Published online Mar 12, 2015. doi: 10.5499/wjr.v5.i1.23
Peer-review started: June 25, 2014
First decision: August 28, 2014
Revised: September 19, 2014
Accepted: October 28, 2014
Article in press: October 29, 2014
Published online: March 12, 2015
Processing time: 267 Days and 0.7 Hours
Rheumatic diseases, characterized by chronic inflammation and damage to various organs and systems, include systemic lupus erythematosus, rheumatoid arthritis, ankylosing spondylitis and other connective tissue diseases. Bone is a target in many inflammatory rheumatic diseases. In recent years, the survival of patients with rheumatic diseases has increased markedly and the relationship between rheumatic diseases and osteoporosis (OP) has become more prominent. OP and related fragility fractures increase the morbidity and mortality of rheumatic disease. The cause of OP in rheumatic diseases is complex. The pathogenesis of OP in rheumatic diseases is multifactorial, including disease and treatment-related factors. Osteoimmunology, a crosstalk between inflammatory and bone cells, provides some insight into the pathogenesis of bone loss in systematic inflammatory diseases. The aim of this article is to review different risk factors in rheumatic diseases. Several factors play a role, such as chronic inflammation, immunological factors, traditional factors, metabolism and drug factors. Chronic inflammation is the most important risk factor and drug treatment is complex in patients with OP and rheumatic disease. Attention should be paid to bone loss in rheumatic disease. Optimal treatment of the underlying rheumatic disease is the first step towards prevention of OP and fractures. Apart from that, a healthy lifestyle is important as well as calcium and vitamin D supplementation. Bisphosphonates or denosumab might be necessary for patients with a low T score.
Core tip: Osteoporosis (OP) and related fractures are one of important complications for patients with rheumatic diseases. The pathogenesis of OP in rheumatic diseases is multifactorial, including disease and treatment-related factors. Chronic inflammation is the most important risk factor and drug treatment is complex in patients with OP and rheumatic disease. Controlling rheumatic disease effectively is an important way to prevent OP.
- Citation: Gao LX, Jin HT, Xue XM, Wang J, Liu DG. Osteoporosis in rheumatic diseases. World J Rheumatol 2015; 5(1): 23-35
- URL: https://www.wjgnet.com/2220-3214/full/v5/i1/23.htm
- DOI: https://dx.doi.org/10.5499/wjr.v5.i1.23
Rheumatic diseases include rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis (SSc), ankylosing spondylitis (AS) and other connective tissue diseases. The characteristics of rheumatic disease are chronic inflammation and damage to various organs and systems. Rheumatic diseases can affect bone, muscle, periarticular attachment and soft tissue. Osteoporosis (OP) is a systemic bone disease characterized by low bone mass and disruption of bone microstructure, increasing skeletal fragility and resulting in fractures occurring easily. Bone mineral density (BMD) is commonly detected by dual-energy X-ray absorptiometry (DEXA). OP is defined by a T score of -2.5 or lower, that is, > 2.5 SD below the average density of a young normal adult.
The survival of patients with rheumatic diseases has increased dramatically during the past few decades. Patients with rheumatic diseases have an increased prevalence of long-term complications, such as cardiovascular diseases and OP[1,2]. Bone is always involved in many inflammatory rheumatic diseases. OP and related fractures are one of the most important complications for patients with rheumatic diseases. Osteoporotic fractures and osteonecrosis increase the morbidity and mortality of rheumatic diseases[3]. The pathogenesis of OP in rheumatic diseases is multifactorial and includes disease and treatment-related factors. Rheumatic diseases could result in bone loss through several mechanisms: inflammation, traditional risk factors and drug-induced factors[4]. OP significantly decreases the quality of life of the person with rheumatic disease but the clinical manifestations of OP are not typical. Glucocorticoid-induced osteoporosis (GIOP) accounts for 5.0%-61.9% of adult rheumatic disease[5]. Although OP has a high rate of prevalence among rheumatic disease patients, most patients do not receive adequate diagnostic evaluation and drug therapy. This article focuses on the relationship between rheumatic diseases and OP.
A systematic search of the literature (from January 1, 1990 to August 31, 2014) was performed using the PubMed and Cochrane databases. We also searched for previously published systematic literature reviews.
The following keywords were used for the search: “rheumatic disease” and “bone mineral mass” or “osteoporosis”; “rheumatoid arthritis” and “bone mineral mass” or “osteoporosis”; “systemic lupus erythematosus” and “bone mineral mass” or ”osteoporosis”; “ankylosing spondylitis” and “bone mineral mass” or ”osteoporosis”; ”systemic sclerosis“ and “bone mineral mass” or ”osteoporosis”.
The literature search was performed independently by two of the authors and a consensus reached. The inclusion criteria for papers were as follows: (1) studies in English; (2) full text of articles available; (3) human patients with rheumatic disease; (4) randomized controlled clinical trials; and (5) diagnostic criteria of different rheumatic diseases met respective international diagnostic criteria. The exclusion criteria were as follows: (1) case reports; (2) reader comments; (3) duplicate publications; (4) literature without original data; and (5) studies with < 20 patients.
SLE is characterized by a variety of clinical manifestations, a spectrum of autoantibodies and a multisystem involvement. There are debates about OP in SLE. The main controversies are about the prevalence of OP and the secondary debate is the dependence of glucocorticoids (GCs). However, all studies have demonstrated that bone loss is more common in patients with SLE than in the healthy human. Several cross-sectional studies have evaluated BMD and the prevalence of OP in SLE patients. There was a difference in the prevalence of OP in these studies but the results suggest a generalized reduction in BMD[6]. The reported prevalence of osteopenia is 25%-74%, while that of OP is 1.4%-68%[7]. SLE influences mainly reproductive females and is affected by the change of sex hormones. For women, osteopenia was found in 40% of patients, while OP was found in only 5%. Low body mass index (BMI), long-term disease damage and corticosteroid treatment were risk factors for low BMD in premenopausal SLE patients. Lumbar and femoral BMD of premenopausal patients with SLE was decreased and related to disease damage and long-term corticosteroid therapy[8-13]. For postmenopausal SLE patients with long-term GC treatment, OP is always a common and terrible problem. The prevalence of lumbar spine OP is as high as 48%[14,15]. Recently, a cross-sectional study investigated BMD in 67 women with SLE in a Mediterranean region and reported that the prevalence of osteopenia was 28%-46% and OP was 3%-9%[16]. For men, although a few studies of OP in SLE have been reported, they have come to different conclusions. Two studies showed that bone mass in men with SLE was not decreased despite corticosteroid therapy[17,18]. Another study reported different results, that low BMD and low body mass were prevalent for males with SLE. When SLE patients were compared with healthy controls, the Z scores of BMD at the femoral neck and spine were significantly lower in SLE[19]. A recent cross-sectional and longitudinal study indicated that juvenile SLE patients had low bone mass and a decreased peak bone mass and juvenile-onset SLE had a high risk of OP in early adulthood[20]. Another longitudinal study of OP in juvenile SLE indicated that BMD had a significant inverse correlation with the cumulative dose of corticosteroids[21].
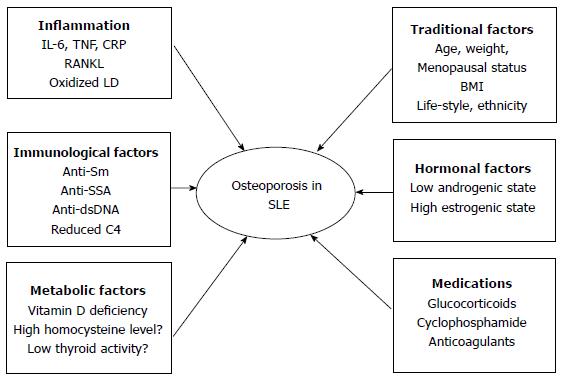
The reason for OP in SLE is considered to be multifactorial and includes inflammation, immune-mediated mechanisms, traditional OP risk factors, metabolic factors, serological factors and drug-induced adverse effects (Figure 1)[7].
