[13] | Registry | Prospective | NR | ETA vs ETA + MTX | Sweden | Mixed | NR | 12 |
| 3 | Cohen et al[14] | Cohort | Retrospective | Therapeutic failure | IFX vs ETA | France | NR | 48 | 3 |
| 4 | Finckh et al[15] | Registry | Prospective | Mixed | ADA vs ETA vs IFX | Switzerland | Mixed | 80 | 12 |
| 5 | Heiberg et al[16] | Cohort | Prospective | Mixed | ADA monotherapy vs ADA + MTX | Norway | Mixed | NR | 12 |
| 6 | Hyrich et al[17,18] | Registry | Prospective | NR | ETA monotherapy vs ETA + MTX vs ETA + DMARD and ADA monotherapy vs ADA + MTX vs ADA + DMARD | England | Pharmaceutical industry | NR | 6 |
| 7 | Kristensen et al[19] | Cohort | Prospective | Naive | ETA vs IFX | Sweden | Mixed | 55 | 36 |
| 8 | Bernal Rivera et al[20] | Cohort | Prospective | Naive | ADA vs ETA vs IFX | Spain | NR | 24 | 12 |
| 9 | Kristensen et al[19] | Cohort | Prospective | Naive | ETA vs IFX | Spain | NR | 72 | 6 |
| 10 | Radstake et al[23] | Cohort | Prospective | NR | IFX vs ADA | The Netherlands | Mixed | NR | 6 |
| 12 | Bazzani et al[24] | Registry | Prospective | Mixed | ADA vs ETA vs IFX | Italy | Pharmaceutical industry | 25.29 | 36 |
| 13 | Greenwood et al[47] | Cohort | Retrospective | NR | ADA vs ETA vs IFX | England | NR | NR | 12 |
| 14 | Laas et al[25] | Cohort | Prospective | Naive | ETA vs ADA | Finland | No pharmaceutical industry | 36 | 3 |
| 15 | Arenere Mendoza et al[26] | Cohort | Retrospective | Mixed | ADA vs ETA vs IFX | Spain | NR | 80 | 12 |
| 16 | Buch et al[46] | Cohort | Prospective | Therapeutic failure | RTX vs anti-TNF | England | NR | NR | 6 |
| 17 | Canhão et al[27] | Registry | Prospective | Naive | ADA vs ETA vs IFX | Portugal | Mixed | NR | 12 |
| 18 | Hetland et al[28] | Registry | Prospective | Naive | ADA vs ETA vs IFX | Denmark | Mixed | 86 | 12 |
| 19 | Blom et al[29] | Registry | Prospective | Therapeutic failure | RTX vs anti-TNF | The Netherlands | Mixed | NR | 12 |
| 20 | Chatzidionysiou et al[30] | Registry | Prospective | Mixed | RTX monotherapy vs RTX + MTX vs RTX + LEF | Europe | Pharmaceutical industry | NR | 12 |
| 21 | Gotenberg et al[45] | Registry | Prospective | Mixed | RTX vs ABAT | France | NR | NR | 6 |
| 22 | Iannone et al[32] | Registry | Prospective | NR | ADA vs ETA vs IFX | Italy | NR | NR | 48 |
| 23 | Leffers et al[33] | Registry | Prospective | Mixed | ABAT vs TOCI | Denmark | Mixed | NR | 48 |
| 24 | Martínez-Pérez et al[44] | Cohort | Retrospective | Mixed | RTX vs IFX | Spain | NR | NR | 12 |
| 25 | Wakabayashi et al[34] | Cohort | Retrospective | Therapeutic failure | TOCI vs ETA | Japan | No pharmaceutical industry | 60 | 12 |
| 26 | Finckh et al[36] | Cohort | Prospective | Therapeutic failure | RTX vs anti-TNF | Switzerland | Mixed | NR | 24 |
| 27 | Gomez-Reino et al[35] | Cohort | Prospective | Therapeutic failure | RTX vs anti-TNF | Spain | Pharmaceutical industry | 36 | 12 |
| 28 | Greenberg et al[37] | Registry | Prospective | Naive | ADA vs ETA vs IFX | Unied States | Mixed | 74 | 24 |
| 29 | Kekow et al[38] | Cohort | Retrospective | Therapeutic failure | RTX vs anti-TNF | Germany | Pharmaceutical industry | NR | 6 |
| 30 | Schabert et al[39] | Cohort | Retrospective | NR | ADA vs ETA vs IFX | Unied States | Mixed | 15 | 12 |
| 31 | Chatzidionysiou et al[40] | Registry | Prospective | Therapeutic failure | Anti-TNF vs ETA vs ADA | Stockholm | NR | NR | 6 |
| 32 | Keystone et al[43] | Cohort | Retrospective | Therapeutic failure | ABAT vs TOCI | Canada | NR | NR | 12 |
| 33 | Emery et al[41] | Cohort | Prospective | Therapeutic failure | RTX vs anti-TNF | Multicentre | Mixed | NR | 12 |
| 34 | Flouri et al[42] | Registry | Prospective | Mixed | ADA vs ETA vs IFX | Greece | Mixed | 60 | 12 |
| 35 | Harrold et al[31] | Registry | Prospective | Therapeutic failure | ABAT vs TOCI | Unied States | Mixed | NR | 12 |
From the 35 analyzed studies, two achieved the highest score on the Newcastle-Ottawa scale, nine stars; 14, eight stars; seven, seven stars; 10, six stars; and two, five stars (Table 3). The funnel plot did not exhibit asymmetry relative to outcomes in the DAS 28 and EULAR response, which indicated the absence of publication bias, and thus of overestimation of the intervention effects calculated in the meta-analysis (data not shown).
| N study | Ref. | Selection | Comparability | Results | Total | |||||
| Representati-veness of the cases | Selection of controls | Ascertain-ment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | |||
| 1 | Geborek et al[12] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (12 mo) | 1 | 8 |
| 2 | Van Vollenhoven et al[13] | 0 | 1 | 1 | 1 | 2 | 0 | 1 (24 mo) | 0 | 6 |
| 3 | Cohen et al[14] | 1 | 1 | 1 | 1 | 1 | 0 | 1 (3 mo) | 0 | 6 |
| 4 | Finckh et al[15] | 1 | 1 | 1 | 0 | 1 | 1 | 1 (12 mo) | 1 | 7 |
| 5 | Heiberg et al[16] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (6 mo) | 1 | 8 |
| 6 | Hyrich et al[17,18] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (6 mo) | 1 | 8 |
| 7 | Kristensen et al[19] | 1 | 1 | 1 | 0 | 2 | 0 | 1 (36 mo) | 0 | 6 |
| 8 | Bernal Rivera et al[20] | 1 | 1 | 1 | 0 | 2 | 0 | 1 (12 mo ) | 1 | 7 |
| 9 | Fernández-Nebro et al[21] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (6 mo) | 1 | 8 |
| 10 | Radstake et al[23] | 0 | 1 | 1 | 1 | 2 | 0 | 1 (6 mo) | 0 | 6 |
| 11 | Kievit et al[22] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (6 mo) | 0 | 7 |
| 12 | Bazzani et al[24] | 1 | 1 | 1 | 0 | 2 | 1 | 1 (6 mo) | 1 | 8 |
| 13 | Greenwood et al[47] | 0 | 1 | 1 | 1 | 2 | 0 | 1 (12 mo) | 0 | 6 |
| 14 | Laas et al[25] | 1 | 1 | 1 | 1 | 2 | 1 | 1 (3 mo) | 1 | 9 |
| 15 | Arenere Mendoza et al[26] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (12 mo) | 0 | 7 |
| 16 | Buch et al[46] | 1 | 1 | 1 | 0 | 2 | 0 | 1 (6 mo) | 0 | 6 |
| 17 | Canhão et al[27] | 1 | 1 | 1 | 0 | 2 | 0 | 1 (12 mo) | 1 | 7 |
| 18 | Hetland et al[28] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (12 mo) | 1 | 8 |
| 19 | Blom et al[29] | 1 | 1 | 1 | 1 | 2 | 1 | 1 (12 mo) | 1 | 9 |
| 20 | Chatzidionysiou et al[30] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (12 mo) | 0 | 7 |
| 21 | Gotenberg et al[45] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (6 mo) | 1 | 8 |
| 22 | Iannone et al[32] | 1 | 1 | 1 | 0 | 2 | 0 | 1 (48 mo) | 0 | 6 |
| 23 | Leffers et al[33] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (12 mo) | 0 | 7 |
| 24 | Martínez-Pérez et al[44] | 0 | 1 | 0 | 1 | 2 | 0 | 1 (12 mo) | 0 | 5 |
| 25 | Wakabayashi et al[34] | 0 | 1 | 1 | 1 | 2 | 1 | 1 (12 mo) | 1 | 8 |
| 26 | Finckh et al[36] | 1 | 1 | 1 | 1 | 1 | 1 | 1 (24 mo) | 1 | 8 |
| 27 | Gomez-Reino et al[35] | 1 | 1 | 1 | 0 | 2 | 1 | 1 (12 mo) | 1 | 8 |
| 28 | Greenberg et al[37] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (24 mo) | 1 | 8 |
| 29 | Kekow et al[38] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (6 mo) | 1 | 8 |
| 30 | Schabert et al[39] | 1 | 1 | 1 | 1 | 1 | 1 | 1 (12 mo) | 1 | 8 |
| 31 | Chatzidionysiou et al[40] | 1 | 1 | 1 | 0 | 2 | 0 | 1 (6 mo) | 0 | 6 |
| 32 | Keystone et al[43] | 0 | 1 | 1 | 0 | 2 | 0 | 1 (12 mo) | 0 | 5 |
| 33 | Emery et al[41] | 1 | 1 | 1 | 0 | 2 | 0 | 1(12 mo) | 0 | 6 |
| 34 | Flouri et al[42] | 1 | 1 | 1 | 1 | 2 | 0 | 1 (12 mo) | 1 | 8 |
| 35 | Harrold et al[31] | 1 | 1 | 1 | 0 | 2 | 0 | 1 (12 mo) | 0 | 6 |
A total of 22 studies assessed the drugs adalimumab, etanercept and infliximab; nine studies assessed anti-TNF naïve patients only and seven anti-TNF naïve participants and cases of therapeutic failure; six studies did not inform whether therapeutic failure had occurred. Nineteen of those studies were included in the meta-analyses of EULAR responses, DAS28, remission acoording to DAS28, CDAI, SDAI, ACR20, 50 and 70, and HAQ (Table 4).
| Intervention | Outcomes | Studies (references) | Partici-pants | Relative risk (95%CI) or other mesure | I2(%) | P value |
| IFX vs ETA | EULAR good response | 10 (18,19,21,22,24, 26,27, 28, 32,42) | 7247 | 0.86 [0.72-1.02] | 76 | < 0.0001 |
| EULAR moderate response | 9 (18,19,21,22,24, 26, 28, 32,42) | 6791 | 0.98 [0.84-1.15] | 78 | < 0.0001 | |
| EULAR no response | 9 (18,19,21,22,24, 26, 28, 32,42) | 6791 | 1.20 [1.05-1.38] | 46 | 0.06 | |
| DAS 28 remission | 7 (21,26,27,28,32,37,42) | 2868 | 0.70 [0.59-0.84] | 0 | 0.51 | |
| DAS 28 | 2 (21,26) | 196 | 0.40 [-0.27- 1.07] | 59 | 0.12 | |