The inflammation associated with active disease contributes to the development of OP in SLE. Recent literature has affirmed an association between low BMD and the inflammatory feature of SLE. Several inflammation markers, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1 and IL-6, can induce osteoclastogenesis which promotes the proliferation of precursor osteoclastic cells or activation of differentiated osteoclasts[22]. Different studies have provided different views of the pathogenesis of inflammatory bone loss but now it is considered that the key osteoclastogenic cytokine, receptor activator of nuclear factor-ĸB ligand (RANKL), plays an important role in the balance of osteoclasts and osteoblasts. Osteoprotegerin (OPG) is the physiological decoy receptor that moderates the biological activity of RANKL[23]. SLE is a systemic autoimmune inflammatory disorder with increasing serum TNF-α, IL-1 and IL-6. These cytokines can increase and induce RANKL expression[23,24]. The serum level of oxidized low-density lipoprotein (LDL) is increased in SLE[25]. Oxidized lipids can induce increased production of RANKL and TNF by activating T cells. Both RANKL and TNF increase the activity and maturation of osteoclasts[26]. A 5 year follow up study demonstrated that SLE patients had significant BMD loss in the femoral neck and hip. Disease activity and new organ damage could result in bone loss and new organ involvement was an independent predictor of bone loss at the femoral neck[27].
Immune-mediated mechanisms are associated with OP of SLE and SLE per se contributes to the deterioration in bone density, cortical microstructure and bone strength. SLE patients without GC treatment have a significantly lower real BMD at the femoral neck and hip and diminished radial total volumetric BMD and cortical area and thickness when compared with controls[28]. SLE is marked by both humoral and cellular abnormalities, including multiple autoantibodies that may participate in the disease. The absence of anti-SSA and presence of anti-Sm were associated with higher BMD in the lumbar spine. The patients with positive anti-SSA were generally advised to avoid sun exposure, which may explain the relationship between the absence of anti-SSA and lower bone loss[14]. Higher serum anti-double-stranded DNA level was an independent predictor of a higher 10 year probability of hip fracture and this reinforced the concept that the inflammatory state as reflected by high SLE disease activity might be an important driver for bone loss[29]. Although clinical studies could not make a conclusion about an association between disease activity score and low BMD, low C4 levels could predict low spine BMD in SLE[30]. The relationship between organ damage and bone loss was reported by several studies, organ damage resulted in bone loss at both the femoral and the lumbar level, and the relationship between cumulative disease damage and reduced BMD is independent of corticosteroid use[31,32].
Metabolic factors are also risk factors for OP. Vitamin D deficiency, hyperhomocysteinemia and low thyroid activities are metabolic conditions that can induce bone loss in SLE. Vitamin D is a secosteroid hormone that regulates calcium homeostasis, bone mineralization and remodeling, as well as neuromuscular function. Many studies in the past decade have reported increased frequency of vitamin D deficiency among patients with SLE[33-37]. The prevalence of vitamin D insufficiency in SLE patients ranged from 16% to 96% and the prevalence of vitamin D deficiency ranged from 4% to 54%[38]. A number of factors contributed, such as avoidance of sunshine as a result of photosensitivity, dark skin pigment, sun screen precautions, disease activity, renal failure, use of drugs, such as GCs, antimalarial and antiepileptic agents, and anti-vitamin D antibodies. Homocysteine (Hcy) modulates bone remodeling via several mechanisms, such as increased osteoclast activity, decreased osteoblast activity and direct action on the bone matrix[39]. SLE patients have an increased level of plasma Hcy[40,41] but no studies demonstrated an association between hyperhomocysteinemia and OP in SLE[42].
The traditional factors, including age, low body weight and postmenopausal status, are all independent risk factors for OP in SLE. It is unclear if sex and ethnicity have an effect on bone loss in SLE; African-American women have lower hip and lumbar spine BMD compared with white women with SLE[43]. The prevalence of OP in Chinese SLE patients with corticosteroids is 4%-6%, less than that reported in Caucasians (12%-18%)[11]. Two studies have shown that white and non-African Caribbean races were a risk factor for OP in SLE patients[44,45]. Daily dietary calcium intake did not correlate with BMD in premenopausal women with SLE[46]. Smoking and alcohol have not been reported as risk factors for OP in lupus[13,47] but alcohol use was associated with low BMD in Hong Kong men with lupus[19].
Hormonal factors, for example, include the significant positive relationship between serum dehydroepiandrosterone sulfate and low BMD[48].
The last factor is drug-induced adverse effects in SLE therapy. GCs are widely used for the therapy of SLE exacerbations and complications. GCs are a double-edged sword with respect to bone loss, are associated with the development of OP and fracture and can trigger significant bone loss. At the same time, they have good effects by controlling disease activity and systemic inflammation in bone[7]. A dose-dependent relationship has been demonstrated between GC use and spinal bone loss in SLE. Significant bone loss was observed in the lumbar spine for SLE patients with a mean prednisolone dose of > 7.5 mg/d, but this phenomenon was not found in the hip[12]. Hydroxychloroquine (HCQ) may act by inhibiting the change of 25-hydroxyvitamin D into 1,25-dihydroxyvitamin D. An earlier study found that patients with SLE treated with HCQ had lower 1,25-dihydroxyvitamin D levels, although there were no differences in circulating 25-hydroxyvitamin D levels between treated and untreated patients[34]. In contrast, some studies have shown that HCQ is a protecting factor for OP[49]. It has been demonstrated that the treatment of HCQ is related to higher levels of 25-hydroxyvitamin D, which was probably a spurious effect of the drug at the expense of reducing the metabolically active form 1,25-dihydroxyvitamin D[50]. Calcineurin inhibitors, such as cyclosporine A and tacrolimus, have been increasingly used in patients with SLE. The use of the calcineurin inhibitors may potentially lead to a vitamin D resistant state, leading to impairment of the normal physiological effects of vitamin D[51,52]. Other drugs used for SLE that play a role in bone loss are methotrexate (MTX), cyclophosphamide, anticonvulsants, oral anticoagulants and heparin[53].
RA is a chronic inflammatory disorder in which an erosive, symmetric joint disorder maintains the center stage accompanied by a variable, but at times prominent, degree of extra-articular involvement. The inflammatory synovitis and damage of cartilage and bone is characteristic of RA patients. Bone involvement includes three types: periarticular osteopenia, bone erosion and systemic OP. There are two types of OP in RA: localized, occurring near to the site of inflamed joints, or generalized, involving the systemic bone. Local or periarticular bone loss is the typical radiographic sign in early RA. Systemic OP is prevalent in RA. So far, the use of biological therapy has not decreased the prevalence of OP in RA. RA patients have a lower bone mass in the appendicular and axial skeleton when compared with healthy controls, according to the conclusion of 10 cross-sectional studies[54]. There was a twofold increase in RA in women aged 20-70 years. The prevalence of OP was 16.8% in the lumbar spine and 14.7% in the femur. It reached 31.5% in the lumbar spine and 28.6% in the femur for women aged 60-70 years[55]. A multicenter cross-sectional study of RA and BMD indicated that the frequency of OP as assessed by DEXA was 28.8% at the lumbar spine and 36.2% at the femoral neck[56]. A longitudinal study indicated that BMD loss was lower in men[57]. A large study with 94 male RA patients concluded that a modest reduction in BMD was found only in patients aged 60-70 years. The percentage of BMD reduction in the femoral neck was 5.2% and the reduction in the hip was 6.9%, with no change in the spine. Despite a moderately low BMD, this report showed that the ratio of reduced BMD in men with RA was nearly twofold higher than in the control group[58].
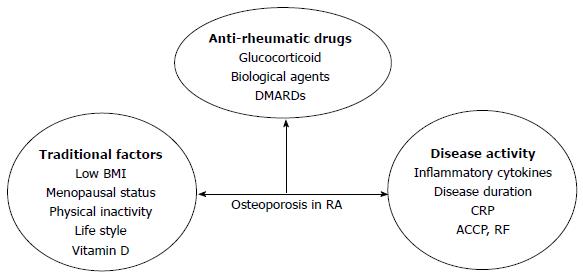
The reason for OP in RA patients is also multifactorial: factors related to the disease itself, antirheumatic drug use and traditional factors, such as low BMI, menopausal status, age and lack of physical exercise (Figure 2).
Local periarticular OP really reflects disease activity in early RA because the acute phase reactants are closely related to this phenomenon, but once periarticular OP appears, it is no longer a sign of disease activity[59]. Generalized OP is a feature of established RA. Some literatures have shown an association between OP and proinflammatory cytokines, such as TNF-α, IL-1 and IL-6[60], and these cytokines were independent risk factors of disease activity. IL-1 and IL-6, secreted by activated macrophages, synovial fibroblasts and T cells, result in synovial inflammation, bone damage and systemic manifestations of RA[61,62]. Inflammation has an uncoupling effect on bone resorption and formation. In patients with active compared to inactive RA, bone resorption was increased, whereas bone formation was decreased. These cytokines are closely associated with osteoclast physiology as they extend survival and improve the activity of mature osteoclasts, mainly through RANKL-mediated and Wnt-signaling pathways[63]. Anti-inflammatory treatment, especially with biological agents, in early RA reduces the rate of bone loss[64]. However, there are some debates about the relationship between inflammation and bone loss in RA and recent data show that bone loss starts before inflammation and clinical disease[65].