| DAS 28 reduction | 2 (15,22) | 1321 | 0.40 [0.04-0.77] | 77 | 0.04 | |
| CDAI remission | 4 (27,28,37,42) | 2293 | 0.90 [0.74-1.09] | 0 | 0.89 | |
| SDAI remission | 2 (27,42) | 840 | 0.87 [0.61-1.26] | 0 | 0.9 | |
| HAQ | 3 (21,26,39) | 495 | 0.14 [0.00-0.27] | 0 | 0.51 | |
| ACR 20 | 2 (19,37) | 1309 | 0.95 [0.86-1.06] | 0 | 0.47 | |
| ACR50 | 3 (19,28,37) | 2315 | 0.92 [0.81-1.03] | 10 | 0.33 | |
| ACR70 | 3 (19,28,37) | 2315 | 0.88 [0.57-1.36] | 79 | 0.009 | |
| ADA vs ETA | EULAR good response | 8 (20,22,24,26,27,28,32,42) | 2492 | 0.97 [0.79-1.20] | 73 | 0.0005 |
| EULAR moderate response | 7 (20,22,24,26,28,32,42) | 2080 | 1.00 [0.89-1.12] | 0 | 0.48 | |
| EULAR no response | 7 (20,22,24,26,28,32,42) | 2080 | 0.90 [0.62-1.32] | 76 | 0.0003 | |
| DAS 28 remission | 6 (26,27,28,32,37,42) | 2412 | 0.93 [0.68-1.26] | 80 | 0.0001 | |
| DAS 28 | 2 (20,26) | 180 | -0.09 [-0.25-0.06] | 0 | 0.73 | |
| DAS 28 reduction | 2 (15,22) | 1392 | 0.17 [-0.19-0.52] | 68 | 0.08 | |
| CDAI remission | 4 (27,28,37,42) | 1883 | 1.16 [0.77-1.74] | 70 | 0.02 | |
| SDAI remission | 2 (27,42) | 641 | 1.40 [0.76-2.59] | 55 | 0.13 | |
| HAQ | 2 (26,39) | 339 | -0.15 [-0.39-0.10] | 49 | 0.16 | |
| HAQ reduction | 2 (22,25) | 653 | -0.07 [-0.16-0.03] | 0 | 0.92 | |
| ACR 20 | 2 (20,37) | 445 | 0.89 [0.71-1.12] | 0 | 0.68 | |
| ACR 50 | 3 (20,28,37) | 1217 | 1.09 [0.91-1.31] | 18 | 0.3 | |
| ACR 70 | 3 (20,28,37) | 1436 | 1.15 [0.92-1.43] | 0 | 0.82 | |
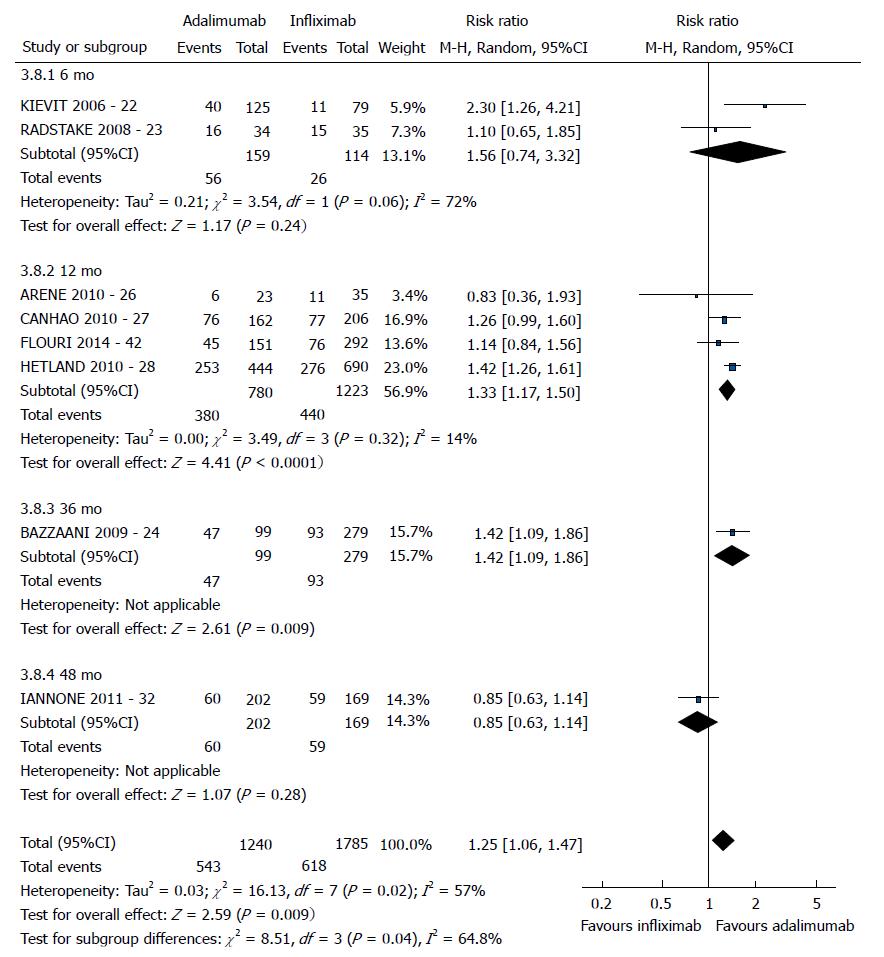
| IFX vs ADA | EULAR good response | 8 (22,23,24,26,27,28,32,42) | 3025 | 1.25 [1.06-1.47] | 57 | 0.02 |
| EULAR moderate response | 7 (22,23,24,26,28,32,42) | 2657 | 0.91 [0.79-1.04] | 31 | 0.19 | |
| EULAR no response | 7 (22,23,24,26,28,32,42) | 2657 | 0.77 [0.56-1.05] | 75 | 0.0006 | |
| DAS 28 remission | 6 (26,27,28,32,37,42) | 2760 | 1.15 [0.91-1.46] | 63 | 0.02 | |
| DAS 28 reduction | 2 (15,22) | 1097 | -0.24 [-0.96-0.48] | 91 | 0.001 | |
| CDAI remission | 4 (27,28,37,42) | 2332 | 1.30 [0.90-1.88] | 68 | 0.02 | |
| SDAI remission | 2 (27,42) | 765 | 1.66 [0.94-2.93] | 61 | 0.11 | |
| HAQ | 2 (26,39) | 182 | -0.33 [-0.53-0.13] | 0 | 0.92 | |
| ACR 50 | 2 (28,37) | 1458 | 1.14 [0.71-1.84] | 79 | 0.03 | |
| ACR 70 | 2 (28,37) | 1458 | 1.41 [0.81-2.44] | 72 | 0.06 |
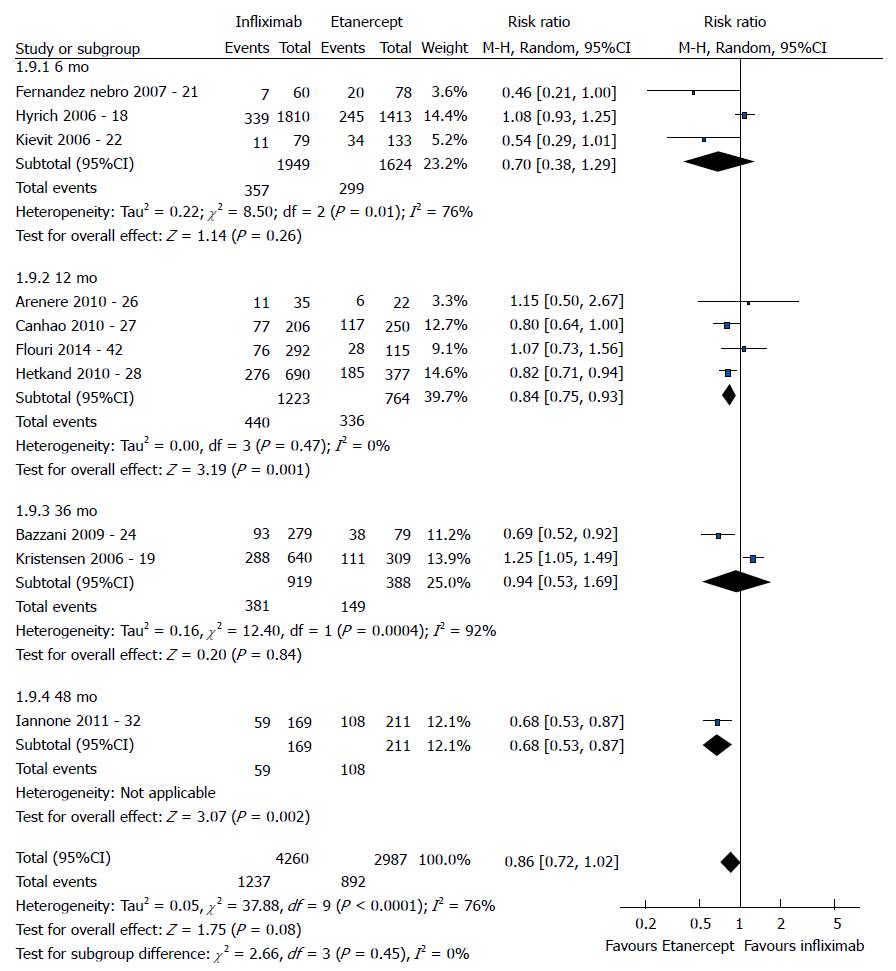
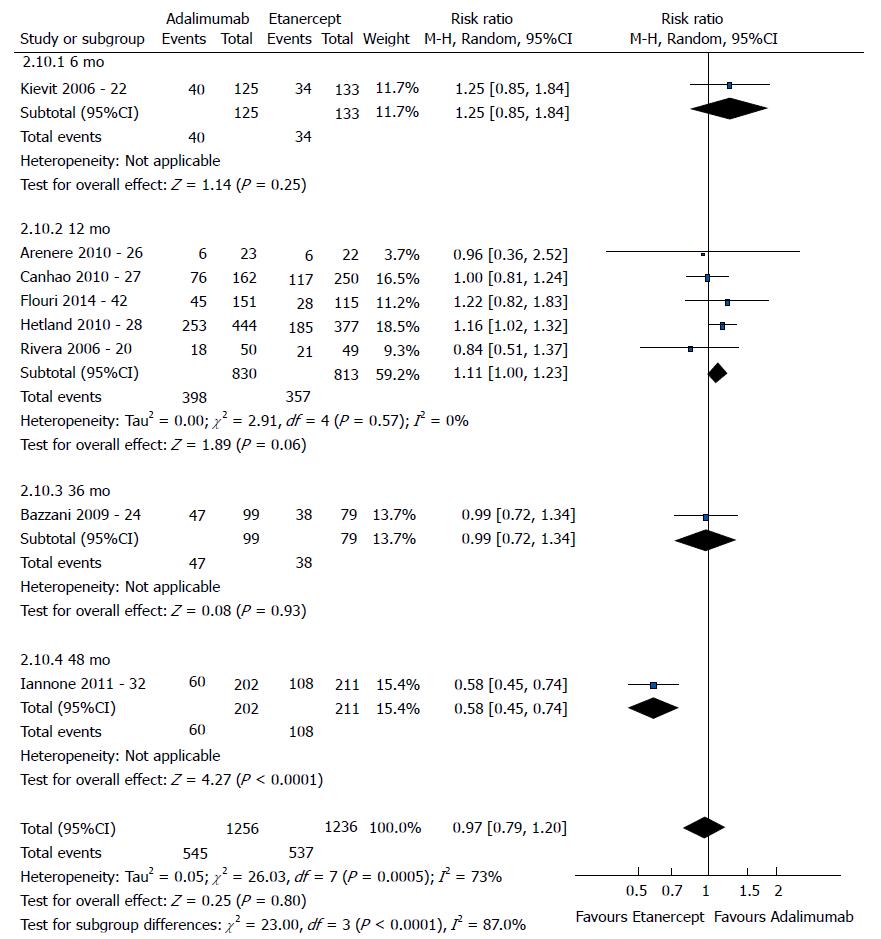
The good EULAR response for the participants who used etanercept was no different as that for the participants who used infliximab (P = 0.08) (Figure 3). However, the meta-analysis exhibited high heterogeneity. Following exclusion of the studies by Kristensen et al[19] (2006) and Hyrich et al[17] (2006), the heterogeneity was lowered, and the results became favorable to etanercept (P < 0.0001). No difference was found between adalimumab and etanercept (P = 0.80) (Figure 4). That meta-analysis also exhibited high heterogeneity; and after the exclusion of the study by Iannone et al[32] (2011), no heterogeneity was detected (P = 0.05). The participants who used adalimumab presented higher good EULAR response compared to those who used infliximab (P = 0.009) (Figure 5). However, that meta-analysis exhibited high heterogeneity. Following exclusion of the study by Iannone et al[32] (2011), the heterogeneity was lowered (P < 0.00001). Comparison of etanercept vs infliximab, adalimumab vs etanercept, and adalimumab vs infliximab found similar results relative to moderate EULAR response (P > 0.05). Regarding the EULAR no response, the results were favorable to infliximab compared to etanercept (P = 0.01), while no difference was detected between adalimumab and infliximab (P = 0.09) or etanercept (P = 0.60). The study by Gottenberg et al[45] (2011), which was not included in the meta-analysis due to the lack of studies comparing abatacept and rituximab, did not detect a difference in the EULAR responses between the two drugs (P > 0.05). Additionally, the study by Leffers et al[33] could not be included in the meta-analysis for the same reason and did not detect a difference in the EULAR responses between abatacept and tocilizumab (P > 0.05).
The participants who used etanercept exhibited greater remission according to DAS28 compared to the participants who used infliximab (P < 0.0001). Comparison of adalimumab and infliximab did not reveal a significant difference (P = 0.23). However, that meta-analysis exhibited moderate heterogeneity. Following exclusion of the study by Iannone et al[32], the heterogeneity was lowered, and the result became favorable to adalimumab (P = 0.001). No significant difference was detected between adalimumab and etanercept (P = 0.63). However, the meta-analysis exhibited high heterogeneity. Following exclusion of the study by Iannone et al[32], heterogeneity was lowered (P = 0.21). The participants who used etanercept exhibited greater reduction in the DAS28 score compared to the participants who used infliximab (P = 0.03). Significant differences were not detected between adalimumab and etanercept (P = 0.36) or infliximab (P = 0.52). Comparison of etanercept vs infliximab or adalimumab did not reveal any statistically significant differences relative to DAS28 (P > 0.05). The study by Arenere Mendoza et al[26] (2010), which was not included in the meta-analysis due to the lack of studies that analyzed the DAS28 outcome, did not report differences between adalimumab and infliximab (P > 0.05). The study by Greenwood et al[47] (2009), which was not included in the meta-analysis due to lack of data, did not report significant differences in the DAS28 response when comparing adalimumab vs etanercept, infliximab vs adalimumab, and infliximab vs etanercept (P > 0.05). The study by Gottenberg et al[45] (2011), which was also not included in meta-analysis, did not report a difference relative to DAS28 outcome between abatacept and rituximab (P > 0.05). The study by Leffers et al[33] (2011) also did not report a difference between abatacept and tocilizumab relative to DAS28 remission (P > 0.05).
In regard to the ACR20 outcome, the comparison of etanercept vs adalimumab or infliximab presented similar results (P > 0.05). Comparisons of etanercept vs infliximab, etanercept vs adalimumab, and infliximab vs adalimumab did not reveal differences relative to the outcomes of CDAI and SDAI remission, ACR50 and ACR70 (P > 0.05).
The HAQ scores of the participants who used adalimumab (P = 0.0009) and etanercept (P =0.04) were better compared to the participants who used infliximab. Adalimumab and etanercept were not different in regard to that outcome (P = 023). Adalimumab and etanercept were also not different in terms of the HAQ reduction outcome (P = 0.16). The study by Martinez-Pérez et al[44] (2011), which was not included in the meta-analysis due to the lack of studies comparing infliximab and rituximab, did not report a difference with respect to HAQ (P > 0.05). The study by Leffers et al[33] (2011), which was also not included in the meta-analysis, did not report a significant difference in HAQ between abatacept and tocilizumab (P > 0.05).
Anti-TNF naïve patients: Nine studies assessed anti-TNF-naïve individuals only, from which seven were included in the meta-analysis that assessed the outcomes of EULAR, remission according to DAS28 and CDAI, and ACR20, 50, 70 (Table 5).
| Intervention | Outcomes | Studies (references) | n | RR (95%CI)or other mesure | I2(%) | P value |
| IFX vs ETA | EULAR good response | 5 (19, 21, 22, 27, 28) | 2822 | 0.82 [0.62-1.09] | 82 | 0.0001 |
| EULAR moderate response | 4 (19, 21, 22, 28) | 2366 | 0.90 [0.61-1.33] | 90 | < 0.00001 | |
| EULAR no response | 4 (19, 21, 22, 28) | 2366 | 1.29 [1.09-1.53] | 27 | 0.25 | |
| DAS 28 remission | 4 (21, 27, 28, 37) | 1804 | 0.82 [0.70-0.95] | 0 | 0.4 | |
| ACR 20 | 2 (19, 37) | 1309 | 0.95 [0.86-1.06] | 0 | 0.47 | |
| ACR50 | 3 (19, 28, 37) | 2315 | 0.92 [0.81-1.03] | 10 | 0.33 | |
| ACR70 | 3 (19, 28, 37) | 2315 | 0.88 [0.57-1.36] | 79 | 0.009 | |
| CDAI remission | 3 (27, 28, 37) | 1876 | 0.88 [0.72-1.08] | 0 | 0.93 | |
| ADA vs ETA | EULAR good response | 4 (20, 22, 27, 28) | 1590 | 1.11 [1.00-1.23] | 0 | 0.4 |
| EULAR moderate response | 3 (20, 22, 28) | 1178 | 1.01 [0.83-1.24] | 19 | 0.29 | |
| EULAR no response | 3 (20, 22, 28) | 1178 | 0.69 [0.53-0.89] | 11 | 0.32 | |
| DAS 28 remission | 3 (27, 28, 37) | 1380 | 1.03 [0.82-1.29] | 37 | 0.21 | |
| ACR 20 | 2 (20, 37) | 445 | 0.89 [0.71-1.12] | 0 | 0.68 | |
| ACR 50 | 3 (20, 28, 37) | 1217 | 1.09 [0.91-1.31] | 18 | 0.3 | |
| ACR 70 | 3 (20, 28, 37) | 1436 | 1.15 [0.92-1.43] | 0 | 0.82 | |
| HAQ reduction | 2 (22, 25) | 653 | -0.07 [-0.16-0.03] | 0 | 0.92 | |
| CDAI remission | 3 (27, 28, 37) | 1601 | 1.02 [0.67-1.56] | 72 | 0.03 | |
| IFX vs ADA | EULAR good response | 3 (22, 27, 28) | 1706 | 1.42 [1.18-1.72] | 42 | 0.18 |
| EULAR moderate response | 2 (22, 28) | 1338 | 0.96 [0.58-1.59] | 80 | 0.03 | |
| EULAR no response | 2 (22, 28) | 1338 | 0.56 [0.45-0.69] | 0 | 0.88 | |
| DAS 28 remission | 3 (27, 28) | 1648 | 1.23 [0.95-1.59] | 48 | 0.15 | |
| ACR 50 | 2 (28, 37) | 1458 | 1.14 [0.71-1.84] | 79 | 0.03 | |
| ACR 70 | 2 (28, 37) | 1458 | 1.41 [0.81-2.44] | 72 | 0.06 | |
| CDAI remission | 3 (27, 28, 37) | 1875 | 1.17 [0.75-1.82] | 75 | 0.02 |
The good EULAR response for the participants who used etanercept were the same as those of the participants who used infliximab (P = 0.17). However, that meta-analysis exhibited high heterogeneity. Following exclusion of the study by Kristensen et al[19] (2006), the heterogeneity was low, and the results became favorable to etanercept (P = 0.0006). No difference was detected between adalimumab and etanercept (P = 0.05). The participants who used adalimumab exhibited greater good EULAR response compared to the participants who used infliximab (P =0.0002). The results relative to the moderate EULAR response outcome were similar in the comparisons of etanercept vs infliximab, adalimumab vs etanercept, and adalimumab vs infliximab (P > 0.05). With regard to the EULAR no response, the results were favorable to infliximab compared to etanercept (P = 0.004) or adalimumab (P < 0.00001) and to etanercept compared to adalimumab (P = 0.004).