Osteoimmunology has attracted increased research attention and RA is also an autoimmune disease. There are many autoantibodies in RA, such as those against citrullinated proteins antibody (ACPA) and rheumatoid factor (RF). There are many data supporting the role of autoimmunity in bone destruction in RA. In RA, ACPA is an important prognostic factor. ACPA has a direct and independent stimulating effect on osteoclasts and induces elevated bone resorption[66]. Bone loss occurs in RA patients displaying ACPA without signs of inflammation[67]. ACPA-positive patients generally have not only higher disease activity and disability, but also more radiological damage[67,68]. Healthy individuals with ACPA have low BMD compared with controls without ACPA and the thickness of cortical bone is significantly lower in healthy individuals positive for ACPA[67]. The frequency of OP and lower BMD is higher in RF-positive patients[55,69] and patients with high C-reactive protein (CRP) levels (> 20 mg/dL) are more likely to have a low BMD in the spine and hips[57]. Immobility related to joint pain or damage aggravates bone loss[70]. Disease-related disability, assessed by Health Assessment Questionnaire (HAQ) score, has nothing to do with BMD in the lumbar spine but is inversely related to BMD in the femoral neck and whole body[71,72]. Disease activity and duration are also risk factors in RA and disease activity is the only reason for BMD loss in the lumbar spine. When active RA lasting more than 2 years is compared to inactive RA, mean bone loss in the former is higher.
The use of antirheumatic drugs plays an important role in OP in RA. There are some debates about the role of GCs in RA: on the one hand, low-dose corticosteroid therapy is associated with increased bone loss and fracture risk, but on the other hand, it effectively controls systemic inflammation. GIOP is the most common form of secondary OP. GCs can affect bone by several direct and indirect ways and affect both bone formation and resorption. Although high doses of GCs are related to bone loss, it is well known that GCs have a strong anti-inflammatory effect and low-dose GCs reduce localized bone loss in the hands. In RA patients with low doses of GCs or with rapidly tapered high-to-moderate dose induction therapy, the direct adverse effect of GCs on bone is counteracted by strong suppression of inflammation by GCs[73-75]. The accumulative dose of steroids is more important for OP and there is no threshold dose. Disease-modifying antirheumatic drugs (DMARDs) have anti-inflammatory and structure-modifying properties, leading to better disease control in RA. Traditional DMARDs include MTX, leflunomide (LEF), sulfasalazine (SSZ), HCQ and gold agents. MTX is considered to be the cornerstone of RA treatment and is the most widely used agent. Osteopathy is reported in patients with malignant diseases treated with high-dose MTX; mostly reported in children with long-term maintenance therapy of MTX for acute leukemia[76]. For postmenopausal women, MTX may be associated with OP because bone biopsy samples are consistent with osteoblast inhibition as a consequence of MTX action on the bone cells in RA patients when given at low doses for prolonged periods[77]. Recently, more and more studies have shown no association between low-dose MTX and bone loss and multivariate covariance analysis has shown that reduced BMD is due to disease severity and activity and not to a direct negative effect of MTX on bone[78-80]. The use of low-dose MTX was not associated with any change in BMD in patients without corticosteroid treatment[81]. MTX seems to have some direct effects on bone metabolism and its anti-inflammatory effects reduce the negative effect of RA on bone. LEF is an isoxazole derivative that inhibits the mitochondrial enzyme dihydroorotate dehydrogenase and prevents bone loss by its active metabolite that can inhibit osteoclastogenesis and osteoclast function[82]. LEF can slow radiographic progression, both in terms of erosion and joint space narrowing. In vitro, SSZ inhibited osteoclastogenesis by acting on osteoclast precursor cells and regulating the RANKL-RANK-OPG interaction, primarily by reducing expression of RANKL on synovial fibroblasts and increasing expression of OPG[83]. No studies have investigated whether LEF and SSZ have a sparing effect on BMD or bone strength in RA. Biological DMARDs dramatically improve inflammatory arthritis treatment and prognosis. Biological agents include TNF-α blockers (infliximab, adalimumab, etanercept, certolizumab and golimumab), agents counteracting B cell activity (rituximab) and T cell activation (abatacept), and anti-IL-6 agents (tocilizumab). All TNF-α blockers reduce the progression and formation of joint erosion and joint space narrowing. Infliximab[84,85], adalimumab[86-88], etanercept[89-91] and rituximab[92] can counteract local bone erosion and generalized bone loss. Tocilizumab has a positive, corrective effect on bone balance. It induces a significant decrease in bone resorption, rebalances bone turnover and increases the BMD of RA patients who have osteopenia at baseline[93,94]. Fundamental studies have elucidated that inflammatory cytokines induce osteoclastogenesis through upregulation of RANKL, with subsequent activation of osteoclastogenesis which plays key role in bone loss in RA. Biological agents improve bone formation and reduce bone resorption by controlling active disease and inflammatory cytokine production[95].
Traditional factors result in osteoporosis in RA. Both postmenopausal women and men with RA have a prevalence of OP. The percentage of OP in postmenopausal women is 55.7% and 50.5% in men, with the prevalence of OP higher than in premenopausal women (18%). OP risk factors are strongly dependent on gender and menopausal state[96]. Female sex, increasing age, years since menopause, low weight, familial OP and low BMI are risk factors for osteopenia in patients with RA[57,97-99].
Spondyloarthritis (SpA) comprises a group of inflammatory diseases, such as psoriatic arthritis (PsA), reactive arthritis (Reiter’s syndrome), enteropathic arthritis, undifferentiated spondyloarthropathy and AS. These diseases have some common characteristics, including imaging features, clinical manifestation and laboratory findings. Both sacroiliac joints and spine can be involved and to different degrees, peripheral joints.
AS is the prototype of SpA and the most frequent subtype. AS mainly involves the axial joints, especially the sacroiliac joints. The spine, peripheral joints and enthuses can be affected to various degrees. Extraosseous new bone formation is considered a hallmark of AS. Although bone formation may affect the detection of BMD, OP always occurs in the early period of AS. The reported prevalence of OP varies from 19% to 62% in AS maybe because new bone formation and age distribution of the study cohorts make detecting BMD difficult[100]. It was reported that the prevalence of OP in patients with early AS within 10 years after diagnosis was unexpected; 13% in the femoral neck and 16% in the lumbar spine[101]. AS mainly affects young men and men with AS have had an annual total bone mass loss of 2.2% in longitudinal studies[102]. Male patients with AS have decreased BMD in their lumbar spine and femoral neck, and femoral neck BMD in male AS patients is 10% lower than in age-matched male controls[103,104].
In women, one study found a slight reduction in BMD in premenopausal women with early AS, but the difference was not significant[105]. Another study to assess BMD of the hip and spine by DEXA and calcaneal quantitative ultrasound in women with AS showed that women with AS had reduced hip BMD and significantly fewer markers of bone formation than controls[106]. In summary, OP is a significant complication in AS and significant OP can occur even in early disease. The spine is more likely to be damaged than the femur, with the spine still the most important site to diagnose OP in AS.
There are several reasons for OP in AS, such as proinflammatory cytokines, acute phase reactants, immobility, vitamin D, sex hormones, age and disease duration.
The systemic inflammatory cytokines are the core of OP during AS. Maybe inflammation of the entheses and synovium increase secretion of proinflammatory cytokines. Cytokines are a link between local and systemic inflammation on the one hand and result in bone resorption and BMD reduction on the other hand. IL-6, IL-1 and TNF-α are well-known osteoclast activators and play an important role in inflammation in AS. The RANK-RANKL system and its natural inhibitor OPG may be the key in bone-cytokine interrelationships. There is a strong correlation between bone turnover, proinflammatory cytokines and acute-phase reactants, for example erythrocyte sedimentation rate (ESR)[107]. Low BMD is related to high biochemical markers of bone resorption, inflammatory activity and low OPG serum levels in AS patients[108]. Longer disease duration, Bath AS Functional Index (BASFI) and Bath AS Disease Activity Index are also factors associated with OP[100]. Low hip BMD was related to low BMI and high BASFI and Bath AS Radiology Index-total (BASRI-t) score and low lateral spinal BMD was associated with BASRI-t score[109]. In addition, high disease burden, immobility and syndesmophyte formation increased the risk of OP[110].