The participants who used etanercept exhibited greater remission according to DAS28 compared to the participants who used infliximab (P = 0.01). No differences were detected between adalimumab and infliximab (P = 0.12) or etanercept (P = 0.79).
With regard to the ACR20 outcome, the results of etanercept vs adalimumab or infliximab were not different (P > 0.05). No differences were detected for the outcomes of ACR50 and 70 and the remission according to CDAI between etanercept and infliximab, etanercept and adalimumab, and infliximab and adalimumab (P > 0.05). Only the study by Greenberg et al[37] (2012) compared the ACR20 outcome between adalimumab and infliximab (P > 0.05). The study by Geborek et al[12] (2002), which was not included in the meta-analysis because it reported graphical data without numerical values; showed that the ACR 20 response was better with etanercept compared to infliximab (P < 0.05), while no differences were detected relative to ACR50 and 70.
The study by Kievit et al[22] (2008) assessed the HAQ reduction outcome and found that the results were better with adalimumab compared to infliximab (P < 0.05). Kievit et al[22] (2008) and Laas et al[25] (2009) did not detect a difference between adalimumab and etanercept (P = 0.16).
Patients with anti-TNF therapeutic failure: Eleven studies assessed anti- TNF therapeutic failure only, from which nine were included in the meta-analyses that assessed the outcomes of EULAR response and DAS28 reduction (Table 6).
| Intervention | Outcomes | Studies (references) | n | Relative risk (95%CI)or other mesure | I2(%) | P value |
| RTX vs anti-TNF | EULAR good response | 4 (35, 38, 40, 46) | 1608 | 0.96 [0.60-1.54] | 74 | 0.009 |
| EULAR moderate response | 5 (29, 35, 38, 40, 46) | 1706 | 1.02 [0.79-1.32] | 66 | 0.02 | |
| EULAR no response | 3 (35, 38, 40) | 1406 | 1.00 [0.53-1.89] | 85 | 0.001 | |
| DAS 28 reduction | 6 (35, 36, 38, 40, 41, 46) | 1584 | 0.42 [-0.65--0.20] | 62 | 0.02 | |
| ETA vs control | EULAR good response | 2 (14, 40) | 173 | 2.11 [1.23-3.62] | 0 | 0.48 |
| IFX | 38 | 1.60 [0.63-4.09] | ||||
| RTX | 135 | 2.42 [1.25-4.68] | ||||
| DAS 28 reduction | 2 (34, 40) | 152 | 0.15 [-0.65-0.95] | 77 | 0.04 | |
| RTX | 113 | -0.22 [-0.64-0.20] | ||||
| TOCI | 39 | 0.60 [-0.05-1.25] |
The participants who used rituximab exhibited greater DAS28 reduction compared to those who used anti-TNF agents (P = 0.0002); however, the EULAR responses did not differ between these groups (P > 0.05). In addition, all of the corresponding meta-analyses exhibited high statistical heterogeneity. The study by Blom et al[29] (2011), which was not included in the meta-analysis because it did not report the DAS28 absolute scores, also detected lower scores for the participants who used rituximab compared to those who used anti-TNF (P = 0.004). Among four studies that assessed HAQ, only the Finckh et al[36] study (2012) found that the participants who used rituximab exhibited greater score reductions compared to those who used anti-TNF, which did not represent a clinically significant improvement (e.g., a reduction of 0.22 points in the HAQ score).
The participants who used etanercept achieved greater good EULAR response compared to those who did not use that drug (P = 0.007); that difference resulted from one study that compared etanercept vs rituximab[37]. With regard to the DAS28 score reduction, no differences were reported between the groups (P = 0.71).
The abstracts of studies that assessed reduction of the CDAI score indicated the superiority of abatacept over rituximab (12.4 vs +1.7) and anti-TNF agents (7.6 vs 8.3); those results could not be assessed in the meta-analysis due to the lack of data[28,41]. Harrold et al[31] (2014) also assessed ACR20 and 50 outcomes and did not detect any differences between the groups treated with abatacept or anti-TNF agents [0.87 (0.59; 1.29) and 0.86 (0.58; 1.27), respectively].
Patients who used bDMARD in monotherapy or in combination with methotrexate: Four studies assessed individuals treated with bDMARD monotherapy or in combination with methotrexate, and three were included in the meta-analysis that assessed EULAR response, DAS28 and HAQ outcomes (Table 7).
| Intervention | Outcomes | Studies (references) | Participants | Relative risk (95%CI) or other mesure | I2(%) | P value |
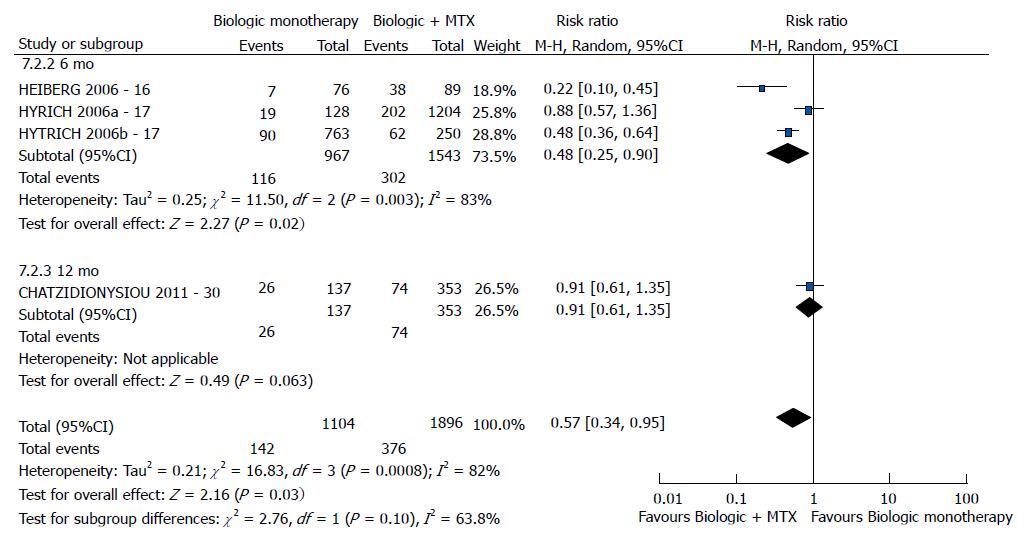
| bDMARD monotherapy vs bDMARD + MTX | EULAR good response | 3 (16,17, 30) | 3000 | 0.57 [0.34-0.95] | 82 | 0.0008 |
| DAS 28 | 3 (17, 20, 30) | 2913 | 0.25 [-0.02-0.52] | 69 | 0.01 | |
| HAQ | 2 (165, 30) | 655 | 0.13 [0.03-0.22] | 0 | 0.43 |
Regarding the good EULAR response, combination with methotrexate was better than bDMARD monotherapy (P = 0.03) (Figure 6). No difference was found relative to DAS28 between bDMARD monotherapy and combination with methotrexate (P = 0.07). However, this meta-analysis exhibited high heterogeneity. Following exclusion of the study by Chatzidionysiou et al[30] (2012)[30], no heterogeneity was detected, and the results became favorable to the combination with methotrexate (P < 0.00001). The study by van Vollenhoven et al[13] (2003), which was not included in the meta-analysis because it reported graphical data without numerical values, reported a difference in DAS28 favorable to combination with methotrexate compared to bDMARD monotherapy (P < 0.05). The study by Heiberg et al[16] (2006), which was not included in the meta-analysis due to the lack of studies that assessed the DAS28 reduction outcome, reported a difference favorable to bDMARD in combination with methotrexate (P < 0.05). With regard to the HAQ score outcome, the best results were exhibited by bDMARD in combination with methotrexate (P = 0.009).
Patients who used adalimumab and etanercept presented similar results among them and better outcomes compared to patients under infliximab therapy. The analysis of subgroup of anti-TNF naïve participants showed better results for adalimumab and etanercept compared to infliximab. The results were similar to the group with all patients (anti-TNF naïve and/or therapeutic failure), probably because most of the participants under treatment were anti-TNF naïve. The use of bDMARD in combination with methotrexate exhibited greater results than bDMARD monotherapy. Rituximab, etanercept and abatacept proved to be effective therapeutic options following therapeutic failure with anti-TNF agents. However, most of the studies on therapeutic failure assessed rituximab; thus, more studies comparing other drugs are needed to contribute to the choice of third-line agents in actual clinical practice.
Systematic reviews that performed indirect comparison meta-analyses of randomized clinical trials that assessed the efficacy of the anti-TNFs adalimumab, etanercept and infliximab reported similar results[48-50]. One meta-analysis found that the efficacy of etanercept was lower compared to that of infliximab and adalimumab; however, the patients selection for the study was different: the study divided patients by those on etanercept (methotrexate-naïve individuals) and other drugs (patients resistant to methotrexate), which makes the comparison of the results between the medicines difficult[51]. The difference of these studies relative to ours might be most likely due to the characteristics of the participants and the low dose of infliximab (3 mg/kg). Some studies reported that patients using infliximab required dose escalation more often compared to those who used etanercept and adalimumab[52,53]. Dose escalation might increase the cost of treatment with infliximab[52] and might thus result in moderate effectiveness[54]. In addition, Pascual-Salcedo et al[55] observed that the production of anti-infliximab antibodies is associated with loss of clinical response[55].
The superiority of the combination of bDMARD and sDMARD compared to bDMARD monotherapy was also reported in other recent meta-analyses[51,56]. In particular, the same pattern was reported for etanercept in combination with methotrexate in a randomized clinical trial[57,58]. The fact that infliximab should be administered in combination with methotrexate is well established[59].
Despite the publication of recent studies on the subject, the definition of the best strategy for patients who exhibit therapeutic failure to at least one anti-TNF agent still poses a challenge[60]. Some studies assessed subgroups in an attempt to identify profiles of patients who will benefit from treatment with rituximab. Thus, whereas testing positive for rheumatoid factor did not induce significant changes in the results[61], rituximab proved to be more effective in individuals who tested positive for rheumatoid factor and for anti-cyclic citrullinated peptide antibody[39].
One of the limitations of systematic reviews with meta-analysis of cohort studies concerns selection bias, which is intrinsic to the design of such studies, as the participants are not randomized but might be allocated to a given treatment based on their patient and physician preferences. A consequence of that limitation was the difference noted among the groups at the onset of treatment that, as a whole, manifested as poorer prognosis in the participants from the rituximab group relative to the numbers of anti-TNF and sDMARD previously used, older age, and greater DAS28 and HAQ scores at baseline[22,36,41].
One further limitation is related to the fact that observational studies are conducted under real-life non-controlled conditions. For that reason, differences were detected in the number of participants among the groups, in the disease activity, and in the lack of dose standardization, especially in the case of infliximab. Moreover, observational studies have the advantage of recruiting large numbers of participants. These types of studies more accurately represent real-life conditions and are able to provide complementary data to the results of randomized clinical studies. Some studies reported that the participants in randomized controlled clinical trials exhibited greater disease activity and fewer associated comorbidities compared to those patients treated in the actual practice setting. The practice of prescribing has been modified over time in real-life. bDMARDs (specially in clinical trials) were prescribed only when patients presented high activity of disease and, now, the medicines are prescribed when the activity is moderate or high[62-64]. Kievit et al[65] (2007) called attention to the reduction of the external validity of randomized clinical trials[65], while another study found similar rates of response in both randomized clinical trials and clinical practice[62].
Nevertheless, all of the assessed therapies were effective to reduce the disease activity and might be considered as therapeutic alternatives as they are proven to exhibit benefits such as greater comfort, less adverse effects and lower cost.
The results of the observational studies included in this review, which reflect the “real-life” use of bDMARD. The best choice for bDMARD treatment-naïve individuals are adalimumab or etanercept in combination with methotrexate. In cases of therapeutic failure with anti-TNF agents rituximab or abatacept (non anti-TNF) or etanercept (as second anti-TNF) might be used; however, more studies of effectiveness were found for rituximab.
Observational studies could provide relevant information for deciding the choice of treatments, the elaboration of clinical protocols, and the formulation of health policies. The present systematic review of biological disease-modifying antirheumatic drugs included cohort observational studies that reported treatment results applied in real-life conditions; thus, these studies are able to fill in gaps in knowledge left by clinical trials.
This study evaluate, by systematic review and metanalysis, the effectiveness of biological disease-modifying antirheumatic drugs (bDMARD) for treatment of rheumatoid arthritis for naïve and therapeutic failure patients.
The innovations of this study is that do not exist others studies evaluating the direct comparison between bDMARD adalimumab, etanercept, infliximab, golimumab, certolizumab pegol, rituximab, tocilizumab and abatacept in the real-world. Furthermore, the study assess naïve and therapeutic failure patients groups.
That study is able to fill in gaps in knowledge left by clinical trials with bDMARD adalimumab, etanercept, infliximab, golimumab, certolizumab pegol, rituximab, tocilizumab and abatacept. Furthermore, do not exist others studies evaluating the direct comparison. Then provide relevant information for deciding the choice of treatments.
The Disease Activity Score (DAS) is a clinical index of rheumatoid arthritis (RA) disease activity that combines information from swollen joints, tender joints, the acute phase response and general health. The EULAR response criteria is a classified response criteria which classifies the patients individual as non, moderate or good responders dependent on the change and the level of the DAS and DAS28. The ACR score represents a percentage. An ACR20 score means that a person’s RA has improved by 20%, an ACR50 score means it has improved by 50%, and an ACR70 score means it has improved by 70%. The CDAI is a clinical index of RA disease activity that combines information from swollen joints, tender joints and general health. The HAQ is one of the first self-report functional status (disability) measures.
The authors present an extensive revision about the effectiveness of the biological treatment for rheumatoid arthritis. The paper is well written. It explores the best treatment options for patients with DMARDs failure and provides useful and practical information for clinicians involved in the care of patients with this disease.