Genetic factors and FokI genotypes of the vitamin D receptor gene were significantly associated with the spine as independent predictors of low BMD, which was also affected by BMI, inflammatory level and degree of pain. CRP and ESR values were also closely related to FokI genotypes in male AS patients[111].
Metabolic factors are also risk factors for OP in AS. Bone loss is correlated with low serum sex steroid hormone levels in AS[107,108,112]. Bone loss in AS is associated with endocrine mechanisms such as parathyroid hormone, impaired calcium and vitamin D absorption[113].
In AS, traditional risk factors including a positive family history, older age, low BMI, Caucasian race, postmenopausal status in women or low androgen levels in men, low dietary calcium intake and vitamin D deficiency are risk factors for OP[107].
Therapy for AS mainly involves nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, biological agents including infliximab, etanercept, adalimumab and anakinra, and conventional DMARDs, including MTX, SSZ and LEF. Some NSAIDs inhibit prostaglandin synthesis which has anabolic effects on bone and is thought to be related to higher BMD in men and women[16,114]. However, 16 patients continued NSAIDs during the 24 mo follow-up period and data were inconclusive on the effect of NSAID use on BMD[115]. Studies have shown that TNF-α blockers prevent systemic inflammation-induced bone loss in AS or SpA[116-119]. In a larger cohort of 106 patients with SpA receiving infliximab or etanercept, patients had great improvement in the spine and hip BMD scores; the mean BMD scores in the lumbar spine reached 5.8% and increased by 2.3% in the total femur after 2 years follow-up[120]. A recent study included seven longitudinal studies and one randomized control trial and studied the effect of TNF inhibitors on BMD in 568 AS patients with a minimum follow-up period of 1 year. They found that lumbar spine BMD increased by 5.1% and total hip BMD increased by 1.8% after 1 year of treatment with TNF inhibitors and lumbar spine BMD increased by 8.6% and total hip BMD increased by 2.5% after 2 years. So, they concluded that TNF inhibitors maintain femoral neck BMD homeostasis and increase BMD in the lumbar spine and hip for up to 2 years[121]. Corticosteroids are used less often in AS so there have been few studies about GCs and OP in AS. We have not searched the literature about change of BMD in AS after using DMARDs treatment until now. Drug-related factors play an important role in OP for AS.
There are only a few studies about bone loss in other forms of SpA such as PsA in OP. The involvement of bone in PsA affects not only mechanisms of bone loss but also bone formation. Periarticular bone loss and general bone loss are present. PsA patients were found to have periarticular bone loss in early disease but overall BMD values are higher than in RA patients[122]. There are conflicting data about bone loss and bone turnover markers in patients with PsA, with some studies showing evidence of association with low BMD and some not, especially systemic OP[123]. Some clinical studies conclude that BMD in patients with PsA has no significant decrease[124-126], but bone biopsies suggest a latent high osteopathy[127]. Recently, some literatures have indicated that the prevalence of OP increased in PsA patients, especially those with longer disease duration and disability[128,129]. When PsA patients were compared with age-matched controls, BMD in the femoral neck and lumbar spine was found to be reduced[130]. Bone demineralization occurs in 11% of young women, 47% of postmenopausal women and 29% men with non-axial PsA. OP is related to HAQ score, reflecting articular function[131].
SSc, dermatomyositis (DM)/polymyositis (PM) and Behcet’s disease (BD) are also rheumatic diseases associated with OP.
SSc is characterized by skin thickening and fibrosis. SSc can be classified into two subsets: diffuse and limited cutaneous SSc. Before 2004, a review concluded that it was unknown if patients with scleroderma have an increased prevalence of OP[132]. Recently, another review analyzed 19 studies about BMD in SSc. Fifteen studies found that the prevalence of BMD was 27%-53.3% and that of OP was 3%-51.1% in SSc patients compared to controls. Ten studies reported a lower BMD in SSc patients and two studies suggested no difference. It was concluded that SSc patients had a risk of low BMD and fracture. The cause of OP was complex, involving traditional factors, SSc-specific risk factors and drug-related factors[133]. After 2012, a Chinese study indicated that the whole body BMD of SSc patients was much lower than controls and there was no association between BMD and the severity of involvement of the skin and other systems, while advanced age, sex, menopause and low BMI were independently correlated with bone loss in the spine or hip in SSc patients[134]. An Italian study reported that the BMD of SSc patients was significantly lower than controls in the lumbar spine, femoral neck and total femur and serum 25 hydroxyvitamin D3 was significantly lower. In scleroderma patients, serum levels of 25 hydroxyvitamin D3 were greatly associated with parathyroid hormone levels, BMD, stiffness index and bone turnover markers[135]. A study about Spanish SSc patients showed that the prevalence of OP/osteopenia was high, reaching 77% in SSc patients, but there was no relationship between vitamin D and low BMD[136]. A cross-sectional study suggested that the prevalence of OP was 30% and fractures was 35% in SSc patients, they were higher than healthy controls (11% and 10%) and the degree was very similar to RA (32% and 33%). Age and vitamin D deficiency were thought to be risk factors for fracture in SSc[137]. So far, SSc patients have a high risk of OP but the risk factors need further study.
DM/PM are uncommon idiopathic and autoimmune myopathies with characteristic clinical symptoms of proximal symmetric muscle weakness, rashes and fatigue. OP/fracture is found in about one quarter of adult DM/PM patients. This bone alteration was correlated with lower BMI[138]. Most studies support decreased bone density in juvenile DM patients[139,140]. Low lean body mass and GC pulse treatment were the important factors for low hip BMD in juvenile DM patients[141] and the RANKL/OPG ratio is elevated in children with juvenile DM[142].
BD is a multisystem vasculitis. BD may be a risk factor for OP because the BMD in the lumbar spine is lower than in healthy controls. The serum levels of cytokines such as IL-1, IL-6, IL-2 and TNF-α are increased in BD and there is a negative correlation between IL-1 levels and femur neck BMD[143]. On the contrary, two studies showed no significant BMD reduction in the lumbar spine and hip of BD patients[144,145].
In summary, inflammatory rheumatic diseases are always accompanied by elevated bone loss and increased fracture rates. Attention should be paid to bone loss in rheumatic disease. OP in rheumatic disease is complex. Several factors take part in this process, such as the disease itself and traditional, metabolic and drug-related factors. Osteoimmunology, a crosstalk between inflammatory and skeletal component cells, has given some perceptions to the pathogenic mechanism of OP in systemic inflammatory diseases. Chronic inflammation plays a key role in OP for rheumatic disease and inflammatory cytokines regulate the homeostasis between bone formation and resorption. Fundamental studies have demonstrated that the RANKL-OPG system plays a major role in bone loss and inflammatory cytokines upregulate RANKL, which further activates osteoclastogenesis, resulting in OP. Clinical studies have shown that effective immunosuppressive therapy prevents bone loss. Thus, the first step to prevent OP and fractures is to control primary rheumatic disease activity. Apart from that, a healthy lifestyle is important with calcium and vitamin D supplementation and prevention of falls. Bisphosphonates or denosumab might be necessary for patients with a low T score.