P- Reviewer: Cavallasca JA, La Montagna G, Sakkas L, Turiel M S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 2. | World Health Organization (WHO). Chronic rheumatic conditions. Available from: http://www.who.int/chp/topics/rheumatic/en/. |
| 3. | da Mota LM, Cruz BA, Brenol CV, Pereira IA, Rezende-Fronza LS, Bertolo MB, de Freitas MV, da Silva NA, Louzada-Júnior P, Giorgi RD. 2012 Brazilian Society of Rheumatology Consensus for the treatment of rheumatoid arthritis. Rev Bras Reumatol. 2012;52:152-174. [PubMed] |
| 4. | Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, Gorter S, Knevel R, Nam J, Schoels M. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964-975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1266] [Cited by in RCA: 1144] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 5. | Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, Moreland LW, O’Dell J, Winthrop KL, Beukelman T. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:625-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1220] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 6. | Salliot C, Finckh A, Katchamart W, Lu Y, Sun Y, Bombardier C, Keystone E. Indirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease-modifying antirheumatic drugs or to an anti-tumour necrosis factor agent: a meta-analysis. Ann Rheum Dis. 2011;70:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Malottki K, Barton P, Tsourapas A, Uthman AO, Liu Z, Routh K, Connock M, Jobanputra P, Moore D, Fry-Smith A. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: a systematic review and economic evaluation. Health Technol Assess. 2011;15:1-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312:1215-1218. [PubMed] |
| 9. | Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated 2011; March]. The Cochrane Collaboration, 2011 Available from: http://www.cochrane-handbook.org. |
| 10. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis PA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13344] [Article Influence: 834.0] [Reference Citation Analysis (0)] |
| 11. | Hartling L, Hamm M, Milne A Vandermeer B, Santaguida PL, Ansari M, Tsertsvadze A, Hempel S, Shekelle P, Dryden DM. Validity and interrater reliability testing of quality assessment instruments [Internet]. Appendix E, decision rules for application of the Newcastle-Ottawa Scale. Rockville, MD: Agency for Healthcare Research and Quality 2012; Available from: http://www.ncbi.nlm.nih.gov/books/NBK92291/. |
| 12. | Geborek P, Crnkic M, Petersson IF, Saxne T. Etanercept, infliximab, and leflunomide in established rheumatoid arthritis: clinical experience using a structured follow up programme in southern Sweden. Ann Rheum Dis. 2002;61:793-798. [PubMed] |
| 13. | van Vollenhoven RF, Ernestam S, Harju A, Bratt J, Klareskog L. Etanercept versus etanercept plus methotrexate: a registry-based study suggesting that the combination is clinically more efficacious. Arthritis Res Ther. 2003;5:R347-R351. [PubMed] |
| 14. | Cohen G, Courvoisier N, Cohen JD, Zaltni S, Sany J, Combe B. The efficiency of switching from infliximab to etanercept and vice-versa in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2005;23:795-800. [PubMed] |
| 15. | Finckh A, Simard JF, Gabay C, Guerne PA. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752. [PubMed] |
| 16. | Heiberg MS, Rødevand E, Mikkelsen K, Kaufmann C, Didriksen A, Mowinckel P, Kvien TK. Adalimumab and methotrexate is more effective than adalimumab alone in patients with established rheumatoid arthritis: results from a 6-month longitudinal, observational, multicentre study. Ann Rheum Dis. 2006;65:1379-1383. [PubMed] |
| 17. | Hyrich KL, Symmons DP, Watson KD, Silman AJ. Comparison of the response to infliximab or etanercept monotherapy with the response to cotherapy with methotrexate or another disease-modifying antirheumatic drug in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:1786-1794. [PubMed] |
| 18. | Hyrich KL, Watson KD, Silman AJ, Symmons DP. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford). 2006;45:1558-1565. [PubMed] |
| 19. | Kristensen LE, Saxne T, Geborek P. The LUNDEX, a new index of drug efficacy in clinical practice: results of a five-year observational study of treatment with infliximab and etanercept among rheumatoid arthritis patients in southern Sweden. Arthritis Rheum. 2006;54:600-606. [PubMed] |
| 20. | Bernal Rivera L, Guerrero Aznar MD, Monzón Moreno A, Beltrán García M, Hernández Cruz B, Colmenero MA. Effectiveness and safety of adalimumab and etanercept for rheumatoid arthritis in a third-level hospital. Farm Hosp. 2006;30:223-229. [PubMed] |
| 21. | Fernández-Nebro A, Irigoyen MV, Ureña I, Belmonte-López MA, Coret V, Jiménez-Núñez FG, Díaz-Cordovés G, López-Lasanta MA, Ponce A, Rodríguez-Pérez M. Effectiveness, predictive response factors, and safety of anti-tumor necrosis factor (TNF) therapies in anti-TNF-naive rheumatoid arthritis. J Rheumatol. 2007;34:2334-2342. [PubMed] |
| 22. | Kievit W, Adang EM, Fransen J, Kuper HH, van de Laar MA, Jansen TL, De Gendt CM, De Rooij DJ, Brus HL, Van Oijen PC. The effectiveness and medication costs of three anti-tumour necrosis factor alpha agents in the treatment of rheumatoid arthritis from prospective clinical practice data. Ann Rheum Dis. 2008;67:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, van Riel PL, Bendtzen K. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1739-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 24. | Bazzani C, Filippini M, Caporali R, Bobbio-Pallavicini F, Favalli EG, Marchesoni A, Atzeni F, Sarzi-Puttini P, Gorla R. Anti-TNFalpha therapy in a cohort of rheumatoid arthritis patients: clinical outcomes. Autoimmun Rev. 2009;8:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Laas K, Peltomaa R, Puolakka K, Kautiainen H, Leirisalo-Repo M. Early improvement of health-related quality of life during treatment with etanercept and adalimumab in patients with rheumatoid arthritis in routine practice. Clin Exp Rheumatol. 2009;27:315-320. [PubMed] |
| 26. | Arenere Mendoza M, Manero Ruiz FJ, Carrera Lasfuentes P, Navarro Aznárez H, Pecondón Español A, Rabanaque Hernández MJ. [Tumour necrosis factor alpha antagonists in established rheumatoid arthritis: effectiveness comparative study]. Med Clin (Barc). 2010;134:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Canhão H, Rodrigues AM, Mourão AF, Martins F, Santos MJ, Canas-Silva J, Polido-Pereira J, Pereira Silva JA, Costa JA, Araújo D. Comparative effectiveness and predictors of response to tumour necrosis factor inhibitor therapies in rheumatoid arthritis. Rheumatology (Oxford). 2012;51:2020-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, Kollerup G, Linde L, Lindegaard HM, Poulsen UE. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 411] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 29. | Blom M, Kievit W, Donders AR, den Broeder AA, Straten VH, Kuper I, Visser H, Jansen TL, Brus HL, Branten AJ. Effectiveness of a third tumor necrosis factor-α-blocking agent compared with rituximab after failure of 2 TNF-blocking agents in rheumatoid arthritis. J Rheumatol. 2011;38:2355-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Chatzidionysiou K, Lie E, Nasonov E, Lukina G, Hetland ML, Tarp U, van Riel PL, Nordström DC, Gomez-Reino J, Pavelka K. Effectiveness of disease-modifying antirheumatic drug co-therapy with methotrexate and leflunomide in rituximab-treated rheumatoid arthritis patients: results of a 1-year follow-up study from the CERERRA collaboration. Ann Rheum Dis. 2012;71:374-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Harrold LR, Reed GW, Kremer JM, Curtis JR, Solomon DH, Hochberg MC, Greenberg JD. The comparative effectiveness of abatacept versus anti-tumour necrosis factor switching for rheumatoid arthritis patients previously treated with an anti-tumour necrosis factor. Ann Rheum Dis. 2015;74:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Iannone F, Gremese E, Atzeni F, Biasi D, Botsios C, Cipriani P, Ferri C, Foschi V, Galeazzi M, Gerli R. Longterm retention of tumor necrosis factor-α inhibitor therapy in a large italian cohort of patients with rheumatoid arthritis from the GISEA registry: an appraisal of predictors. J Rheumatol. 2012;39:1179-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Leffers HC, Ostergaard M, Glintborg B, Krogh NS, Foged H, Tarp U, Lorenzen T, Hansen A, Hansen MS, Jacobsen MS. Efficacy of abatacept and tocilizumab in patients with rheumatoid arthritis treated in clinical practice: results from the nationwide Danish DANBIO registry. Ann Rheum Dis. 2011;70:1216-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Wakabayashi H, Hasegawa M, Nishioka Y, Sudo A, Nishioka K. Which subgroup of rheumatoid arthritis patients benefits from switching to tocilizumab versus etanercept after previous infliximab failure? A retrospective study. Mod Rheumatol. 2012;22:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Gomez-Reino JJ, Maneiro JR, Ruiz J, Roselló R, Sanmarti R, Romero AB. Comparative effectiveness of switching to alternative tumour necrosis factor (TNF) antagonists versus switching to rituximab in patients with rheumatoid arthritis who failed previous TNF antagonists: the MIRAR Study. Ann Rheum Dis. 2012;71:1861-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Finckh A, Möller B, Dudler J, Walker UA, Kyburz D, Gabay C. Evolution of radiographic joint damage in rituximab-treated versus TNF-treated rheumatoid arthritis cases with inadequate response to TNF antagonists. Ann Rheum Dis. 2012;71:1680-1685. [PubMed] |