P- Reviewer: Korovessis P, Luo XH S- Editor: Tian YL L- Editor: Roemmele A E- Editor: Wu HL
| 1. | Dougados M, Soubrier M, Antunez A, Balint P, Balsa A, Buch MH, Casado G, Detert J, El-Zorkany B, Emery P. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. 2014;73:62-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 578] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 2. | Sineglazova AV. Coronary atherosclerosis and osteoporosis in rheumatoid arthritis. Vestn Rentgenol Radiol. 2013;25-28. [PubMed] |
| 3. | Gladman DD, Chaudhry-Ahluwalia V, Ibañez D, Bogoch E, Urowitz MB. Outcomes of symptomatic osteonecrosis in 95 patients with systemic lupus erythematosus. J Rheumatol. 2001;28:2226-2229. [PubMed] |
| 4. | Bultink IE, Vis M, van der Horst-Bruinsma IE, Lems WF. Inflammatory rheumatic disorders and bone. Curr Rheumatol Rep. 2012;14:224-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Pereira RM, Carvalho JF, Canalis E. Glucocorticoid-induced osteoporosis in rheumatic diseases. Clinics (Sao Paulo). 2010;65:1197-1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | García-Carrasco M, Mendoza-Pinto C, Escárcega RO, Jiménez-Hernández M, Etchegaray Morales I, Munguía Realpozo P, Rebollo-Vázquez J, Soto-Vega E, Delezé M, Cervera R. Osteoporosis in patients with systemic lupus erythematosus. Isr Med Assoc J. 2009;11:486-491. [PubMed] |
| 7. | Bultink IE. Osteoporosis and fractures in systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2012;64:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Pons F, Peris P, Guañabens N, Font J, Huguet M, Espinosa G, Ingelmo M, Muñoz-Gomez J, Setoain J. The effect of systemic lupus erythematosus and long-term steroid therapy on bone mass in pre-menopausal women. Br J Rheumatol. 1995;34:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Kipen Y, Buchbinder R, Forbes A, Strauss B, Littlejohn G, Morand E. Prevalence of reduced bone mineral density in systemic lupus erythematosus and the role of steroids. J Rheumatol. 1997;24:1922-1929. [PubMed] |
| 10. | Formiga F, Moga I, Nolla JM, Pac M, Mitjavila F, Roig-Escofet D. Loss of bone mineral density in premenopausal women with systemic lupus erythematosus. Ann Rheum Dis. 1995;54:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Li EK, Tam LS, Young RP, Ko GT, Li M, Lau EM. Loss of bone mineral density in Chinese pre-menopausal women with systemic lupus erythematosus treated with corticosteroids. Br J Rheumatol. 1998;37:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Jardinet D, Lefèbvre C, Depresseux G, Lambert M, Devogelaer JP, Houssiau FA. Longitudinal analysis of bone mineral density in pre-menopausal female systemic lupus erythematosus patients: deleterious role of glucocorticoid therapy at the lumbar spine. Rheumatology (Oxford). 2000;39:389-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Mendoza-Pinto C, García-Carrasco M, Sandoval-Cruz H, Escárcega RO, Jiménez-Hernández M, Etchegaray-Morales I, Soto-Vega E, Muñoz-Guarneros M, López-Colombo A, Delezé-Hinojosa M. Risks factors for low bone mineral density in pre-menopausal Mexican women with systemic lupus erythematosus. Clin Rheumatol. 2009;28:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Mok CC, Mak A, Ma KM. Bone mineral density in postmenopausal Chinese patients with systemic lupus erythematosus. Lupus. 2005;14:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Bhattoa HP, Bettembuk P, Balogh A, Szegedi G, Kiss E. Bone mineral density in women with systemic lupus erythematosus. Clin Rheumatol. 2002;21:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Salman-Monte TC, Torrente-Segarra V, Muñoz-Ortego J, Mojal S, Carbonell-Abelló J. Prevalence and predictors of low bone density and fragility fractures in women with systemic lupus erythematosus in a Mediterranean region. Rheumatol Int. 2015;35:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Formiga F, Nolla JM, Mitjavila F, Bonnin R, Navarro MA, Moga I. Bone mineral density and hormonal status in men with systemic lupus erythematosus. Lupus. 1996;5:623-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Bhattoa HP, Kiss E, Bettembuk P, Balogh A. Bone mineral density, biochemical markers of bone turnover, and hormonal status in men with systemic lupus erythematosus. Rheumatol Int. 2001;21:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Mok CC, Ying SK, To CH, Ma KM. Bone mineral density and body composition in men with systemic lupus erythematosus: a case control study. Bone. 2008;43:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Stagi S, Cavalli L, Bertini F, Matucci Cerinic M, Luisa Brandi M, Falcini F. Cross-sectional and longitudinal evaluation of bone mass and quality in children and young adults with juvenile onset systemic lupus erythematosus (JSLE): role of bone mass determinants analyzed by DXA, PQCT and QUS. Lupus. 2014;23:57-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Trapani S, Civinini R, Ermini M, Paci E, Falcini F. Osteoporosis in juvenile systemic lupus erythematosus: a longitudinal study on the effect of steroids on bone mineral density. Rheumatol Int. 1998;18:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1138] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 23. | Weitzmann MN. The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Scientifica (Cairo). 2013;2013:125705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Robak E, Sysa-Jedrzejewska A, Dziankowska B, Torzecka D, Chojnowski K, Robak T. Association of interferon gamma, tumor necrosis factor alpha and interleukin 6 serum levels with systemic lupus erythematosus activity. Arch Immunol Ther Exp (Warsz). 1998;46:375-380. [PubMed] |
| 25. | Frostegård J, Svenungsson E, Wu R, Gunnarsson I, Lundberg IE, Klareskog L, Hörkkö S, Witztum JL. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum. 2005;52:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Svenungsson E, Fei GZ, Jensen-Urstad K, de Faire U, Hamsten A, Frostegard J. TNF-alpha: a link between hypertriglyceridaemia and inflammation in SLE patients with cardiovascular disease. Lupus. 2003;12:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Qian BP, Qiu Y, Wang B, Zhu ZZ, Wang WJ, Ma WW. Unusual association of ankylosing spondylitis with congenital spinal deformity. Spine (Phila Pa 1976). 2010;35:E1512-E1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Tang XL, Griffith JF, Qin L, Hung VW, Kwok AW, Zhu TY, Kun EW, Leung PC, Li EK, Tam LS. SLE disease per se contributes to deterioration in bone mineral density, microstructure and bone strength. Lupus. 2013;22:1162-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Mak A, Lim JQ, Liu Y, Cheak AA, Ho RC. Significantly higher estimated 10-year probability of fracture in lupus patients with bone mineral density comparable to that of healthy individuals. Rheumatol Int. 2013;33:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Petri M. Musculoskeletal complications of systemic lupus erythematosus in the Hopkins Lupus Cohort: an update. Arthritis Care Res. 1995;8:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 113] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Lee C, Almagor O, Dunlop DD, Manzi S, Spies S, Chadha AB, Ramsey-Goldman R. Disease damage and low bone mineral density: an analysis of women with systemic lupus erythematosus ever and never receiving corticosteroids. Rheumatology (Oxford). 2006;45:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Mendoza-Pinto C, García-Carrasco M, Sandoval-Cruz H, Muñoz-Guarneros M, Escárcega RO, Jiménez-Hernández M, Munguía-Realpozo P, Sandoval-Cruz M, Delezé-Hinojosa M, López-Colombo A. Risk factors of vertebral fractures in women with systemic lupus erythematosus. Clin Rheumatol. 2009;28:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Borba VZ, Vieira JG, Kasamatsu T, Radominski SC, Sato EI, Lazaretti-Castro M. Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporos Int. 2009;20:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Huisman AM, White KP, Algra A, Harth M, Vieth R, Jacobs JW, Bijlsma JW, Bell DA. Vitamin D levels in women with systemic lupus erythematosus and fibromyalgia. J Rheumatol. 2001;28:2535-2539. [PubMed] |
| 35. | Toloza SM, Cole DE, Gladman DD, Ibañez D, Urowitz MB. Vitamin D insufficiency in a large female SLE cohort. Lupus. 2010;19:13-19. [PubMed] |