| 37. | Greenberg JD, Reed G, Decktor D, Harrold L, Furst D, Gibofsky A, Dehoratius R, Kishimoto M, Kremer JM. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Ann Rheum Dis. 2012;71:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 38. | Kekow J, Mueller-Ladner U, Schulze-Koops H. Rituximab is more effective than second anti-TNF therapy in rheumatoid arthritis patients and previous TNFα blocker failure. Biologics. 2012;6:191-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Schabert VF, Bruce B, Ferrufino CF, Globe DR, Harrison DJ, Lingala B, Fries JF. Disability outcomes and dose escalation with etanercept, adalimumab, and infliximab in rheumatoid arthritis patients: a US-based retrospective comparative effectiveness study. Curr Med Res Opin. 2012;28:569-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Chatzidionysiou K, van Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Emery P, Gottenberg JE, Rubbert-Roth A, Sarzi-Puttini P, Choquette D, Martínez Taboada VM, Barile-Fabris L, Moots RJ, Ostor A, Andrianakos A. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2014;Jan 29; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 42. | Flouri I, Markatseli TE, Voulgari PV, Boki KA, Papadopoulos I, Settas L, Zisopoulos D, Skopouli FN, Iliopoulos A, Bertsias GK. Comparative effectiveness and survival of infliximab, adalimumab, and etanercept for rheumatoid arthritis patients in the Hellenic Registry of Biologics: Low rates of remission and 5-year drug survival. Semin Arthritis Rheum. 2014;43:447-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Keystone E, Weber D, Xiong J, et al. Rituximab versus abatacept in rheumatoid arthritis patients with an inadequate response to prior biologic therapy: a retrospective, single-center study (EULAR Abstracts 2013; FRI0226). Available from: http://www.newevidence.com/rheumatology/entries/Rituximab_versus_abatacept_in_RA_patients_with/. |
| 44. | Martínez-Pérez R, Rodríguez-Montero S, Muñoz A, León M, Gallo F, Velloso ML, Marenco JL. Impact of two biological treatments in the functional capacity of a cohort of patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:A1-A94. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Gottenberg J, Ravaud P, Bardin T, Cacoub P, Cantagrel AG, Combe BG, Dougados M. Comparative Effectiveness Of Rituximab And Abatacept In 1192 Patients With Rheumatoid Arthritis Included In The French Society Of Rheumatology AIR And ORA Registries. Arthritis Rheum. 2011;63 Suppl 10:438 Available from: http://www.blackwellpublishing.com/acrmeeting/abstract.asp?MeetingID=781&id=95188. |
| 46. | Buch M, Vital EM, Dass S, Das S, Brayer D, Emery P. Switching to rituximab and an alternative TNF inhibitor in patients with rheumatoid arthritis that have failed previous TNF inhibitor(s) are both effective treatment options with good maintenance rates. Ann Rheum Dis. 2010;69 Suppl 3:379. |
| 47. | Greenwood MC, Donnelly SP, Rooney MM, Hakim AJ, Tahir H. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Rheumatology (Oxford). 2009;48 Suppl 1:i58-i71. |
| 48. | Gartlehner G, Hansen RA, Jonas BL, Thieda P, Lohr KN. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol. 2006;33:2398-2408. [PubMed] |
| 49. | Hochberg MC, Tracy JK, Hawkins-Holt M, Flores RH. Comparison of the efficacy of the tumour necrosis factor alpha blocking agents adalimumab, etanercept, and infliximab when added to methotrexate in patients with active rheumatoid arthritis. Ann Rheum Dis. 2003;62 Suppl 2:ii13-ii16. [PubMed] |
| 50. | Donahue KE, Gartlehner G, Jonas DE, Lux LJ, Thieda P, Jonas BL, Hansen RA, Morgan LC, Lohr KN. Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann Intern Med. 2008;148:124-134. [PubMed] |
| 51. | Lee YH, Woo JH, Rho YH, Choi SJ, Ji JD, Song GG. Meta-analysis of the combination of TNF inhibitors plus MTX compared to MTX monotherapy, and the adjusted indirect comparison of TNF inhibitors in patients suffering from active rheumatoid arthritis. Rheumatol Int. 2008;28:553-559. [PubMed] |
| 52. | Ariza-Ariza R, Navarro-Sarabia F, Hernández-Cruz B, Rodríguez-Arboleya L, Navarro-Compán V, Toyos J. Dose escalation of the anti-TNF-alpha agents in patients with rheumatoid arthritis. A systematic review. Rheumatology (Oxford). 2007;46:529-532. [PubMed] |
| 53. | Ollendorf DA, Klingman D, Hazard E, Ray S. Differences in annual medication costs and rates of dosage increase between tumor necrosis factor-antagonist therapies for rheumatoid arthritis in a managed care population. Clin Ther. 2009;31:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Harley CR, Frytak JR, Tandon N. Treatment compliance and dosage administration among rheumatoid arthritis patients receiving infliximab, etanercept, or methotrexate. Am J Manag Care. 2003;9:S136-S143. [PubMed] |
| 55. | Pascual-Salcedo D, Plasencia C, Ramiro S, Nuño L, Bonilla G, Nagore D, Ruiz Del Agua A, Martínez A, Aarden L, Martín-Mola E. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology (Oxford). 2011;50:1445-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 56. | Machado MA, Maciel AA, de Lemos LL, Costa JO, Kakehasi AM, Andrade EI, Cherchiglia ML, Acurcio Fde A, Sampaio-Barros PD. Adalimumab in rheumatoid arthritis treatment: a systematic review and meta-analysis of randomized clinical trials. Rev Bras Reumatol. 2013;53:419-430. [PubMed] |
| 57. | Maini SR. Infliximab treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 2004;30:329-347, vii. [PubMed] |
| 58. | van der Heijde D, Klareskog L, Singh A, Tornero J, Melo-Gomes J, Codreanu C, Pedersen R, Freundlich B, Fatenejad S. Patient reported outcomes in a trial of combination therapy with etanercept and methotrexate for rheumatoid arthritis: the TEMPO trial. Ann Rheum Dis. 2006;65:328-334. [PubMed] |
| 59. | Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, Martín Mola E, Pavelka K, Sany J, Settas L. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681. [PubMed] |
| 60. | Moots RJ, Naisbett-Groet B. The efficacy of biologic agents in patients with rheumatoid arthritis and an inadequate response to tumour necrosis factor inhibitors: a systematic review. Rheumatology (Oxford). 2012;51:2252-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Finckh A, Ciurea A, Brulhart L, Kyburz D, Möller B, Dehler S, Revaz S, Dudler J, Gabay C. B cell depletion may be more effective than switching to an alternative anti-tumor necrosis factor agent in rheumatoid arthritis patients with inadequate response to anti-tumor necrosis factor agents. Arthritis Rheum. 2007;56:1417-1423. [PubMed] |
| 62. | Hetland ML, Lindegaard HM, Hansen A, Pødenphant J, Unkerskov J, Ringsdal VS, Østergaard M, Tarp U. Do changes in prescription practice in patients with rheumatoid arthritis treated with biological agents affect treatment response and adherence to therapy? Results from the nationwide Danish DANBIO Registry. Ann Rheum Dis. 2008;67:1023-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Zink A, Strangfeld A, Schneider M, Herzer P, Hierse F, Stoyanova-Scholz M, Wassenberg S, Kapelle A, Listing J. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum. 2006;54:3399-3407. [PubMed] |
| 64. | Hjardem E, Hetland ML, Østergaard M, Krogh NS, Kvien TK. Prescription practice of biological drugs in rheumatoid arthritis during the first 3 years of post-marketing use in Denmark and Norway: criteria are becoming less stringent. Ann Rheum Dis. 2005;64:1220-1223. [PubMed] |
| 65. | Kievit W, Fransen J, Oerlemans AJ, Kuper HH, van der Laar MA, de Rooij DJ, De Gendt CM, Ronday KH, Jansen TL, van Oijen PC. The efficacy of anti-TNF in rheumatoid arthritis, a comparison between randomised controlled trials and clinical practice. Ann Rheum Dis. 2007;66:1473-1478. [PubMed] |