| 36. | Yeap SS, Othman AZ, Zain AA, Chan SP. Vitamin D levels: its relationship to bone mineral density response and disease activity in premenopausal Malaysian systemic lupus erythematosus patients on corticosteroids. Int J Rheum Dis. 2012;15:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Sumethkul K, Boonyaratavej S, Kitumnuaypong T, Angthararuk S, Cheewasat P, Manadee N, Sumethkul V. The predictive factors of low serum 25-hydroxyvitamin D and vitamin D deficiency in patients with systemic lupus erythematosus. Rheumatol Int. 2013;33:1461-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Mok CC. Vitamin D and systemic lupus erythematosus: an update. Expert Rev Clin Immunol. 2013;9:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Vacek TP, Kalani A, Voor MJ, Tyagi SC, Tyagi N. The role of homocysteine in bone remodeling. Clin Chem Lab Med. 2013;51:579-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 40. | Sabio JM, Vargas-Hitos JA, Martinez-Bordonado J, Navarrete-Navarrete N, Díaz-Chamorro A, Olvera-Porcel C, Zamora-Pasadas M, Jiménez-Alonso J. Relationship between homocysteine levels and hypertension in systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2014;66:1528-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Galeazzi M, Laghi Pasini F. Hyperhomocysteinemia: a cardiovascular risk factor in autoimmune diseases? Lupus. 2007;16:852-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Rhew EY, Lee C, Eksarko P, Dyer AR, Tily H, Spies S, Pope RM, Ramsey-Goldman R. Homocysteine, bone mineral density, and fracture risk over 2 years of followup in women with and without systemic lupus erythematosus. J Rheumatol. 2008;35:230-236. [PubMed] |
| 43. | Lee C, Almagor O, Dunlop DD, Chadha AB, Manzi S, Spies S, Ramsey-Goldman R. Association between African American race/ethnicity and low bone mineral density in women with systemic lupus erythematosus. Arthritis Rheum. 2007;57:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Lakshminarayanan S, Walsh S, Mohanraj M, Rothfield N. Factors associated with low bone mineral density in female patients with systemic lupus erythematosus. J Rheumatol. 2001;28:102-108. [PubMed] |
| 45. | Yee CS, Crabtree N, Skan J, Amft N, Bowman S, Situnayake D, Gordon C. Prevalence and predictors of fragility fractures in systemic lupus erythematosus. Ann Rheum Dis. 2005;64:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Chong HC, Chee SS, Goh EM, Chow SK, Yeap SS. Dietary calcium and bone mineral density in premenopausal women with systemic lupus erythematosus. Clin Rheumatol. 2007;26:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Almehed K, Forsblad d’Elia H, Kvist G, Ohlsson C, Carlsten H. Prevalence and risk factors of osteoporosis in female SLE patients-extended report. Rheumatology (Oxford). 2007;46:1185-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Formiga F, Moga I, Nolla JM, Navarro MA, Bonnin R, Roig-Escofet D. The association of dehydroepiandrosterone sulphate levels with bone mineral density in systemic lupus erythematosus. Clin Exp Rheumatol. 1997;15:387-392. [PubMed] |
| 49. | Boyanov M, Robeva R, Popivanov P. Bone mineral density changes in women with systemic lupus erythematosus. Clin Rheumatol. 2003;22:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Ruiz-Irastorza G, Egurbide MV, Olivares N, Martinez-Berriotxoa A, Aguirre C. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology (Oxford). 2008;47:920-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 51. | Lee CT, Ng HY, Lien YH, Lai LW, Wu MS, Lin CR, Chen HC. Effects of cyclosporine, tacrolimus and rapamycin on renal calcium transport and vitamin D metabolism. Am J Nephrol. 2011;34:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Grenet O, Bobadilla M, Chibout SD, Steiner S. Evidence for the impairment of the vitamin D activation pathway by cyclosporine A. Biochem Pharmacol. 2000;59:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Di Munno O, Mazzantini M, Delle Sedie A, Mosca M, Bombardieri S. Risk factors for osteoporosis in female patients with systemic lupus erythematosus. Lupus. 2004;13:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Deodhar AA, Woolf AD. Bone mass measurement and bone metabolism in rheumatoid arthritis: a review. Br J Rheumatol. 1996;35:309-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 153] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum. 2000;43:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 56. | Sinigaglia L, Nervetti A, Mela Q, Bianchi G, Del Puente A, Di Munno O, Frediani B, Cantatore F, Pellerito R, Bartolone S. A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. Italian Study Group on Bone Mass in Rheumatoid Arthritis. J Rheumatol. 2000;27:2582-2589. [PubMed] |
| 57. | Gough AK, Lilley J, Eyre S, Holder RL, Emery P. Generalised bone loss in patients with early rheumatoid arthritis. Lancet. 1994;344:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 405] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 58. | Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Reduced bone mineral density in male rheumatoid arthritis patients: frequencies and associations with demographic and disease variables in ninety-four patients in the Oslo County Rheumatoid Arthritis Register. Arthritis Rheum. 2000;43:2776-2784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Dequeker J, Maenaut K, Verwilghen J, Westhovens R. Osteoporosis in rheumatoid arthritis. Clin Exp Rheumatol. 1995;13 Suppl 12:S21-S26. [PubMed] |
| 60. | Dischereit G, Lange U. Osteoporosis - inflammatory effects on bone metabolism and fracture risk. Z Orthop Unfall. 2014;152:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Abdel Meguid MH, Hamad YH, Swilam RS, Barakat MS. Relation of interleukin-6 in rheumatoid arthritis patients to systemic bone loss and structural bone damage. Rheumatol Int. 2013;33:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Abramson SB, Amin A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford). 2002;41:972-980. [PubMed] |
| 63. | Maeda K, Takahashi N, Kobayashi Y. Roles of Wnt signals in bone resorption during physiological and pathological states. J Mol Med (Berl). 2013;91:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Haugeberg G, Helgetveit KB, Førre Ø, Garen T, Sommerseth H, Prøven A. Generalized bone loss in early rheumatoid arthritis patients followed for ten years in the biologic treatment era. BMC Musculoskelet Disord. 2014;15:289. [PubMed] |
| 65. | Kleyer A, Schett G. Arthritis and bone loss: a hen and egg story. Curr Opin Rheumatol. 2014;26:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 66. | Mustila A, Korpela M, Haapala AM, Kautiainen H, Laasonen L, Möttönen T, Leirisalo-Repo M, Ilonen J, Järvenpää S, Luukkainen R. Anti-citrullinated peptide antibodies and the progression of radiographic joint erosions in patients with early rheumatoid arthritis treated with FIN-RACo combination and single disease-modifying antirheumatic drug strategies. Clin Exp Rheumatol. 2011;29:500-505. [PubMed] |
| 67. | Kleyer A, Finzel S, Rech J, Manger B, Krieter M, Faustini F, Araujo E, Hueber AJ, Harre U, Engelke K. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis. 2014;73:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 68. | Syversen SW, Goll GL, van der Heijde D, Landewé R, Lie BA, Odegård S, Uhlig T, Gaarder PI, Kvien TK. Prediction of radiographic progression in rheumatoid arthritis and the role of antibodies against mutated citrullinated vimentin: results from a 10-year prospective study. Ann Rheum Dis. 2010;69:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 69. | Güler-Yüksel M, Bijsterbosch J, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Ronday HK, Peeters AJ, de Jonge-Bok JM, Breedveld FC, Dijkmans BA, Allaart CF. Bone mineral density in patients with recently diagnosed, active rheumatoid arthritis. Ann Rheum Dis. 2007;66:1508-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 70. | Laan RF, Buijs WC, Verbeek AL, Draad MP, Corstens FH, van de Putte LB, van Riel PL. Bone mineral density in patients with recent onset rheumatoid arthritis: influence of disease activity and functional capacity. Ann Rheum Dis. 1993;52:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Dao HH, Do QT, Sakamoto J. Bone mineral density and frequency of osteoporosis among Vietnamese women with early rheumatoid arthritis. Clin Rheumatol. 2011;30:1353-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Book C, Karlsson M, Akesson K, Jacobsson L. Disease activity and disability but probably not glucocorticoid treatment predicts loss in bone mineral density in women with early rheumatoid arthritis. Scand J Rheumatol. 2008;37:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Boers M, Verhoeven AC, Markusse HM, van de Laar MA, Westhovens R, van Denderen JC, van Zeben D, Dijkmans BA, Peeters AJ, Jacobs P. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997;350:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 698] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 74. | Haugeberg G, Morton S, Emery P, Conaghan PG. Effect of intra-articular corticosteroid injections and inflammation on periarticular and generalised bone loss in early rheumatoid arthritis. Ann Rheum Dis. 2011;70:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Haugeberg G, Strand A, Kvien TK, Kirwan JR. Reduced loss of hand bone density with prednisolone in early rheumatoid arthritis: results from a randomized placebo-controlled trial. Arch Intern Med. 2005;165:1293-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 76. | Ragab AH, Frech RS, Vietti TJ. Osteoporotic fractures secondary to methotrexate therapy of acute leukemia in remission. Cancer. 1970;25:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 77. | Preston SJ, Diamond T, Scott A, Laurent MR. Methotrexate osteopathy in rheumatic disease. Ann Rheum Dis. 1993;52:582-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Cranney AB, McKendry RJ, Wells GA, Ooi DS, Kanigsberg ND, Kraag GR, Smith CD. The effect of low dose methotrexate on bone density. J Rheumatol. 2001;28:2395-2399. [PubMed] |
| 79. | di Munno O, Mazzantini M, Sinigaglia L, Bianchi G, Minisola G, Muratore M, la Corte R, di Matteo L, Canesi B, Caminiti M. Effect of low dose methotrexate on bone density in women with rheumatoid arthritis: results from a multicenter cross-sectional study. J Rheumatol. 2004;31:1305-1309. [PubMed] |
| 80. | Kita K, Sierakowski S. The effect of low dose methotrexate treatment on bone mineral density in patients with rheumatoid arthritis. Pol Merkur Lekarski. 2002;12:122-125. [PubMed] |
| 81. | Buckley LM, Leib ES, Cartularo KS, Vacek PM, Cooper SM. Effects of low dose methotrexate on the bone mineral density of patients with rheumatoid arthritis. J Rheumatol. 1997;24:1489-1494. [PubMed] |
| 82. | Kobayashi Y, Ueyama S, Arai Y, Yoshida Y, Kaneda T, Sato T, Shin K, Kumegawa M, Hakeda Y. The active metabolite of leflunomide, A771726, inhibits both the generation of and the bone-resorbing activity of osteoclasts by acting directly on cells of the osteoclast lineage. J Bone Miner Metab. 2004;22:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 83. | Lee CK, Lee EY, Chung SM, Mun SH, Yoo B, Moon HB. Effects of disease-modifying antirheumatic drugs and antiinflammatory cytokines on human osteoclastogenesis through interaction with receptor activator of nuclear factor kappaB, osteoprotegerin, and receptor activator of nuclear factor kappaB ligand. Arthritis Rheum. 2004;50:3831-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 84. | Korczowska I, Lacki JK, Hrycaj P. Influence of infliximab on cytokines network and markers of bone remodeling in rheumatoid arthritis patients. Yonsei Med J. 2013;54:183-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Musacchio E, Valvason C, Botsios C, Ostuni F, Furlan A, Ramonda R, Modesti V, Sartori L, Punzi L. The tumor necrosis factor-{alpha}-blocking agent infliximab inhibits interleukin 1beta (IL-1beta) and IL-6 gene expression in human osteoblastic cells. J Rheumatol. 2009;36:1575-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Krieckaert CL, Nurmohamed MT, Wolbink G, Lems WF. Changes in bone mineral density during long-term treatment with adalimumab in patients with rheumatoid arthritis: a cohort study. Rheumatology (Oxford). 2013;52:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 87. | Hoff M, Kvien TK, Kälvesten J, Elden A, Haugeberg G. Adalimumab therapy reduces hand bone loss in early rheumatoid arthritis: explorative analyses from the PREMIER study. Ann Rheum Dis. 2009;68:1171-1176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Wijbrandts CA, Klaasen R, Dijkgraaf MG, Gerlag DM, van Eck-Smit BL, Tak PP. Bone mineral density in rheumatoid arthritis patients 1 year after adalimumab therapy: arrest of bone loss. Ann Rheum Dis. 2009;68:373-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 89. | Tanida A, Kishimoto Y, Okano T, Hagino H. Etanercept Promotes Bone Formation via Suppression of Dickkopf-1 Expression in Rats with Collagen-Induced Arthritis. Yonago Acta Med. 2013;56:13-19. [PubMed] |
| 90. | Lisbona M, Maymó J, Solano A, Almirall M, Navallas M, Ares J, Carbonell J. Repair of erosions in patients with rheumatoid arthritis treated with etanercept: magnetic resonance imaging findings after 1 year of follow-up. Scand J Rheumatol. 2013;42:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Ozaki T, Hashizume K, Nakahara R, Nishida K. Radiographic remodeling of the shoulder joint in a patient with rheumatoid arthritis after 4 years of treatment with etanercept. Mod Rheumatol. 2012;22:635-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 92. | Hein G, Eidner T, Oelzner P, Rose M, Wilke A, Wolf G, Franke S. Influence of Rituximab on markers of bone remodeling in patients with rheumatoid arthritis: a prospective open-label pilot study. Rheumatol Int. 2011;31:269-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Kume K, Amano K, Yamada S, Kanazawa T, Ohta H, Hatta K, Amano K, Kuwaba N. The effect of tocilizumab on bone mineral density in patients with methotrexate-resistant active rheumatoid arthritis. Rheumatology (Oxford). 2014;53:900-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 94. | Karsdal MA, Schett G, Emery P, Harari O, Byrjalsen I, Kenwright A, Bay-Jensen AC, Platt A. IL-6 receptor inhibition positively modulates bone balance in rheumatoid arthritis patients with an inadequate response to anti-tumor necrosis factor therapy: biochemical marker analysis of bone metabolism in the tocilizumab RADIATE study (NCT00106522). Semin Arthritis Rheum. 2012;42:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 95. | Vis M, Güler-Yüksel M, Lems WF. Can bone loss in rheumatoid arthritis be prevented? Osteoporos Int. 2013;24:2541-2553. [PubMed] |
| 96. | Oelzner P, Schwabe A, Lehmann G, Eidner T, Franke S, Wolf G, Hein G. Significance of risk factors for osteoporosis is dependent on gender and menopause in rheumatoid arthritis. Rheumatol Int. 2008;28:1143-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Tourinho TF, Stein A, Castro JA, Brenol JC. Rheumatoid arthritis: evidence for bone loss in premenopausal women. J Rheumatol. 2005;32:1020-1025. [PubMed] |
| 98. | Ørstavik RE, Haugeberg G, Uhlig T, Mowinckel P, Falch JA, Halse JI, Kvien TK. Self reported non-vertebral fractures in rheumatoid arthritis and population based controls: incidence and relationship with bone mineral density and clinical variables. Ann Rheum Dis. 2004;63:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Lodder MC, de Jong Z, Kostense PJ, Molenaar ET, Staal K, Voskuyl AE, Hazes JM, Dijkmans BA, Lems WF. Bone mineral density in patients with rheumatoid arthritis: relation between disease severity and low bone mineral density. Ann Rheum Dis. 2004;63:1576-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 100. | Ghozlani I, Ghazi M, Nouijai A, Mounach A, Rezqi A, Achemlal L, Bezza A, El Maghraoui A. Prevalence and risk factors of osteoporosis and vertebral fractures in patients with ankylosing spondylitis. Bone. 2009;44:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 101. | van der Weijden MA, Claushuis TA, Nazari T, Lems WF, Dijkmans BA, van der Horst-Bruinsma IE. High prevalence of low bone mineral density in patients within 10 years of onset of ankylosing spondylitis: a systematic review. Clin Rheumatol. 2012;31:1529-1535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 102. | Bronson WD, Walker SE, Hillman LS, Keisler D, Hoyt T, Allen SH. Bone mineral density and biochemical markers of bone metabolism in ankylosing spondylitis. J Rheumatol. 1998;25:929-935. [PubMed] |
| 103. | Will R, Palmer R, Bhalla AK, Ring F, Calin A. Osteoporosis in early ankylosing spondylitis: a primary pathological event? Lancet. 1989;2:1483-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 119] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 104. | Jun JB, Joo KB, Her MY, Kim TH, Bae SC, Yoo DH, Kim SK. Femoral bone mineral density is associated with vertebral fractures in patients with ankylosing spondylitis: a cross-sectional study. J Rheumatol. 2006;33:1637-1641. [PubMed] |
| 105. | Juanola X, Mateo L, Nolla JM, Roig-Vilaseca D, Campoy E, Roig-Escofet D. Bone mineral density in women with ankylosing spondylitis. J Rheumatol. 2000;27:1028-1031. [PubMed] |
| 106. | Speden DJ, Calin AI, Ring FJ, Bhalla AK. Bone mineral density, calcaneal ultrasound, and bone turnover markers in women with ankylosing spondylitis. J Rheumatol. 2002;29:516-521. [PubMed] |
| 107. | Magrey M, Khan MA. Osteoporosis in ankylosing spondylitis. Curr Rheumatol Rep. 2010;12:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 108. | Franck H, Meurer T, Hofbauer LC. Evaluation of bone mineral density, hormones, biochemical markers of bone metabolism, and osteoprotegerin serum levels in patients with ankylosing spondylitis. J Rheumatol. 2004;31:2236-2241. [PubMed] |
| 109. | Ulu MA, Batmaz İ, Dilek B, Çevik R. Prevalence of osteoporosis and vertebral fractures and related factors in patients with ankylosing spondylitis. Chin Med J (Engl). 2014;127:2740-2747. [PubMed] |
| 110. | Klingberg E, Lorentzon M, Mellström D, Geijer M, Göthlin J, Hilme E, Hedberg M, Carlsten H, Forsblad-d’Elia H. Osteoporosis in ankylosing spondylitis - prevalence, risk factors and methods of assessment. Arthritis Res Ther. 2012;14:R108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 111. | Obermayer-Pietsch BM, Lange U, Tauber G, Frühauf G, Fahrleitner A, Dobnig H, Hermann J, Aglas F, Teichmann J, Neeck G. Vitamin D receptor initiation codon polymorphism, bone density and inflammatory activity of patients with ankylosing spondylitis. Osteoporos Int. 2003;14:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 112. | Aydin T, Karacan I, Demir SE, Sahin Z. Bone loss in males with ankylosing spondylitis: its relation to sex hormone levels. Clin Endocrinol (Oxf). 2005;63:467-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 113. | Lange U, Jung O, Teichmann J, Neeck G. Relationship between disease activity and serum levels of vitamin D metabolites and parathyroid hormone in ankylosing spondylitis. Osteoporos Int. 2001;12:1031-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 114. | Morton DJ, Barrett-Connor EL, Schneider DL. Nonsteroidal anti-inflammatory drugs and bone mineral density in older women: the Rancho Bernardo study. J Bone Miner Res. 1998;13:1924-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 115. | Kaya A, Ozgocmen S, Kamanli A, Ardicoglu O. Bone loss in ankylosing spondylitis: does syndesmophyte formation have an influence on bone density changes? Med Princ Pract. 2009;18:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 116. | Visvanathan S, van der Heijde D, Deodhar A, Wagner C, Baker DG, Han J, Braun J. Effects of infliximab on markers of inflammation and bone turnover and associations with bone mineral density in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68:175-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 117. | Briot K, Garnero P, Le Henanff A, Dougados M, Roux C. Body weight, body composition, and bone turnover changes in patients with spondyloarthropathy receiving anti-tumour necrosis factor {alpha} treatment. Ann Rheum Dis. 2005;64:1137-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 118. | Allali F, Breban M, Porcher R, Maillefert JF, Dougados M, Roux C. Increase in bone mineral density of patients with spondyloarthropathy treated with anti-tumour necrosis factor alpha. Ann Rheum Dis. 2003;62:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 119. | Brandt J, Haibel H, Cornely D, Golder W, Gonzalez J, Reddig J, Thriene W, Sieper J, Braun J. Successful treatment of active ankylosing spondylitis with the anti-tumor necrosis factor alpha monoclonal antibody infliximab. Arthritis Rheum. 2000;43:1346-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 120. | Briot K, Gossec L, Kolta S, Dougados M, Roux C. Prospective assessment of body weight, body composition, and bone density changes in patients with spondyloarthropathy receiving anti-tumor necrosis factor-alpha treatment. J Rheumatol. 2008;35:855-861. [PubMed] |
| 121. | Haroon NN, Sriganthan J, Al Ghanim N, Inman RD, Cheung AM. Effect of TNF-alpha inhibitor treatment on bone mineral density in patients with ankylosing spondylitis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 122. | Harrison BJ, Hutchinson CE, Adams J, Bruce IN, Herrick AL. Assessing periarticular bone mineral density in patients with early psoriatic arthritis or rheumatoid arthritis. Ann Rheum Dis. 2002;61:1007-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 123. | Husni ME, Mease PJ. Managing comorbid disease in patients with psoriatic arthritis. Curr Rheumatol Rep. 2010;12:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 124. | Dheda K, Cassim B, Patel N, Mody GM. A comparison of bone mineral density in Indians with psoriatic polyarthritis and healthy Indian volunteers. Clin Rheumatol. 2004;23:89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 125. | Grisar J, Bernecker PM, Aringer M, Redlich K, Sedlak M, Wolozcszuk W, Spitzauer S, Grampp S, Kainberger F, Ebner W. Ankylosing spondylitis, psoriatic arthritis, and reactive arthritis show increased bone resorption, but differ with regard to bone formation. J Rheumatol. 2002;29:1430-1436. [PubMed] |
| 126. | Nolla JM, Fiter J, Rozadilla A, Gomez-Vaquero C, Mateo L, Rodriguez-Moreno J, Roig-Escofet D. Bone mineral density in patients with peripheral psoriatic arthritis. Rev Rhum Engl Ed. 1999;66:457-461. [PubMed] |
| 127. | Hein G, Abendroth K, Müller A, Wessel G. Studies on psoriatic osteopathy. Clin Rheumatol. 1991;10:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 128. | Grazio S, Cvijetić S, Vlak T, Grubišić F, Matijević V, Nemčić T, Punda M, Kusić Z. Osteoporosis in psoriatic arthritis: is there any? Wien Klin Wochenschr. 2011;123:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 129. | Pedreira PG, Pinheiro MM, Szejnfeld VL. Bone mineral density and body composition in postmenopausal women with psoriasis and psoriatic arthritis. Arthritis Res Ther. 2011;13:R16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 130. | Borman P, Babaoğlu S, Gur G, Bingol S, Bodur H. Bone mineral density and bone turnover in patients with psoriatic arthritis. Clin Rheumatol. 2008;27:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 131. | Frediani B, Allegri A, Falsetti P, Storri L, Bisogno S, Baldi F, Filipponi P, Marcolongo R. Bone mineral density in patients with psoriatic arthritis. J Rheumatol. 2001;28:138-143. [PubMed] |
| 132. | Loucks J, Pope JE. Osteoporosis in scleroderma. Semin Arthritis Rheum. 2005;34:678-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 133. | Omair MA, Pagnoux C, McDonald-Blumer H, Johnson SR. Low bone density in systemic sclerosis. A systematic review. J Rheumatol. 2013;40:1881-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 134. | Mok CC, Chan PT, Chan KL, Ma KM. Prevalence and risk factors of low bone mineral density in Chinese patients with systemic sclerosis: a case-control study. Rheumatology (Oxford). 2013;52:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 135. | Atteritano M, Sorbara S, Bagnato G, Miceli G, Sangari D, Morgante S, Visalli E, Bagnato G. Bone mineral density, bone turnover markers and fractures in patients with systemic sclerosis: a case control study. PLoS One. 2013;8:e66991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 136. | Rios-Fernández R, Callejas-Rubio JL, Fernández-Roldán C, Simeón-Aznar CP, García-Hernández F, Castillo-García MJ, Fonollosa Pla V, Barnosi Marín AC, González-Gay MÁ, Ortego-Centeno N. Bone mass and vitamin D in patients with systemic sclerosis from two Spanish regions. Clin Exp Rheumatol. 2012;30:905-911. [PubMed] |
| 137. | Avouac J, Koumakis E, Toth E, Meunier M, Maury E, Kahan A, Cormier C, Allanore Y. Increased risk of osteoporosis and fracture in women with systemic sclerosis: a comparative study with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1871-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 138. | de Andrade DC, de Magalhães Souza SC, de Carvalho JF, Takayama L, Borges CT, Aldrighi JM, Pereira RM. High frequency of osteoporosis and fractures in women with dermatomyositis/polymyositis. Rheumatol Int. 2012;32:1549-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 139. | Alsufyani KA, Ortiz-Alvarez O, Cabral DA, Tucker LB, Petty RE, Nadel H, Malleson PN. Bone mineral density in children and adolescents with systemic lupus erythematosus, juvenile dermatomyositis, and systemic vasculitis: relationship to disease duration, cumulative corticosteroid dose, calcium intake, and exercise. J Rheumatol. 2005;32:729-733. [PubMed] |
| 140. | Castro TC, Terreri MT, Szejnfeld VL, Len C, Fonseca AS, Hilário MO. Bone mineral density of Brazilian girls with juvenile dermatomyositis. Braz J Med Biol Res. 2005;38:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 141. | Santiago RA, Silva CA, Caparbo VF, Sallum AM, Pereira RM. Bone mineral apparent density in juvenile dermatomyositis: the role of lean body mass and glucocorticoid use. Scand J Rheumatol. 2008;37:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 142. | Rouster-Stevens KA, Langman CB, Price HE, Seshadri R, Shore RM, Abbott K, Pachman LM. RANKL: osteoprotegerin ratio and bone mineral density in children with untreated juvenile dermatomyositis. Arthritis Rheum. 2007;56:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 143. | Kirnap M, Calis M, Kaya N, Muhtaroglu S. Is the Behcet’s disease a risk factor for osteoporosis and is relation to cytokines? Bratisl Lek Listy. 2010;111:340-344. [PubMed] |
| 144. | Tekin NS, Ozdolap S, Sarikaya S, Esturk E, Gumustas S. Bone mineral density and bone turnover markers of patients with Behçet’s disease. J Eur Acad Dermatol Venereol. 2007;21:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 145. | Bicer A, Tursen U, Kaya TI, Ozer C, Camdeviren H, Ikizoglu G, Erdogan C. Bone mineral density in patients with Behçet’s disease. Rheumatol Int. 2004;24:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |